Nanocomposites of Conducting Polymers and 2D Materials for Flexible Supercapacitors
Abstract
1. Introduction
2. Nanocomposites of Conducting Polymers and 2D Materials
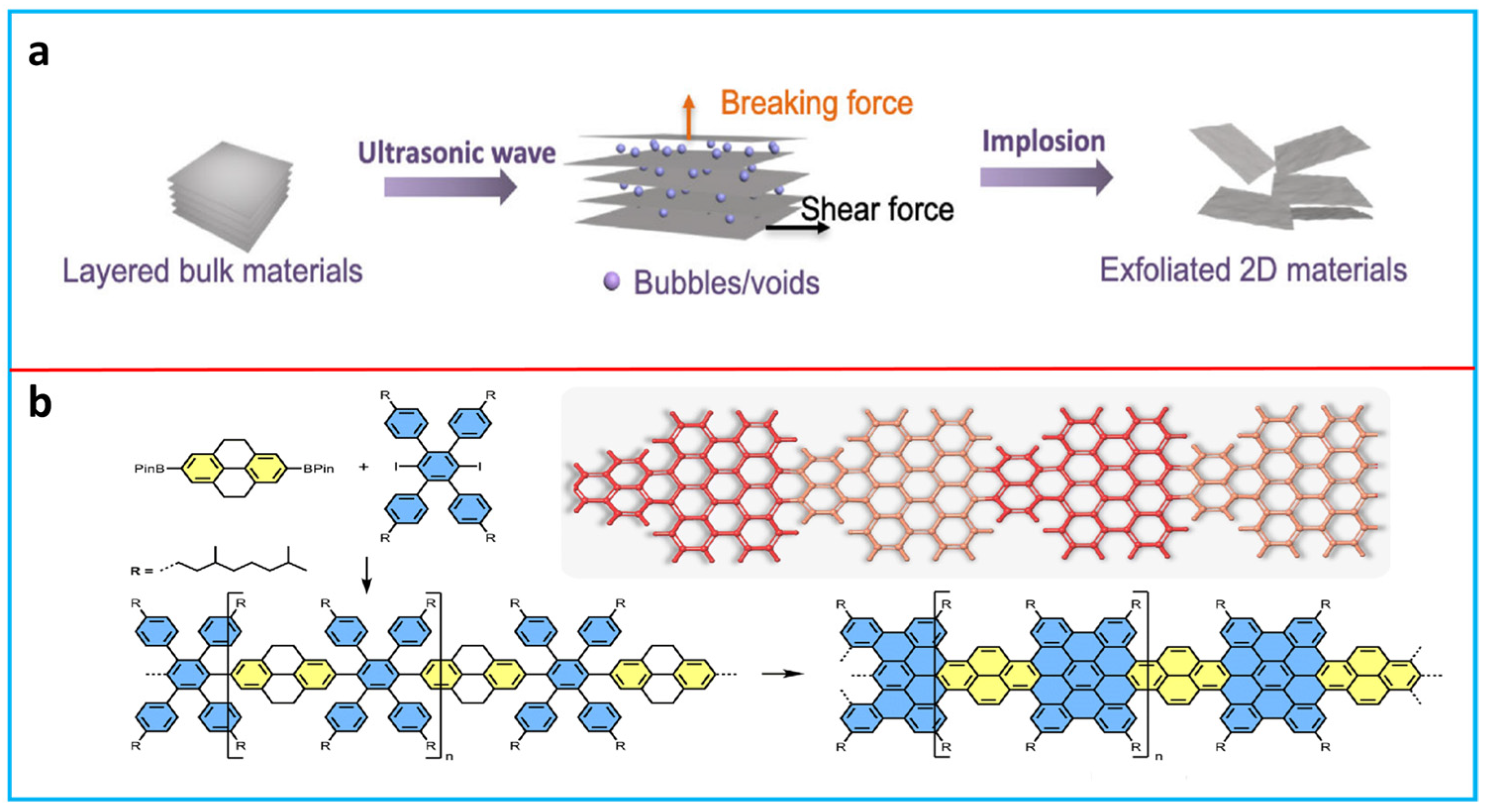
2.1. General Synthesis Routes
2.2. Macroscopic Assemblies of Nanocomposites
2.2.1. Fibers
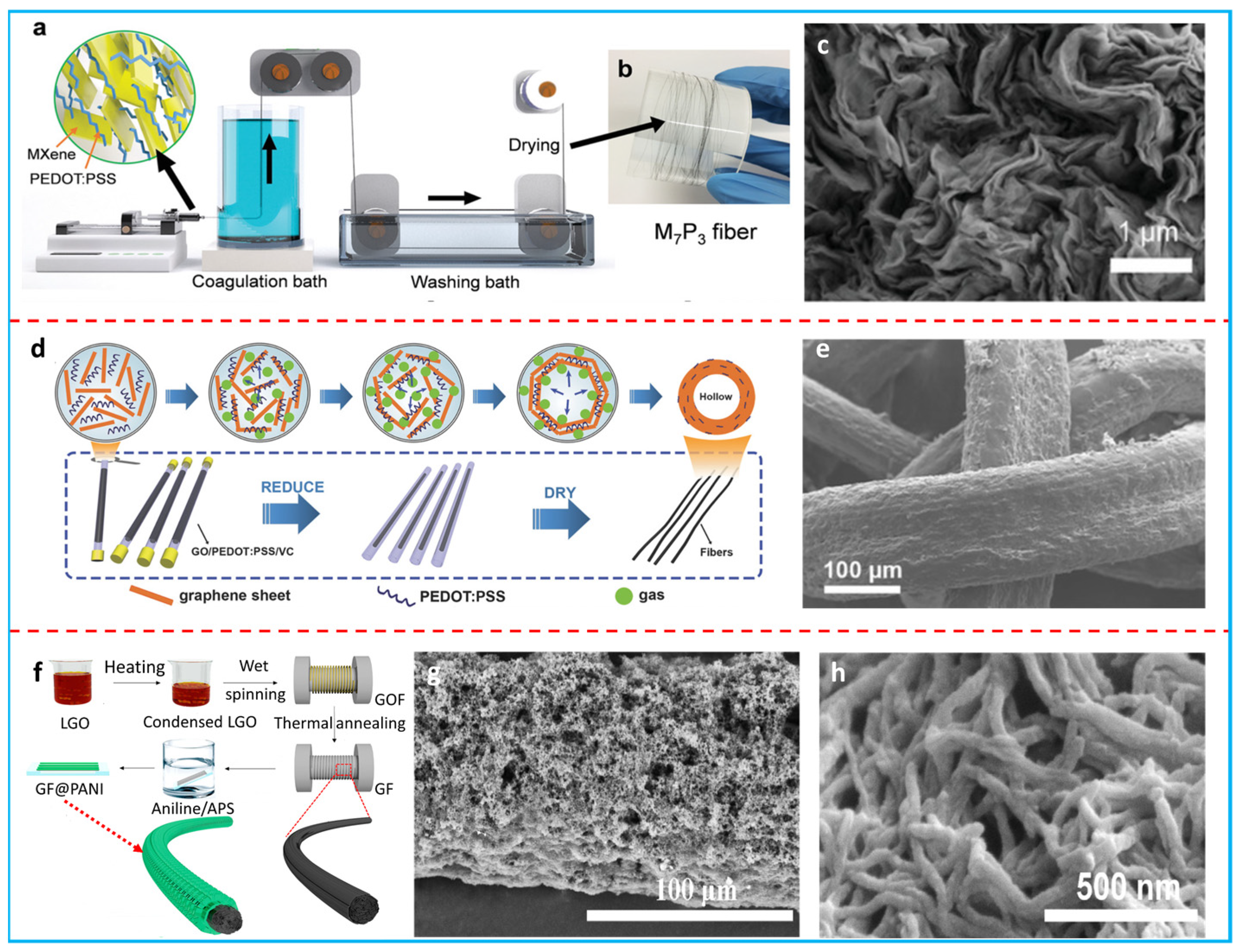
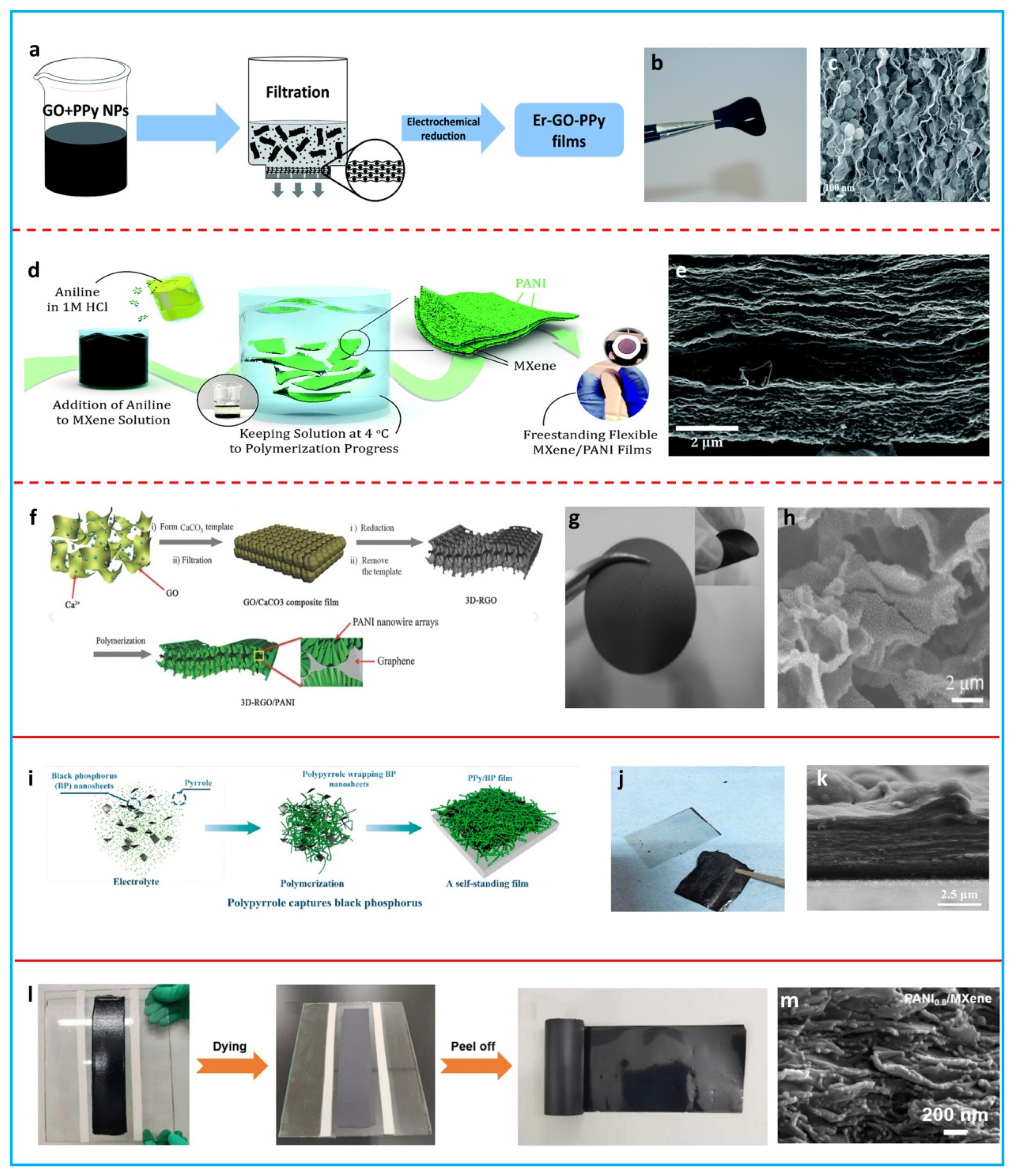
2.2.2. Films
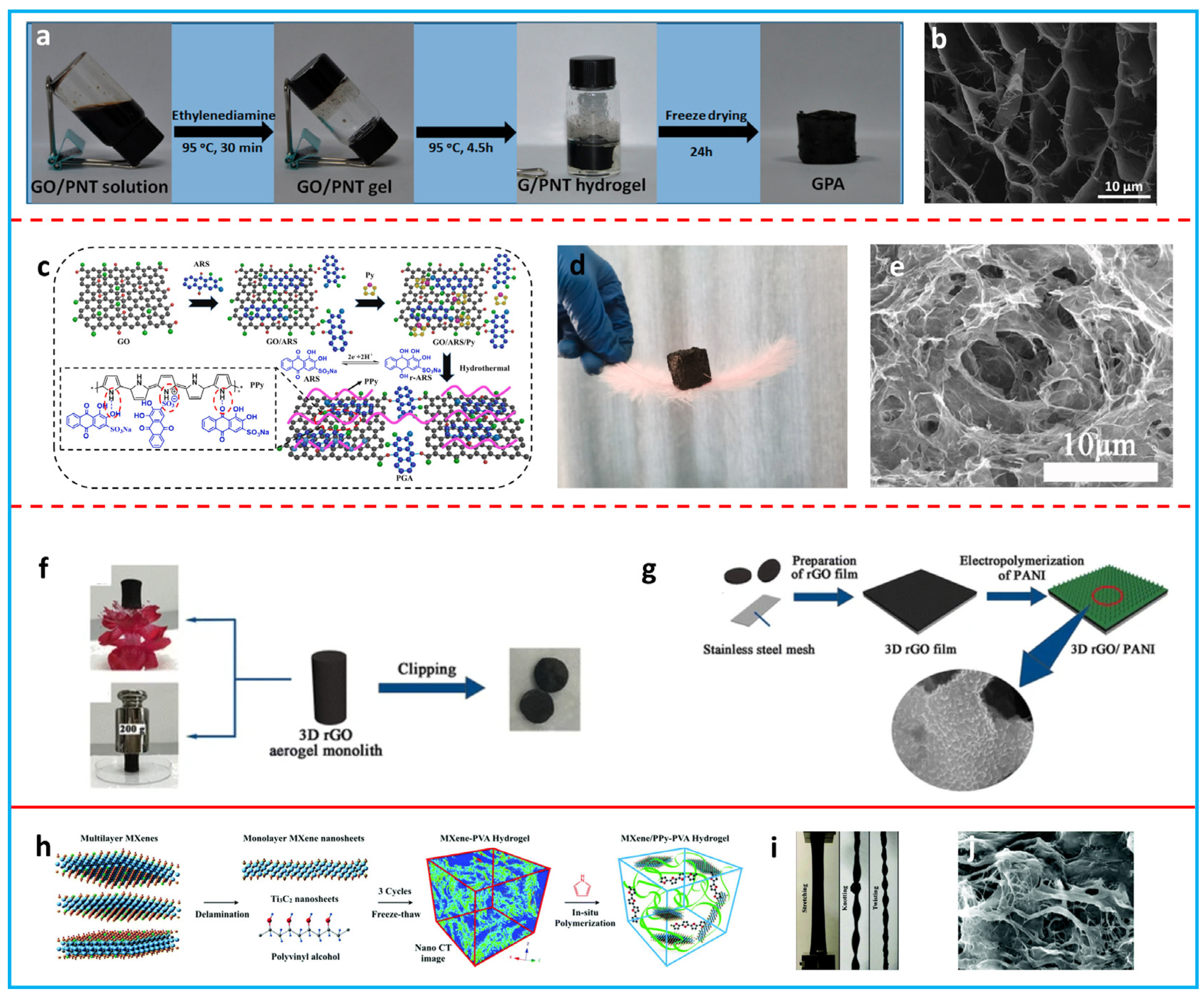
2.3. Aerogels/Hydrogels
3. FSCs Based on Macroscopic Assemblies of Nanocomposites
3.1. Fiber-Shaped FSCs
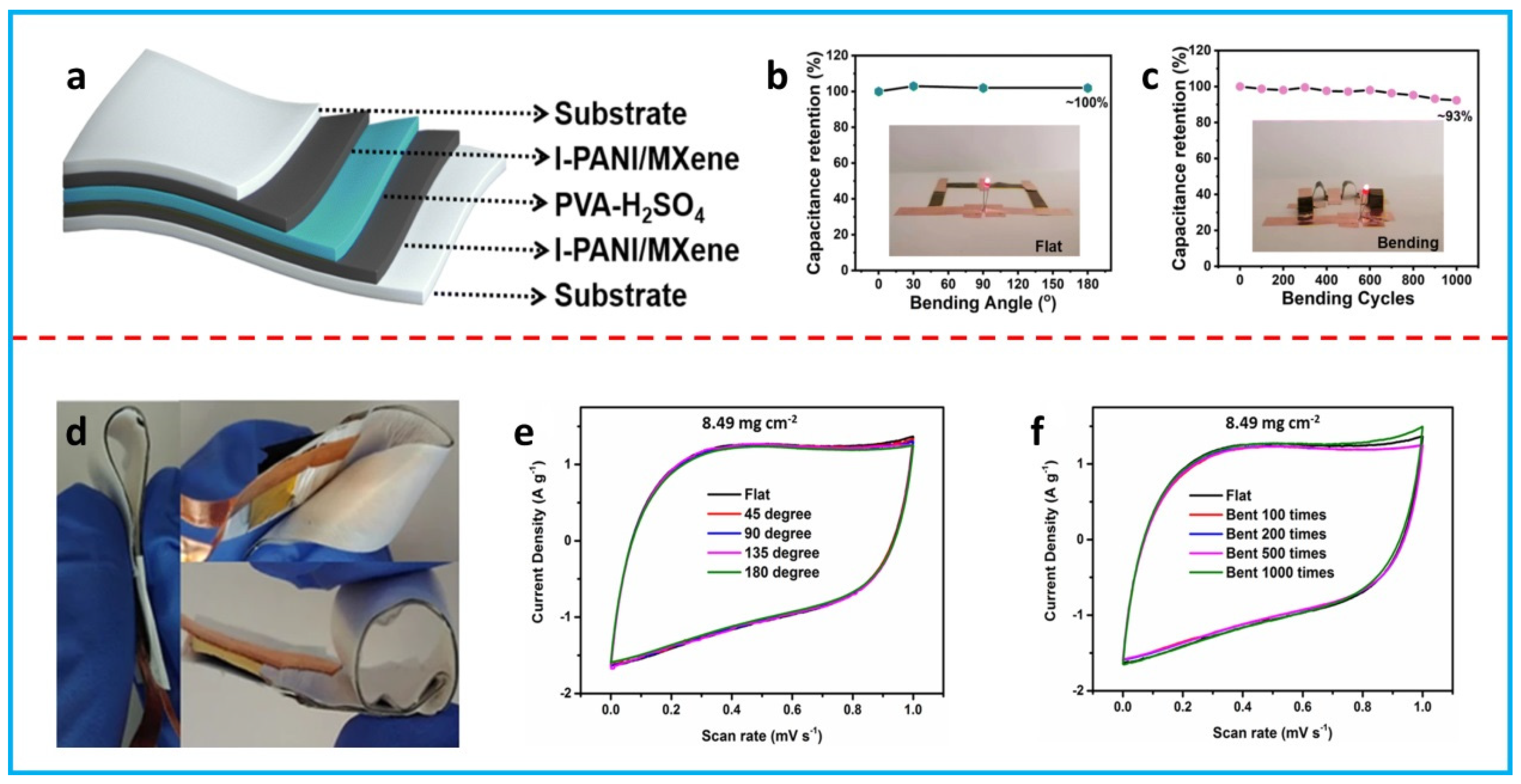
3.2. FSCs with a Sandwiched Structure
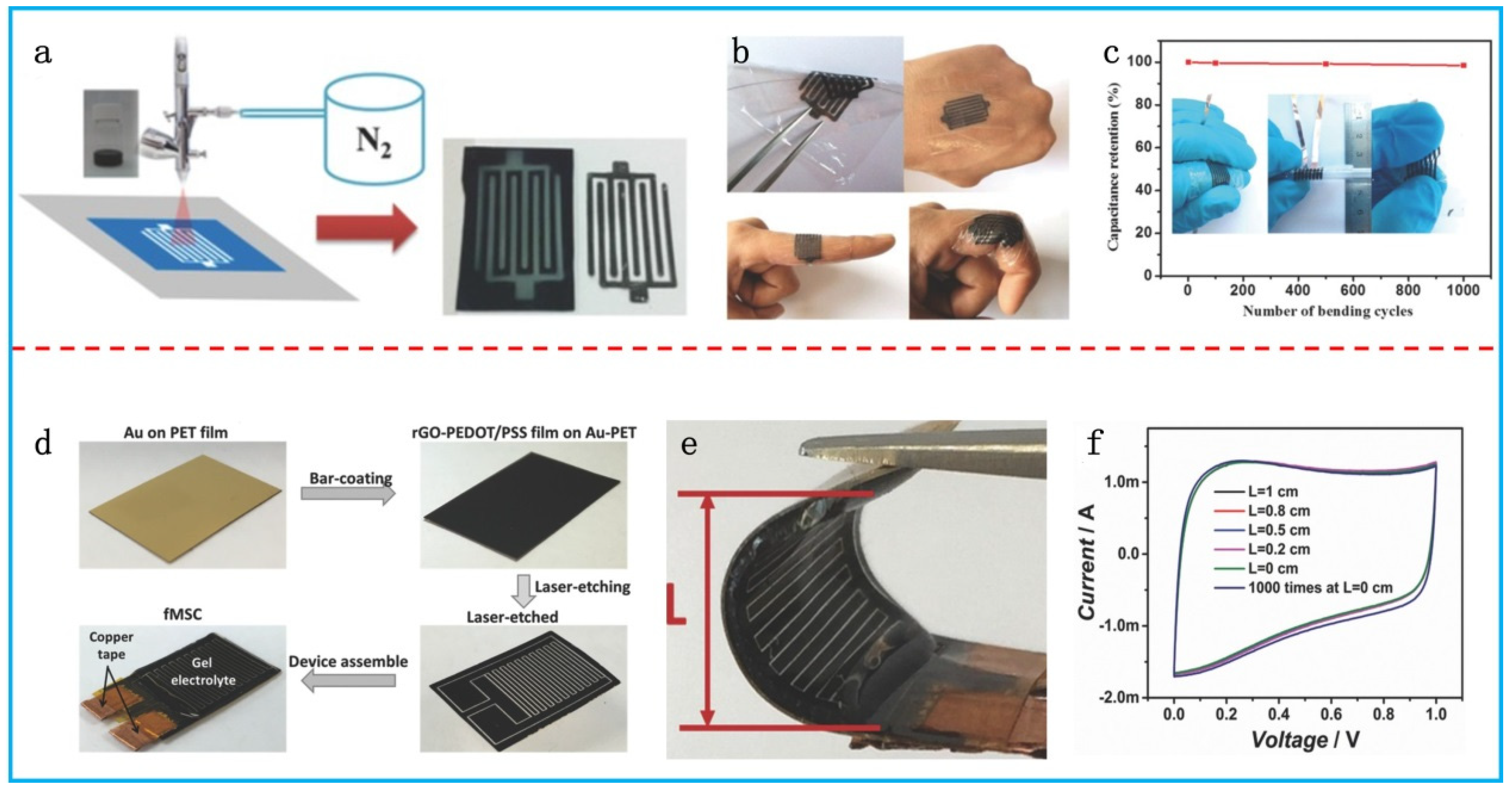
3.3. Flexible Micro-Supercapacitors
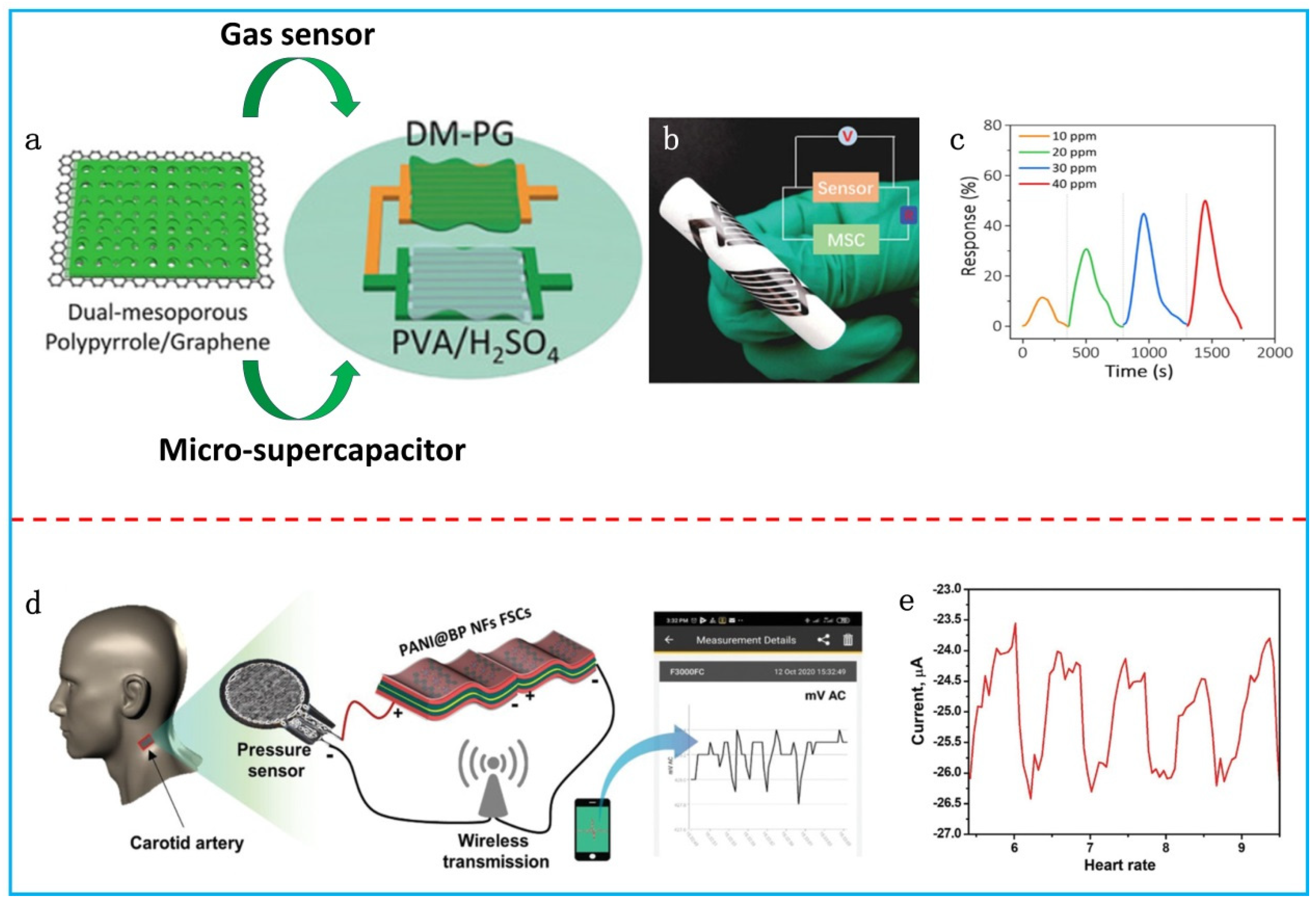
4. Integrated Systems
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, F.; Cheng, F.; Sun, Y.; Yang, X.; Lu, W.; Amal, R.; Dai, L. Recent advances in flexible batteries: From materials to applications. Nano Res. 2023, 16, 4821–4854. [Google Scholar] [CrossRef]
- Tareen, A.K.; Khan, K.; Iqbal, M.; Zhang, Y.; Long, J.; Mahmood, A.; Mahmood, N.; Xie, Z.; Li, C.; Zhang, H. Recent advance in two-dimensional MXenes: New horizons in flexible batteries and supercapacitors technologies. Energy Storage Mater. 2022, 53, 783–826. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Y.; Li, N.; Zhang, H.; Zhi, C. Recent Progress on Flexible and Wearable Supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yang, G.; Kim, C.-H.; Mahajan, R.L.; Lee, S.-Y.; Park, S.-J. Flexible solid-state hybrid supercapacitors for the internet of everything (IoE). Energy Environ. Sci. 2022, 15, 2233–2258. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, C.; Liang, J.; Wu, W. Electrode materials and device architecture strategies for flexible supercapacitors in wearable energy storage. J. Mater. Chem. A 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Sabahi Namini, A.; Le, Q.V.; Shokouhimehr, M.; Shahedi Asl, M.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Han, Y.; Dai, L. Conducting Polymers for Flexible Supercapacitors. Macromol. Chem. Phys. 2019, 220, 1800355. [Google Scholar] [CrossRef]
- Cheng, M.; Meng, Y.-N.; Wei, Z.-X. Conducting Polymer Nanostructures and their Derivatives for Flexible Supercapacitors. Isr. J. Chem. 2018, 58, 1299–1314. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ul Hoque, M.I.; Holze, R. Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors. Polymers 2023, 15, 730. [Google Scholar] [CrossRef] [PubMed]
- del Valle, M.A.; Gacitúa, M.A.; Hernández, F.; Luengo, M.; Hernández, L.A. Nanostructured Conducting Polymers and Their Applications in Energy Storage Devices. Polymers 2023, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, L.; Ciesielski, A.; Samorì, P. 2D Materials Beyond Graphene for High-Performance Energy Storage Applications. Adv. Energy Mater. 2016, 6, 1600671. [Google Scholar] [CrossRef]
- Augustyn, V.; Gogotsi, Y. 2D Materials with Nanoconfined Fluids for Electrochemical Energy Storage. Joule 2017, 1, 443–452. [Google Scholar] [CrossRef]
