The Crystallization Morphology and Conformational Changes of Polypropylene Random Copolymer Induced by a Novel β-Nucleating Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Characterizations and Measurements
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Wide-Angle X-ray Diffraction (WAXD)
2.3.3. Polarizing Optical Microscopy (POM)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. High-Resolution In Situ FTIR Spectroscopy
3. Results and Discussion
3.1. Measurement of β-Crystal
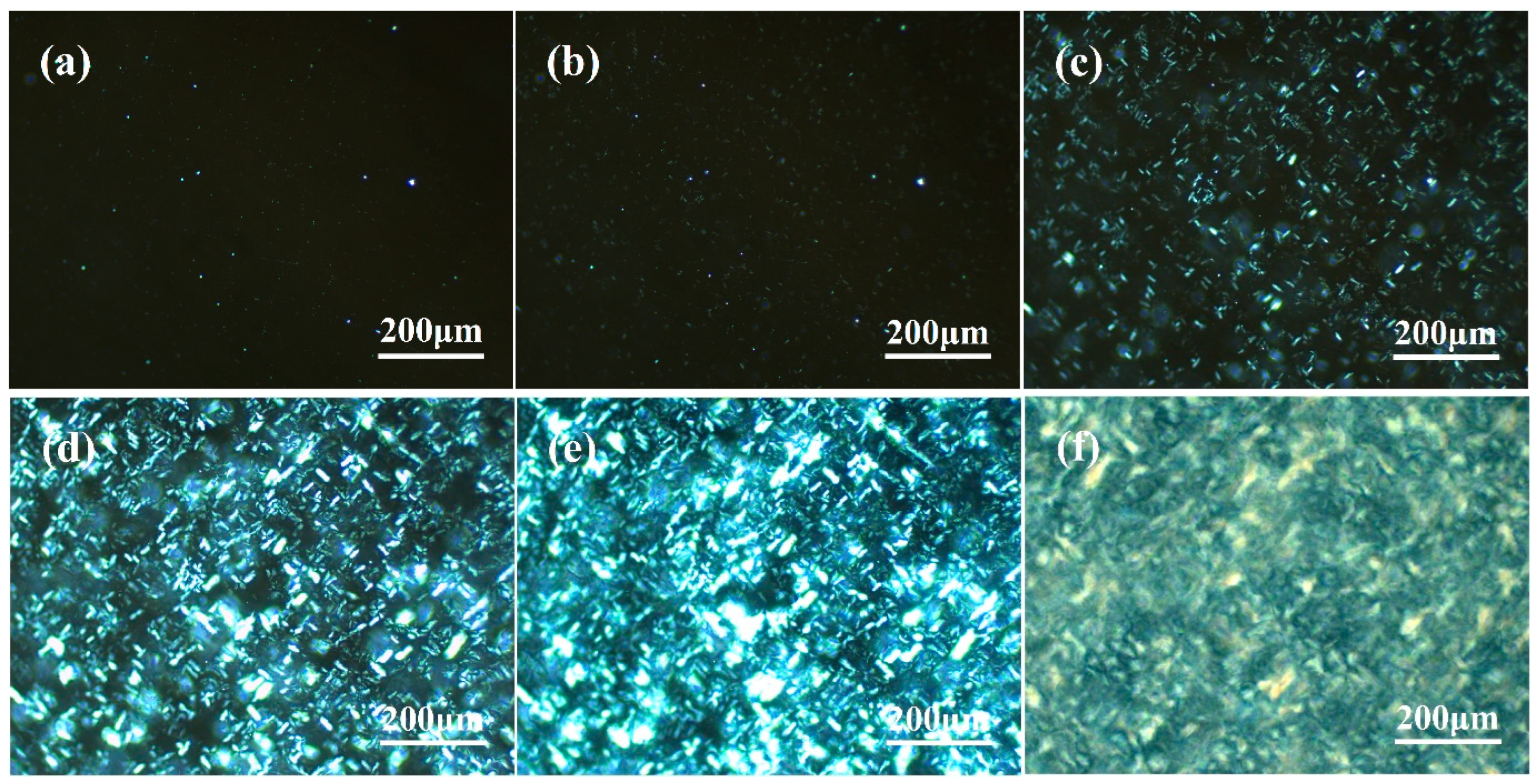
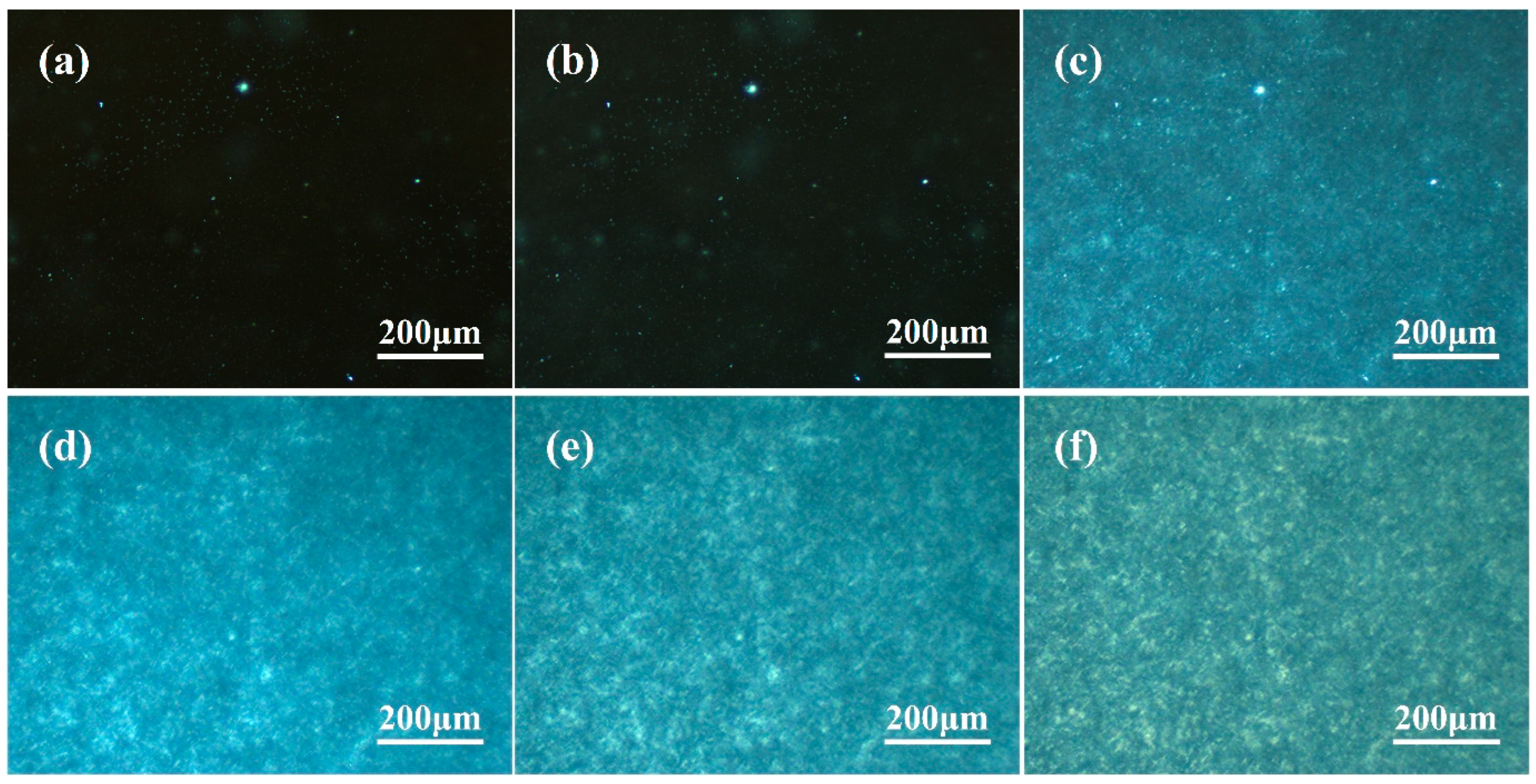
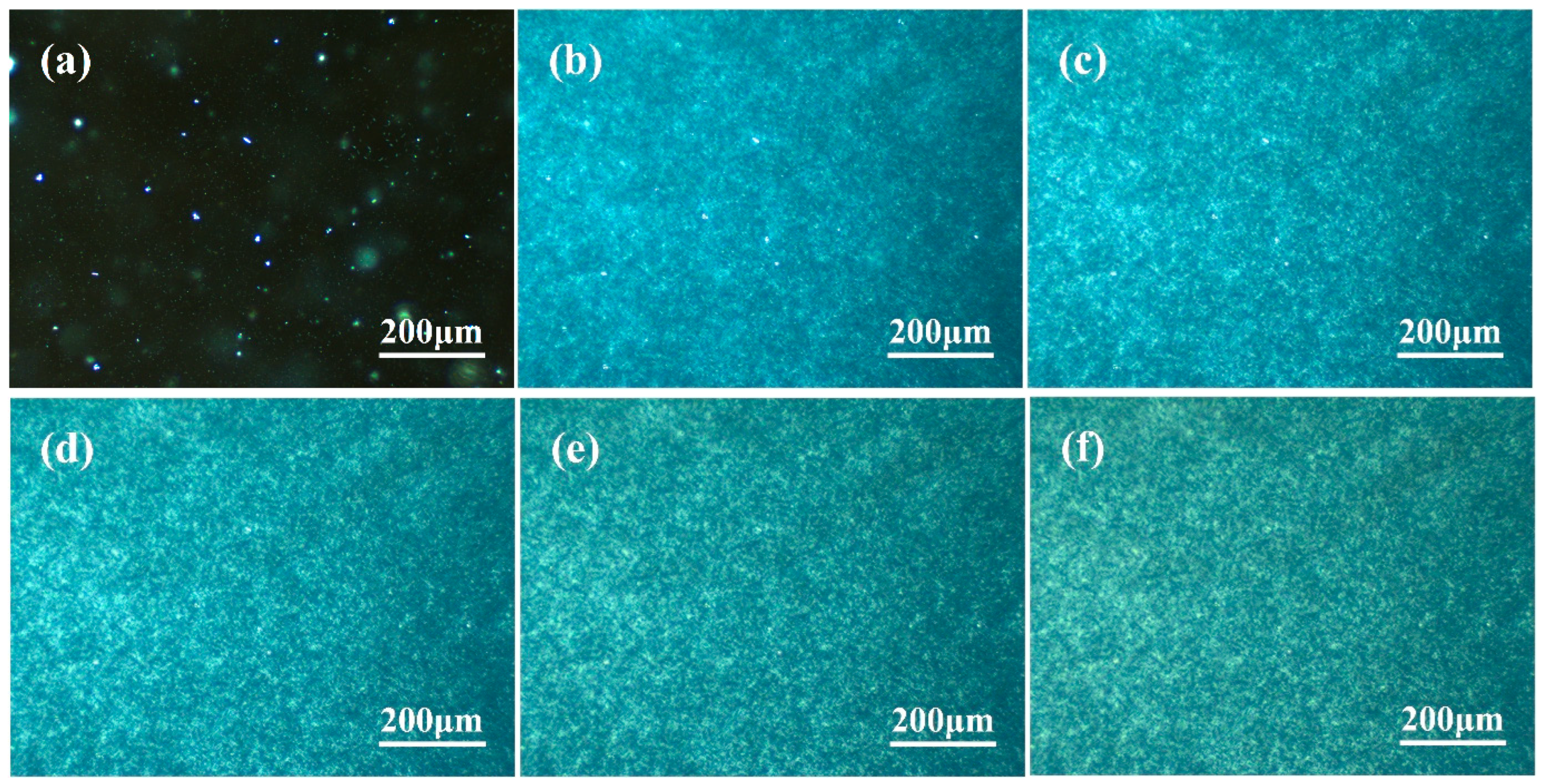
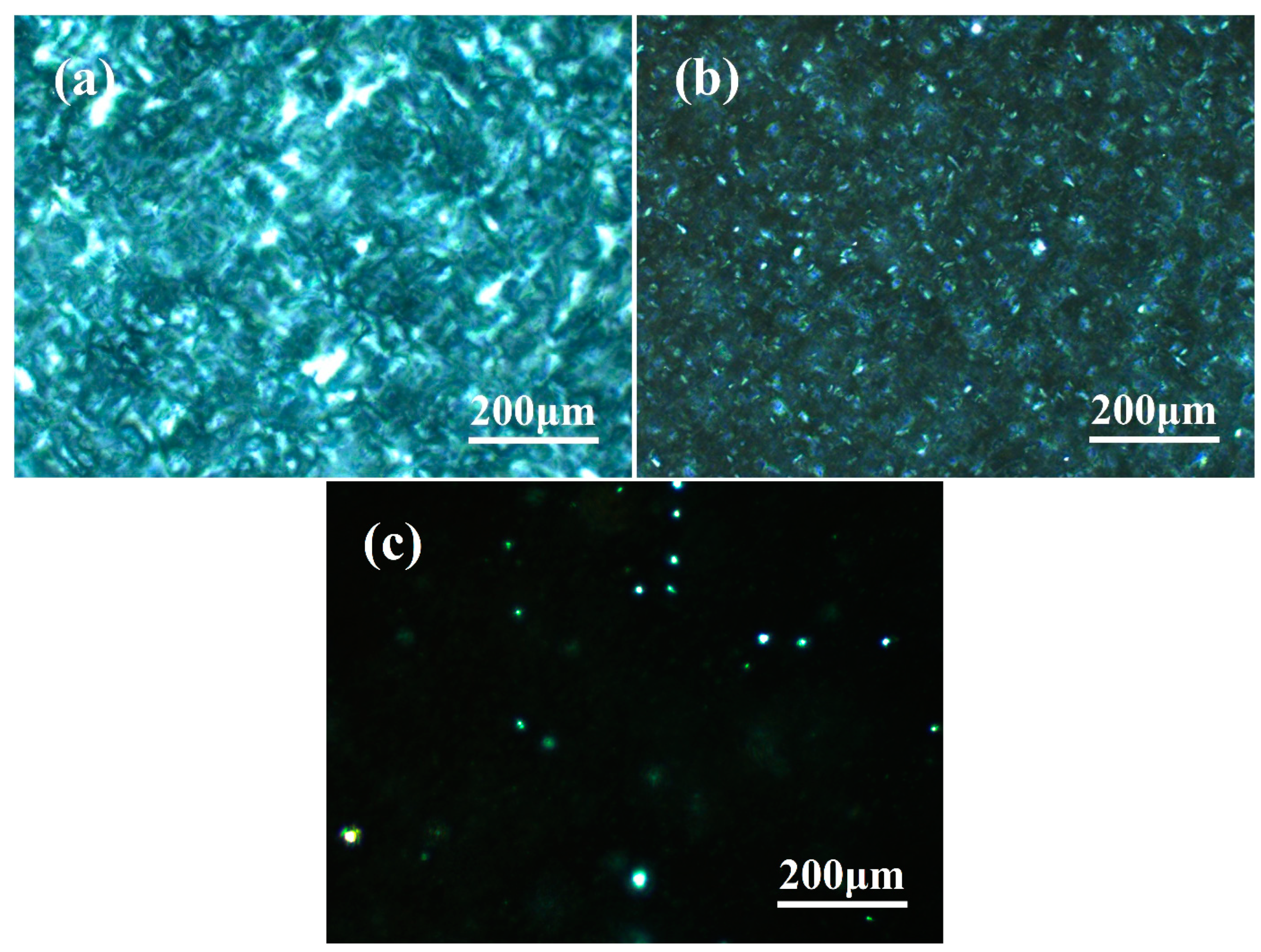
3.2. Observation of β-Crystal Morphology
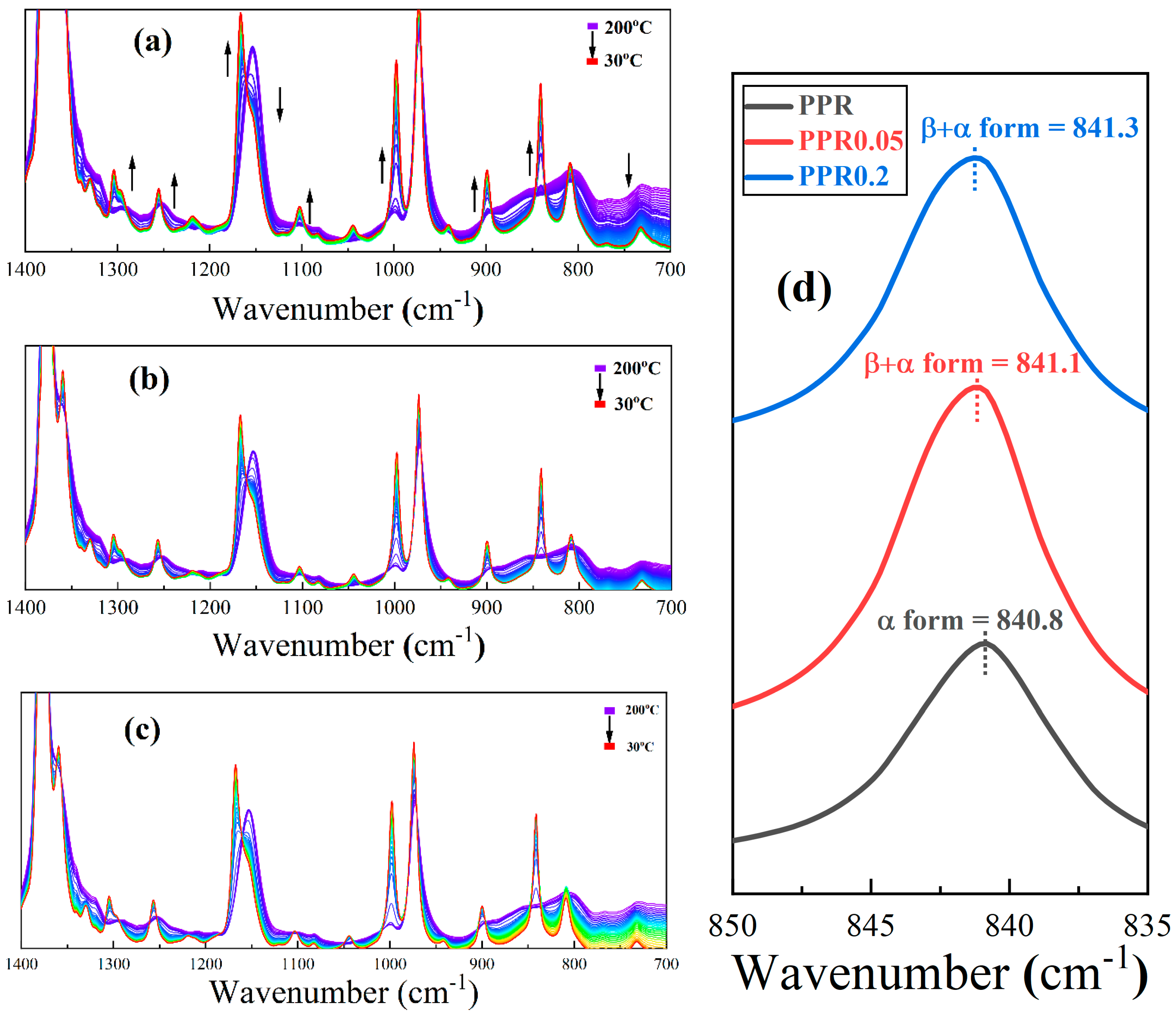
3.3. Analysis of High-Resolution In Situ FTIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Q.; Fan, J.; Zhang, Q.; Yi, J.; Feng, J. Toughened polypropylene random copolymer with olefin block copolymer. Mater. Des. 2016, 107, 295–301. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Xu, J.; Liu, C.; Xu, L. Experiment on frost heave failure mechanism of PPR water pipe. Eng. Fail. Anal. 2020, 117, 104831. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Bikiaris, D.N.; Chrissafis, K. Effect of crystalline structure of polypropylene random copolymers on mechanical properties and thermal degradation kinetics. Thermochim. Acta 2012, 543, 288–294. [Google Scholar] [CrossRef]
- Rabbani, F.A.; Sulaiman, M.; Tabasum, F.; Yasin, S.; Iqbal, T.; Shahbaz, M.; Mujtaba, M.A.; Bashir, S.; Fayaz, H.; Saleel, C.A. Investigation of tribo-mechanical performance of alkali treated rice-husk and polypropylene-random-copolymer based biocomposites. Heliyon 2023, 9, e22028. [Google Scholar] [CrossRef]
- Wu, B.; Zheng, X.; Xu, W.; Ren, Y.; Leng, H.; Liang, L.; Zheng, D.; Chen, J.; Jiang, H. β-Nucleated Polypropylene: Preparation, Nucleating Efficiency, Composite, and Future Prospects. Polymers 2023, 15, 3107. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wu, C.; Cheng, S.; Zhou, F.; Yan, H. Effects of toughening propylene/ethylene graft copolymer on the crystallization behavior and mechanical properties of polypropylene random-copolymerized with a small amount of ethylene. Polym. Test 2015, 41, 252–263. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Nie, M.; Wang, Q.; Lu, X. Step crystal growth on β-nucleated polypropylene homocomposites governing the crystalline compositions. J. Mater. Res. Technol. 2023, 25, 7192–7202. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nie, M.; Wang, Q. High-value recycling of isotactic polypropylene-based plastic waste as a crystallization promoter for high-performance polypropylene random copolymers. ACS Sustain. Chem. Eng. 2022, 10, 860–867. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Gong, H.; Xin, Z.; Shi, Y.; Zhou, S. The Crystallization Behavior of Isotactic Polypropylene Induced by a Novel Antinucleating Agent and Its Inhibition Mechanism of Nucleation. Ind. Eng. Chem. Res. 2015, 54, 7650–7657. [Google Scholar] [CrossRef]
- Zheng, L.; Fernandez-Ballester, L.; Peters, G.W.M.; Ma, Z. Concomitant Crystallization in Propylene/Ethylene Random Copolymer with Strong Flow at Elevated Temperatures. Ind. Eng. Chem. Res. 2018, 57, 6870–6877. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, F.; Shangguan, Y.; Lin, Y.; Zheng, Q.; Wang, X. Toughening mechanism in impact polypropylene copolymer containing a β-nucleating agent. RSC Adv. 2016, 6, 23117–23125. [Google Scholar] [CrossRef]
- Chen, H.B.; Karger-Kocsis, J.; Wu, J.S.; Varga, J. Fracture toughness of α- and β-phase polypropylene homopolymers and random- and block-copolymers. Polymer 2002, 43, 6505–6514. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Papageorgiou, G.Z.; Zhuravlev, E.; Bikiaris, D.; Schick, C.; Chrissafis, K. Competitive Crystallization of a Propylene/Ethylene Random Copolymer Filled with a β-Nucleating Agent and Multi-Walled Carbon Nanotubes. Conventional and Ultrafast DSC Study. J. Phys. Chem. B 2013, 117, 14875–14884. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhu, Y.; Wang, K.; Deng, H.; Chen, F.; Zhang, Q.; Fu, Q. Enhancement of β-nucleated crystallization in polypropylene random copolymer via adding isotactic polypropylene. Polymer 2012, 53, 4861–4870. [Google Scholar] [CrossRef]
- Varga, J. Supermolecular structure of isotactic polypropylene. J. Mater. Sci. 1992, 27, 2557–2579. [Google Scholar] [CrossRef]
