Specific FRET Probes Sensitive to Chitosan-Based Polymeric Micelles Formation, Drug-Loading, and Fine Structural Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
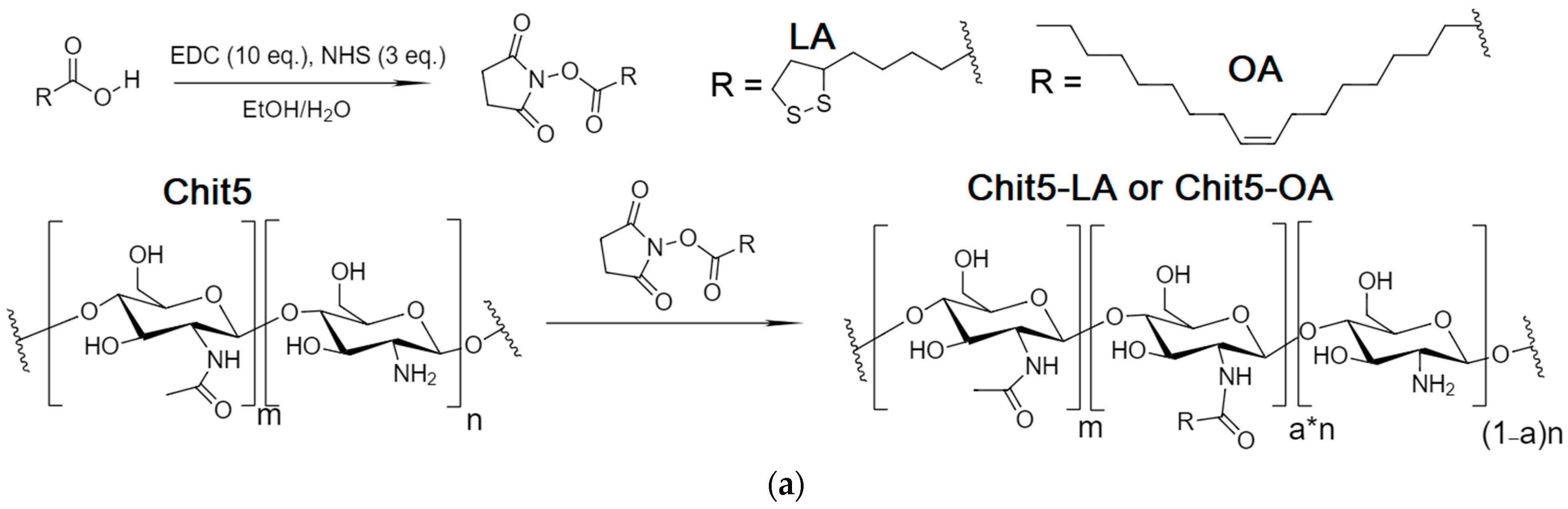
2.2. Synthesis of Chitosan Grafted with Lipoic Acid (Chit5-LA) and Oleic Acid (Chit5-OA)—Micelles Preparation
2.3. Characterization of Chitosan Grafted with Lipoic Acid (Chit5-LA)
2.4. FRET Probes for Determination of CMC for Micelles Formed from Surfactants and Chit5-LA
2.4.1. Determination of CMC for Micelles Formed from Surfactants
2.4.2. Determination of CMC for Polymeric Micelles
2.4.3. Flow Cytometry for Micelle Formation Study
2.4.4. Release of R6G from Micelles by Addition of Reduced Glutathione as Thiol-Disulfide Exchange Agent (Tumor Microenvironment Model)
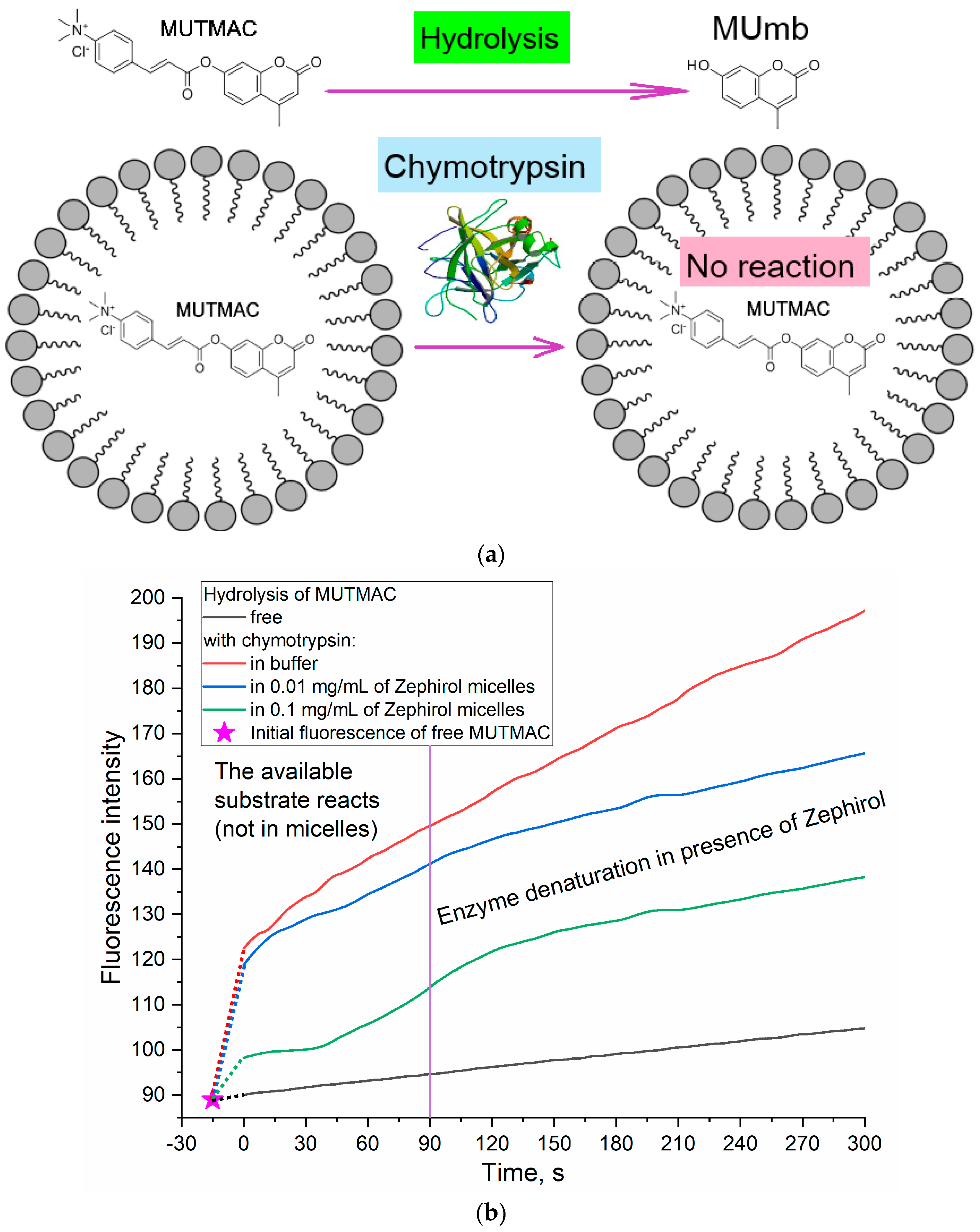
2.5. Enzyme Activity Studies for Determination of the Fluorophore Inclusion Degree in Micelles
3. Results
3.1. Article Design
3.2. FRET as an Indicator of Micelle Formation in Surfactants Solution
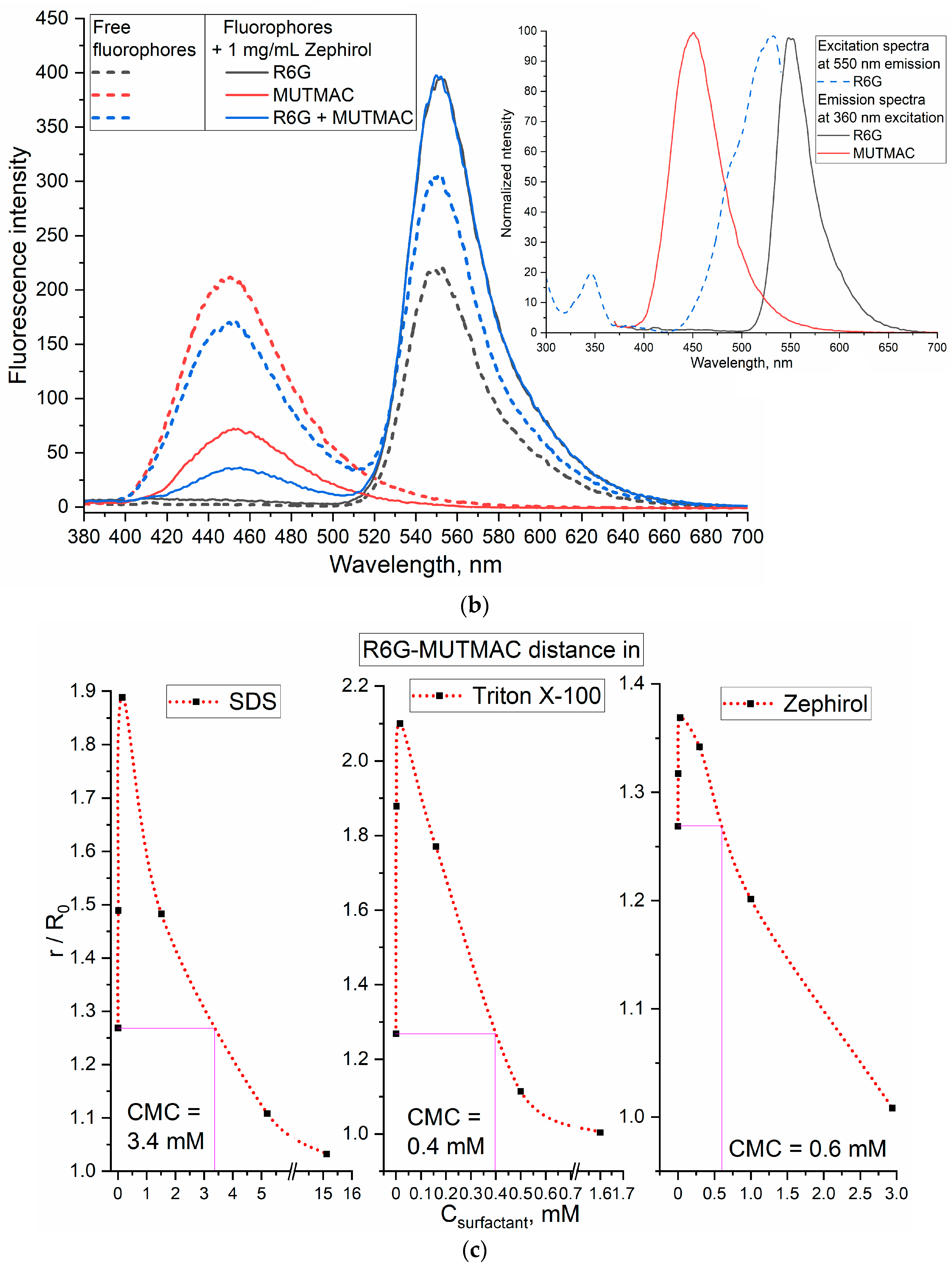
3.2.1. MUTMAC–R6G Pair
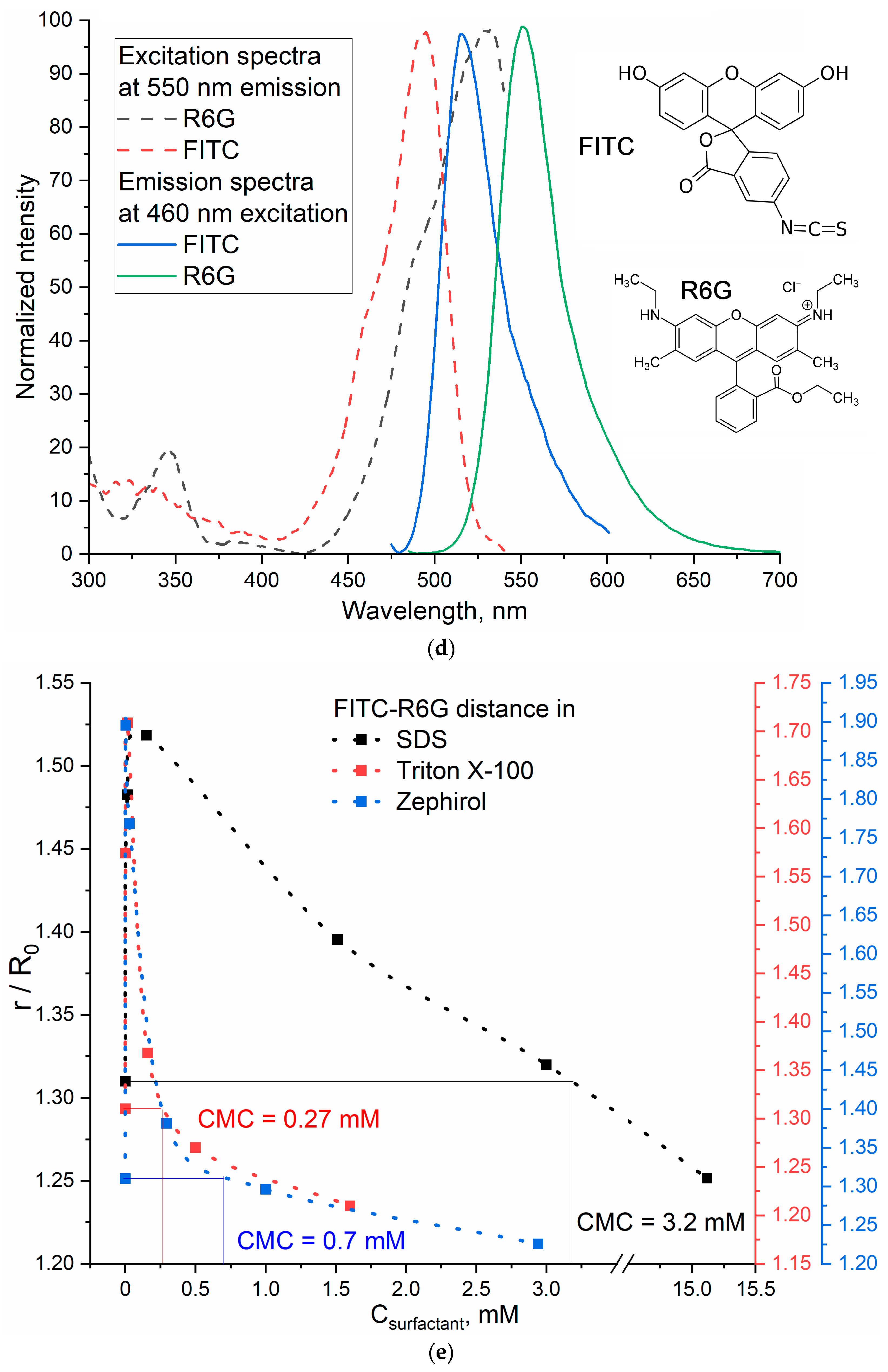
3.2.2. FITC–R6G Pair
3.2.3. Comparison of CMC Values Obtained Using Two FRET Probes and the Literature Data
| Surfactant | CPMC, µM | CMC, mM | ||
|---|---|---|---|---|
| FRET Probe 1: MUTMAC + R6G | FRET Probe 2: FITC + R6G | Literature Data | ||
| SDS (sodium dodecyl sulfate) | 15 ± 4 | 3.4 ± 0.1 | 3.2 ± 0.3 | 3.32 ± 0.01 [51] |
| Triton X-100 | 16 ± 3 | 0.39 ± 0.05 | 0.27 ± 0.08 | 0.3 ± 0.01 [51] |
| Zephirol (benzalkonium chloride) | 4 ± 1 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.6 mM [52] |
3.3. Determination of the Fluorophore Inclusion Degree in Micelles by Enzymatic Activity
3.4. Formation of Polymeric Micelles as Assessed by FRET Probes
3.4.1. Self-Assembled Amphiphilic Chitosan Grafted with Lipoic and Oleic Acid Residues
3.4.2. Polymeric Micelles Formation and S-S Stitching Detection Using MUTMAC–R6G Probe