- Zhai, S.; Wei, L.; Karahan, H.E.; Chen, X.; Wang, C.; Zhang, X.; Chen, J.; Wang, X.; Chen, Y. 2D materials for 1D electrochemical energy storage devices. Energy Storage Mater. 2019, 19, 102–123. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.; Liu, D.; Li, X.; Wallace, G.G.; Zhang, S. Engineering 2D Materials: A Viable Pathway for Improved Electrochemical Energy Storage. Adv. Energy Mater. 2020, 10, 2002621. [Google Scholar] [CrossRef]
- Wu, M.; Zheng, W.; Hu, X.; Zhan, F.; He, Q.; Wang, H.; Zhang, Q.; Chen, L. Exploring 2D Energy Storage Materials: Advances in Structure, Synthesis, Optimization Strategies, and Applications for Monovalent and Multivalent Metal-Ion Hybrid Capacitors. Small 2022, 18, 2205101. [Google Scholar] [CrossRef]
- Lu, X.; Cai, M.; Wu, X.; Zhang, Y.; Li, S.; Liao, S.; Lu, X. Controllable Synthesis of 2D Materials by Electrochemical Exfoliation for Energy Storage and Conversion Application. Small 2023, 19, 2206702. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Lin, L.; Lei, W.; Zhang, S.; Liu, Y.; Wallace, G.G.; Chen, J. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Storage Mater. 2019, 19, 408–423. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, L.; Xiang, S.; Yu, S.; Johnson, H.M.; Wang, S.; Yin, J.; Zhao, J.; Luo, Y.; Chu, P.K. Unleashing the Potential of MXene-Based Flexible Materials for High-Performance Energy Storage Devices. Adv. Sci. 2024, 11, 2304874. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, W.; Xu, M.; Bai, S.; Chen, Y.; Tang, Z.; Wang, C.; Yang, Y.; Zhang, X.; Yuan, Y.; et al. Two-dimensional black phosphorus: Properties, fabrication and application for flexible supercapacitors. Chem. Eng. J. 2021, 412, 128744. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, X.; Shu, K.; Yu, C.; Wallace, G.G.; Wang, C. Conducting polymer composites for unconventional solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 4677–4699. [Google Scholar] [CrossRef]
- He, B.-L.; Zhou, Y.-K.; Zhou, W.-J.; Dong, B.; Li, H.-L. Preparation and characterization of ruthenium-doped polypyrrole composites for supercapacitor. Mater. Sci. Eng. A 2004, 374, 322–326. [Google Scholar] [CrossRef]
- Du, X.; Hao, X.; Wang, Z.; Ma, X.; Guan, G.; Abuliti, A.; Ma, G.; Liu, S. Highly stable polypyrrole film prepared by unipolar pulse electro-polymerization method as electrode for electrochemical supercapacitor. Synth. Met. 2013, 175, 138–145. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X. Preparation and characterization of polypyrrole films for three-dimensional micro supercapacitor. J. Power Sources 2009, 193, 924–929. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Zhang, D.; Hu, X.; Zhang, L.; Zhao, C.; He, Y.-S.; Zhang, W.; Yang, N.; Ma, Z.-F. Structural Tuning of a Flexible and Porous Polypyrrole Film by a Template-Assisted Method for Enhanced Capacitance for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2021, 13, 17726–17735. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, X.; Wang, C.; Zhou, Y.; Jia, X.; Jiang, L.; Liu, X.; Wallace, G.G. A smart cyto-compatible asymmetric polypyrrole membrane for salinity power generation. Nano Energy 2018, 53, 475–482. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Prokeš, J.; Zemek, J. In-situ polymerized polyaniline films. Synth. Met. 1999, 105, 195–202. [Google Scholar] [CrossRef]
