Improvement in Crystallization, Thermal, and Mechanical Properties of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic with Zinc Phenylphosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLLA-PEG-PLLA/PPZn Composites
2.3. Characterization of PLLA-PEG-PLLA/PPZn Composites
3. Results
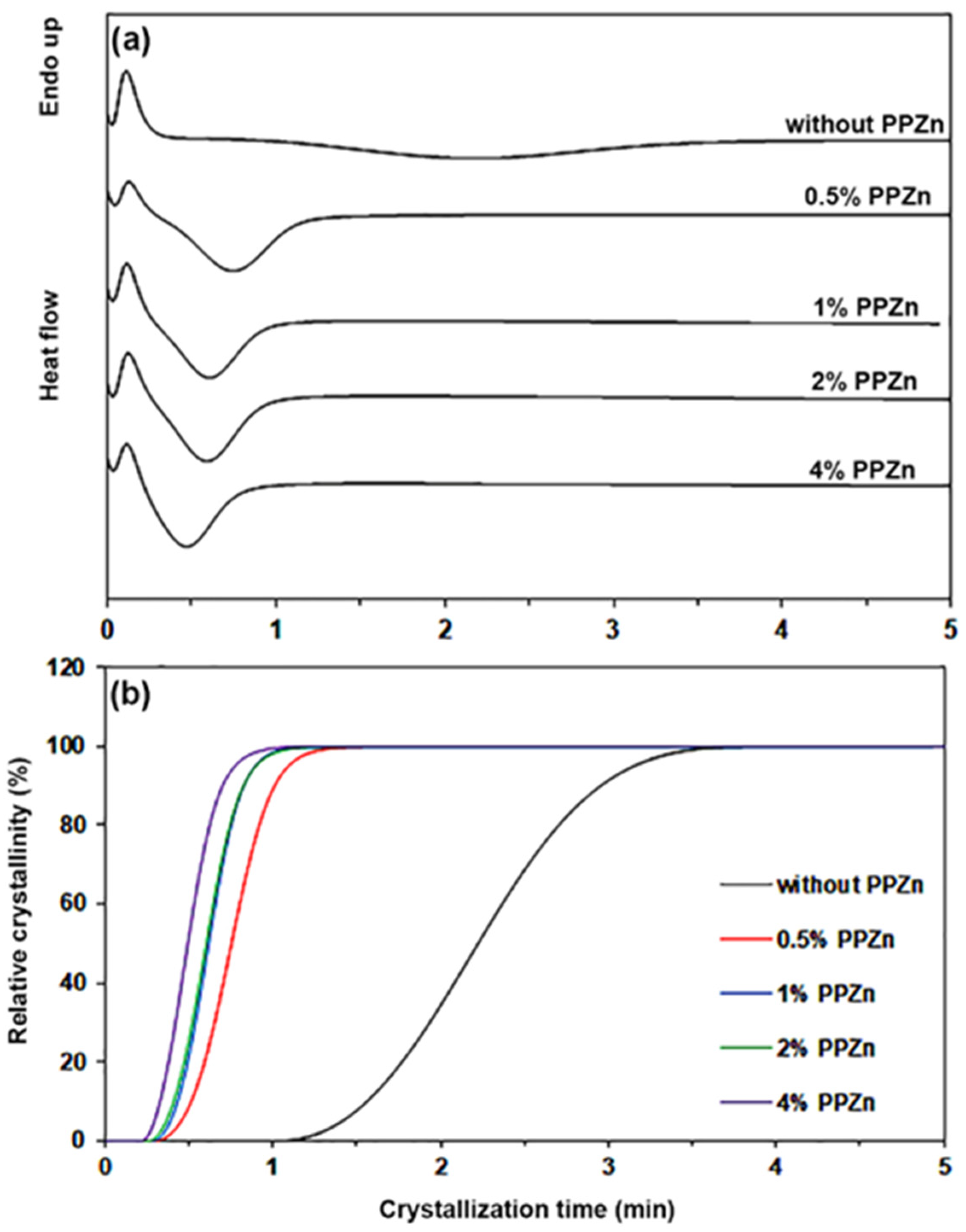
3.1. Thermal Transition Properties
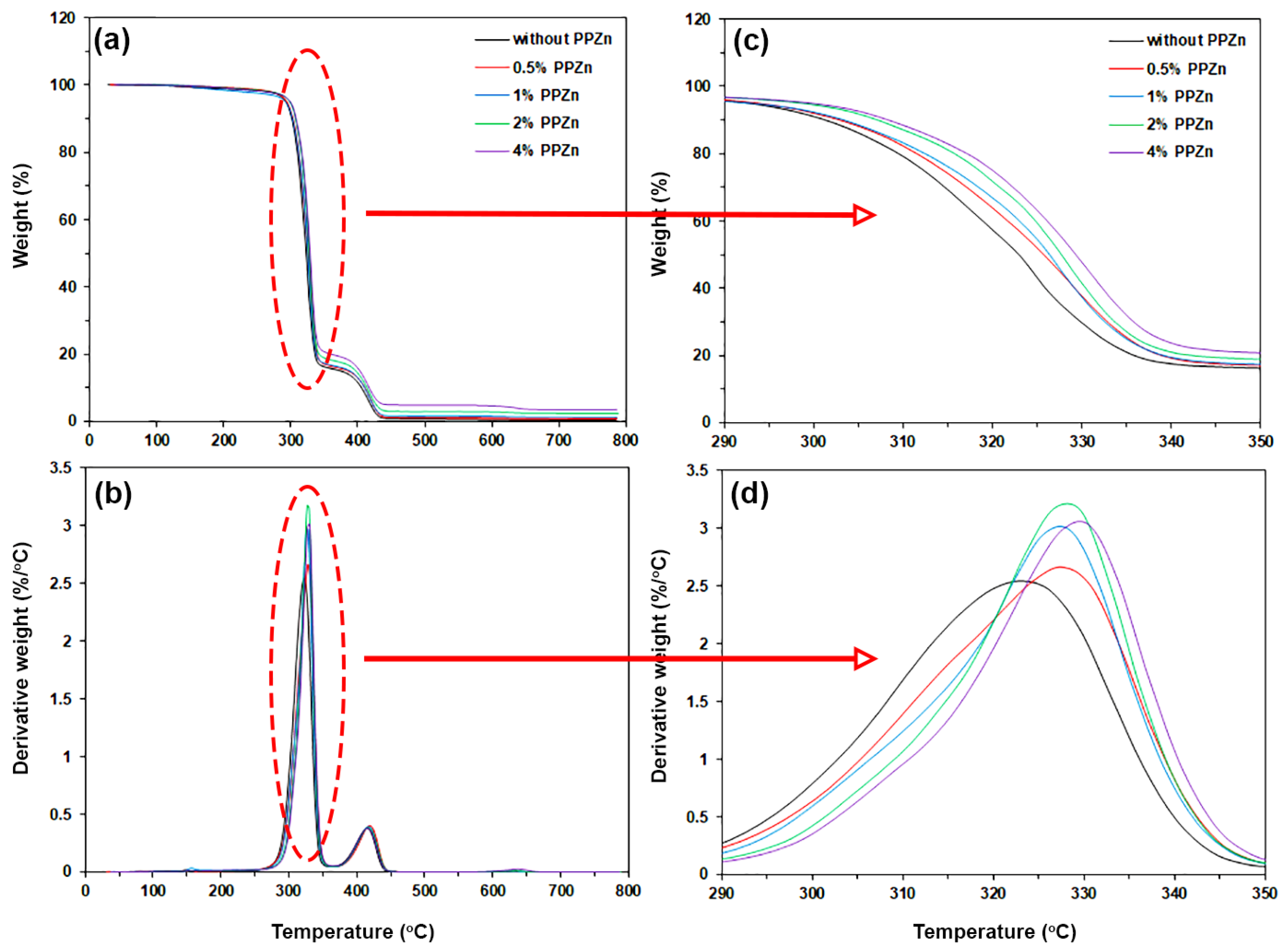
3.2. Thermal Decomposition Behaviors
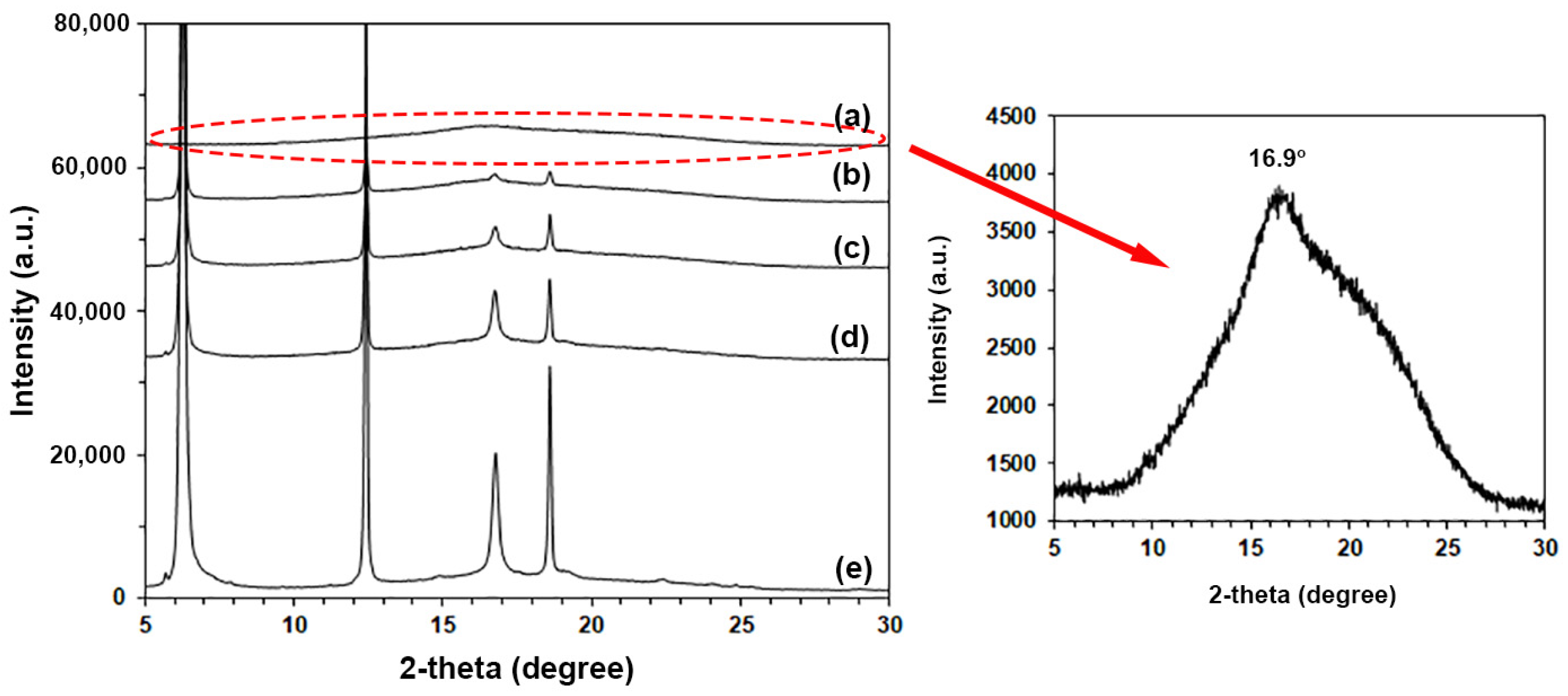
3.3. Crystalline Structures
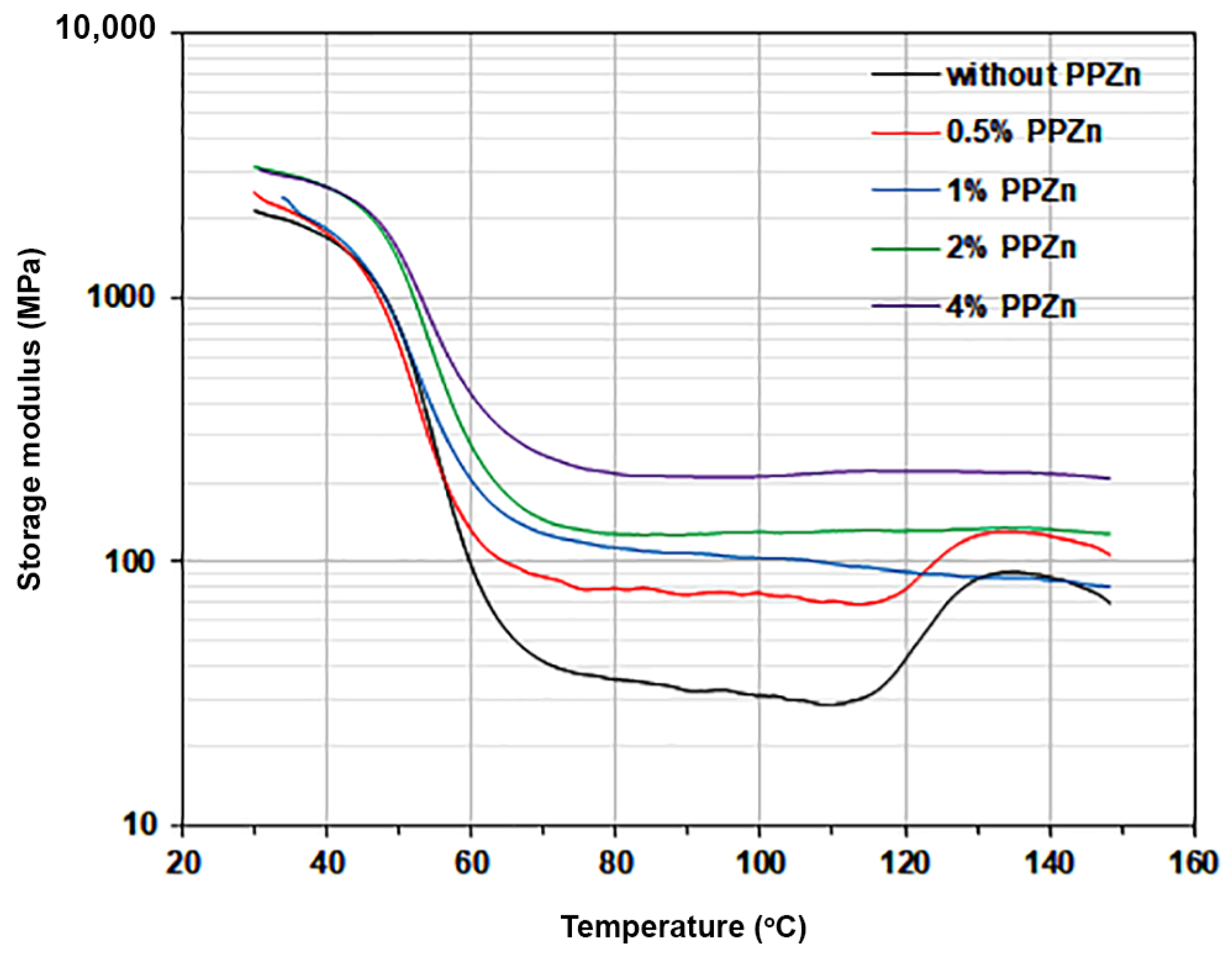
3.4. Thermo-Mechanical Properties
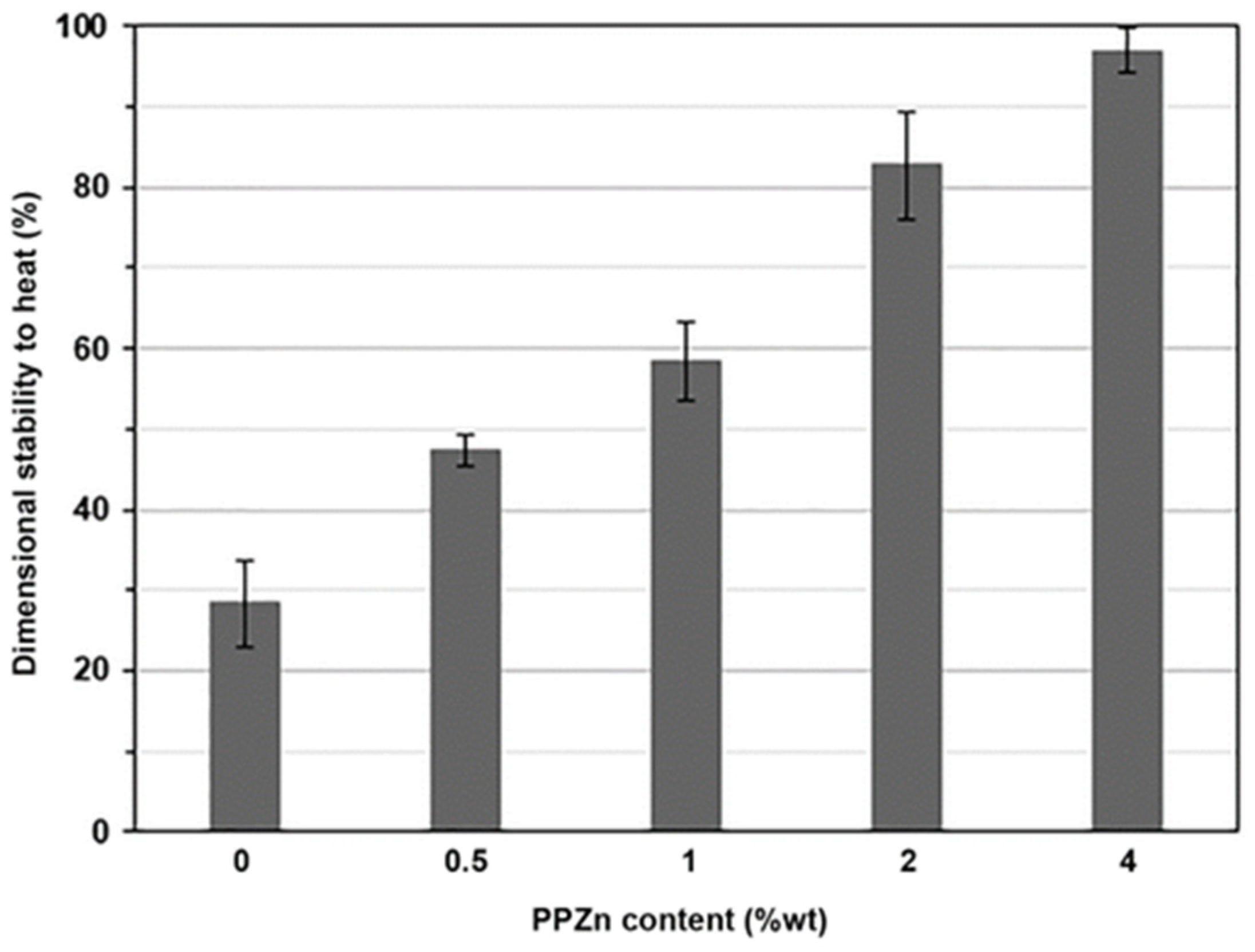
3.5. Dimensional Stability to Heat
3.6. Phase Compatibility
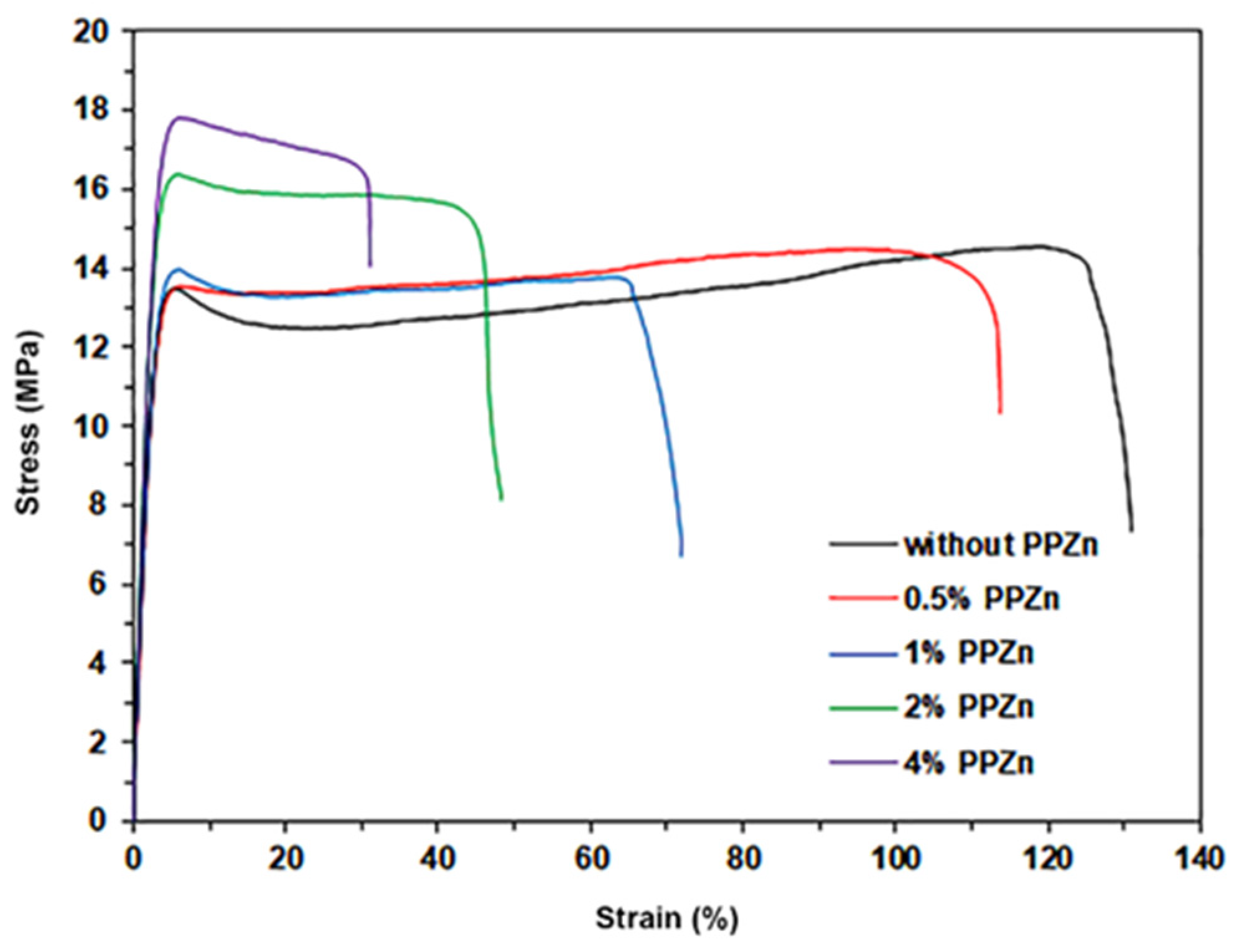
3.7. Tensile Properties
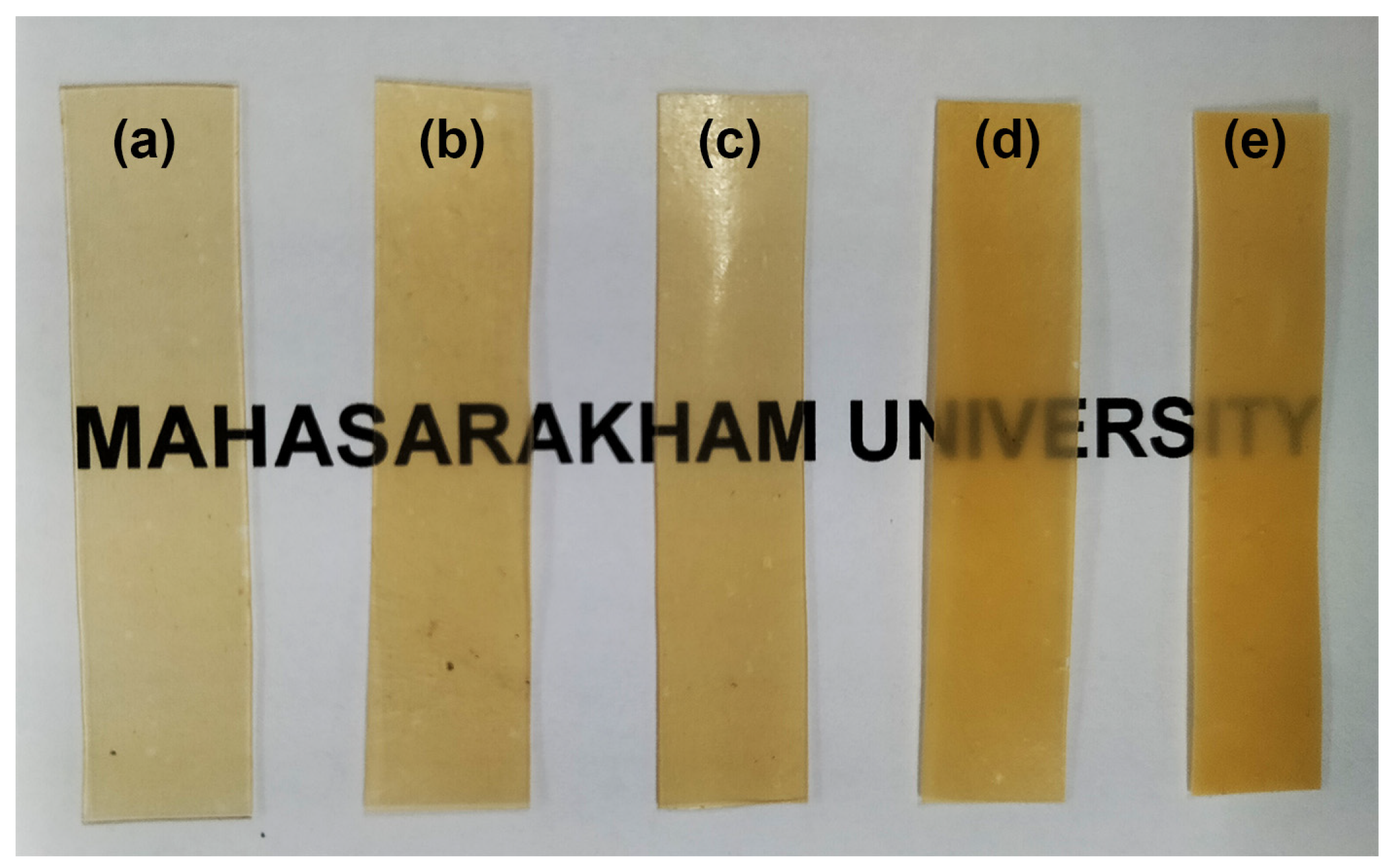
3.8. Film’s Opacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)–mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Aleksanyan, K.V. Recent approaches to the plasticization of poly(lactic acid) (PLA) (A review). Polymers 2024, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- de França, J.O.C.; Lima, Q.d.S.; Barbosa, M.M.d.M.; Fonseca, A.L.F.; Machado, G.d.F.; Dias, S.C.L.; Dias, J.A. Sonochemical synthesis of magnetite/poly(lactic acid) nanocomposites. Polymers 2023, 15, 4662. [Google Scholar] [CrossRef]
- Guo, W.; Bu, W.; Mao, Y.; Wang, E.; Yang, Y.; Liu, C.; Guo, F.; Mai, H.; You, H.; Long, Y. Magnesium hydroxide as a versatile nanofiller for 3D-printed PLA bone scaffolds. Polymers 2024, 16, 198. [Google Scholar] [CrossRef] [PubMed]
- Jamnongkan, T.; Sirichaicharoenkol, K.; Kongsomboon, V.; Srinuan, J.; Srisawat, N.; Pangon, A.; Mongkholrattanasit, R.; Tammasakchai, A.; Huang, C.-F. Innovative electrospun nanofiber mats based on polylactic acid composited with silver nanoparticles for medical applications. Polymers 2024, 16, 409. [Google Scholar] [CrossRef]
