Analysis of Resin-Based Dental Materials’ Composition Depending on Their Clinical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Studied
2.2. Search Strategy
2.3. Data Analysis
3. Results
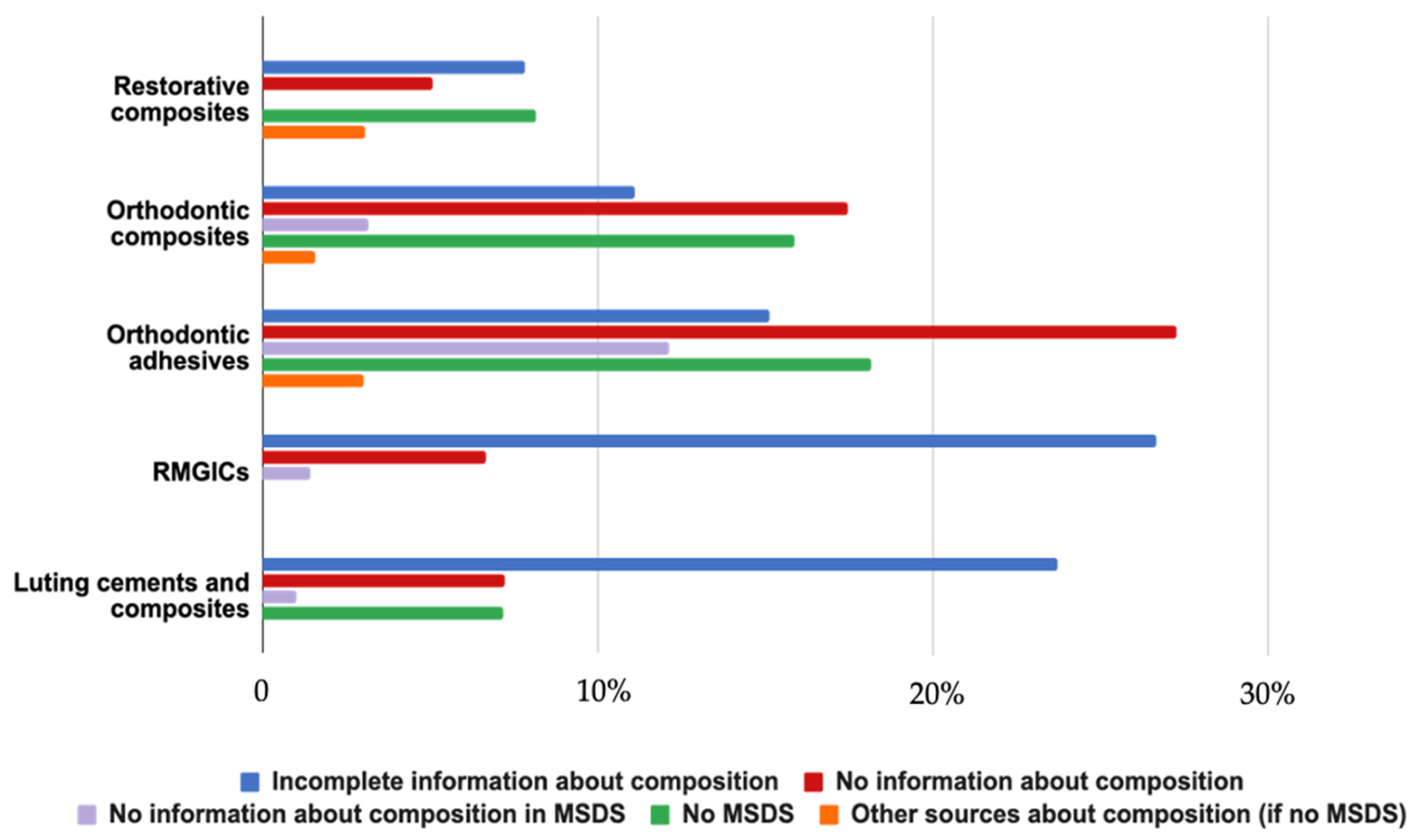
3.1. Materials Identified and Source of Information
- -
- A total of 305 restorative composite resins;
- -
- A total of 49 core build-up composite resins;
- -
- A total of 66 orthodontic composite resins;
- -
- A total of 142 restorative adhesive systems;
- -
- A total of 33 orthodontic adhesive systems;
- -
- A total of 32 sealants;
- -
- A total of 16 restorative resin-modified glass ionomer cements;
- -
- A total of 100 luting resin-modified glass ionomer cements and composites.
