Copper-Containing Bionanocomposites Based on Natural Raw Arabinogalactan as Effective Vegetation Stimulators and Agents against Phytopathogens
Abstract
1. Introduction
2. Results
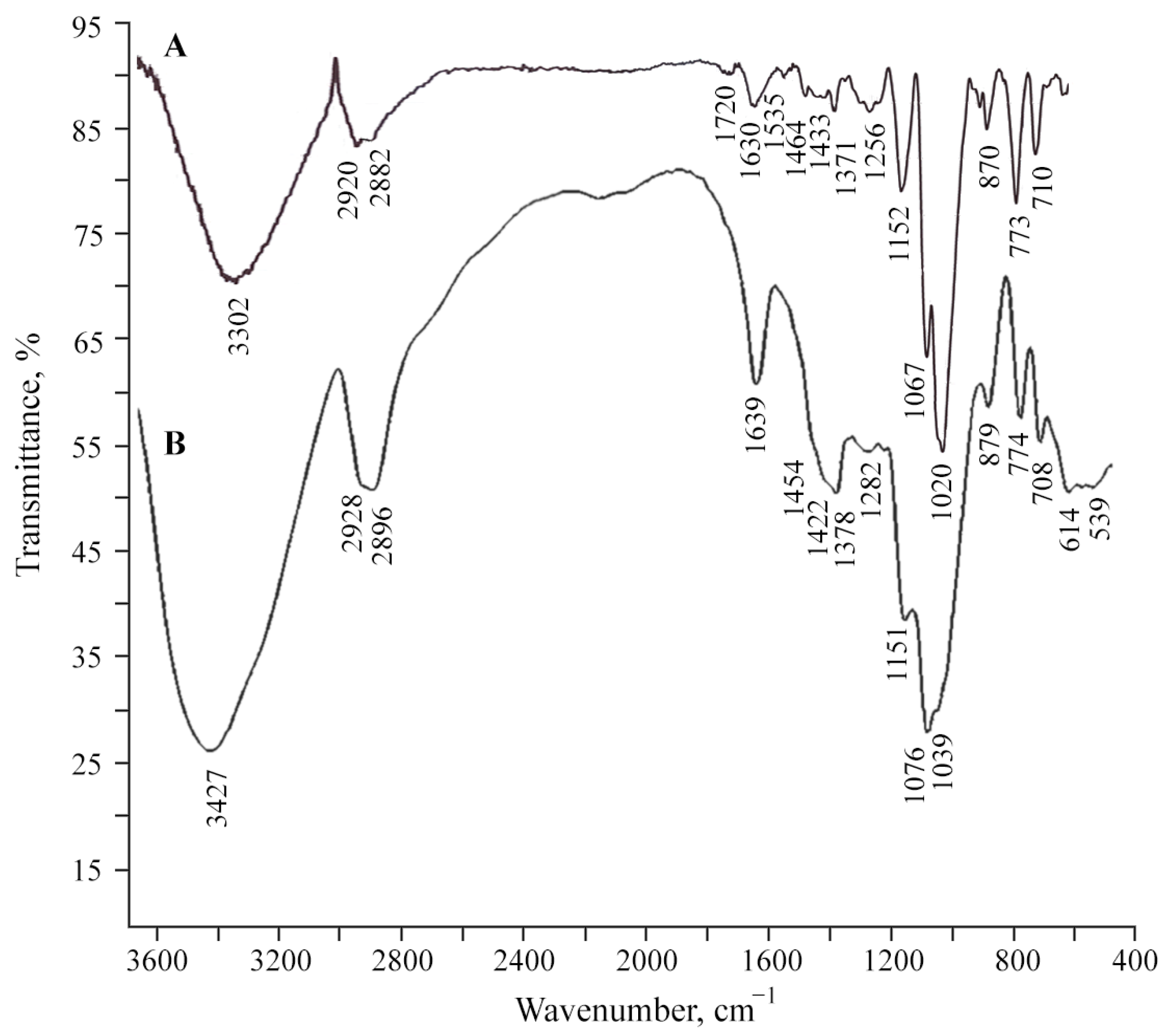
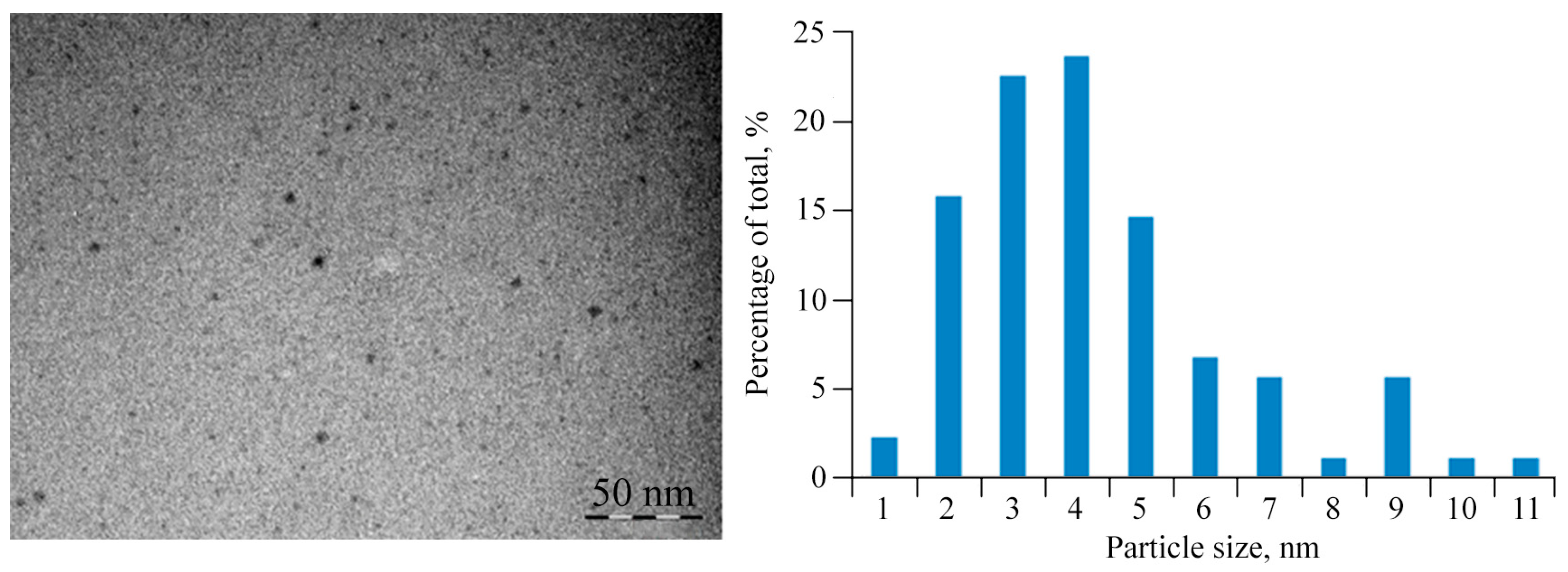
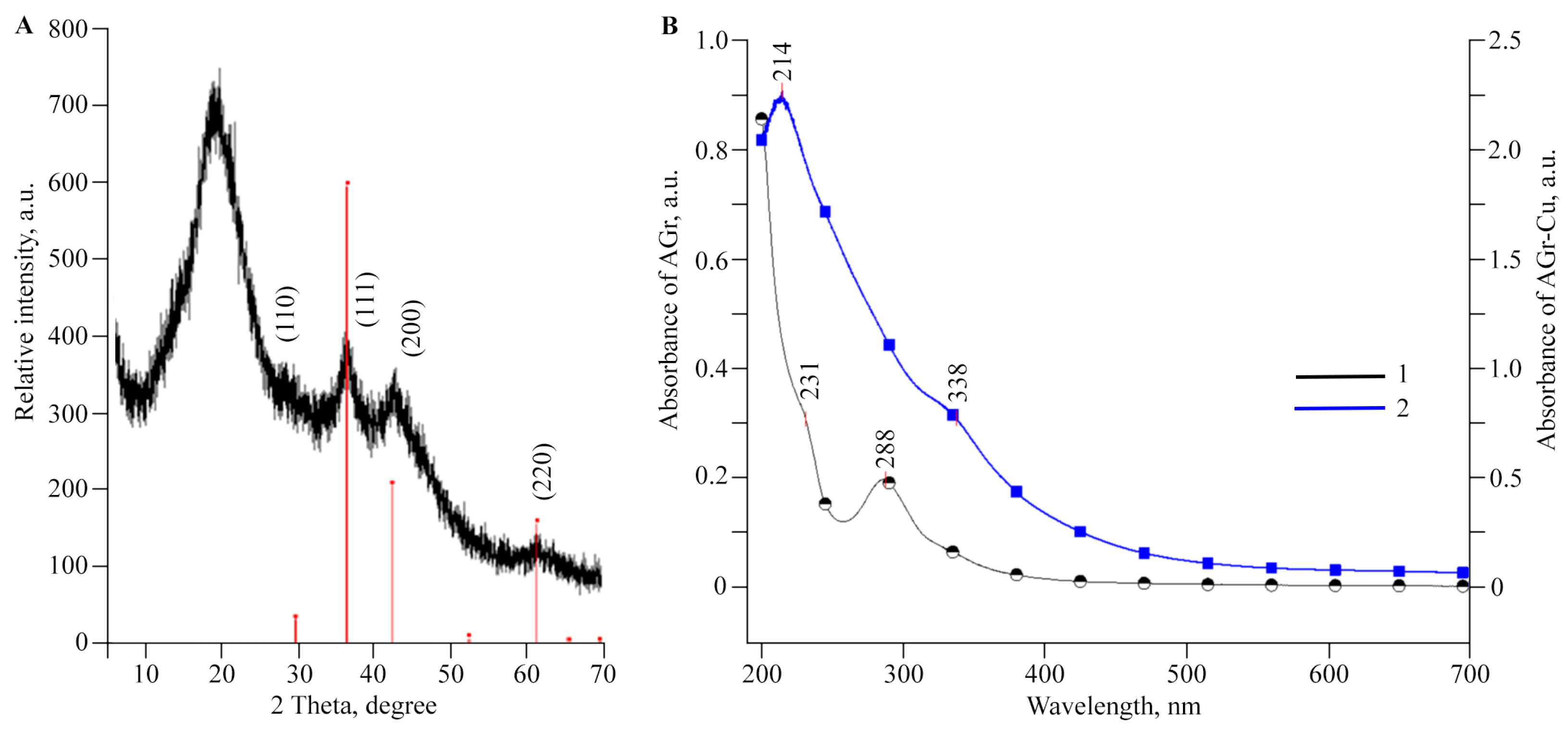
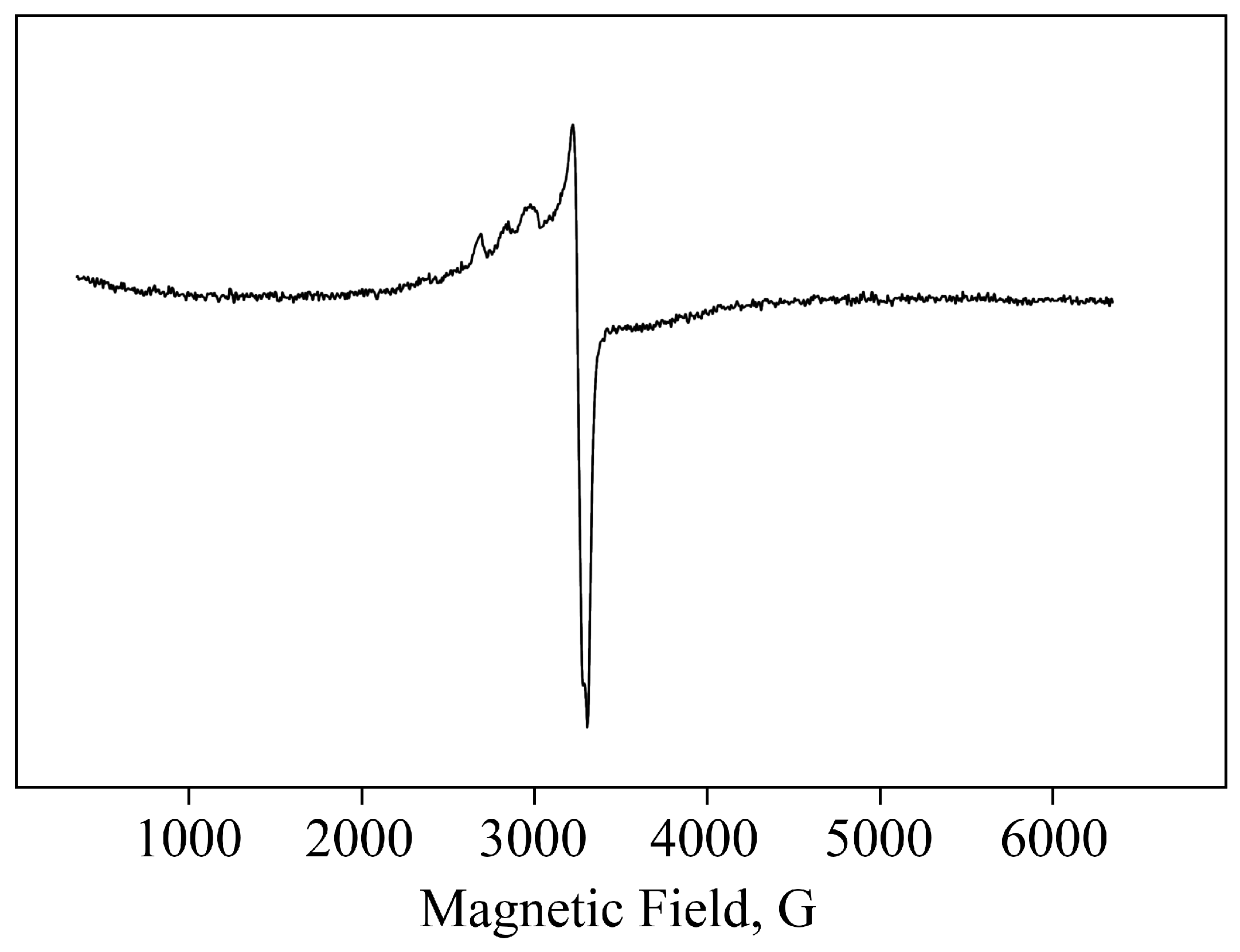
2.1. Structural Features and Physicochemical Properties of Obtained AGr-Cu
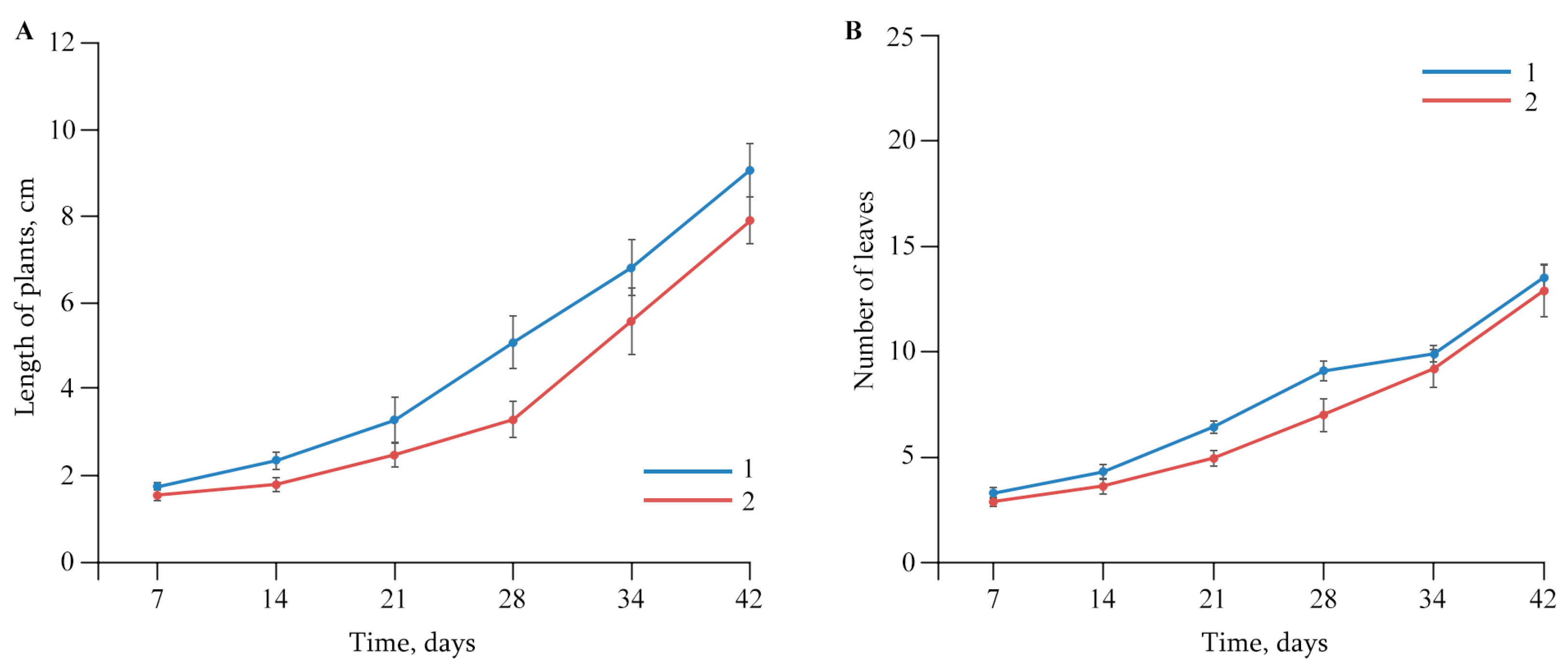
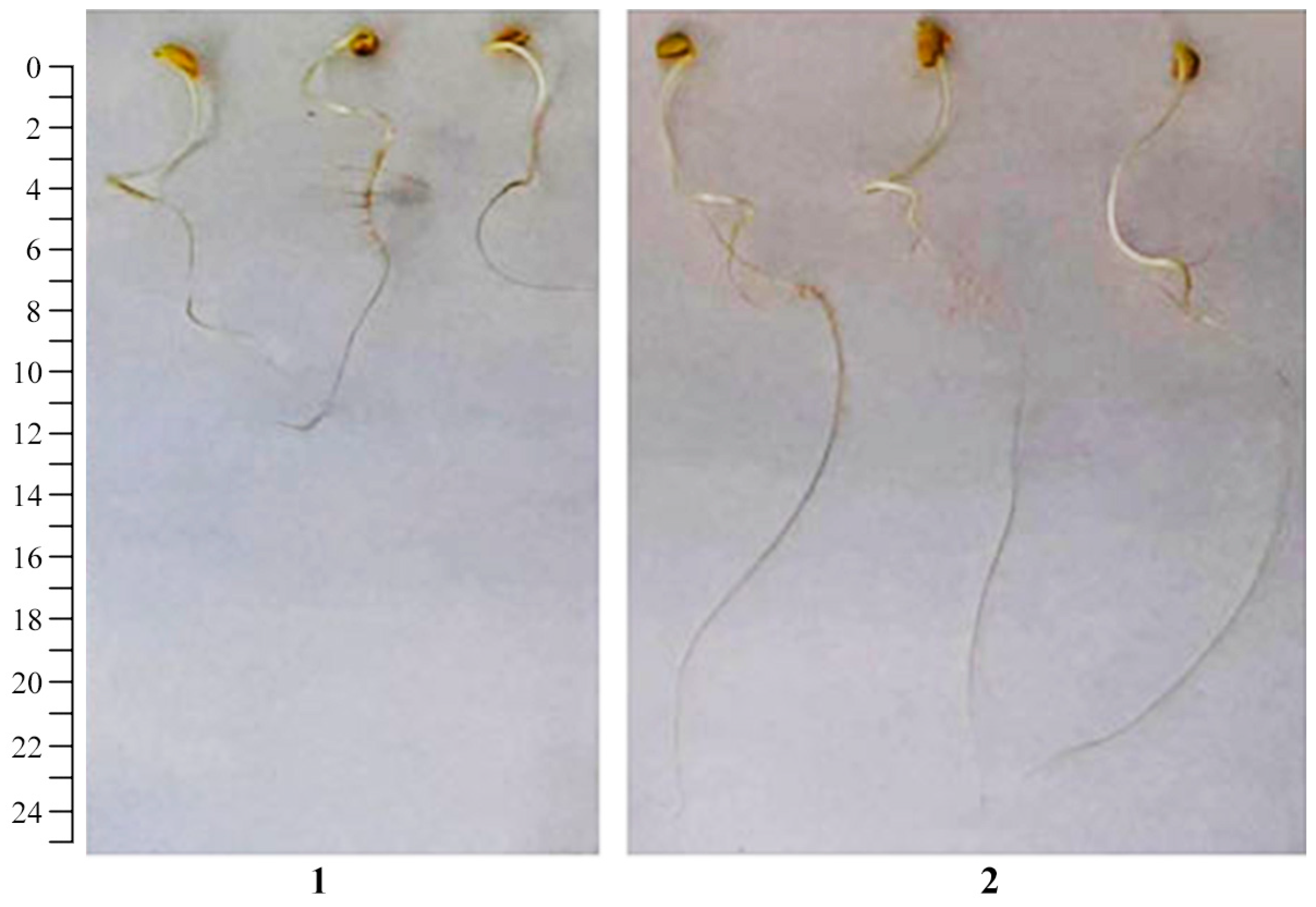
2.2. The Effect of AGr-Cu on the Growth and Development of Solanum tuberosum L.
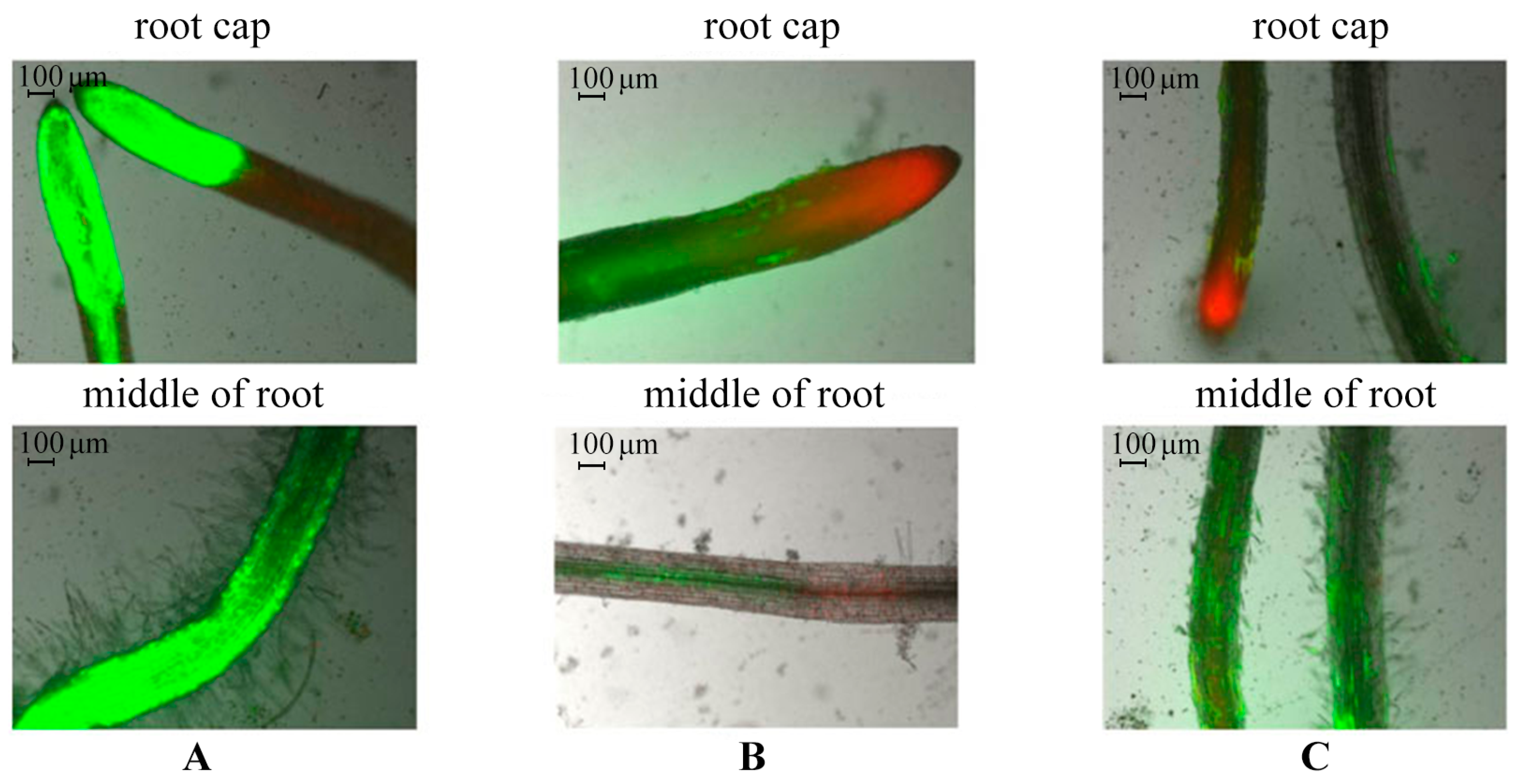
2.3. Evaluation of Potato Root Viability In Vitro in the Presence of AGr-Cu
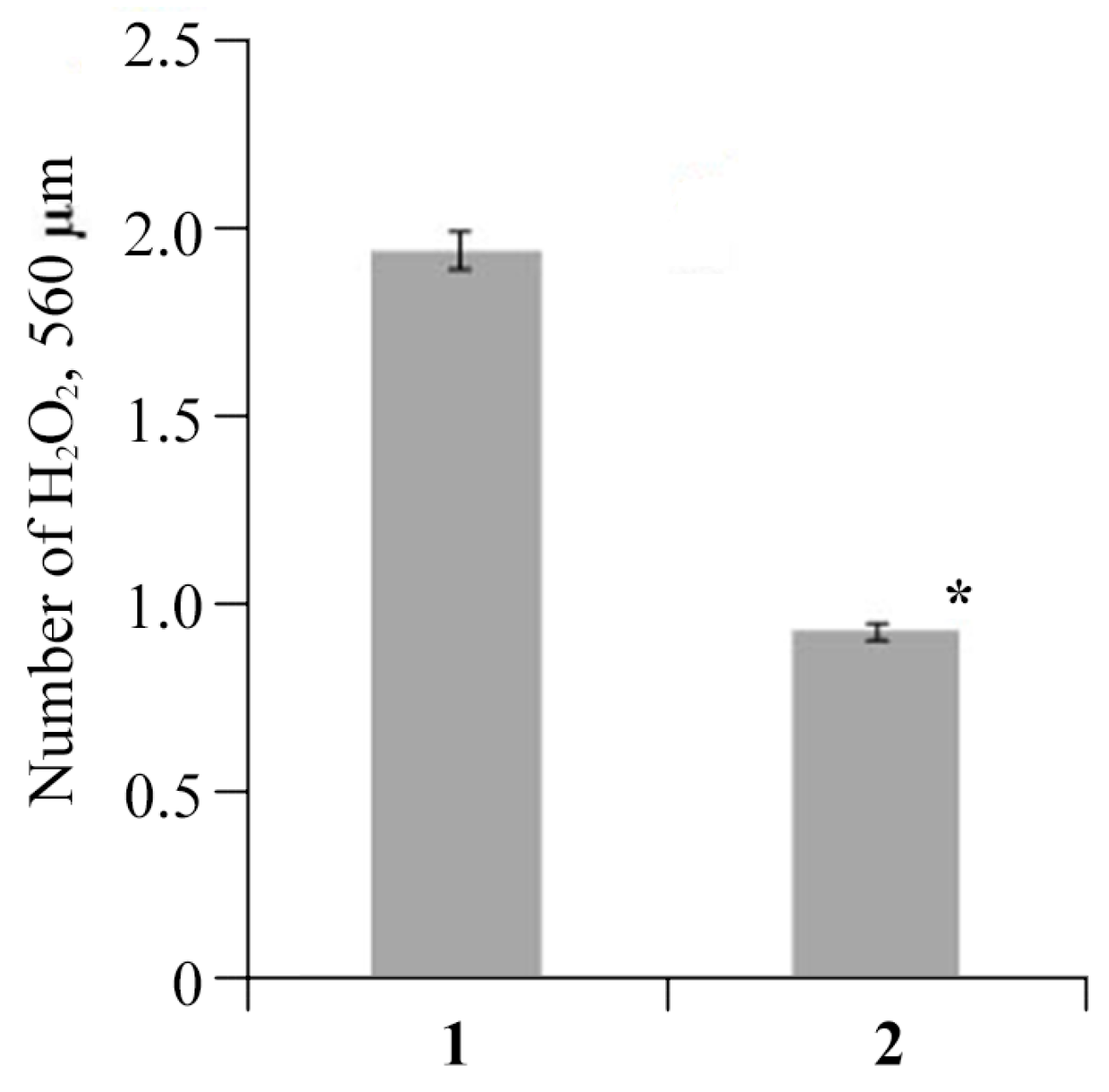
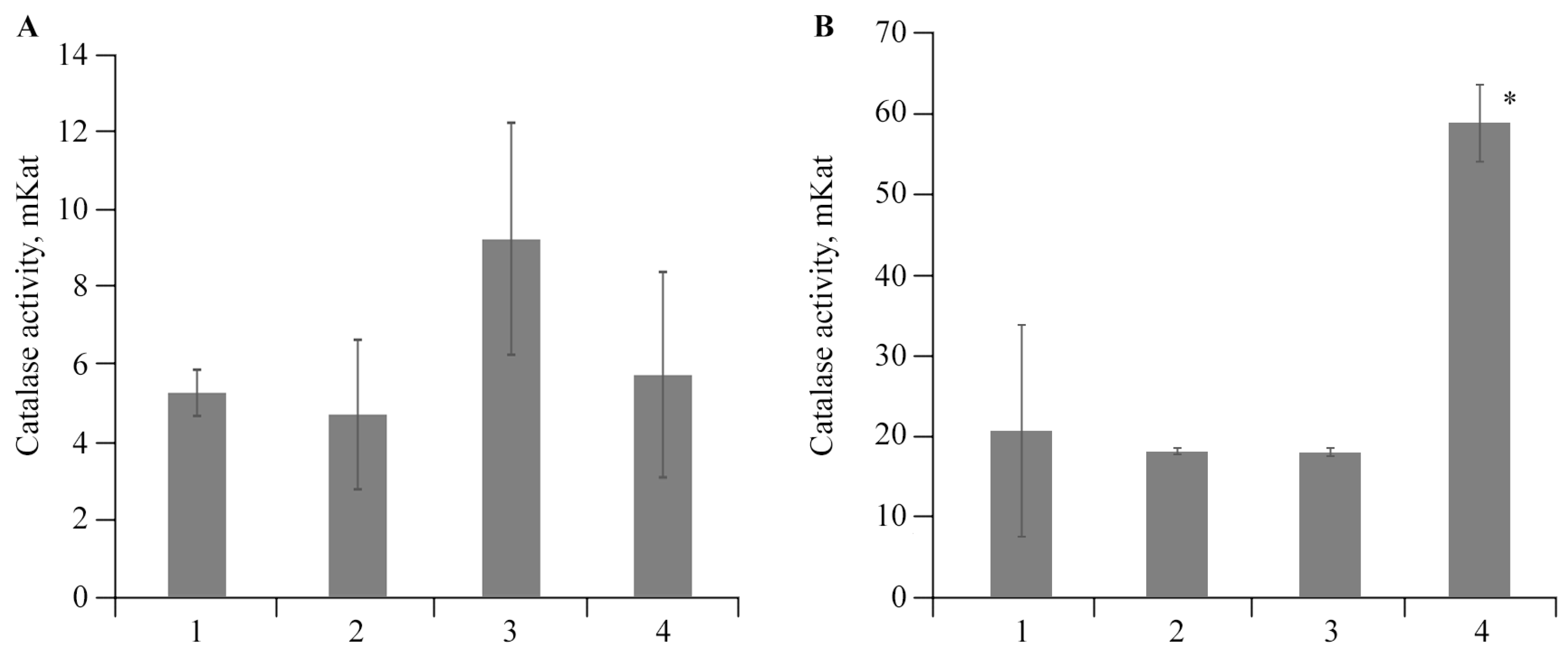
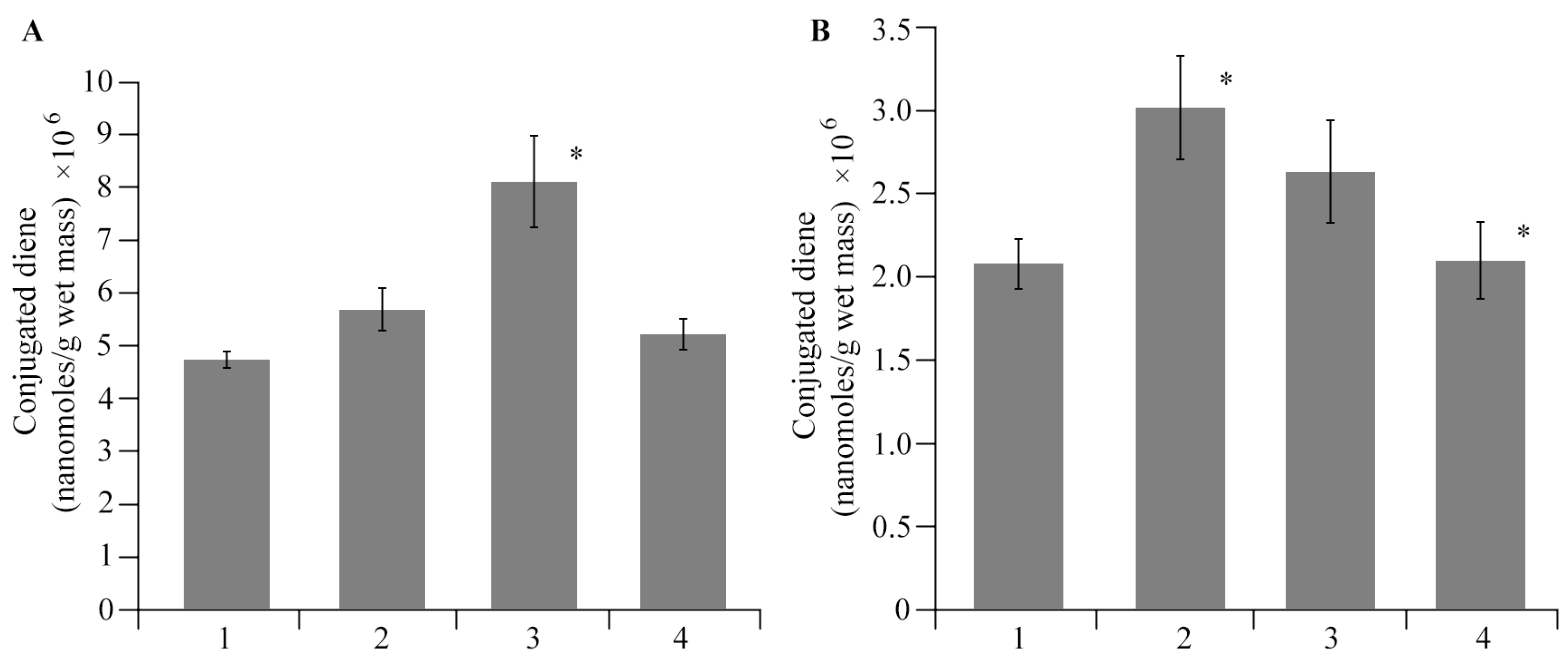
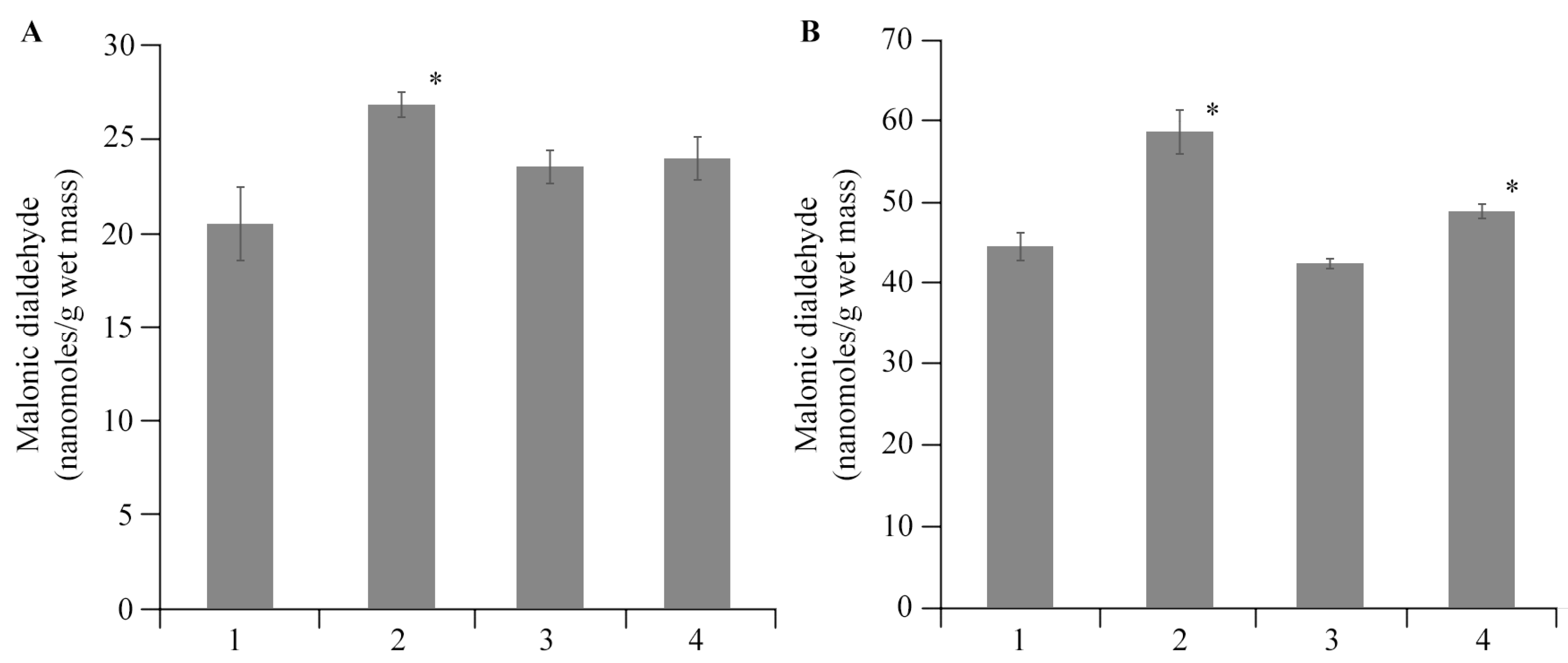
2.4. The Effect of AGr-Cu on the Stress Resistance of Solanum tuberosum L.
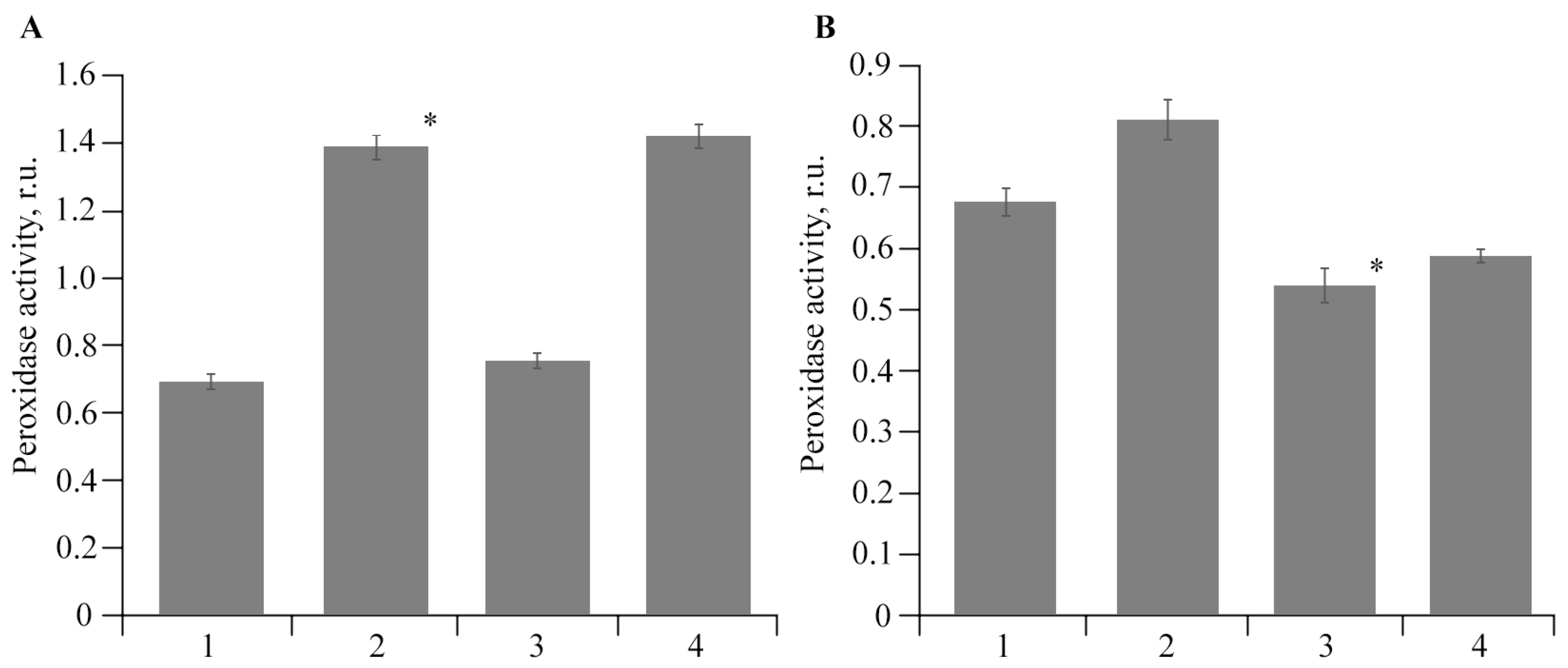
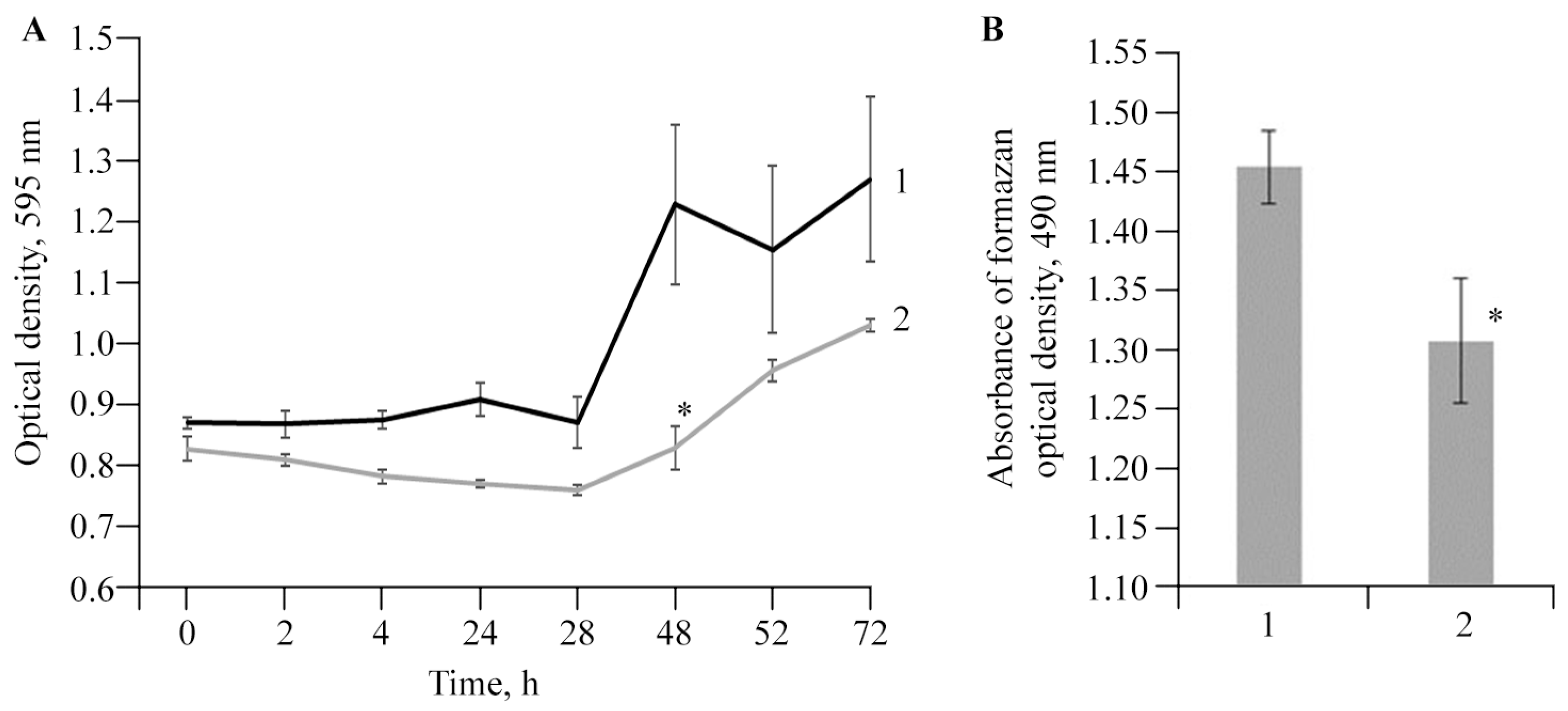
2.5. Antibacterial Activity of AGr-Cu against Cms Phytopathogen
2.6. The Effect of NC on the Growth and Development of Glycine max L.
3. Discussion
4. Materials and Methods
4.1. Synthesis of Copper-Containing Nanocomposites
4.2. Physicochemical Measurements
4.3. Plant Material
4.4. Stress Resistance Experiments
4.5. Bactericidal and Bacteriostatic Effect of the AGr-Cu
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandini, B.; Mawale, K.S.; Giridhar, P. Nanomaterials in agriculture for plant health and food safety: A comprehensive review on the current state of agro-nanoscience. 3 Bioteh 2023, 13, 73. [Google Scholar] [CrossRef]
- Gupta, G.S.; Kumar, A.; Verma, N. Bacterial homoserine lactones as a nanocomposite fertilizer and defense regulator for chickpeas. Environ. Sci. Nano 2019, 6, 1246–1258. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotech. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Perfileva, A.I.; Nozhkina, O.A.; Ganenko, T.V.; Krutovsky, K.V. Novel nanobiocomposites based on natural polysaccharides as universal trophic low-dose micronutrients. Int. J. Mol. Sci. 2021, 22, 12006. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, characterization and application of polysaccharide-based metallic nanoparticles: A review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, B.A.; Tenne, R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Ochsner, A.; Shokuhfar, A. New Frontiers of Nanoparticles and Nanocomposite Materials. Novel Principles and Techniques; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mavelil-Sam, R.; Ouseph, E.M.; Morreale, M.; Scaffaro, R.; Thomas, S. Recent developments and formulations for hydrophobic modification of carrageenan bionanocomposites. Polymers 2023, 15, 1650. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Ganenko, T.V.; Graskova, I.A.; Sukhov, B.G.; Artem’ev, A.V.; Trofimov, B.A.; Krutovsky, K.V. Selenium nanocomposites in natural matrices as potato recovery agent. Int. J. Mol. Sci. 2021, 22, 4576. [Google Scholar] [CrossRef] [PubMed]
