Fluorine-Containing Ionogels with Stretchable, Solvent-Resistant, Wide Temperature Tolerance, and Transparent Properties for Ionic Conductors
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of the Ionogels
2.2. Characterization of the Ionogels
2.3. Mechanical Tests
2.4. Contact Angle Measurement
2.5. Swelling Property of the Ionogels
2.6. Thermal Analysis
2.7. Impedance Tests
2.8. Cyclic Tensile Load-Unload Test
2.9. Fabricating of the Ionogel-Based Sensors
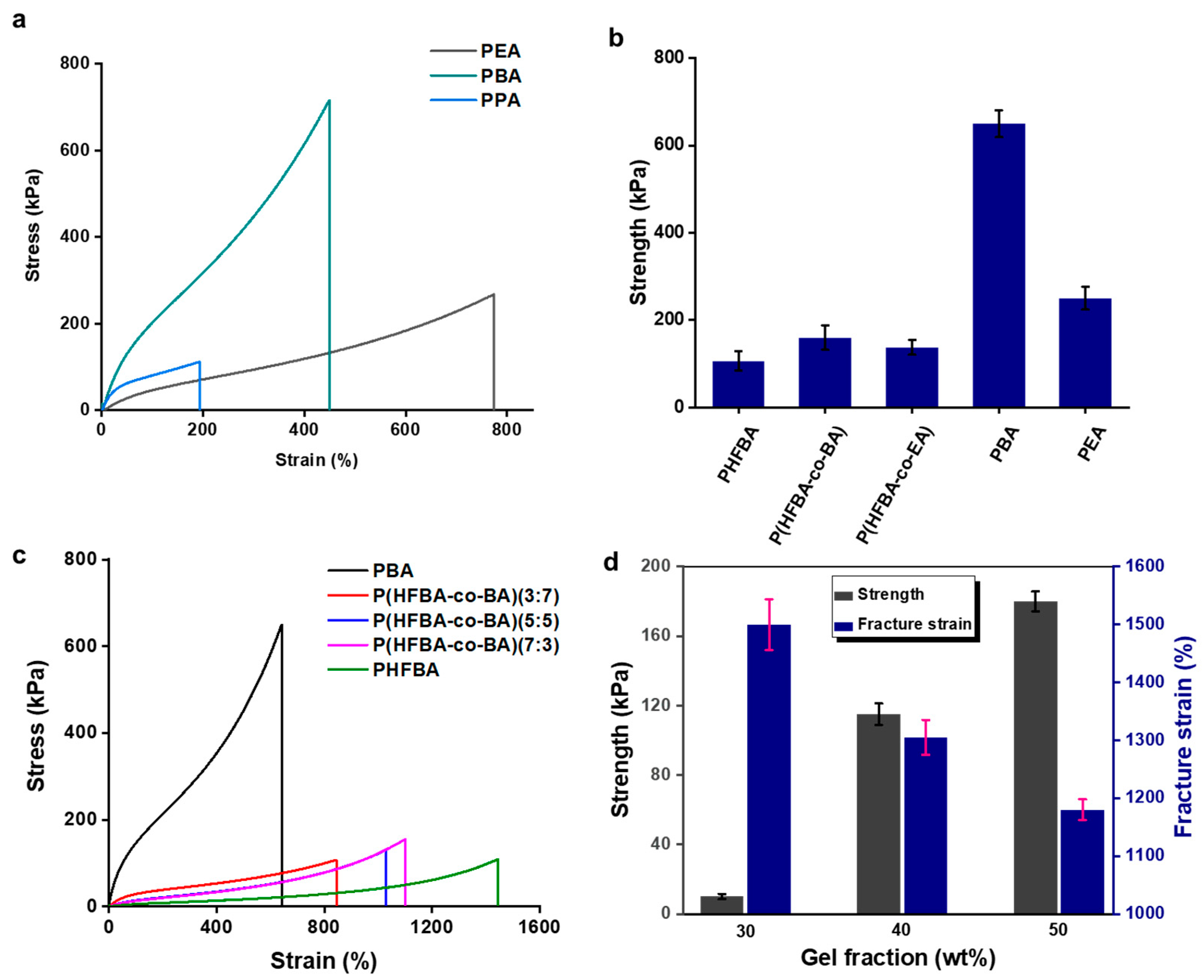
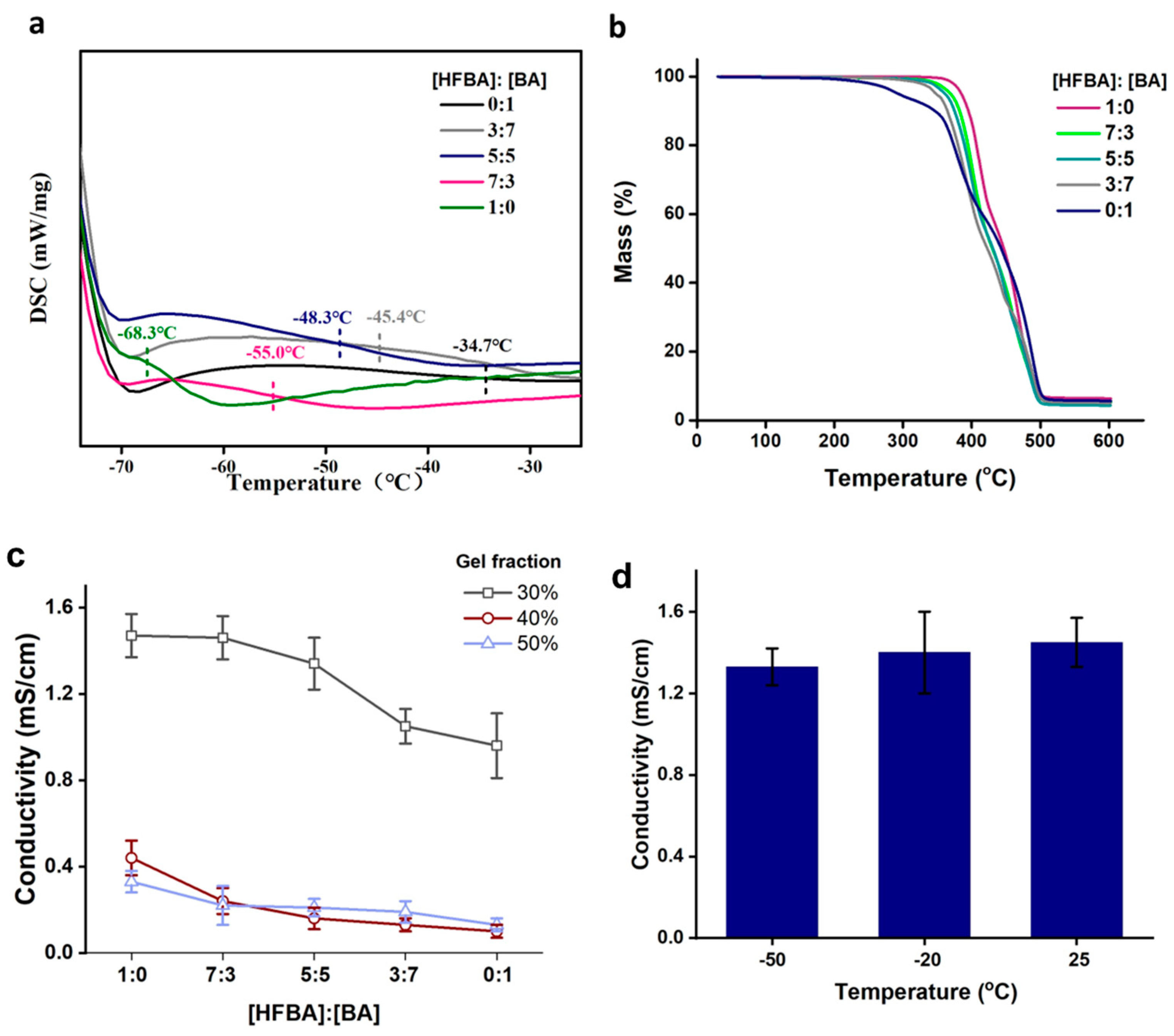
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Hu, J.; Dickey, M.D. Tough Ionogels: Synthesis, Toughening Mechanisms, and Mechanical Properties—A Perspective. JACS Au 2022, 2, 2645–2657. [Google Scholar] [CrossRef] [PubMed]
- Hyun, W.J.; Thomas, C.M.; Hersam, M.C. Nanocomposite Ionogel Electrolytes for Solid-State Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 2002135. [Google Scholar] [CrossRef]
- Hyun, W.J.; Thomas, C.M.; Luu, N.S.; Hersam, M.C. Layered Heterostructure Ionogel Electrolytes for High-Performance Solid-State Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2007864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, Z.; Wu, P. Mechanically Adaptative and Environmentally Stable Ionogels for Energy Harvest. Adv. Sci. 2023, 10, 2300253. [Google Scholar] [CrossRef]
- Li, H.; Xu, F.; Guan, T.T.; Li, Y.; Sun, J. Mechanically and environmentally stable triboelectric nanogenerator based on high-strength and anti-compression self-healing ionogel. Nano Energy 2021, 90, 106645. [Google Scholar] [CrossRef]
- Cheng, H.; He, X.; Fan, Z.; Ouyang, J. Flexible Quasi-Solid State Ionogels with Remarkable Seebeck Coefficient and High Thermoelectric Properties. Adv. Energy Mater. 2019, 9, 1901085. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, Y.; Zhi, X.; Guo, W.; Yang, B.; Zhang, S.; Li, M.; Wang, X.; Pan, C. Transparent Self-Healing Anti-Freezing Ionogel for Monolayered Triboelectric Nanogenerator and Electromagnetic Energy-Based Touch Panel. Adv. Mater. 