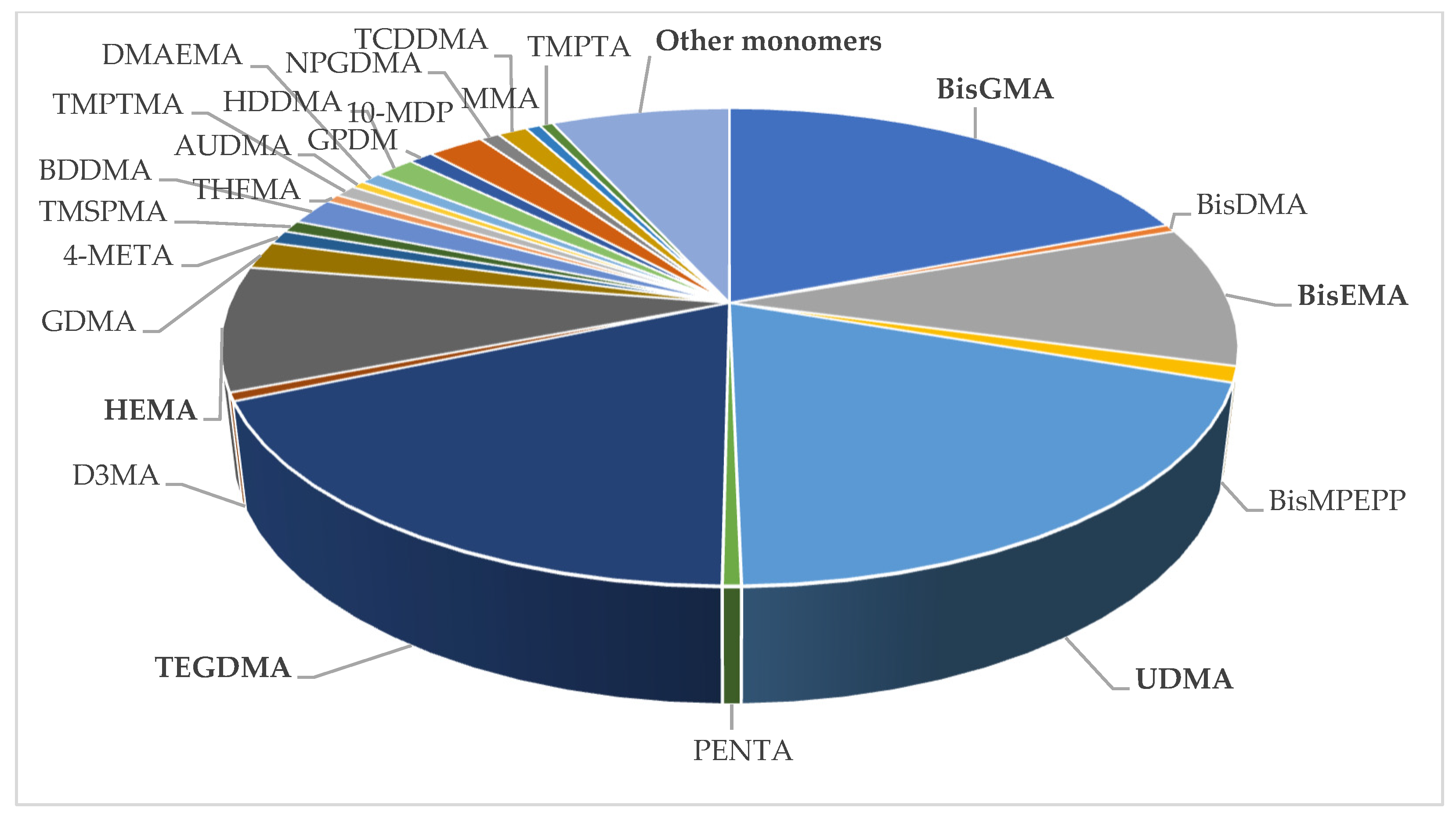
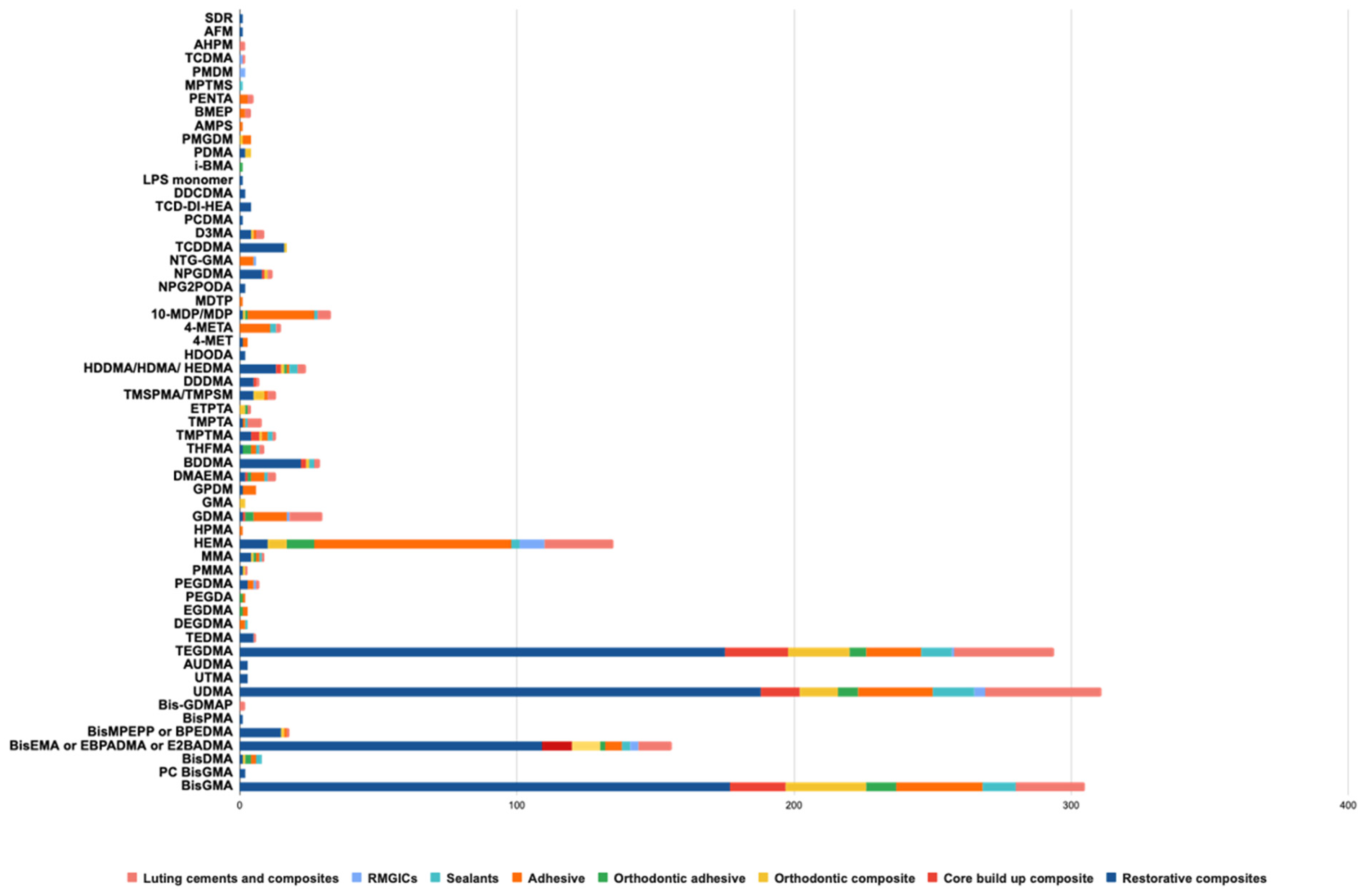
3.2. Monomers Identified
4. Discussion
4.1. Source of Information
4.2. Concerns about Bisphenol A-Derivative Monomers
4.3. Other Types of Monomers
4.4. Limitations of the Study and Perspectives
4.5. Clinical Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Naaman, R.; El-Housseiny, A.A.; Alamoudi, N. The use of pit and fissure sealants—A literature review. Dent. J. 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierczyk, D.; Małkiewicz, K. Orthodontic adhesive systems–over half a century of research and experience. J. Stomatol. 2019, 2, 179–183. [Google Scholar] [CrossRef]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental luting cements: An updated comprehensive review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Fischer, N.G.; Nojiri, K.; Nagura, Y.; Takamizawa, T.; Latta, M.A.; Miazaki, M. Wear of resin composites: Current insights into underlying mechanisms, evaluation methods and influential factors. Jpn. Dent. Sci. Rev. 2018, 54, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Raszewski, Z.; Alamoush, R.A.; Chojnacka, K.; Mikulewicz, M.; Haider, J. Influence of Storing Composite Filling Materials in a Low-pH Artificial Saliva on Their Mechanical Properties—An In Vitro Study. J. Funct. Biomater. 2023, 14, 328. [Google Scholar] [CrossRef]
- Oilo, G. Biodegradation of dental composites/glass-ionomer cements. Adv. Dent. Res. 1992, 6, 50–54. [Google Scholar] [CrossRef]
- Shahi, S.M.; Özcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Pongprueksa, P.; Munck, J.D.; Duca, R.C.; Poels, K.; Covaci, A.; Hoet, P.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Monomer elution in relation to degree of conversion for different types of composites. J. Dent. 2015, 43, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Romo-Huerta, M.J.; Cervantes-Urenda, A.D.R.; Velasco-Neri, J.; Torres-Bugarín, O.; Valdivia, A.D.C.M. Genotoxicity associated with residual monomers in restorative dentistry: A systematic review. Oral. Health Prev. Dent. 2021, 19, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Chopra, R.; Sachdev, V. Allergic reactions to dental materials-a systematic review. J. Clin. Diagn. Res. 2015, 9, ZE04–ZE09. [Google Scholar] [CrossRef] [PubMed]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Scientific Opinion on the re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, 6857392. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef]
- De Angelis, F.; Sarteur, N.; Buonvivere, M.; Vadini, M.; Šteffl, M.; D’Arcangelo, C. Meta-analytical analysis on components released from resin-based dental materials. Clin. Oral Investig. 2022, 26, 6015–6041. [Google Scholar] [CrossRef]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A as degradation product of monomers used in resin-based dental materials. Dent. Mater. 2021, 37, 1020–1029. [Google Scholar] [CrossRef]