- Bashal, A.H.; Riyadh, S.M.; Alharbi, W.; Alharbi, K.H.; Farghaly, T.A.; Khalil, K.D. Bio-based (chitosan-ZnO) nanocomposite: Synthesis, characterization, and its use as recyclable, ecofriendly biocatalyst for synthesis of thiazoles tethered azo groups. Polymers 2022, 14, 386. [Google Scholar] [CrossRef]
- Tunik, T.V.; Nemchenko, U.M.; Ganenko, T.V.; Yurinova, G.V.; Dzhioev, Y.P.; Sukhov, B.G.; Zlobin, V.I.; Trofimov, B.A. Synthesis and spectral characterization of new biodegradable arabinogalactan derivatives for diagnosis and therapy. Bull. Russ. Acad. Sci. Phys. 2019, 83, 343–349. [Google Scholar] [CrossRef]
- Pogodaeva, N.N.; Medvedeva, S.A.; Sukhov, B.G.; Larina, L.I. Spectroscopic study of the reaction of a natural arabinogalactan polysaccharide with 3-hydroxyflavones in aqueous solutions. Chem. Nat. Compd. 2012, 48, 723–727. [Google Scholar] [CrossRef]
- Pogodaeva, N.N.; Sukhov, B.G.; Medvedeva, S.A. Enantioselective keto-enol tautomerism of 3-hydroxyflavones upon molecular complex formation of their α-diketo forms with carbohydrates in aqueous solutions. Chem. Nat. Compd. 2016, 52, 579–584. [Google Scholar] [CrossRef]
- Ganenko, T.V.; Tantsyrev, A.P.; Sapozhnikov, A.N.; Khutsishvili, S.S.; Vakul’skaya, T.I.; Fadeeva, T.V.; Sukhov, B.G.; Trofimov, B.A. Nanocomposites of silver with arabinogalactan sulfate: Preparation, structure, and antimicrobial activity. Russ. J. Gen. Chem. 2015, 85, 477–484. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Mulder, E.G. Mineral nutrition in relation to the biochemistry and physiology of potatoes. Plant Soil 1949, 2, 59–121. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Churilov, D.; Churilova, V.; Stepanova, I.; Polischuk, S.; Gusev, A.; Zakharova, O.; Arapov, I.; Churilov, G. Size-dependent biological effects of copper nanopowders on mustard seedlings. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012008. [Google Scholar] [CrossRef]
- Mohamed-Faisal, M.; Umarani, R.; Parthiban, K.T. Poor seed germination of Melia dubia—Unraveling the biological causes and designing an appropriate seed treatment protocol. J. Trop. For. Sci. 2022, 34, 371–382. [Google Scholar]