- Durpekova, S.; Bergerova, E.D.; Hanusova, D.; Dusankova, M.; Sedlarik, V. Eco-friendly whey/polysaccharide-based hydrogel with poly(lactic acid) for improvement of agricultural soil quality and plant growth. Int. J. Biol. Macromol. 2022, 212, 85–96. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable polylactic acid and its composites: Characteristics, processing, and sustainable applications in sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Smart packaging based on polylactic acid: The effects of antibacterial and antioxidant agents from natural extracts on physical–mechanical properties, colony reduction, perishable food shelf life, and future prospective. Polymers 2023, 15, 4103. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)—Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef]
- An, L.; Perkins, P.; Yi, R.; Ren, T. Development of polylactic acid based antimicrobial food packaging films with N-halamine modified microcrystalline cellulose. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124685. [Google Scholar] [CrossRef]
- Alexeeva, O.V.; Olkhov, A.A.; Konstantinova, M.L.; Podmasterev, V.V.; Petrova, T.V.; Martirosyan, L.Y.; Karyagina, O.K.; Kozlov, S.S.; Lomakin, S.M.; Tretyakov, I.V.; et al. A novel approach for glycero-(9,10-trioxolane)-trialeate incorporation into poly(lactic acid)/poly(ε-caprolactone) blends for biomedicine and packaging. Polymers 2024, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Puglia, D.; Iannoni, A.; Terenzi, A.; Kenny, J.M.; Torre, L. Processing conditions, thermal and mechanical responses of stretchable poly(lactic acid)/poly(butylene succinate) films. Materials 2017, 10, 809. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Ferrari, F. Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties. J. Therm. Anal. Calorim. 2021, 146, 131–141. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(l-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Sungsanit, K.; Kao, N.; Bhattacharya, S.N. Properties of linear poly(lactic acid)/polyethylene glycol blends. Polym. Eng. Sci. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Ma, P.; Shen, T.; Lin, L.; Dong, W.; Chen, M. Cellulose-g-poly(d-lactide) nanohybrids induced significant low melt viscosity and fast crystallization of fully bio-based nanocomposites. Carbohydr. Polym. 2017, 155, 498–506. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Garcia-Garcia, D.; Dominici, F.; Torre, L.; Sanchez-Nacher, L.; Balart, R. PLA films with improved flexibility properties by using maleinized cotton-seed oil. Eur. Polym. J. 2017, 91, 248–259. [Google Scholar] [CrossRef]
- Yun, X.; Li, X.; Jin, Y.; Sun, W.; Dong, T. Fast crystallization and toughening of poly(L-lactic acid) by incorporating with poly(ethylene glycol) as a middle block chain. Polym. Sci. Ser. A 2018, 60, 141–155. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymorama, N. Improvement in melt flow property and flexibility of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) by chain extension reaction for potential use as flexible bioplastics. Mater. Des. 2018, 154, 73–80. [Google Scholar] [CrossRef]
- Srihanam, P.; Thongsomboon, W.; Baimark, Y. Phase Morphology, mechanical, and thermal properties of calcium carbonate-reinforced poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastics. Polymers 2023, 15, 301. [Google Scholar] [CrossRef]
- Cailloux, J.; Santona, O.O.; Franco-Urquiza, E.; Bou, J.J.; Carrasco, F.; Gamez-Perez, J.; Maspoch, M.L. Sheets of branched poly(lactic acid) obtained by one step reactive extrusion calendaring process: Melt rheology analysis. Express Polym. Lett. 2013, 7, 304–318. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Li, G.; Liang, N.; Zhang, J.; Zhu, Z.; Wang, R. Effect of nucleating agents on the crystallization behavior and heat resistance of poly(L-lactide). J. Appl. Polym. Sci. 2016, 133, 42999. [Google Scholar] [CrossRef]
- Srisuwan, S.; Baimark, Y. Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic. e-Polymers 2022, 22, 389–398. [Google Scholar] [CrossRef]