2023, 36, e2308424. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.; Lee, H.H.; Oh, K.H.; Sun, J.Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Guo, Z.; Xi, R.; Xu, L.; Zhao, Z.; Ge, Y.; Jiang, X.; Yang, W.; Jiang, L. Fabrication of submicron linewidth silver grid/ionogel hybrid films for highly stable flexible transparent electrodes via asymmetric wettability template-assisted self-assembly. Chem. Eng. J. 2023, 469, 144065. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Li, S.; Liu, X.; Sun, J. Mechanically Robust, Elastic, and Healable Ionogels for Highly Sensitive Ultra-Durable Ionic Skins. Adv. Mater. 2020, 32, 2002706. [Google Scholar] [CrossRef]
- Lee, H.; Jang, J.; Lee, J.; Shin, M.; Lee, J.S.; Son, D. Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties. Polymers 2023, 15, 3852. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, X.; Majidi, C.; Gu, G. Cutaneous Ionogel Mechanoreceptors for Soft Machines, Physiological Sensing, and Amputee Prostheses. Adv. Mater. 2021, 33, 2102069. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Sun, S.; Wu, P. Non-equilibrium-Growing Aesthetic Ionic Skin for Fingertip-Like Strain-Undisturbed Tactile Sensation and Texture Recognition. Adv. Mater. 2023, 35, 2300593. [Google Scholar] [CrossRef] [PubMed]
- Yiming, B.; Han, Y.; Han, Z.; Zhang, X.; Li, Y.; Lian, W.; Zhang, M.; Yin, J.; Sun, T.; Wu, Z.; et al. A Mechanically Robust and Versatile Liquid-Free Ionic Conductive Elastomer. Adv. Mater. 2021, 33, 202006111. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Li, X.; Huang, X.; Cao, C.; Ai, L.; Wang, X.; Ravi, S.K.; Yao, X. Microphase-Separated Elastic and Ultrastretchable Ionogel for Reliable Ionic Skin with Multimodal Sensation. Adv. Mater. 2023, e2309821. [Google Scholar] [CrossRef] [PubMed]
- Rotjanasuworapong, K.; Lerdwijitjarud, W.; Sirivat, A. Dual electro- and magneto-induced bending actuators of magnetite-loaded agarose ionogels. Carbohydr. Polym. 2023, 310, 120741. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, Z.; Ye, L.; Zhao, X. Construction of poly (vinyl alcohol)-based ionogels with continuous ion transport channels enables high performance ionic soft actuators. J. Mater. Chem. A 2023, 11, 19981–19995. [Google Scholar] [CrossRef]