- Lopes-Rocha, L.; Ribeiro-Gonçalves, L.; Henriques, B.; Özcan, M.; Tiritan, M.E.; Souza, J.C.M. An integrative review on the toxicity of Bisphenol A (BPA) released from resin composites used in dentistry. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1942–1952. [Google Scholar] [CrossRef]
- Dursun, E.; Fron-Chabouis, H.; Attal, J.P.; Raskin, A. Bisphenol A release: Survey of the composition of dental composite resins. Open Dent. J. 2016, 10, 446–453. [Google Scholar] [CrossRef]
- Małkiewicz, K.; Turło, J.; Marciniuk-Kluska, A.; Grzech-Leśniak, K.; Gąsior, M.; Kluska, M. Release of bisphenol A and its derivatives from orthodontic adhesive systems available on the European market as a potential health risk factor. Ann. Agric. Environ. Med. 2015, 22, 172–177. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Jedeon, K.; De la Dure-Molla, M.; Brookes, S.J.; Loiodice, S.; Marciano, C.; Kirkham, K.; Canivenc-Lavier, M.-C.; Boudalia, S.; Bergès, R.; Harada, H.; et al. Enamel defects reflect perinatal exposure to bisphenol A. Am. J. Pathol. 2013, 183, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Maserejian, N.N.; Trachtenberg, F.L.; Wheaton, O.B.; Calafat, A.M.; Ranganathan, G.; Kim, H.-Y.; Hauser, R. Changes in urinary bisphenol A concentrations associated with placement of dental composite restorations in children and adolescents. J. Am. Dent. Assoc. 2016, 147, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.L.L.; Lygre, G.B.; Jönsson, B.A.G.; Lindh, C.H.; Björkman, L. Bisphenol A concentration in human saliva related to dental polymer-based fillings. Clin. Oral Investig. 2017, 21, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Hampe, T.; Wiessner, A.; Frauendorf, H.; Alhussein, M.; Karlovsky, P.; Bürgers, R.; Krohn, S. Monomer Release from Dental Resins: The current status on study setup, detection and quantification for in vitro testing. Polymers 2022, 14, 1790. [Google Scholar] [CrossRef]
- American Dental Association. Bisphenol A Released from Resin Based Dental Sealants. ADA Prof. Prod. Rev. 2016, 11, 1–6. [Google Scholar]
- Jung, Y.H.; Wanga, H.L.V.; Ruiza, D.; Bixlera, B.J.; Linsenbauma, H.; Xianga, J.F.; Forestier, S.; Shafik, A.M.; Jin, P.; Corces, V.G. Recruitment of CTCF to an Fto enhancer is responsible for transgenerational inheritance of BPA-induced obesity. PNAS 2022, 119, e2214988119. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Gayrard, V.; Lacroix, M.Z.; Collet, S.H.; Viguié, C.; Bousquet-Melou, A.; Toutain, P.L.; Picard-Hagen, N. High bioavailability of bisphenol A from sublingual exposure. Environ. Health Perspect. 2013, 21, 951–956. [Google Scholar] [CrossRef]