- Wu, N.; Wang, H. Effect of zinc phenylphosphonate on the crystallization behavior of poly(L-lactide). J. Appl. Polym. Sci. 2013, 130, 2744–2752. [Google Scholar] [CrossRef]
- Tabi, T.; Ageyeva, T.; Kovacs, J.G. The influence of nucleating agents, plasticizers, and molding conditions on the properties of injection molded PLA products. Mater. Today Commun. 2022, 32, 103936. [Google Scholar] [CrossRef]
- Ageyeva, T.; Kovács, J.G.; Tabi, T. Comparison of the efficiency of the most effective heterogeneous nucleating agents for poly(lactic acid). J. Therm. Anal. Calorim. 2022, 147, 8199–8211. [Google Scholar] [CrossRef]
- Tsuji, H.; Takai, H.; Saha, S.K. Isothermal and non-isothermal crystallization behavior of poly(L-lactic acid): Effects of stereocomplex as nucleating agent. Polymer 2006, 47, 3826–3837. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Baimark, Y. Improvement in thermal stability of flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastic by blending with native cassava starch. Polymers 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, X.; He, Y.; Dong, Z.; Zhang, X.; Chen, X.; Liu, T. Effect of poly(lactic acid) crystallization on its mechanical and heat resistance performances. Polymer 2021, 212, 123280. [Google Scholar] [CrossRef]
- Thongsomboon, W.; Srihanam, P.; Baimark, Y. Preparation of flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/talcum/thermoplastic starch ternary composites for use as heat-resistant and single-use bioplastics. Int. J. Biol. Macromol. 2023, 230, 123172. [Google Scholar] [CrossRef] [PubMed]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
- Srihanam, P.; Srisuwan, Y.; Phromsopha, T.; Manphae, A.; Baimark, Y. Improvement in phase compatibility and mechanical properties of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/thermoplastic starch blends with citric acid. Polymers 2023, 15, 3966. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, Z.Q.; Bao, R.