- Fernandes, J.C.; Henriques, F.S. Biochemical, physiological, and structural effects of excess copper in plants. Bot. Rev. 1991, 57, 246–273. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric nanocomposites for environmental and industrial applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Churilova, V.V.; Churilov, G.I.; Polishchuk, S.D. Agent for Presowing Treatment of Seeds of Agricultural Plants and Method of Its Use. Patent RU 2735268 C1, 29 October 2020. [Google Scholar]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Reed, D.W.; Bosch, C.A.V. Engineering perspectives of growing plants in space. J. Indian Inst. Sci. 2023, 103, 797–805. [Google Scholar] [CrossRef]
- Poulet, L.; Fontaine, J.-P.; Dussap, C.-G. Plant’s response to space environment: A comprehensive review including mechanistic modelling for future space gardeners. Bot. Lett. 2016, 163, 337–347. [Google Scholar] [CrossRef]
- Eichenlaub, R.; Gartemann, K.-H. The Clavibacter michiganensis subspecies: Molecular investigation of gramm-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tambong, J.; Yuan, K.X.; Chen, W.; Xu, H.; Lévesque, C.A.; De Boer, S.H. Re-classification of Clavibacter michiganensis subspecies on the basis of whole-genome and multi-locus sequence analyses. Int. J. Syst. Evol. Microbiol. 2018, 68, 234–240. [Google Scholar] [CrossRef]
- Stevens, L.H.; Tom, J.Y.; van der Zouwen, P.S.; Mendes, O.; Poleij, L.M.; van der Wolf, J.M. Effect of temperature treatments on the viability of Clavibacter sepedonicus in infected potato tissue. Potato Res. 2021, 64, 535–552. [Google Scholar] [CrossRef]
- Papkina, A.V.; Perfileva, A.I.; Zhivetev, M.A.; Borovskiy, G.B.; Graskova, I.A.; Lesnichaya, M.V.; Klimenkov, I.V.; Sukhov, B.G.; Trofimov, B.A. Effect of selenium and arabinogalactan nanocomposite on viability of the phytopathogen Clavibacter michiganensis subsp. sepedonicus. Dokl. Biol. Sci. 2015, 461, 89–91. [Google Scholar] [CrossRef]
- Lesnichaya, M.; Gazizova, A.; Perfileva, A.; Nozhkina, O.; Graskova, I.; Sukhov, B. Starch-capped sulfur nanoparticles synthesized from bulk powder sulfur and their antiphytopathogenic activity against Clavibacter sepedonicus. IET Nanobiotechnol. 2021, 15, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Djanaguiraman, M. Encyclopedia of Applied Plant Sciences, 2nd ed.; Academic Press: Cambridge, UK, 2017; pp. 246–255. [Google Scholar]
- Farias, J.G.; Nunes, S.T.; Sausen, D.; Nunes, M.A.G.; Neis, F.A.; Garlet, L.C.; Nunes, P.A.A.; Dressler, V.L.; Schetinger, M.R.C.; Rossato, L.V.; et al. Agricultural contamination: Effect of copper excess on physiological parameters of potato genotypes and food chain security. J. Appl. Bot. Food Qual. 2018, 91, 249–259. [Google Scholar] [CrossRef]