- Lan, J.; Li, Y.; Yan, B.; Yin, C.; Ran, R.; Shi, L.Y. Transparent Stretchable Dual-Network Ionogel with Temperature Tolerance for High-Performance Flexible Strain Sensors. ACS Appl. Mater. Interfaces 2020, 12, 37597–37606. [Google Scholar] [CrossRef]
- Sun, J.; Lu, G.; Zhou, J.; Yuan, Y.; Zhu, X.; Nie, J. Robust Physically Linked Double-Network Ionogel as a Flexible Bimodal Sensor. ACS Appl. Mater. Interfaces 2020, 12, 14272–14279. [Google Scholar] [CrossRef]
- Dinh Xuan, H.; Timothy, B.; Park, H.Y.; Lam, T.N.; Kim, D.; Go, Y.; Kim, J.; Lee, Y.; Ahn, S.; Jim, S.; et al. Super Stretchable and Durable Electroluminescent Devices Based on Double-Network Ionogels. Adv. Mater. 2021, 33, 202008849. [Google Scholar] [CrossRef]
- Su, A.; Guo, P.; Li, J.; Kan, D.; Pang, Q.; Li, T.; Sun, J.; Chen, G.; Wei, Y. An organic–inorganic semi-interpenetrating network ionogel electrolyte for high-voltage lithium metal batteries. J. Mater. Chem. A 2020, 8, 4775–4783. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Ji, T.; Bai, R.; Zhu, H. Highly Ionic Conductive, Stretchable, and Tough Ionogel for Flexible Solid-State Supercapacitor. Small 2023, e2307019. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Zheng, S.; Liu, Z.; Zou, X.; Sun, Z.; Guo, J.; Yan, F. Recyclable, Healable, and Tough Ionogels Insensitive to Crack Propagation. Adv. Mater. 2022, 34, 202203049. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, Y.; Tjiu, W.W.; Zhang, C.; Liu, T. Highly Stretchable, Fast Self-Healing, and Waterproof Fluorinated Copolymer Ionogels with Selectively Enriched Ionic Liquids for Human-Motion Detection. ACS Appl. Mater. Interfaces 2021, 13, 49358–49368. [Google Scholar] [CrossRef]
- Ma, C.; Wei, J.; Zhang, Y.; Chen, X.; Liu, C.; Diao, S.; Matyjaszewski, K.; Liu, H. Highly Processable Ionogels with Mechanical Robustness. Adv. Funct. Mater. 2023, 33, 202211771. [Google Scholar] [CrossRef]