3.5. A Comparison of the Proposed FRET Probe Technique with Other Techniques Described in the Literature to Study the Properties of Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Hohng, S.; Ha, T. A practical guide to single-molecule FRET. Nat. Methods 2008, 5, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.-P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Y.; Song, Y.; Liu, Y. A new trend to determine biochemical parameters by quantitative FRET assays. Acta Pharmacol. Sin. 2015, 36, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhu, X.; Zhang, L.; Liu, M. Switchable Circularly Polarized Luminescence in Supramolecular Gels through Photomodulated FRET. ACS Appl. Mater. Interfaces 2021, 13, 15501–15508. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Watrob, H.M.; Pan, C.P.; Barkley, M.D. Two-step FRET as a structural tool. J. Am. Chem. Soc. 2003, 125, 7336–7343. [Google Scholar] [CrossRef]
- Shrestha, D.; Jenei, A.; Nagy, P.; Vereb, G.; Szöllősi, J. Understanding FRET as a research tool for cellular studies. Int. J. Mol. Sci. 2015, 16, 6718–6756. [Google Scholar] [CrossRef]
- Panniello, A.; Trapani, M.; Cordaro, M.; Dibenedetto, C.N.; Tommasi, R.; Ingrosso, C.; Fanizza, E.; Grisorio, R.; Collini, E.; Agostiano, A.; et al. High-Efficiency FRET Processes in BODIPY-Functionalized Quantum Dot Architectures. Chem.—A Eur. J. 2021, 27, 2371–2380. [Google Scholar] [CrossRef]
- Ngan, V.T.T.; Chiou, P.Y.; Ilhami, F.B.; Bayle, E.A.; Shieh, Y.T.; Chuang, W.T.; Chen, J.K.; Lai, J.Y.; Cheng, C.C. A CO2-Responsive Imidazole-Functionalized Fluorescent Material Mediates Cancer Chemotherapy. Pharmaceutics 2023, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Ilhami, F.B.; Bayle, E.A.; Cheng, C.C. Complementary nucleobase interactions drive co-assembly of drugs and nanocarriers for selective cancer chemotherapy. Pharmaceutics 2021, 13, 1929. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yuan, H.; Yu, Y.; Gao, Z.