- Wei, D.; Lin, X.; Li, L.; Shang, S.; Yuen, M.C.-w.; Yan, G.; Yu, X. Controlled growth of polypyrrole hydrogels. Soft Matter 2013, 9, 2832–2836. [Google Scholar] [CrossRef]
- Temmer, R.; Kiefer, R.; Aabloo, A.; Tamm, T. Direct chemical synthesis of pristine polypyrrole hydrogels and their derived aerogels for high power density energy storage applications. J. Mater. Chem. A 2013, 1, 15216–15219. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Słoniewska, A.; Pałys, B. Supramolecular polyaniline hydrogel as a support for urease. Electrochim. Acta 2014, 126, 90–97. [Google Scholar] [CrossRef]
- Guo, H.; He, W.; Lu, Y.; Zhang, X. Self-crosslinked polyaniline hydrogel electrodes for electrochemical energy storage. Carbon 2015, 92, 133–141. [Google Scholar] [CrossRef]
- Zhang, X.; Goux, W.J.; Manohar, S.K. Synthesis of Polyaniline Nanofibers by “Nanofiber Seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Processable stabilizer-free polyaniline nanofiber aqueous colloids. Chem. Commun. 2005, 3286–3288. [Google Scholar] [CrossRef]
- Wu, A.; Kolla, H.; Manohar, S.K. Chemical Synthesis of Highly Conducting Polypyrrole Nanofiber Film. Macromolecules 2005, 38, 7873–7875. [Google Scholar] [CrossRef]
- Li, M.; Wei, Z.; Jiang, L. Polypyrrole nanofiber arrays synthesized by a biphasic electrochemical strategy. J. Mater. Chem. 2008, 18, 2276–2280. [Google Scholar] [CrossRef]
- Zang, J.; Li, C.M.; Bao, S.-J.; Cui, X.; Bao, Q.; Sun, C.Q. Template-Free Electrochemical Synthesis of Superhydrophilic Polypyrrole Nanofiber Network. Macromolecules 2008, 41, 7053–7057. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Facile fabrication of polypyrrole nanotubes using reverse microemulsion polymerization. Chem. Commun. 2003, 720–721. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, Z.; Dai, T.; Lu, Y. Facile Fabrication of Functional Polypyrrole Nanotubes via a Reactive Self-Degraded Template. Macromol. Rapid Commun. 2005, 26, 1736–1740. [Google Scholar] [CrossRef]
- Zhang, X.; Manohar, S.K. Narrow Pore-Diameter Polypyrrole Nanotubes. J. Am. Chem. Soc. 2005, 127, 14156–14157. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wan, M.; Matthews, B.; Dai, L. Conducting Polyaniline Nanotubes by Template-Free Polymerization. Macromolecules 2001, 34, 675–677. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.; Wan, M. Formation Mechanism of Self-Assembled Polyaniline Micro/Nanotubes. Langmuir 2002, 18, 917–921. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, M. Chiral nanotubes of polyaniline synthesized by a template-free method. J. Mater. Chem. 2002, 12, 897–901. [Google Scholar] [CrossRef]