- Guo, P.; Su, A.; Wei, Y.; Liu, X.; Li, Y.; Guo, F.; Li, J.; Hu, Z.; Sun, J. Healable, Highly Conductive, Flexible, and Nonflammable Supramolecular Ionogel Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 19413–19420. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, H.; Li, S.; Xu, M.; Li, T.; Yang, X.; Song, H. Stretchable, Low-Hysteresis, and Recyclable Ionogel by Ionic Liquid Catalyst and Mixed Ionic Liquid-Induced Phase Separation. ACS Sustain. Chem. Eng. 2023, 11, 15031–15042. [Google Scholar] [CrossRef]
- Weng, D.; Xu, F.; Li, X.; Li, S.; Li, Y.; Sun, J. Polymeric Complex-Based Transparent and Healable Ionogels with High Mechanical Strength and Ionic Conductivity as Reliable Strain Sensors. ACS Appl. Mater. Interfaces 2020, 12, 57477–57485. [Google Scholar] [CrossRef]
- Lai, J.; Zhou, H.; Jin, Z.; Li, S.; Liu, H.; Jin, X.; Luo, C.; Ma, A.; Chen, W. Fatigue-Resistant, Electrically Conductive, and Temperature-Tolerant Ionogels for High-Performance Flexible Sensors. ACS Appl. Mater. Interfaces 2019, 11, 26412–26420. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Liu, Y.; Luo, X.; He, Y.; Niu, Y.; Xu, Q. Transparent, stretchable, self-healing, and self-adhesive ionogels for flexible multifunctional sensors and encryption systems. Chem. Eng. J. 2024, 484, 149632. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; He, Z.; Fan, L.; Yao, D.; Zheng, Y. Preparation of tough and stiff ionogels via phase separation. Mater. Horiz. 2024, 11, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhang, X.; Wen, J.; Sun, X.; He, D.; Li, W. Multifunctional visualized electronic skin based on a solvatochromic poly (ionic liquid) ionogel. Chem. Eng. J. 2023, 477, 147182. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Sun, H.; Wang, F.; Lei, B.; Wang, W.; Wang, Q.; Yang, Y.; Shao, J.; Dong, X. Self-Healing Transparent Ionogel Polymerized by Liquid Metal for Strain Sensor. Chem. Eng. J. 2023, 478, 147321. [Google Scholar] [CrossRef]