- Barišić, M.L.; Sarajlija, H.; Klarić, E.; Knežević, A.; Sabol, I.; Pandurić, V. Detection of leachable components from conventional and dental bulk-fill resin composites (high and low viscosity) using liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Polymers 2023, 15, 627. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chiang, C.Y.; Chiang, Y.W.; Lee, M.W.; Lee, C.Y.; Chen, H.Y.; Lin, H.-W.; Kuan, Y.-H. Toxic effects of urethane dimethacrylate on macrophages through caspase activation, mitochondrial dysfunction, and reactive oxygen species generation. Polymers 2020, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, M.C.; Wang, H.H.; Huang, G.F.; Lee, Y.L.; Wang, Y.L.; Chan, C.-P.; Yeung, S.-Y.; Tseng, S.-K.; Jeng, J.-H. Urethane dimethacrylate induces cytotoxicity and regulates cyclooxygenase 2; hemeoxygenase and carboxylesterase expression in human dental pulp cells. Acta Biomater. 2014, 10, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska-Jarosinska, M.; Poplawski, T.; Chojnacki, C.J.; Pawlowska, E.; Krupa, R.; Szczepanska, J.; Blasiak, J. Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol. Biol. Rep. 2011, 38, 4603. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, B.W.; Simon-Santamaria, J.; Örtengren, U.; Jensen, E.; Bruun, J.A.; Michelsen, V.B.; Sørensen, K.K. Dose- and time-dependent effects of triethylene glycol dimethacrylate on the proteome of human THP-1 monocytes. Eur. J. Oral. Sci. 2018, 126, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Ahn, Y.J.; Seo, H.; Seo, A.; Lee, H.; Lee, S.H.; Shon, W.-J.; Park, Y. Comprehensive assessment of the estrogenic activity of resin composites. Chemosphere 2023, 343, 140104. [Google Scholar] [CrossRef]
- Gallorini, M.; Cataldi, A.; di Giacomo, V. HEMA-induced cytotoxicity: Oxidative stress, genotoxicity and apoptosis. Int. Endod. J. 2014, 47, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.S.; Hadis, M.A.; Palin, W.M. Alternative co-initiators for photocurable dental resins: Polymerisation, quantum yield of conversion and cytotoxicity. Dent. Mater. 2022, 38, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.A.; Schuster, G.S.; Rueggeberg, F.A.; Tamareselvy, K.; Knoernschild, K.L. Responses of oral epithelial cells to dental resin components. J. Biomater. Sci. Polym. 1996, 7, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Komurcuoglu, E.; Olmez, S.; Vural, N. Evaluation of residual monomer elimination methods in three different fissure sealants in vitro. J. Oral Rehabil. 2005, 32, 116–121. [Google Scholar] [CrossRef]
- Tkáčiková, S.; Sabo, J. Release of monomers from dental composite materials into saliva and the possibility of reducing the toxic risk for the patient. Medicina 2023, 59, 1204. [Google Scholar] [CrossRef]
- Hampe, T.; Liersch, J.; Wiechens, B.; Wassmann, T.; Schubert, A.; Alhussein, M.; Bürgers, R.; Krohn, S. A pilot study on monomer and bisphenol A (BPA) release from UDMA-based and conventional indirect veneering composites. Polymers 2022, 14, 4580. [Google Scholar] [CrossRef] [PubMed]