- Zaafarany, I.; Gobouri, A.; Hassan, R. Oxidation of some sulfated carbohydrates: Kinetics and mechanism of oxidation of chondroitin-4-sulfate by alkaline permanganate with novel synthesis of coordination biopolymer precursor. J. Mater. Sci. Res. 2013, 2, 23–36. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Ganenko, T.V.; Sukhov, B.G. Formation and paramagnetic properties of manganese-containing bionanocomposites based on natural polysaccharide matrices. J. Carbohydr. Chem. 2021, 40, 211–225. [Google Scholar] [CrossRef]
- Tajmir-Riahi, H.A. Carbohydrate metal ion complexes. Interaction of D-glucono-1,5-lactone with Zn(II), Cd(II), and Hg(II) ions in the solid and aqueous solution, studied by 13C-NMR, FT-IR, and X-ray powder diffraction measurements. Can. J. Chem. 1989, 67, 651–654. [Google Scholar] [CrossRef]
- De Souza, R.F.V.; De Giovani, W.F. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta A 2005, 61, 1985–1990. [Google Scholar] [CrossRef]
- Nikolić, G.S.; Cakić, M.D. Analysis of bioactive oligosaccharide-metal complexes by modern FTIR spectroscopy: Copper complexes. In Fourier Transforms—New Analytical Aproaches and FTIR Strategies; Nikolić, G.S., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Lis, T.; Matuszewski, J. Manganese(II) malonate dihydrate: A reinvestigation. Acta Cryst. B 1979, 35, 2212–2214. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Wang, J.; Yang, Z.; Long, M.; Hu, N.; Zhang, Y. Preparation of hollow porous Cu2O microspheres and photocatalytic activity under visible light irradiation. Nanoscale Res. Lett. 2012, 7, 347. [Google Scholar] [CrossRef]
- Barret, C.A.; Massalsky, T.B. Structure of Metals; McGraw-Hill: New York, NY, USA, 1966. [Google Scholar]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef]
- Khanehzaei, H.; Ahmad, M.B.; Shameli, K.; Ajdari, Z. Synthesis and characterization of Cu@Cu2O core shell nanoparticles prepared in seaweed Kappaphycus alvarezii media. Int. J. Electrochem. Sci. 2014, 9, 8189–8198. [Google Scholar] [CrossRef]
- Sukhov, B.G.; Pogodaeva, N.N.; Kuznetsov, S.V.; Kupriyanovich, Y.N.; Yurinova, G.V.; Selivanova, D.S.; Pristavka, A.A.; Dzhioev, Y.P.; Popkova, S.M.; Rakova, E.B.; et al. Prebiotic effect of native noncovalent arabinogalactan-flavonoid conjugates on bifidobacteria. Russ. Chem. Bull. 2014, 63, 2189–2194. [Google Scholar] [CrossRef]
- Biler, M.; Biedermann, D.; Valentová, K.; Křen, V.; Kubala, M. Quercetin and its analogues: Optical and acido–basic properties. Phys. Chem. Chem. Phys. 2017, 19, 26870–26879. [Google Scholar] [CrossRef] [PubMed]