- Meng, L.; Lu, Y.; Wang, X.; Zhang, J.; Duan, Y.; Li, C. Facile Synthesis of Straight Polyaniline Nanostick in Hydrogel. Macromolecules 2007, 40, 2981–2983. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Yang, L.; Chen, W.; Yu, Q.; Liu, B. Mass production of two-dimensional materials beyond graphene and their applications. Nano Res. 2021, 14, 1583–1597. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.-M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z.; et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, G.; Liu, Z.; Ma, X.; Chen, J.; Zhang, Z.; Ma, X.; Li, F.; Cheng, H.-M.; Ren, W. Scalable Clean Exfoliation of High-Quality Few-Layer Black Phosphorus for a Flexible Lithium Ion Battery. Adv. Mater. 2016, 28, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, Y.; Zhu, H.; Preston, C.; Wan, J.; Fang, Z.; Hu, L. Scalable, printable, surfactant-free graphene ink directly from graphite. Nanotechnology 2013, 24, 205304. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Yang, M.; Chang, B.; Ai, Z.; Zhang, K.; Shao, Y.; Wang, S.; Wu, Y.; Hao, X. Ultrasonic-Ball Milling: A Novel Strategy to Prepare Large-Size Ultrathin 2D Materials. Small 2020, 16, 1906734. [Google Scholar] [CrossRef] [PubMed]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-Scale Production of Size-Controlled MoS2 Nanosheets by Shear Exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, J.; Pan, Y.; Cai, X.; Zou, X.; Cheng, H.-M.; Liu, B. Mass production of 2D materials by intermediate-assisted grinding exfoliation. Natl. Sci. Rev. 2019, 7, 324–332. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-Layer Semiconducting Nanosheets: High-Yield Preparation and Device Fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of Graphene Films on Copper Foils by Chemical Vapor Deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef]
- Obermann, S.; Zheng, W.; Melidonie, J.; Böckmann, S.; Osella, S.; Arisnabarreta, N.; Guerrero-León, L.A.; Hennersdorf, F.; Beljonne, D.; Weigand, J.J.; et al. Curved graphene nanoribbons derived from tetrahydropyrene-based polyphenylenes via one-pot K-region oxidation and Scholl cyclization. Chem. Sci. 2023, 14, 8607–8614. [Google Scholar] [CrossRef]
- Han, J.; Dou, Y.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Flexible CoAl LDH@PEDOT Core/Shell Nanoplatelet Array for High-Performance Energy Storage. Small 2013, 9, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.-P.; Chen, J.-F.; Yu, S.-H. Graphene-based macroscopic assemblies and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef] [PubMed]
- Usman, K.A.S.; Qin, S.; Henderson, L.C.; Zhang, J.; Hegh, D.Y.; Razal, J.M. Ti3C2Tx MXene: From dispersions to multifunctional architectures for diverse applications. Mater. Horiz. 2021, 8, 2886–2912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Seyedin, S.; Qin, S.; Wang, Z.; Moradi, S.; Yang, F.; Lynch, P.A.; Yang, W.; Liu, J.; Wang, X.; et al. Highly Conductive Ti3C2Tx MXene Hybrid Fibers for Flexible and Elastic Fiber-Shaped Supercapacitors. Small 2019, 15, 1804732. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cheng, J.; Wang, Z.; Li, Y.; Ni, W.; Wang, B. Highly-wrinkled reduced graphene oxide-conductive polymer fibers for flexible fiber-shaped and interdigital-designed supercapacitors. J. Power Sources 2018, 376, 117–124. [Google Scholar] [CrossRef]