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. FRET-based analysis on the structural stability of polymeric micelles: Another key attribute beyond PEG coverage and particle size affecting the blood clearance. J. Control. Release 2023, 360, 734–746. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Bhat, P.A.; Chat, O.A.; Dar, A.A. Exploiting Co-solubilization of Warfarin, Curcumin, and Rhodamine B for Modulation of Energy Transfer: A Micelle FRET On/Off Switch. ChemPhysChem 2016, 17, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Cao, Z.; Du, J.; Yan, L.; Wang, J. Investigation of the in vivo integrity of polymeric micelles via large Stokes shift fluorophore-based FRET. J. Control. Release 2020, 324, 47–54. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Hiebner, D.W.; Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. J. Nanobiotechnol. 2021, 19, 104. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Le-Vinh, B.; Le, N.M.N.; Nazir, I.; Matuszczak, B.; Bernkop-Schnürch, A. Chitosan based micelle with zeta potential changing property for effective mucosal drug delivery. Int. J. Biol. Macromol. 2019, 133, 647–655. [Google Scholar] [CrossRef]
- Parra, A.; Jarak, I.; Santos, A.; Veiga, F.; Figueiras, A. Polymeric micelles: A promising pathway for dermal drug delivery. Materials 2021, 14, 7278. [Google Scholar] [CrossRef]
- He, L.; Qin, X.; Fan, D.; Feng, C.; Wang, Q.; Fang, J. Dual-Stimuli Responsive Polymeric Micelles for the Effective Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 21076–21086. [Google Scholar] [CrossRef]
- Kumar, R.; Sirvi, A.; Kaur, S.; Samal, S.K.; Roy, S.; Sangamwar, A.T. Polymeric micelles based on amphiphilic oleic acid modified carboxymethyl chitosan for oral drug delivery of bcs class iv compound: Intestinal permeability and pharmacokinetic evaluation. Eur. J. Pharm. Sci. 2020, 153, 105466. [Google Scholar] [CrossRef]