- Whyatt, R.M.; Rundle, A.G.; Perzanowski, M.S.; Just, A.C.; Donohue, K.M.; Calafat, A.M.; Hoepner, L.; Perera, F.P.; Miller, R.L. Prenatal phthalate and early childhood bisphenol A exposures increase asthma risk in inner-city children. J. Allergy Clin. Immunol. 2014, 134, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Reina-Pérez, I.; Olivas-Martínez, A.; Mustieles, V.; Ruiz-Ojeda, F.J.; Molina-Molina, J.M.; Olea, N.; Fernández, M.F. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem. Toxicol. 2021, 152, 112216. [Google Scholar] [CrossRef] [PubMed]

| Manufacturer | Restorative Composite Resins | Core Build-Up Composite Resins | Orthodontic Composite Resins | Restorative Adhesive Systems | Orthodontic Adhesive Systems | Sealants | Restorative RMGICs | Luting Cements and Composites |
|---|---|---|---|---|---|---|---|---|
| Apol | 5 | / | / | 1 | / | / | / | / |
| American orthodontics | / | / | 1 | / | 1 | / | / | / |
| Bisico | 12 | 3 | / | 6 | / | 1 | / | 4 |
| BJM | / | / | 2 | / | 1 | / | / | / |
| Cavex | 3 | / | / | 2 | / | / | / | / |
| Coltene | 11 | 1 | / | 5 | / | / | / | 3 |
| Cosmedent | 8 | 1 | / | / | / | 2 | / | 2 |
| CyberTech | 2 | 1 | / | / | / | / | / | / |
| Dentaurum | / | / | 3 | / | / | / | / | / |
| DenMat | 2 | 1 | / | 6 | / | 1 | 2 | / |
| Dental Technologies | 5 | 2 | 2 | 4 | 1 | 2 | / | 2 |
| Dentsply | 14 | 2 | / | 4 | / | 1 | / | 3 |
| DMG | 6 | 2 | / | 4 | / | / | / | 2 |
| Exotec | 2 | / | / | / | / | / | / | / |
| FGM | 5 | 1 | / | / | / | / | / | 2 |
| GC | 20 | 1 | 2 | 3 | / | / | 2 | 10 |
| Gestenco | / | / | 1 | / | / | / | / | / |
| Henry Schein | 7 | / | / | 2 | / | 1 | / | 1 |
| Itena | 2 | 1 | / | 2 | / | 1 | / | 3 |
| Ivoclar-Vivadent | 15 | 2 | 1 | 7 | / | 4 | / | 4 |
| Jeneric Pentron | 5 | 2 | / | 2 | / | / | / | / |
| Kerr | 11 | / | / | 5 | / | / | / | 4 |
| Kettenbach Dental | 2 | / | / | / | / | / | / | 1 |
| Kulzer | 15 | / | / | 3 | / | / | / | / |
| Kuraray | 6 | 3 | / | / | / | 1 | / | 1 |
| 3M | 12 | / | 6 | 8 | 2 | 2 | 5 | 3 |
| Micerium | 5 | / | / | / | / | 1 | / | 4 |
| Ormco | / | / | 4 | / | 1 | / | / | / |
| Ortho Technology | / | / | 3 | / | / | / | / | / |
| Parkell | 2 | 2 | / | 2 | / | / | / | 2 |
| Reliance | / | / | 13 | / | 10 | / | / | / |
| RMO | / | / | 3 | / | 1 | / | / | / |
| R and S | 3 | / | / | 1 | / | / | / | / |
| Saremco | 6 | / | / | 4 | / | 1 | / | 1 |
| Schütz Dental | 8 | / | / | 1 | / | / | / | 1 |
| Septodont | 7 | / | / | 1 | / | / | / | / |
| Shofu | 10 | / | / | / | / | 1 | / | 2 |
| SDI | 11 | / | / | / | / | / | / | / |
| Sun Medical | 3 | / | / | / | / | / | / | 1 |
| Tokuyama | 11 | / | / | 3 | / | / | / | 2 |
| TP Orthodontics | / | / | 1 | / | 1 | / | / | / |
| Ultradent | 3 | 1 | 2 | 3 | / | 2 | / | 4 |