- Celiz, G.; Suarez, S.A.; Arias, A.; Molina, J.; Brondino, C.D.; Doctorovich, F. Synthesis, structural elucidation and antiradical activity of a copper (II) naringenin complex. Biometals 2019, 32, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Artem’ev, A.V.; Vysotskaya, O.V.; Oparina, L.A.; Bogomyakov, A.S.; Khutsishvili, S.S.; Sterkhova, I.V.; Ovcharenko, V.I.; Trofimov, B.A. New heterospin chain-polymers based on Cu(hfac)2 complex with TEMPO derivatives bearing b-(oxy)acrylate moiety: Synthesis, structural and magnetic properties. Polyhedron 2016, 119, 293–299. [Google Scholar] [CrossRef]
- Taiwo, F.A. Electron paramagnetic resonance spectroscopic studies of iron and copper proteins. J. Spectrosc. 2003, 17, 673567. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Perfileva, A.I.; Nozhkina, O.A.; Dyrkach, A.Y. EPR Study of accumulation and toxic effect of iron and copper during the development of Solanum tuberosum L. in vitro. J. Appl. Spectr. 2022, 89, 288–295. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signaling events in apoplastic oxidative burst in suspension cultured french bean cells involve camp and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Torres, A.; Kasturiarachi, N.; DuPont, M.; Cooper, V.S.; Bomberger, J.; Zemke, A. NADH dehydrogenases in Pseudomonas aeruginosa growth and virulence. Front. Microbiol. 2019, 10, 75. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Goellner, E.M.; Utermoehlen, J.; Kramer, R.; Classen, B. Structure of arabinogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinogalactans. Carbohydr. Polym. 2011, 86, 1739–1744. [Google Scholar] [CrossRef]
- Zhao, D.; Tu, C.-M.; Hu, X.-J.; Zhang, N. Notable in situ surface transformation of Cu2O nanomaterials leads to dramatic activity enhancement for CO oxidation. RSC Adv. 2017, 7, 37596–37603. [Google Scholar] [CrossRef]
- Trofimova, N.N.; Babkin, V.A.; Kiselev, O.I. Complex compounds of zinc and copper(II) ions with dihydroquercetin and their antiviral activity. Russ. Chem. Bull. 2015, 64, 1430–1436. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Root system architecture, copper uptake and tissue distribution in soybean (Glycine max (L.) Merr.) grown in copper oxide nanoparticle (CuONP)-amended soil and implications for human nutrition. Plants 2020, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Tambasco, G.; Sauvé, S.; Cook, N.; McBride, M.; Hendershot, W. Phytoavailability of Cu and Zn to lettuce (Lactuca sativa) in contaminated urban soils. Can. J. Soil Sci. 2000, 80, 309–317. [Google Scholar] [CrossRef]
- Anwaar, S.; Maqbool, Q.; Jabeen, N.; Nazar, M.; Abbas, F.; Nawaz, B.; Hussain, T.; Hussain, S.Z. The effect of green synthesized CuO nanoparticles on callogenesis and regeneration of Oryza sativa L. Front. Plant Sci. 2016, 7, 1330. [Google Scholar] [CrossRef]