- Jiang, G.B.; Quan, D.; Liao, K.; Wang, H. Preparation of polymeric micelles based on chitosan bearing a small amount of highly hydrophobic groups. Carbohydr. Polym. 2006, 66, 514–520. [Google Scholar] [CrossRef]
- Luo, T.; Han, J.; Zhao, F.; Pan, X.; Tian, B.; Ding, X.; Zhang, J. Redox-sensitive micelles based on retinoic acid modified chitosan conjugate for intracellular drug delivery and smart drug release in cancer therapy. Carbohydr. Polym. 2019, 215, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz, S.; Sarmento, B. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater. Sci. Eng. C 2020, 112, 110920. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Brzeziński, M.; Cieślak, M.; Królewska-Golińska, K.; Makowski, T.; Socka, M.; Biela, T. Stereocomplexed micelles based on polylactides with β-cyclodextrin core as anti-cancer drug carriers. Eur. Polym. J. 2019, 120, 109271. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, characterization and antioxidant properties of curcumin encapsulated chitosan/lignosulfonate micelles. Carbohydr. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef] [PubMed]
- Stern, T.; Kaner, I.; Laser Zer, N.; Shoval, H.; Dror, D.; Manevitch, Z.; Chai, L.; Brill-Karniely, Y.; Benny, O. Rigidity of polymer micelles affects interactions with tumor cells. J. Control. Release 2017, 257, 40–50. [Google Scholar] [CrossRef]
- Kim, M.P.; Kim, H.J.; Kim, B.J.; Yi, G.R. Structured nanoporous surfaces from hybrid block copolymer micelle films with metal ions. Nanotechnology 2015, 26, 095302. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Savchenko, I.V.; Kudryashova, E.V. Fluorescent Probes with Förster Resonance Energy Transfer Function for Monitoring the Gelation and Formation of Nanoparticles Based on Chitosan Copolymers. J. Funct. Biomater. 2023, 14, 401. [Google Scholar] [CrossRef]