| Vericom | / | / | 1 | / | 1 | / | / | / |
| Voco | 27 | 3 | 2 | 2 | / | 4 | 1 | 5 |
| Total | 266 | 29 | 47 | 81 | 19 | 24 | 10 | 67 |
| Terms Mentioned in MSDS (for Materials with Incomplete Compositions) | Number of Materials Concerned |
|---|---|
| Methacrylates | 18 |
| Hydrophobic aromatic dimethacrylate | 10 |
| Dimethacrylates | 8 |
| Uncured methacrylate ester monomers | 7 |
| Blend of multifunctional methacrylates | 6 |
| Hydrophobic aliphatic dimethacrylate | 6 |
| Acid adhesive monomer | 6 |
| Hydrophilic aliphatic methacrylate | 6 |
| Acidic monomer | 6 |
| Hydrophilic dimethacrylates | 4 |
| Acrylic monomers | 4 |
| Phosphoric acid monomer | 4 |
| Uncured acrylate ester monomers | 4 |
| Trade secret | 3 |
| Other | 3 |
| Phosphonic acid type monomer | 3 |
| Carboxilic acid type monomer | 3 |
| Hydrophilic amide monomer | 3 |
| Dimethacrylate cross linker | 3 |
| Copolymer of acrylic and itaconic acid | 3 |
| Aliphatic dimethacrylate | 2 |
| Uncured methacrylate resin mixture | 2 |
| Phosphatic methacrylate monomer | 2 |
| Mixture of uncured methacrylate ester monomers | 2 |
| Acidic and hydrophilic methacrylic monomers | 2 |
| Acrylates | 2 |
| Hydrophilic acidic monomer | 2 |
| Other bifunctional methacrylate monomers | 1 |
| Aromatic dimethacrylate | 1 |
| Aliphatic trimethacrylate | 1 |
| Matrix of methacrylic monomers | 1 |
| Methacrylate ester monomer | 1 |
| Polymerizable dimethacrylate resin | 1 |
| Polymerizable trimethacrylate resin | 1 |
| Citric acid methacrylate oligomer | 1 |
| Multifunctional monomers | 1 |
| Hydrophobic aromatic methacrylate | 1 |
| Proprietary methacrylate | 1 |
| Mixture of methacrylate monomers | 1 |
| Monomer Abbreviation | Monomer Name and/or Chemical Name |
|---|---|
| BisGMA | Bisphenol A Glycidyl Methacrylate or 2,2-bis[4-(3-methacryloxy-2-hydroxypropoxy)phenyl]propane |
| PC BisGMA | Polycarbonate-modified bis-GMA |
| BisDMA | Bisphenol A Dimethacrylate or 2,2-bis-(4-(methacryloxy) phenyl) propane |
| BisEMA or EBPADMA or E2BADMA | Ethoxylated Bisphenol A glycol dimethacrylate |
| BisMPEPP or BPEDMA | Bisphenol A polyethoxy dimethacrylate or 2,2-bis(4-methacryloxy poly-ethoxyphenyl)propane |
| BisPMA | Propoxylated Bisphenol A-Dimethacrylate |
| BisGDMAP | Bis(Glyceryl Dimethacrylate) Phosphate or 2-methacryl acid phosphinicobis (oxy-2,1,3-propanetriyl) ester |
| UDMA/UDMA modified | Urethane dimethacrylate or 1,6-di(methacryloyloxyethylcarbamoyl)-3,3,5-trimethylhexane |
| UTMA | Urethane trimethacrylate |
| AUDMA | Aromatic urethane dimethacrylate |
| TEGDMA | Triethylene glycol dimethacrylate |
| TEDMA | Triethylene dimethacrylate |
| DEGDMA | Diethylene glycol dimethacrylate |
| EGDMA | Ethylene glycol dimethacrylate |
| PEGDMA | Polyethylene glycol dimethacrylate |
| PEGDA | Polyethylene glycol diacrylate |
| PMMA | Polymethyl methacrylate |