- Li, H.; Jiang, S.; Wang, J.; Wang, D.; Yan, S. Optical microscopic study on the morphologies of isotactic polypropylene induced by its homogeneity fibers. Macromolecules 2003, 36, 2802–2807. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Wang, J.; Yan, S. Shear-induced interfacial structure of isotactic polypropylene (iPP) in iPP/fiber composites. Macromolecules 2006, 39, 8720–8726. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.B. Unusual hierarchical distribution of β-crystals and improved mechanical properties of injection-molded bars of isotactic polypropylene. RSC Adv. 2014, 4, 25135–25147. [Google Scholar] [CrossRef]
- Varga, J.; Ehrenstein, G.W. High-temperature hedritic crystallization of the β-modification of isotactic polypropylene. Colloid Polym. Sci. 1997, 275, 511–519. [Google Scholar] [CrossRef]
- Han, R.; Nie, M.; Wang, Q. Control over β-form hybrid shish-kebab crystals in polypropylene pipe via coupled effect of self-assembly β nucleating agent and rotation extrusion. J. Taiwan Inst. Chem. Eng. 2015, 52, 158–164. [Google Scholar] [CrossRef]
- Norton, D.R.; Keller, A. The spherulitic and lamellar morphology of melt-crystallized isotactic polypropylene. Polymer 1985, 26, 704–716. [Google Scholar] [CrossRef]
- Varga, J. β-modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. J. Macromol. Sci. Part B Phys. 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Qin, W.; Xin, Z.; Pan, C.; Sun, S.; Jiang, X.; Zhao, S. In situ formation of zinc phthalate as a highly dispersed β-nucleating agent for mechanically strengthened isotactic polypropylene. Chem. Eng. J. 2019, 358, 1243–1252. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Zhang, C.; Dong, Z.; Wang, K.; Wang, D. Morphological characteristics of β-nucleating agents governing the formation of the crystalline structure of isotactic polypropylene. Macromolecules 2021, 54, 6824–6834. [Google Scholar] [CrossRef]
- Horváth, F.; Bodrogi, D.; Hilt, B.; Pregi, E.; Menyhárd, A. Organogelators with dual β-and α-nucleating ability in isotactic polypropylene. J. Therm. Anal. Calorim. 2022, 147, 9451–9468. [Google Scholar] [CrossRef]