- Yu, D.; Goh, K.; Wang, H.; Wei, L.; Jiang, W.; Zhang, Q.; Dai, L.; Chen, Y. Scalable synthesis of hierarchically structured carbon nanotube–graphene fibres for capacitive energy storage. Nat. Nanotechnol. 2014, 9, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Cheng, J.; Li, X.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A Fiber Supercapacitor with High Energy Density Based on Hollow Graphene/Conducting Polymer Fiber Electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef]
- Qiu, Y.; Xie, F.; Ji, Y.; Jia, X.; Li, H.; Zhang, M. Anchoring polypyrrole on nitrogen and sulfur codoped graphene fibers to construct flexible supercapacitors. Synth. Met. 2024, 301, 117519. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, T.; Zhang, P.; Wei, X.; Zhang, L.; Li, H. Polyaniline/graphene hybrid fibers as electrodes for flexible supercapacitors. Synth. Met. 2020, 268, 116484. [Google Scholar] [CrossRef]
- Zheng, X.; Yao, L.; Qiu, Y.; Wang, S.; Zhang, K. Core–Sheath Porous Polyaniline Nanorods/Graphene Fiber-Shaped Supercapacitors with High Specific Capacitance and Rate Capability. ACS Appl. Energy Mater. 2019, 2, 4335–4344. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wang, Q.; Du, P.; Wei, W.; Liu, P. PANI coated microporous graphene fiber capable of subjecting to external mechanical deformation for high performance flexible supercapacitors. Carbon 2019, 143, 147–153. [Google Scholar] [CrossRef]
- Teng, W.; Zhou, Q.; Lv, G.; Hu, P.; Du, Y.; Li, H.; Hu, Y.; Liu, W.; Wang, J. Hierarchical Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate)/Reduced graphene oxide/Polypyrrole hybrid electrode with excellent rate capability and cycling stability for fiber-shaped supercapacitor. J. Colloid Interface Sci. 2023, 636, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lv, G.; Wang, X.; Teng, W.; Hu, P.; Du, Y.; Li, H.; Hu, Y.; Liu, W.; Wang, J. Constructing a Hierarchical Ternary Hybrid of PEDOT:PSS/rGO/MoS2 as an Efficient Electrode for a Flexible Fiber-Shaped Supercapacitor. ACS Appl. Energy Mater. 2023, 6, 5797–5805. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, C.; Shu, K.; Zhao, C.; Jia, X.; Gambhir, S.; Wallace, G.G. A facile approach for fabrication of mechanically strong graphene/polypyrrole films with large areal capacitance for supercapacitor applications. RSC Adv. 2015, 5, 102643–102651. [Google Scholar] [CrossRef]
- Ge, Y.; Jalili, R.; Wang, C.; Zheng, T.; Chao, Y.; Wallace, G.G. A robust free-standing MoS2/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) film for supercapacitor applications. Electrochim. Acta 2017, 235, 348–355. [Google Scholar] [CrossRef]