- Panyuta, O.; Belava, V.; Fomaidi, S.; Kalinichenko, O.; Volkogon, M.; Taran, N. The effect of pre-sowing seed treatment with metal nanoparticles on the formation of the defensive reaction of wheat seedlings infected with the eyespot causal agent. Nanoscale Res. Lett. 2016, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; De Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Feigl, G. The impact of copper oxide nanoparticles on plant growth: A comprehensive review. J. Plant Physiol. 2023, 18, 2243098. [Google Scholar] [CrossRef]
- Ma, Z.-Q.; Xu, Y.-C.; Fan, Z.-J.; Hou, D.-Y.; Xu, Q.-Y. Impacts of cuprous oxide nanoparticles on wheat root morphology and genotoxicity. Chin. J. Appl. Ecol. 2021, 3, 1105–1111. [Google Scholar] [CrossRef]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidat activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.-F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.-G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard Mater. 2021, 417, 125900. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, G.M.; Wu, H.; Santana, I.; Giraldo, J.P. Catalytic scavenging of plant reactive oxygen species in vivo by anionic cerium oxide nanoparticles. J. Vis. Exp. 2018, 138, 58373. [Google Scholar] [CrossRef]
- Baker, D.H.A. An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: A comprehensive review based on up to date. Toxicol. Rep. 2022, 9, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.S.; Büyük, İ.; Gündüzer, E.G.; Büyük, B.P.; Kandemir, İ.; Cansaran-Duman, D.; Aras, S. Effects of lead and cadmium elements on lipid peroxidation, catalase enzyme activity and catalase gene expression profile in tomato plants. J. Agric. Sci. 2016, 22, 539–547. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.J.; Huang, Y.W.; Chen, H.C.; Tsao, L.I.; Chang Chien, C.F.; Singh, B.; Liu, B.R. Effect of size and concentration of copper nanoparticles on the antimicrobial activity in Escherichia coli through multiple mechanisms. Nanomaterials 2022, 12, 3715. [Google Scholar] [CrossRef]
- Kasana, R.C.; Panwar, N.R.; Kaul, R.K.; Kumar, P. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Lett. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Graskova, I.A.; Sidorov, A.V.; Lesnichaya, M.V.; Aleksandrova, G.P.; Dolmaa, G.; Klimenkov, I.V.; Sukhov, B.G. Synthesis of selenium and silver nanobiocomposites and their influence on phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus. Russ. Chem. Bull. 2018, 67, 157–163. [Google Scholar] [CrossRef]
- Lambert, M.J. Preparation of Plant Material for Estimating a Wide Range of Elements; Forestry Commission of New South Wales: Sydney, Australia, 1976. [Google Scholar]