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Poly(L-lactic acid)-polyethylene glycol-poly(L-lactic acid) triblock copolymer: A novel macromolecular plasticizer to enhance the crystallization of poly(L-lactic acid). Eur. Polym. J. 2017, 97, 272–281. [Google Scholar] [CrossRef]
- Baimark, Y.; Kittipoom, S. Influence of chain-extension reaction on stereocomplexation, mechanical properties and heat resistance of compressed stereocomplex-polylactide bioplastic films. Polymers 2018, 10, 1218. [Google Scholar] [CrossRef]
- Baimark, Y.; Pasee, S.; Rungseesantivanon, W.; Prakymoramas, N. Flexible and high heat-resistant stereocomplex PLLA-PEG-PLLA/PDLA blends prepared by melt process: Effect of chain extension. J. Polym. Res. 2019, 26, 218. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Seven, K.M.; Cogen, J.M.; Gilchrist, J.F. Nucleating agents for high-density polyethylene—A review. Polym. Eng. Sci. 2016, 56, 541–554. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, G.; Phuong, T.V.; Lazzeri, A. Synergistic effects of nucleating agents and plasticizers on the crystallization behavior of poly(lactic acid). Molecules 2015, 20, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhang, D.; Guo, H.; Zhao, J.; Wang, Z.; Hu, H.; Xu, J.; Fu, C. Functionalized boron nitride nanosheets/poly(L-lactide) nanocomposites and their crystallization behavior. Polymers 2019, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.N.; Huang, Z.G.; Weng, Y.X. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Chen, P.; Yu, K.; Wang, Y.; Wang, W.; Zhou, H.; Li, H.; Mi, J.; Wang, X. The effect of composite nucleating agent on the crystallization behavior of branched poly(lactic acid). J. Polym. Environ. 2018, 26, 3718–3730. [Google Scholar] [CrossRef]