- Horváth, F.; Bihari, L.; Bodrogi, D.; Gombár, T.; Hilt, B.; Keszei, B.; Krain, T.; Simon, A.; Menyhárd, A. Effect of N, N′-dicyclohexyldicarboxamide homologues on the crystallization and properties of isotactic polypropylene. ACS Omega 2021, 6, 9053–9065. [Google Scholar] [CrossRef]
- Mishra, S.; Sonawane, S.H.; Singh, R.P. Studies on characterization of nano CaCO3 prepared by the in situ deposition technique and its application in PP-nano CaCO3 composites. J. Polym. Sci. Pt. B Polym. Phys. 2005, 43, 107–113. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H. Heterogeneous nucleation and self-nucleation of isotactic polypropylene with addition of nano-ZnO. J. Therm. Anal. Calorim. 2021, 146, 2115–2126. [Google Scholar] [CrossRef]
- Feng, J.; Chen, M.; Huang, Z.; Guo, Y.; Hu, H. Effects of mineral additives on the β-crystalline form of isotactic polypropylene. J. Appl. Polym. Sci. 2002, 85, 1742–1748. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.P.; Jin, B.Q.; Sheng, Q.; Wang, T.; Zhang, J. Influence of morphology evolution on the mechanical properties of beta nucleated isotactic polypropylene in presence of polypropylene random copolymer. Polym. Test 2016, 51, 13–19. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, T. Effect of entanglement upon branching on dispersibility, β-nucleating and mechanically strengthening ability of polystyrene in isotactic polypropylene. Polym. Bull. 2021, 78, 3259–3274. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Liu, Q.; Huang, Y.; Kong, M.; Yang, Q.; Li, G. Polydopamine particles as a β-nucleating agent and antioxidant for isotactic polypropylene. Chem. Eng. J. 2019, 363, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, G. The effect of a β-nucleator on the crystallization and melting behavior of ethylene/propylene random and block copolymers. Thermochim. Acta 1994, 235, 49–59. [Google Scholar] [CrossRef]