- Shi, L.; Jia, K.; Gao, Y.; Yang, H.; Ma, Y.; Lu, S.; Gao, G.; Bu, H.; Lu, T.; Ding, S. Highly Stretchable and Transparent Ionic Conductor with Novel Hydrophobicity and Extreme-Temperature Tolerance. Research 2020, 2020, 2505619. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, T.; Gao, G.; Zhang, X.; Wei, W.; Liu, W.; Ding, S. Highly stretchable and transparent ionic conducting elastomers. Nat. Commun. 2018, 9, 2630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Song, H.; Duan, X.; Wang, Z.; Liu, J.; Ba, X. Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries. Polymers 2019, 11, 444. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Z.; Deng, Z.; Du, Z.; Sun, T.L.; Guo, Z.H.; Yue, K. A Transparent, Highly Stretchable, Solvent-Resistant, Recyclable Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Reliable Strain Sensors. Adv. Mater. 2021, 33, 202105306. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Feng, W.; Wang, S.; Chen, Y.; Zheng, W.J.; Yan, J. Fluorine-Containing Ionogels with Stretchable, Solvent-Resistant, Wide Temperature Tolerance, and Transparent Properties for Ionic Conductors. Polymers 2024, 16, 1013. https://doi.org/10.3390/polym16071013
Fan X, Feng W, Wang S, Chen Y, Zheng WJ, Yan J. Fluorine-Containing Ionogels with Stretchable, Solvent-Resistant, Wide Temperature Tolerance, and Transparent Properties for Ionic Conductors. Polymers. 2024; 16(7):1013. https://doi.org/10.3390/polym16071013
Chicago/Turabian StyleFan, Xiaoxi, Wenlong Feng, Shuang Wang, Yinpeng Chen, Wen Jiang Zheng, and Jie Yan. 2024. "Fluorine-Containing Ionogels with Stretchable, Solvent-Resistant, Wide Temperature Tolerance, and Transparent Properties for Ionic Conductors" Polymers 16, no. 7: 1013. https://doi.org/10.3390/polym16071013
APA StyleFan, X., Feng, W., Wang, S., Chen, Y., Zheng, W. J., & Yan, J. (2024). Fluorine-Containing Ionogels with Stretchable, Solvent-Resistant, Wide Temperature Tolerance, and Transparent Properties for Ionic Conductors. Polymers, 16(7), 1013. https://doi.org/10.3390/polym16071013