- Li, Y.; Han, C. Isothermal and nonisothermal cold crystallization behaviors of asymmetric poly(L-lactide)/poly(D-lactide) blends. Ind. Eng. Chem. Res. 2012, 51, 15927–15935. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Elkoun, S. Effect of thermal history on nucleation and crystallization of poly(lactic acid). J. Mater. Sci. 2016, 51, 7768–7779. [Google Scholar] [CrossRef]
- Gao, P.; Alanazi, S.; Masato, D. Crystallization of polylactic acid with organic nucleating agents under quiescent conditions. Polymers 2024, 16, 320. [Google Scholar] [CrossRef]
- Vidovic, E.; Faraguna, F.; Jukic, A. Influence of inorganic fillers on PLA crystallinity and thermal properties. J. Therm. Anal. Calorim. 2017, 127, 371–380. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Kim, D.-K.; Han, W.; Park, J.-Y.; Kim, K.-W.; Kim, B.-J. Effect of nucleating agents addition on thermal and mechanical properties of natural fiber-reinforced polylactic acid composites. Polymers 2022, 14, 4263. [Google Scholar] [CrossRef]
- Srithep, Y.; Nealey, P.; Turng, L.S. Effects of annealing time and temperature on the crystallinity and heat resistance behavior of injection-molded poly(lactic acid). Polym. Mater. Sci. Eng. 2013, 53, 580–588. [Google Scholar] [CrossRef]
- Chauliac, D.; Pullammanappallil, P.C.; Ingram, L.O.; Shanmugam, K.T. A combined thermochemical and microbial process for recycling polylactic acid polymer to optically pure L-lactic acid for reuse. J. Polym. Environ. 2020, 28, 1503–1512. [Google Scholar] [CrossRef]
- Yin, H.-Y.; Wei, X.-F.; Bao, R.-Y.; Dong, Q.-X.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Enhancing thermomechanical properties and heat distortion resistance of poly(L-lactide) with high crystallinity under high cooling rate. ACS Sustain. Chem. Eng. 2015, 3, 654–661. [Google Scholar] [CrossRef]
- Vadori, R.; Mohanty, A.K.; Misra, M. The effect of mold temperature on the performance of injection molded poly(lactic acid)-based bioplastic. Macromol. Mater. Eng. 2013, 298, 981–990. [Google Scholar] [CrossRef]