- Romanenko, A.S.; Riffel, A.A.; Graskova, I.A.; Rachenko, M.A. The role of extracellular pH-homeostasis in potato resistance to ring rot pathogen. J. Phytopathol. 1999, 147, 679–686. [Google Scholar] [CrossRef]
- Gavrilenko, V.F.; Ladygina, M.E.; Khandobina, L.M. Large Workshop on Plant Physiology; Higher School: Moscow, Russia, 1975. [Google Scholar]
- Yamori, W.; Kogami, H.; Masuzawa, T. Freezing tolerance in alpine plants as assessed by the FDA-staining method. Polar Biol. 2005, 18, 73–81. [Google Scholar] [CrossRef]
- Zelentsov, S.V.; Moshnenko, E.V.; Trunova, M.V.; Bubnova, L.A.; Budnikov, E.N.; Lukomets, A.V.; Savichenko, V.G.; Dorofeev, N.V.; Katysheva, N.B.; Pomortsev, A.V. A cold-resistant soybean cultivar of the northern ecotype Sayana. Oil Crops 2021, 1, 95–102. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Kharasova, A.R.; Nozhkina, O.A.; Sidorov, A.V.; Graskova, I.A.; Krutovsky, K.V. Effect of nanopriming with selenium nanocomposites on potato productivity in a field experiment, soybean germination and viability of Pectobacterium carotovorum. Horticulturae 2023, 9, 458. [Google Scholar] [CrossRef]
- Bolland, J.L.; Koch, H.P. The course of antioxidant reaction in polyisoprenes and allied compounds. Part IX. The primary thermal oxidation product of ethyl linoleate. J. Chem. Soc. 1945, 7, 445–447. [Google Scholar] [CrossRef]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Samonova, A.Y.; Tychkov, A.Y.; Bochkareva, S.S.; Moskovkin, A.A.; Kuzmenko, T.P. The influence of oxidative stress and natural antioxidants on morphometric parameters of red blood cells, the hemoglobin oxygen binding capacity, and the activity of antioxidant enzyme. BioMed Res. Int. 2019, 2019, 2109269. [Google Scholar] [CrossRef] [PubMed]
- Sharlaeva, E.A.; Chirkova, V.Y. The impact of short-wave UV radiation on peroxidase activity in soft wheat seeds. IOP Conf. Ser. Earth Environ. Sci. 2021, 670, 012008. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Br. 2016, 6, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Roozen, N.J.M.; Van Vuurde, J.W.L. Development of a semi-selective medium and an immunofluorescence colony-staining procedure for the detection of Clavibacter michiganensis subsp. sepedonicus in cattle manure slurry. Neth. J. Plant Pathol. 1991, 97, 321–334. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Tsivileva, O.M.; Nozhkina, O.A.; Karepova, M.S.; Graskova, I.A.; Ganenko, T.V.; Sukhov, B.G.; Krutovsky, K.V. Effect of natural polysaccharide matrix-based selenium nanocomposites on Phytophthora cactorum and rhizospheric microor-ganisms. Nanomaterials 2021, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Enikeev, A.G.; Vysotskaya, E.F.; Leonova, L.A.; Gamburg, K.Z. Viability assay with 2,3,5-triphenyltetrazolium chloride in plant cell cultures. Russ. J. Plant Physiol. 1995, 42, 372–375. [Google Scholar]