- Grosso, R.; De-Paz, M.V. Thiolated-polymer-based nanoparticles as an avant-garde approach for anticancer therapies—Reviewing thiomers from chitosan and hyaluronic acid. Pharmaceutics 2021, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, P.; Mishra, B.; Mudavath, S.L.; Patel, R.R.; Chaurasia, S.; Sundar, S.; Suvarna, V.; Monteiro, M. Mannose-conjugated curcumin-chitosan nanoparticles: Efficacy and toxicity assessments against Leishmania donovani. Int. J. Biol. Macromol. 2018, 111, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, J.P.; Weikert, T.; Hoffmann, S.; Vila-Sanjurjo, C.; Moerschbacher, B.M.; Goycoolea, F.M.; Kolkenbrock, S. Affinity protein-based FRET tools for cellular tracking of chitosan nanoparticles and determination of the polymer degree of acetylation. Biomacromolecules 2014, 15, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.A.; Schramm, J.; Fenaroli, F.; Siemer, S.; Seidl, C.I.; Rosenauer, C.; Bleul, R.; Stauber, R.H.; Koynov, K.; Maskos, M.; et al. Complex Structures Made Simple—Continuous Flow Production of Core Cross-Linked Polymeric Micelles for Paclitaxel Pro-Drug-Delivery. Adv. Mater. 2023, 35, 2210704. [Google Scholar] [CrossRef]
- Mustafai, A.; Zubair, M.; Hussain, A.; Ullah, A. Recent Progress in Proteins-Based Micelles as Drug Delivery Carriers. Polymers 2023, 15, 836. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Gaucher, G.; Satturwar, P.; Jones, M.C.; Furtos, A.; Leroux, J.C. Polymeric micelles for oral drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R.R. Polymeric Micelles for Drug Delivery. Int. J. Drug Dev. 2017, 9, 12–13. [Google Scholar]
- Zlotnikov, I.D.; Davydova, M.P.; Danilov, M.R.; Krylov, S.S.; Belogurova, N.G.; Kudryashova, E.V. Covalent Conjugates of Allylbenzenes and Terpenoids as Antibiotics Enhancers with the Function of Prolonged Action. Pharmaceuticals 2023, 16, 1102. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Dobryakova, N.V.; Kudryashova, E.V. Disulfide Cross-Linked Polymeric Redox-Responsive Nanocarrier Based on Heparin, Chitosan and Lipoic Acid Improved Drug Accumulation, Increased Cytotoxicity and Selectivity to Leukemia Cells by Tumor Targeting via “Aikido” Principle. Gels 2024, 10, 157. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A.; Kudryashova, E.V. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E.V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility, Cytostatic Activity and Selectivity against Cancer Cells. Pharmaceutics 2023, 15, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E.V. Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. Int. J. Mol. Sci. 2022, 23, 16144. [Google Scholar] [CrossRef]
- Bales, B.L.; Messina, L.; Vidal, A.; Peric, M.; Nascimento, O.R. Precision relative aggregation number determinations of SDS micelles using a spin probe. A model of micelle surface hydration. J. Phys. Chem. B 1998, 102, 10347–10358. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977, 81, 1075–1078. [Google Scholar] [CrossRef]
- Wu, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Determining the Critical Micelle Concentration of Surfactants by a Simple and Fast Titration Method. Anal. Chem. 2020, 92, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Deutschle, T.; Porkert, U.; Reiter, R.; Keck, T.; Riechelmann, H. In vitro genotoxicity and cytotoxicity of benzalkonium chloride. Toxicol. Vitr. 2006, 20, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Agardan, N.B.; Sarisozen, C.; Torchilin, V.P. Cytotoxicity of Novel Redox Sensitive PEG2000-S-S-PTX Micelles against Drug-Resistant Ovarian and Breast Cancer Cells. Pharm. Res. 2020, 37, 65. [Google Scholar] [CrossRef] [PubMed]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar] [PubMed]
- Zhong, P.; Zhang, J.; Deng, C.; Cheng, R.; Meng, F.; Zhong, Z. Glutathione-Sensitive Hyaluronic Acid-SS-Mertansine Prodrug with a High Drug Content: Facile Synthesis and Targeted Breast Tumor Therapy. Biomacromolecules 2016, 17, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, K.; Chauhan, S.; Chauhan, M.S. Conductometric and spectrophotometric studies of self-aggregation behavior of streptomycin sulphate in aqueous solution: Effect of electrolytes. J. Mol. Liq. 2020, 297, 111782. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Characterization of chitosan-oleic acid composite films. Food Hydrocoll. 2009, 23, 536–547. [Google Scholar] [CrossRef]