- Li, S.; Zhao, C.; Shu, K.; Wang, C.; Guo, Z.; Wallace, G.G.; Liu, H. Mechanically strong high performance layered polypyrrole nano fibre/graphene film for flexible solid state supercapacitor. Carbon 2014, 79, 554–562. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors Based on Flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef]
- Luo, W.; Wei, Y.; Zhuang, Z.; Lin, Z.; Li, X.; Hou, C.; Li, T.; Ma, Y. Fabrication of Ti3C2Tx MXene/polyaniline composite films with adjustable thickness for high-performance flexible all-solid-state symmetric supercapacitors. Electrochim. Acta 2022, 406, 139871. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; El Ghazaly, A.; Fernandez-Rodriguez, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. High-Performance Ultrathin Flexible Solid-State Supercapacitors Based on Solution Processable Mo1.33C MXene and PEDOT:PSS. Adv. Funct. Mater. 2018, 28, 1703808. [Google Scholar] [CrossRef]
- Shu, K.; Chao, Y.; Chou, S.; Wang, C.; Zheng, T.; Gambhir, S.; Wallace, G.G. A “Tandem” Strategy to Fabricate Flexible Graphene/Polypyrrole Nanofiber Film Using the Surfactant-Exfoliated Graphene for Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 22031–22041. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 22123–22133. [Google Scholar] [CrossRef]
- Davies, A.; Audette, P.; Farrow, B.; Hassan, F.; Chen, Z.; Choi, J.-Y.; Yu, A. Graphene-Based Flexible Supercapacitors: Pulse-Electropolymerization of Polypyrrole on Free-Standing Graphene Films. J. Phys. Chem. C 2011, 115, 17612–17620. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, K.; Zhang, Y.; Wei, Z. Hierarchical Porous Graphene/Polyaniline Composite Film with Superior Rate Performance for Flexible Supercapacitors. Adv. Mater. 2013, 25, 6985–6990. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, X.; Li, S.; Urbankowski, P.; Li, J.; Xu, Y.; Gogotsi, Y. An Ultrafast Conducting Polymer@MXene Positive Electrode with High Volumetric Capacitance for Advanced Asymmetric Supercapacitors. Small 2020, 16, 1906851. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, D.; Zhang, M.; Sun, Y.; Jiang, M.; Du, Y.; Liu, J. Ice crystal-assisted intercalation of PANI within Ti3C2Tx MXene thin films for flexible supercapacitor electrodes with simultaneously high mechanical strength and rate performance. J. Mater. Chem. A 2023, 11, 1419–1429. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Huang, Y.; Wang, Z.; Li, H.; Huang, Q.; et al. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, J.; Zou, J.; He, Z.; Xu, C.; Liu, F.; Huang, Y.; Dong, L.; Wang, L.; Zhang, H. Self-Standing Polypyrrole/Black Phosphorus Laminated Film: Promising Electrode for Flexible Supercapacitor with Enhanced Capacitance and Cycling Stability. ACS Appl. Mater. Interfaces 2018, 10, 3538–3548. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, B.; Razal, J.M.; Xu, Q.; Zhao, C.; Hou, Y.; Seyedin, S.; Jalili, R.; Wallace, G.G.; Chen, J. High-Performance Flexible All-Solid-State Supercapacitor from Large Free-Standing Graphene-PEDOT/PSS Films. Sci. Rep. 2015, 5, 17045. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, X.; Bai, Y.; Xiao, H.; Liu, Y.; Yuan, G. Scalable fabrication of polyaniline nanodots decorated MXene film electrodes enabled by viscous functional inks for high-energy-density asymmetric supercapacitors. Chem. Eng. J. 2021, 405, 126664. [Google Scholar] [CrossRef]