| Treatment | Roots | Vegetation Part |
|---|---|---|
| Control | 0.144 ± 0.022 | 0.346 ± 0.019 |
| AGr-Cu | 0.154 ± 0.022 | 0.344 ± 0.029 |
| Treatment | Roots | Stems | Leaves |
|---|---|---|---|
| Control | 0.15 ± 0.06 | 0.07 ± 0.06 | 0.02 ± 0.01 |
| AGr-Cu | 0.09 ± 0.04 | 0.03 ± 0.01 | 0.05 ± 0.03 |
| Treatment | Chlorophyll a | Chlorophyll b | Chlorophyll a and b | Caratenoid |
|---|---|---|---|---|
| Control | 0.80 ± 0.03 | 0.55 ± 0.08 | 1.35 ± 0.33 | 1.30 ± 0.24 |
| Cms | 0.53 ± 0.01 * | 0.17 ± 0.06 * | 0.70 ± 0.16 * | 1.22 ± 0.17 |
| AGr-Cu | 1.00 ± 0.02 * | 0.48 ± 0.01 | 1.44 ± 0.19 | 1.31 ± 0.03 |
| AGr-Cu + Cms | 0.72 ± 0.03 | 0.37 ± 0.06 | 1.08 ± 0.06 | 1.41 ± 0.06 * |
| Treatment | Length, cm | Mass, g | Content of DC, nmoles/g Wet Mass | |||
|---|---|---|---|---|---|---|
| Root | Stem | Root | Stem | Root | Stem | |
| Control | 11.43 ± 1.11 | 4.59 ± 0.28 | 0.065 ± 0.007 | 0.085 ± 0.005 | 5.45 ± 0.73 | 6.06 ± 1.01 |
| AGr-Cu | 11.12 ± 1.12 | 5.78 ± 0.35 * | 0.124 ± 0.007 * | 0.122 ± 0.011 * | 1.52 ± 0.13 * | 3.68 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khutsishvili, S.S.; Perfileva, A.I.; Kon’kova, T.V.; Lobanova, N.A.; Sadykov, E.K.; Sukhov, B.G. Copper-Containing Bionanocomposites Based on Natural Raw Arabinogalactan as Effective Vegetation Stimulators and Agents against Phytopathogens. Polymers 2024, 16, 716. https://doi.org/10.3390/polym16050716
Khutsishvili SS, Perfileva AI, Kon’kova TV, Lobanova NA, Sadykov EK, Sukhov BG. Copper-Containing Bionanocomposites Based on Natural Raw Arabinogalactan as Effective Vegetation Stimulators and Agents against Phytopathogens. Polymers. 2024; 16(5):716. https://doi.org/10.3390/polym16050716
Chicago/Turabian StyleKhutsishvili, Spartak S., Alla I. Perfileva, Tatyana V. Kon’kova, Natalya A. Lobanova, Evgeniy K. Sadykov, and Boris G. Sukhov. 2024. "Copper-Containing Bionanocomposites Based on Natural Raw Arabinogalactan as Effective Vegetation Stimulators and Agents against Phytopathogens" Polymers 16, no. 5: 716. https://doi.org/10.3390/polym16050716
APA StyleKhutsishvili, S. S., Perfileva, A. I., Kon’kova, T. V., Lobanova, N. A., Sadykov, E. K., & Sukhov, B. G. (2024). Copper-Containing Bionanocomposites Based on Natural Raw Arabinogalactan as Effective Vegetation Stimulators and Agents against Phytopathogens. Polymers, 16(5), 716. https://doi.org/10.3390/polym16050716