- Fluksman, A.; Benny, O. A robust method for critical micelle concentration determination using coumarin-6 as a fluorescent probe. Anal. Methods 2019, 11, 3810–3818. [Google Scholar] [CrossRef]
- Lesemann, M.; Thirumoorthy, K.; Kim, Y.J.; Jonas, J.; Paulaitis, M.E. Pressure dependence of the critical micelle concentration of a nonionic surfactant in water studied by 1H-NMR. Langmuir 1998, 14, 5339–5341. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Hamed, N.A.; Mansour, F.R. Spectroscopic methods for determination of critical micelle concentrations of surfactants; a comprehensive review. Appl. Spectrosc. Rev. 2023, 58, 206–234. [Google Scholar] [CrossRef]
- Ollmann, M.; Galla, H.J.; Schwarzmann, G.; Sandhoff, K. Pyrene-Labeled Gangliosides: Micelle Formation in Aqueous Solution, Lateral Diffusion, and Thermotropic Behavior in Phosphatidylcholine Bilayers. Biochemistry 1987, 26, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Talbiersky, P.; Korth, H.G.; Sustmann, R.; Boese, R.; Bläser, D.; Rehage, H. A new pyrene-based fluorescent probe for the determination of critical micelle concentrations. J. Phys. Chem. B 2007, 111, 12985–12992. [Google Scholar] [CrossRef]
- Berghmans, M.; Govaers, S.; Berghmans, H.; De Schryver, F.C. Study of polymer gelation by fluorescence spectroscopy. Polym. Eng. Sci. 1992, 32, 1466–1470. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; YT, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]

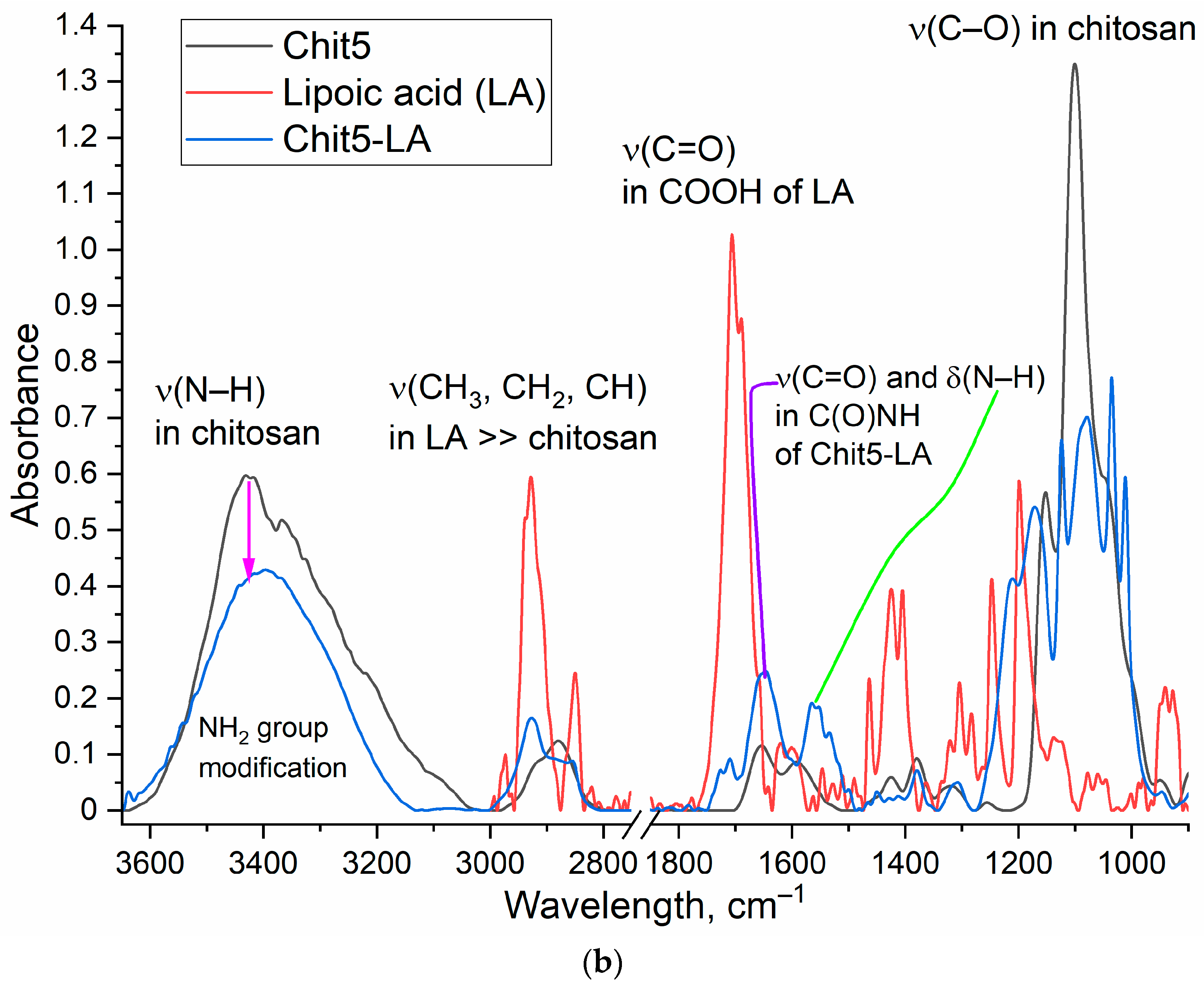


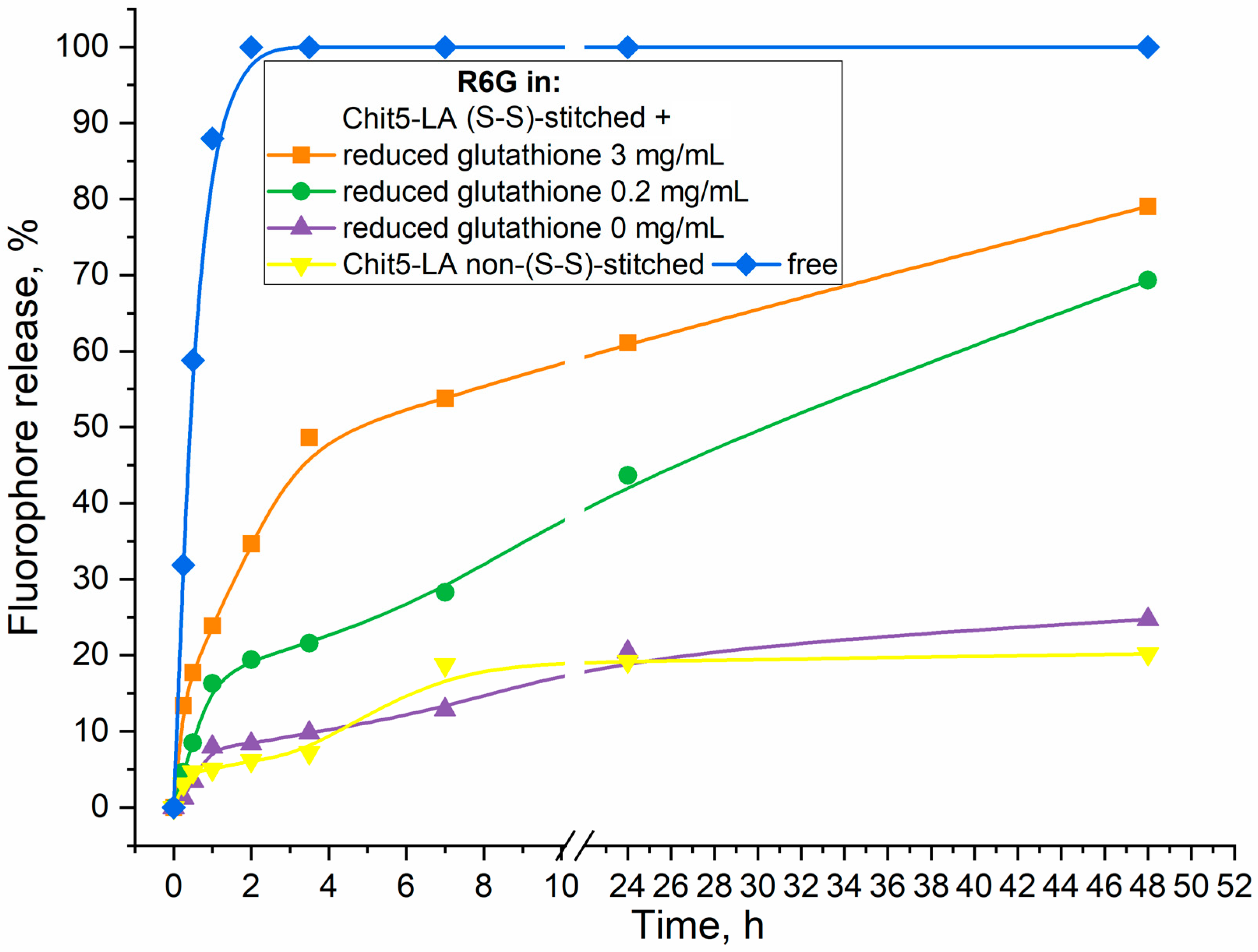
| Micelle * | Grafting Degree, % | Mw of One Polymeric Unit, kDa | CMC, nM | Hydrodynamic Diameter **, nm | Zeta Potential, mV |
|---|---|---|---|---|---|
| Chit5-OA | 18 ± 2 | 6.7 ± 0.8 | 8 ± 2 | 300–450 | +5 ± 1 |