- Si, W.-J.; An, X.-P.; Zeng, J.-B.; Chen, Y.-K.; Wang, Y.-Z. Fully biobased, highly toughened and heat-resistant poly(L-lactide) ternary blends via dynamic vulcanization with poly(D-lactide) and unsaturated bioelastomer. Sci. China Mater. 2017, 60, 1008–1022. [Google Scholar] [CrossRef]
- Masutani, K.; Kobayashi, K.; Kimura, Y.; Lee, C.W. Properties of stereo multi-block polylactides obtained by chain-extension of stereo tri-block polylactides consisting of poly(L-lactide) and poly(D-lactide). J. Polym. Res. 2018, 25, 74. [Google Scholar] [CrossRef]
- Tabi, T.; Ageyeva, T.; Kovacs, J.G. Improving the ductility and heat deflection temperature of injection molded poly(lactic acid) products: A comprehensive review. Polym. Test. 2021, 101, 107282. [Google Scholar] [CrossRef]
- Bindhu, B.; Renisha, R.; Roberts, L.; Varghese, T.O. Boron Nitride reinforced polylactic acid composites film for packaging: Preparation and properties. Polym. Test. 2018, 66, 172–177. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent semi-crystalline polymeric materials and their nanocomposites: A review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
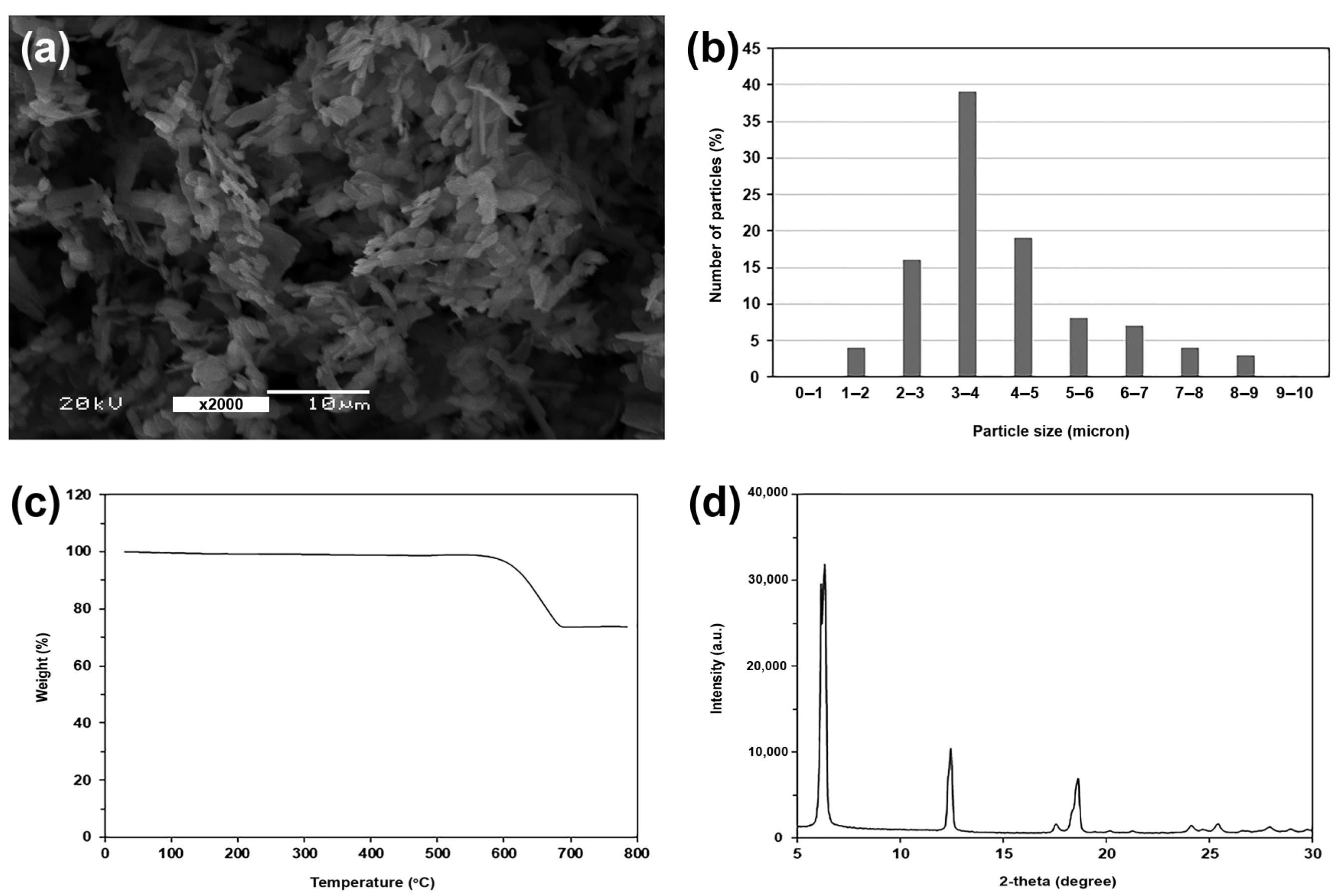
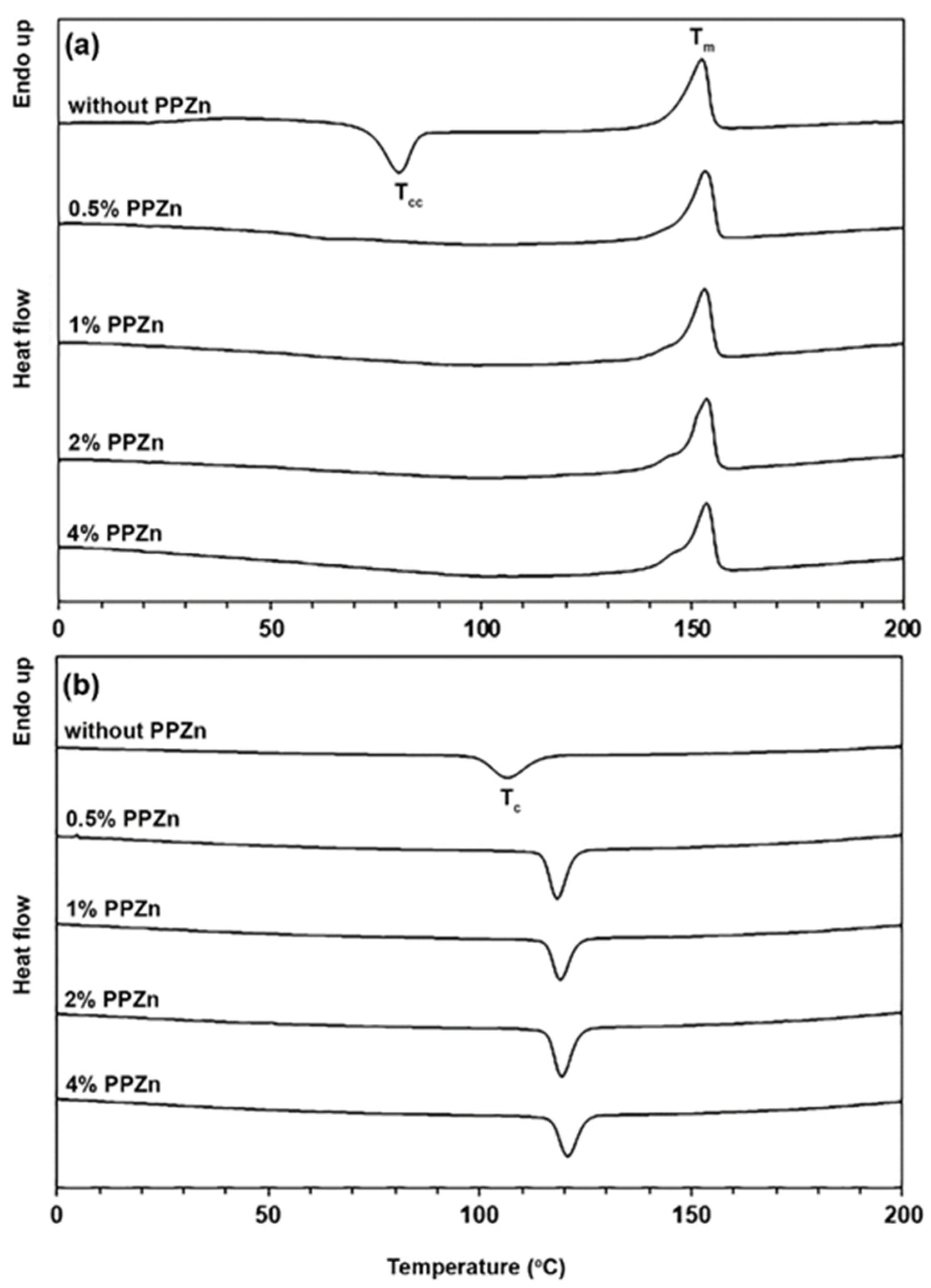


| PPZn Content (%wt) | WPLLA1 | Tg 2 (°C) | Tcc 2 (°C) | ΔHcc 2 (J/g) | Tm 2 (°C) | ΔHm 2 (J/g) | DSC-Xc 2 (%) | Tc 3 (°C) |
|---|---|---|---|---|---|---|---|---|
| - | 0.830 | 31 | 81 | 16.5 | 152 | 26.1 | 12.4 | 107 |
| 0.5 | 0.826 | - | - | - | 153 | 32.6 | 42.2 | 118 |
| 1 | 0.822 | - | - | - | 153 | 33.4 | 43.4 | 119 |
| 2 | 0.813 | - | - | - | 153 | 34.7 | 45.6 | 120 |
| 4 | 0.797 | - | - | - | 153 | 37.0 | 49.6 | 120 |
| PPZn Content (%wt) | t1/2 (min) | n | k (min−k) | R2 |
|---|---|---|---|---|
| - | 2.18 | 3.8569 | 0.0208 | 0.9986 |
| 0.5 | 0.75 | 3.2228 | 2.2596 | 0.9968 |
| 1 | 0.69 | 3.2092 | 4.2982 | 0.9983 |
| 2 | 0.58 | 3.2012 | 4.6353 | 0.9955 |
| 4 | 0.47 | 2.5142 | 5.9607 | 0.9970 |
| PPZn Content (%wt) | Residue Weight at 800 °C 1 (%) | PLLA-Td,max2 (°C) | PEG-Td,max2 (°C) |
|---|---|---|---|
| - | 0.42 | 323 | 416 |