- Fan, J.; Feng, J. Study on β-nucleated controlled-rheological polypropylene random copolymer: Crystallization behavior and a possible degradation mechanism. Ind. Eng. Chem. Res. 2013, 52, 761–770. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Chen, X.; Zhao, Y.; Dong, X.; Müller, A.J.; Wang, D. Ethylene Comonomer-Directed Epitaxial Nucleation and Growth of β-Nucleated Isotactic Polypropylene. Macromolecules 2023, 56, 1965–1972. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.; Zhou, M.; Hong, R.; Zhang, J. The α-, β-, and γ-polymorphs of polypropylene–polyethylene random copolymer modified by two kinds of β-nucleating agent. Polym. Bull. 2019, 76, 865–881. [Google Scholar] [CrossRef]
- Zheng, H.; Zeng, F.; Chen, Z.; Kang, J.; Chen, J.; Cao, Y.; Xiang, M. Exploring the roles of molecular structure on the β-crystallization of polypropylene random copolymer. J. Polym. Res. 2017, 24, 225. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, F.; Lv, W.; Wu, H.; He, X.; Zhao, S. Effect of an active β-nucleating agent on the crystallization behavior of polypropylene random copolymer. J. Polym. Res. 2022, 29, 4. [Google Scholar] [CrossRef]
- Nyden, M.R.; Vanderhart, D.L.; Alamo, R.G. The conformational structures of defect-containing chains in the crystalline regions of isotactic polypropylene. Comput. Theor. Polym. Sci. 2001, 11, 175–189. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, G.; Vittoria, V. Structural and Morphological Changes during UV Irradiation of the Crystalline Helical Form of Syndiotactic Polypropylene. Macromolecules 2004, 37, 9826–9834. [Google Scholar] [CrossRef]
- Matsuba, G.; Kaji, K.; Nishida, K.; Kanaya, T.; Imai, M. Conformational Change and Orientation Fluctuations of Isotactic Polystyrene Prior to Crystallization. Polym. J. 1999, 31, 722–727. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, D.; Yao, H.; Zhu, P. In situ FTIR spectroscopic study of the regularity bands and partial-order melts of isotactic poly(propylene). Macromol. Rapid Commun. 2000, 21, 354–357. [Google Scholar] [CrossRef]
- Wang, X.X.; Yi, J.J.; Wang, L.; Yuan, Y.; Feng, J.C. Investigating the Nucleation Effect of DMDBS on Syndiotactic Polypropylene from the Perspective of Chain Conformation. Chin. J. Polym. Sci. 2020, 38, 1355–1364. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, D.; Fang, Y. In Situ FTIR Spectroscopic Study of the Conformational Change of Isotactic Polypropylene during the Crystallization Process. J. Phys. Chem. B 2001, 105, 12461–12463. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Zhao, L.; Yuan, W. In situ FT-IR spectroscopic study on the conformational changes of isotactic polypropylene in the presence of supercritical CO2. Eur. Polym. J. 2008, 44, 2619–2624. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, G.; Cong, Y.; Bai, L.; Li, L.; Yang, C. Shear-Induced Nucleation and Growth of Long Helices in Supercooled Isotactic Polypropylene. Macromolecules 2009, 42, 4751–4757. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, Y.; Yan, D. A possible explanation to the structure change of isotactic polypropylene occurring at about 135 °C. Polymer 2001, 42, 8595–8598. [Google Scholar] [CrossRef]
- He, P.; Xiao, Y.; Zhang, P.; Zhu, N.; Zhu, X.; Yan, D. In Situ Fourier transform infrared spectroscopic study of the thermal degradation of isotactic poly (propylene). Appl. Spectrosc. 2005, 59, 33–38. Available online: https://opg.optica.org/as/abstract.cfm?URI=as-59-1-33 (accessed on 18 February 2024). [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Yan, D.; Zhu, P.; Lu, Q. Influence of the order of polymer melt on the crystallization behavior: I. Double melting endotherms of isotactic polypropylene. Colloid Polym. Sci. 2001, 279, 292–296. [Google Scholar] [CrossRef]
- Kobayashi, M.; Akita, K.; Tadokoro, H. Infrared spectra and regular sequence lengths in isotactic polymer chains. Macromol. Chem. Phys. 1968, 118, 324–342. [Google Scholar] [CrossRef]
- Hanna, L.A.; Hendra, P.J.; Maddams, W.; Willis, H.A.; Zichy, V.; Cudby, M.E.A. Vibrational spectroscopic study of structural changes in isotactic polypropylene below the melting point. Polymer 1988, 29, 1843–1847. [Google Scholar] [CrossRef]
- Jones, B.A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Macromol. Chem. Phys. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Olley, R.H.; Bassett, D.C. An improved permanganic etchant for polyolefines. Polymer 1982, 23, 1707–1710. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Inoue, Y.; Onai, T.; Doshu, C.; Takahashi, H.; Uehara, H. Deconvolution Analyses of Differential Scanning Calorimetry Profiles of β-Crystallized Polypropylenes with Synchronized X-ray Measurements. Macromolecules 2007, 40, 2745–2750. [Google Scholar] [CrossRef]