| Chit5-LA nonstitched | 24 ± 3 | 6.4 ± 0.3 | 50 ± 10 | 300–350 | +20 ± 3 |
| Chit5-LA S-S stitched | 45 ± 6 (is about 7 residues of Chit5-branches) | 16 ± 2 | 230–280 | +15 ± 2 |
| Method | CMC Determination | CPMC Determination | Aggregation Number Determination | Size Determination | Robustness | Applicability to Different Types of Micelles | Expressiveness |
|---|---|---|---|---|---|---|---|
| Conductometry [39,58,59] | ± | − | − | − | + | ± | + |
| Surface tension [51,60,61] | + | − | − | − | ± | − | + |
| Densitometry [59] | ± | − | − | − | ± | − | + |
| NMR spectrometry [62] | + | ± | ± | − | + | ± | − |
| UV/VIS spectroscopy [63] | + | − | − | − | + | ± | + |
| Fluorometric methods (including pyrene probe) [32,61,64,65,66] | ++ | + | ± | ± | + | + | + |
| Atomic force and electron microscopy [67] | ± | − | − | ++ | ± | + | − |
| FRET probes | + | + | ± | ± | + | ++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlotnikov, I.D.; Savchenko, I.V.; Kudryashova, E.V. Specific FRET Probes Sensitive to Chitosan-Based Polymeric Micelles Formation, Drug-Loading, and Fine Structural Features. Polymers 2024, 16, 739. https://doi.org/10.3390/polym16060739
Zlotnikov ID, Savchenko IV, Kudryashova EV. Specific FRET Probes Sensitive to Chitosan-Based Polymeric Micelles Formation, Drug-Loading, and Fine Structural Features. Polymers. 2024; 16(6):739. https://doi.org/10.3390/polym16060739
Chicago/Turabian StyleZlotnikov, Igor D., Ivan V. Savchenko, and Elena V. Kudryashova. 2024. "Specific FRET Probes Sensitive to Chitosan-Based Polymeric Micelles Formation, Drug-Loading, and Fine Structural Features" Polymers 16, no. 6: 739. https://doi.org/10.3390/polym16060739
APA StyleZlotnikov, I. D., Savchenko, I. V., & Kudryashova, E. V. (2024). Specific FRET Probes Sensitive to Chitosan-Based Polymeric Micelles Formation, Drug-Loading, and Fine Structural Features. Polymers, 16(6), 739. https://doi.org/10.3390/polym16060739