| 0.5 | 0.97 | 328 | 417 |
| 1 | 1.22 | 328 | 418 |
| 2 | 2.15 | 328 | 416 |
| 4 | 3.66 | 329 | 415 |
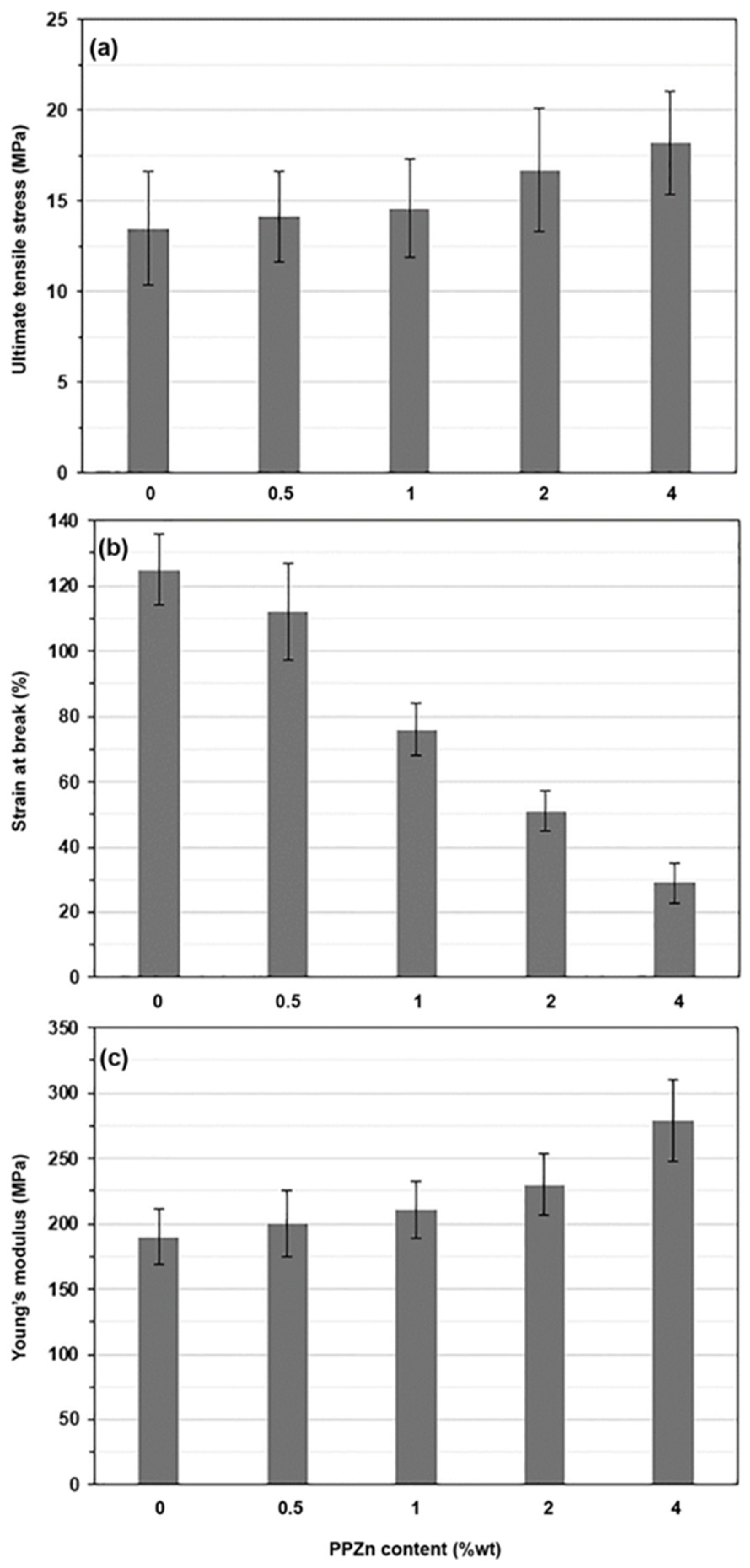
| PPZn Content (%wt) | Ultimate Tensile Stress (MPa) | Strain at Break (%) | Young’s Modulus (Mpa) | Opacity (mm−1) |
|---|---|---|---|---|
| - | 13.5 ± 3.1 | 125 ± 11 | 190 ± 21 | 0.402 ± 0.088 |
| 0.5 | 14.1 ± 2.5 | 112 ± 15 | 200 ± 25 | 0.754 ± 0.045 |
| 1 | 14.6 ± 2.7 | 76 ± 8 | 211 ± 22 | 0.936 ± 0.027 |
| 2 | 16.7 ± 3.4 | 51 ± 6 | 230 ± 24 | 1.960 ± 0.067 |
| 4 | 18.2 ± 2.8 | 29 ± 6 | 279 ± 31 | 3.829 ± 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakkethati, K.; Srihanam, P.; Manphae, A.; Rungseesantivanon, W.; Prakymoramas, N.; Lan, P.N.; Baimark, Y. Improvement in Crystallization, Thermal, and Mechanical Properties of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic with Zinc Phenylphosphate. Polymers 2024, 16, 975. https://doi.org/10.3390/polym16070975
Pakkethati K, Srihanam P, Manphae A, Rungseesantivanon W, Prakymoramas N, Lan PN, Baimark Y. Improvement in Crystallization, Thermal, and Mechanical Properties of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic with Zinc Phenylphosphate. Polymers. 2024; 16(7):975. https://doi.org/10.3390/polym16070975
Chicago/Turabian StylePakkethati, Kansiri, Prasong Srihanam, Apirada Manphae, Wuttipong Rungseesantivanon, Natcha Prakymoramas, Pham Ngoc Lan, and Yodthong Baimark. 2024. "Improvement in Crystallization, Thermal, and Mechanical Properties of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic with Zinc Phenylphosphate" Polymers 16, no. 7: 975. https://doi.org/10.3390/polym16070975
APA StylePakkethati, K., Srihanam, P., Manphae, A., Rungseesantivanon, W., Prakymoramas, N., Lan, P. N., & Baimark, Y. (2024). Improvement in Crystallization, Thermal, and Mechanical Properties of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic with Zinc Phenylphosphate. Polymers, 16(7), 975. https://doi.org/10.3390/polym16070975