- Wang, J.; Gahleitner, M.; Gloger, D.; Bernreitner, K. β-Nucleation of isotactic polypropylene: Chain structure effects on the effectiveness of two different nucleating agents. Express Polym. Lett. 2020, 14, 491–502. [Google Scholar] [CrossRef]
- Lotz, B. α and β phases of isotactic polypropylene: A case of growth kinetics ‘phase reentrency’ in polymer crystallization. Polymer 1998, 39, 4561–4567. [Google Scholar] [CrossRef]
- Yang, R.; Ding, L.; Chen, W.; Chen, L.; Zhang, X.; Li, J. Chain Folding in Main-Chain Liquid Crystalline Polyester with Strong π−π Interaction: An Efficient β-Nucleating Agent for Isotactic Polypropylene. Macromolecules 2017, 50, 1610–1617. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Wu, T.; Fu, Q. Yielding behavior of isotactic polypropylene at elevated temperature understood at the spherulite level. Polymer 2023, 281, 126150. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujiwara, Y.; Asano, T. Plastic deformation of oriented lamellae: 3. Drawing behaviour of β-phase isotactic polypropylene. Polymer 1983, 24, 925–929. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Zhao, Y.; Wang, K.; Dong, X.; Li, Z.; Wang, L.; Wang, D. Exploring the polymorphic behavior of a β-nucleated propylene-ethylene random copolymer under shear flow. Polym. Cryst. 2020, 3, e1015. [Google Scholar] [CrossRef]
- Wang, X.; Dai, J.; Chen, J.; Duan, J.; Yang, J.; Zhang, J.; Wang, Y. Abnormal Tensile Creep Behavior of Annealed β-Nucleated Isotactic Polypropylene. Ind. Eng. Chem. Res. 2015, 54, 4976–4987. [Google Scholar] [CrossRef]
- Qian, C.; Zhao, Y.; Wang, Z.; Liu, L.; Wang, D. Probing the difference of crystalline modifications and structural disorder of isotactic polypropylene via high-resolution FTIR spectroscopy. Polymer 2021, 224, 123722. [Google Scholar] [CrossRef]
- Varga, J.; Fujiwara, Y.; Ille, A. βα-bifurcation of growth during the spherulitic crystallization of polypropylene. Period. Polytech. Chem. Eng. 1990, 34, 255–271. Available online: https://pp.bme.hu/ch/article/view/2731/1836 (accessed on 18 February 2024).