| MMA | Methyl methacrylate |
| HEMA | Hydroxyethyl methacrylate or -Propenoic acid, 2-methyl-, 2-hydroxyethyl ester or 2-hydroxyethyl methacrylate |
| HPMA | 2-Hydroxypropyl methacrylate |
| GDMA | Glycerol dimethacrylate |
| GMA | Glycidyl methacrylate |
| GPDM | Gycerol phosphate dimethacrylate |
| DMAEMA | 2-(Dimethylamino)ethyl methacrylate or Methacrylic acid 2-(dimethylamino)ethyl ester |
| BDDMA | 1,4-Butanediol Dimethacrylate or Tetramethylene dimethacrylate |
| THFMA | Tetrahydrofurfuryl methacrylate or 2-Propenoic acid, 2-methyl-, (tetrahydro-2-furanyl)methyl ester |
| TMPTMA | Trimethylolpropane trimethacrylate |
| TMPTA | Triméthyllolpropane triacrylate or 2-propenoic acid, 2-ethyl-2-((1-oxo-2-propenyl)oxy)methyl)-1,3-propanediyl ester |
| TMPSM or TMSPMA | 3-(Trimethoxysilyl)propyl methacrylate or 3-Methacryloxypropyltrimethoxysilane |
| HDODA | 1,6-Hexanediol diacrylate |
| 4-MET | 4-methacryloxyethyl trimellitic acid |
| 4-META | 4-methacryloyloxyethy trimellitate anhydride |
| 10-MDP | 10-Methacryloyloxydecyl dihydrogen phosphate |
| MDTP | 10-methacryloyloxydecyl dihydrogen thiophosphate |
| NPG2PODA | Neopentyl glycol propoxylate diacrylate |
| NPGDMA | Neopentyl glycol Dimethacrylate or 2,2-dimethylpropane-1,1-diyl bis(2-methylprop-2-enoate) |
| NTGGMA | N-(2-hydroxy-3-((2-methyl-1-oxo-2-propenyl) oxy) propyl)-N-tolyl glycine |
| TCDDMA or TCDMA | Tricyclodecane dimethanol dimethacrylate or 2-propenoic acid,(octahydro-4,7-methano-1h-indene-5,1-diyl)bis(methylene) ester |
| D3MA | decanediol 1,10-dimethacrylate |
| PCDMA | Polycarbonate dimethacrylate |
| TCD-DI-HEA | 2-propenoic acid; (octahydro-4,7-methano-1H-indene-5-diyl) bis(methyleneiminocarbonyloxy-2,1-ethanediyl) ester |
| DDCDMA | Dimer dicarbamate dimethacrylate |
| LPS monomer | proprietary monomer |
| IBMA | Isobutyl methacrylate |
| PDMA | Polybutanediol dimethacrylate 600 |
| PMGDM | Pyromellitic dianhydride glycerol dimethacrylate |
| AMPS | 2-Acrylamido-2-methylpropane sulfonic acid ou 2-Acrylamido-2-methylpropane sulfonic acid |
| BMEP | Bis[2-(methacryloyloxy)ethyl] phosphate |
| PENTA | Dipentaerythritol penta-acrylate phosphate |
| MPTMS | 3-Mercaptopropyl trimethoxysilane |
| PMDM | Pyromellitic dimethacrylate |
| TCDDA | Tricyclodecane dimethanol diacrylate or Tricyclo[5.2.1.02,6]decanedimethanol diacrylate |
| AHPM | 3-(acryloyloxy)-2-hydroxypropyl methacrylate |
| PPTTA | ethoxylated (5.0) pentaerythritol tetraacrylate |
| AFM | Proprietary monomer |
| SDR | Proprietary monomer |
| DDDMA | 1,12-Dodecanediol dimethacrylate or 12-(2-methylprop-2-enoyloxy)dodecyl 2-methylprop-2-enoate |
| HDMA or HDDMA or HEDMA | 1,6 Hexanediol dimethacrylate |
| ETPTA | Trimethylolpropane ethoxylate triacrylate |
|
Monomer
Composition | Restorative Composites |
Core Build-Up
Composites | Orthodontic Composites |