- Ye, S.; Feng, J. Self-Assembled Three-Dimensional Hierarchical Graphene/Polypyrrole Nanotube Hybrid Aerogel and Its Application for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9671–9679. [Google Scholar] [CrossRef]
- He, Y.; Bai, Y.; Yang, X.; Zhang, J.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.-H. Holey graphene/polypyrrole nanoparticle hybrid aerogels with three-dimensional hierarchical porous structure for high performance supercapacitor. J. Power Sources 2016, 317, 10–18. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Li, W.; Wang, S.; Liu, L.; Li, T.; Han, Y. Facile synthesis of polypyrrole/graphene composite aerogel with Alizarin Red S as reactive dopant for high-performance flexible supercapacitor. J. Power Sources 2022, 517, 230737. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, Y.; Li, J.; Wei, G.; Klyui, N.I.; Han, W. Flexible Supercapacitors Based on Polyaniline Arrays Coated Graphene Aerogel Electrodes. Nanoscale Res. Lett. 2017, 12, 394. [Google Scholar] [CrossRef]
- Wu, X.; Lian, M. Highly flexible solid-state supercapacitor based on graphene/polypyrrole hydrogel. J. Power Sources 2017, 362, 184–191. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Chen, Y.; Cheng, K.; Yan, J.; Zhu, K.; Ye, K.; Wang, G.; Zhou, L.; Cao, D. Freestanding 3D Polypyrrole@reduced graphene oxide hydrogels as binder-free electrode materials for flexible asymmetric supercapacitors. J. Colloid Interface Sci. 2019, 536, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, J.; Zhang, W.; Zhang, P.; He, W.; Chen, J.; Sun, Z. A multidimensional nanostructural design towards electrochemically stable and mechanically strong hydrogel electrodes. Nanoscale 2020, 12, 6637–6643. [Google Scholar] [CrossRef]
- Deng, Y.; Shang, T.; Wu, Z.; Tao, Y.; Luo, C.; Liang, J.; Han, D.; Lyu, R.; Qi, C.; Lv, W.; et al. Fast Gelation of Ti3C2Tx MXene Initiated by Metal Ions. Adv. Mater. 2019, 31, 1902432. [Google Scholar] [CrossRef]
- Yu, D.; Qian, Q.; Wei, L.; Jiang, W.; Goh, K.; Wei, J.; Zhang, J.; Chen, Y. Emergence of fiber supercapacitors. Chem. Soc. Rev. 2015, 44, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, L.; Ren, J.; Qiu, L.; Lin, H.; Peng, H. Flexible, weavable and efficient microsupercapacitor wires based on polyaniline composite fibers incorporated with aligned carbon nanotubes. J. Mater. Chem. A 2013, 1, 258–261. [Google Scholar] [CrossRef]
- Liu, N.; Gao, Y. Recent Progress in Micro-Supercapacitors with In-Plane Interdigital Electrode Architecture. Small 2017, 13, 1701989. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, Z.-S.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible In-Plane Micro-Supercapacitors by Direct Printing of Solution-Processable Electrochemically Exfoliated Graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Kurra, N.; Hota, M.K.; Alshareef, H.N. Conducting polymer micro-supercapacitors for flexible energy storage and Ac line-filtering. Nano Energy 2015, 13, 500–508. [Google Scholar] [CrossRef]