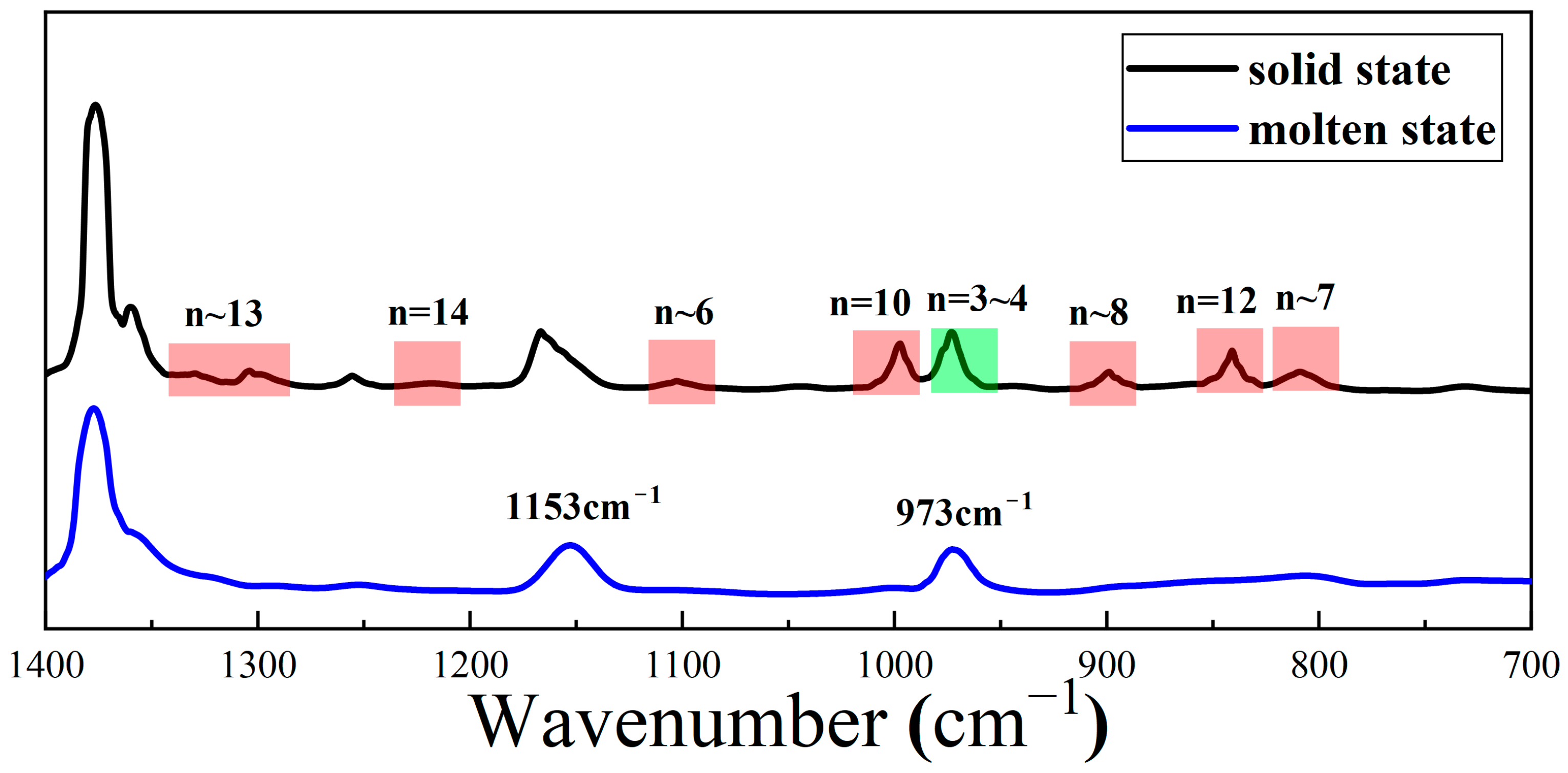

| Wave Number (cm−1) | Vibrational Mode | Corresponding Number of Monomers in Helices |
|---|---|---|
| 808 | v(C-C); c(CH) | ~7 |
| 841 | v(C-C); r(CH2); r(CH3) | 12 |
| 900 | v(C-C); c(CH) | ~8 |
| 940 | v(C-C); c(CH) | >14 |
| 973 | v(C-C); r(CH2); r(CH3) | 3–4 |
| 998 | v(C-C); r(CH2); r(CH3) | 10 |
| 1100 | v(C-CH3); r(CH3); δ(CH) | ~6 |
| 1167 | v(C-C); w(CH3) | ~6 |
| 1220 | t(CH2); δ(CH); v(C-CH3) | 14 |
| 1303 | δ(CH) | ~13 |
| 1330 | δ(CH); t(CH2) | ~13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Zheng, X.; Ren, Y.; Yu, H.; Wang, Y.; Jiang, H. The Crystallization Morphology and Conformational Changes of Polypropylene Random Copolymer Induced by a Novel β-Nucleating Agent. Polymers 2024, 16, 827. https://doi.org/10.3390/polym16060827
Wu B, Zheng X, Ren Y, Yu H, Wang Y, Jiang H. The Crystallization Morphology and Conformational Changes of Polypropylene Random Copolymer Induced by a Novel β-Nucleating Agent. Polymers. 2024; 16(6):827. https://doi.org/10.3390/polym16060827
Chicago/Turabian StyleWu, Bo, Xian Zheng, Yanwei Ren, Hailong Yu, Yubo Wang, and Huanfeng Jiang. 2024. "The Crystallization Morphology and Conformational Changes of Polypropylene Random Copolymer Induced by a Novel β-Nucleating Agent" Polymers 16, no. 6: 827. https://doi.org/10.3390/polym16060827
APA StyleWu, B., Zheng, X., Ren, Y., Yu, H., Wang, Y., & Jiang, H. (2024). The Crystallization Morphology and Conformational Changes of Polypropylene Random Copolymer Induced by a Novel β-Nucleating Agent. Polymers, 16(6), 827. https://doi.org/10.3390/polym16060827