Restorative
Adhesives |
Orthodontic
Adhesives | Sealants |
Restorative
RMGICs |
Luting
Cements and Composites | Total |
|---|---|---|---|---|---|---|---|---|---|
| With BPA derivatives | 223 (83.8%) | 24 (82.8%) | 37 (78.7%) | 36 (44.4%) | 12 (63.2%) | 16 (66.7%) | 3 (30%) | 31 (46.3%) | 382 (70.3%) |
| With BisGMA | 177 (66.5%) | 20 (69%) | 29 (61.7%) | 31 (38.3%) | 11 (57.9%) | 12 (50%) | 0 | 25 (37.3%) | 305 (56.2%) |
| With BisEMA | 109 (41%) | 11 (37.9%) | 7 (14.9%) | 5 (6.2%) | 2 (10.5%) | 3 (12.5%) | 3 (30%) | 12 (17.9%) | 152 (28%) |
| With BisDMA | 1 (0.4%) | 0 | 1 (2.1%) | 2 (2.5%) | 2 (10.5%) | 2 (8.3%) | 0 | 0 | 8 (1.5%) |
| With BisMPEPP | 15 (5.6%) | 0 | 0 | 1 (1.2%) | 0 | 0 | 0 | 1 (1.5%) | 17 (3.1%) |
| With BisPMA | 3 (1.1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0.6%) |
| With PC BisGMA | 2 (0.8%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.4%) |
| With UDMA | 188 (70.7%) | 14 (48.3%) | 12 (25.5%) | 27 (33.3%) | 7 (36.8%) | 15 (62.5%) | 4 (40%) | 42 (62.7%) | 309 (56.9%) |
| With TEGDMA | 175 (65.8%) | 23 (79.3%) | 20 (42.6%) | 20 (24.7%) | 6 (31.6%) | 11 (45.8%) | 1 (10%) | 36 (53.7%) | 292 (53.8%) |
| With HEMA | 10 (3.8%) | 0 | 6 (12.8%) | 71 (87.7%) | 10 (52.6%) | 3 (12.5%) | 9 (90%) | 25 (37.3%) | 134 (24.7%) |
| Without BPA derivatives | 43 (16.2%) | 5 (17.2%) | 10 (21.3%) | 45 (55.6%) | 7 (36.8%) | 8 (33.3%) | 7 (70%) | 36 (53.7%) | 161 (29.7%) |
| Without BPA derivatives or UDMA, TEGDMA and HEMA | 7 (2.6%) | 0 | 3 (6,4%) | 6 (7.4%) | 1 (5.3%) | 2 (8.3%) | 1 (10%) | 1 (1.5%) | 21 (3.9%) |
| Total | 266 | 29 | 47 | 81 | 19 | 24 | 10 | 67 | 543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantagnan, C.-A.; Babajko, S.; Nassif, A.; Houari, S.; Jedeon, K.; François, P.; Dursun, E.; Attal, J.-P.; Bosco, J. Analysis of Resin-Based Dental Materials’ Composition Depending on Their Clinical Applications. Polymers 2024, 16, 1022. https://doi.org/10.3390/polym16081022
Dantagnan C-A, Babajko S, Nassif A, Houari S, Jedeon K, François P, Dursun E, Attal J-P, Bosco J. Analysis of Resin-Based Dental Materials’ Composition Depending on Their Clinical Applications. Polymers. 2024; 16(8):1022. https://doi.org/10.3390/polym16081022
Chicago/Turabian StyleDantagnan, Claire-Adeline, Sylvie Babajko, Ali Nassif, Sophia Houari, Katia Jedeon, Philippe François, Elisabeth Dursun, Jean-Pierre Attal, and Julia Bosco. 2024. "Analysis of Resin-Based Dental Materials’ Composition Depending on Their Clinical Applications" Polymers 16, no. 8: 1022. https://doi.org/10.3390/polym16081022
APA StyleDantagnan, C.-A., Babajko, S., Nassif, A., Houari, S., Jedeon, K., François, P., Dursun, E., Attal, J.-P., & Bosco, J. (2024). Analysis of Resin-Based Dental Materials’ Composition Depending on Their Clinical Applications. Polymers, 16(8), 1022. https://doi.org/10.3390/polym16081022