- Gao, C.; Gao, J.; Shao, C.; Xiao, Y.; Zhao, Y.; Qu, L. Versatile origami micro-supercapacitors array as a wind energy harvester. J. Mater. Chem. A 2018, 6, 19750–19756. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, B.; Xu, Q.; Hou, Y.; Zhao, C.; Beirne, S.; Shu, K.; Jalili, R.; Wallace, G.G.; Razal, J.M.; et al. Facile Fabrication of Flexible Microsupercapacitor with High Energy Density. Adv. Mater. Technol. 2016, 1, 1600166. [Google Scholar] [CrossRef]
- Liu, L.; Ye, D.; Yu, Y.; Liu, L.; Wu, Y. Carbon-based flexible micro-supercapacitor fabrication via mask-free ambient micro-plasma-jet etching. Carbon 2017, 111, 121–127. [Google Scholar] [CrossRef]
- Salles, P.; Quain, E.; Kurra, N.; Sarycheva, A.; Gogotsi, Y. Automated Scalpel Patterning of Solution Processed Thin Films for Fabrication of Transparent MXene Microsupercapacitors. Small 2018, 14, 1802864. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Xie, Z.; Zhang, L.; Ma, X.; Wu, X.; Guo, Y.; Song, W.; Li, Z.; Cao, T. Microfluidic etching for fabrication of flexible and all-solid-state micro supercapacitor based on MnO2 nanoparticles. Nanoscale 2011, 3, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Gao, J.; Shi, X.; Chang, J.; Dong, Y.; Zheng, S.; Wang, X.; Feng, L.; Wu, Z.-S. Hierarchical Ordered Dual-Mesoporous Polypyrrole/Graphene Nanosheets as Bi-Functional Active Materials for High-Performance Planar Integrated System of Micro-Supercapacitor and Gas Sensor. Adv. Funct. Mater. 2020, 30, 1909756. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Křípalová, K.; Hermanová, S.; Mayorga-Martinez, C.C.; Pumera, M. Real-Time Biomonitoring Device Based on 2D Black Phosphorus and Polyaniline Nanocomposite Flexible Supercapacitors. Small 2021, 17, 2102337. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Xu, R.; Wan, T.; Yuan, W.; Shu, K.; Boonprakob, N.; Zhao, C. Nanocomposites of Conducting Polymers and 2D Materials for Flexible Supercapacitors. Polymers 2024, 16, 756. https://doi.org/10.3390/polym16060756
Zhu H, Xu R, Wan T, Yuan W, Shu K, Boonprakob N, Zhao C. Nanocomposites of Conducting Polymers and 2D Materials for Flexible Supercapacitors. Polymers. 2024; 16(6):756. https://doi.org/10.3390/polym16060756
Chicago/Turabian StyleZhu, Haipeng, Ruiqi Xu, Tao Wan, Wenxiong Yuan, Kewei Shu, Natkritta Boonprakob, and Chen Zhao. 2024. "Nanocomposites of Conducting Polymers and 2D Materials for Flexible Supercapacitors" Polymers 16, no. 6: 756. https://doi.org/10.3390/polym16060756
APA StyleZhu, H., Xu, R., Wan, T., Yuan, W., Shu, K., Boonprakob, N., & Zhao, C. (2024). Nanocomposites of Conducting Polymers and 2D Materials for Flexible Supercapacitors. Polymers, 16(6), 756. https://doi.org/10.3390/polym16060756






