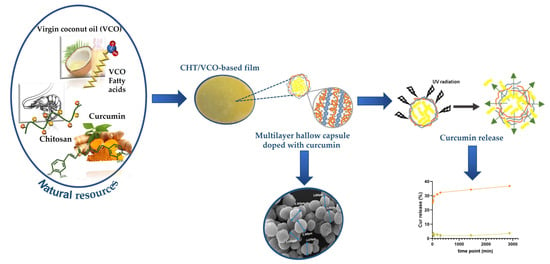
Chitosan/Virgin Coconut Oil-Based Emulsions Doped with Photosensitive Curcumin Loaded Capsules: A Functional Carrier to Topical Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
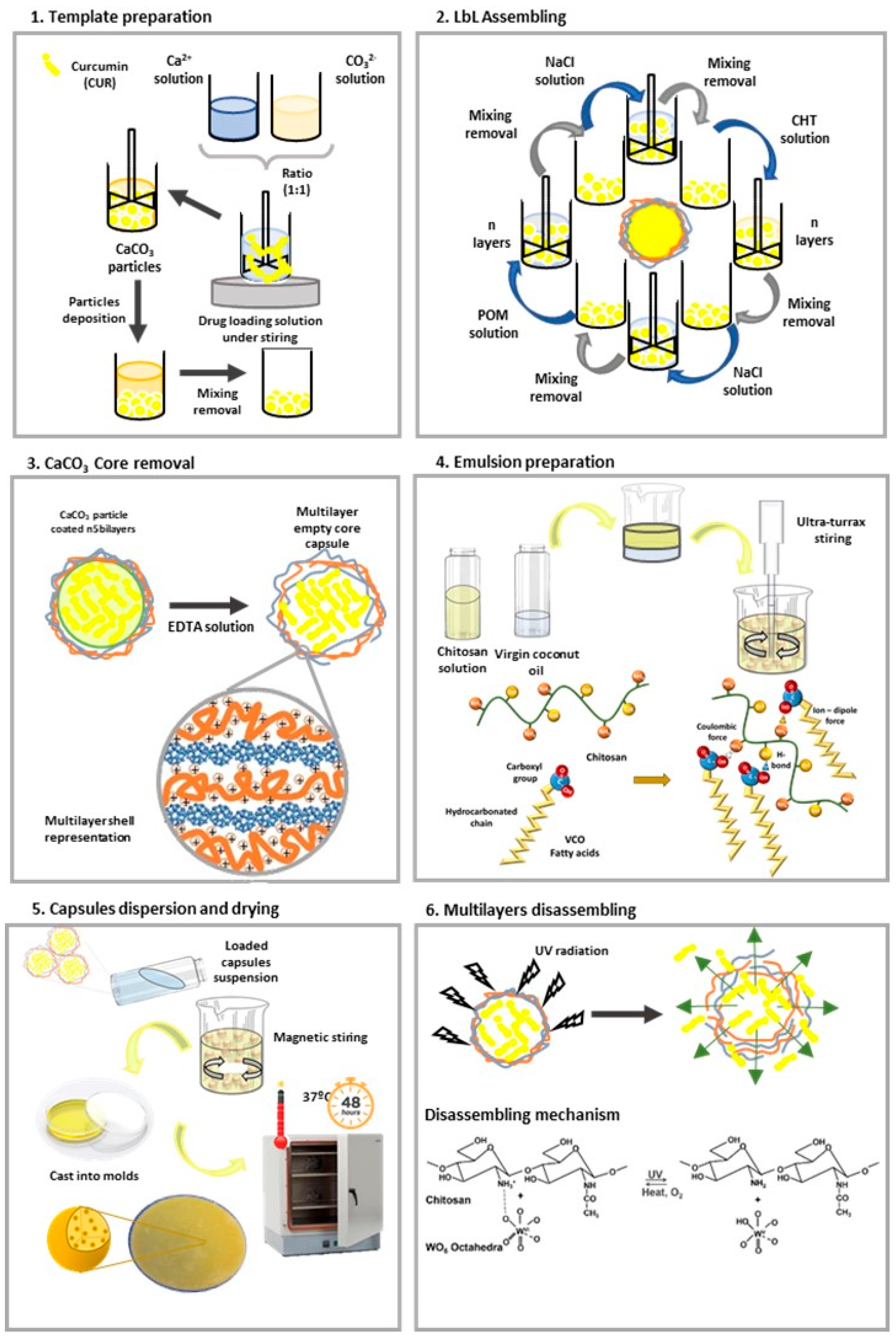
2.2.1. Solutions Preparation
2.2.2. Hollow Capsules Preparation
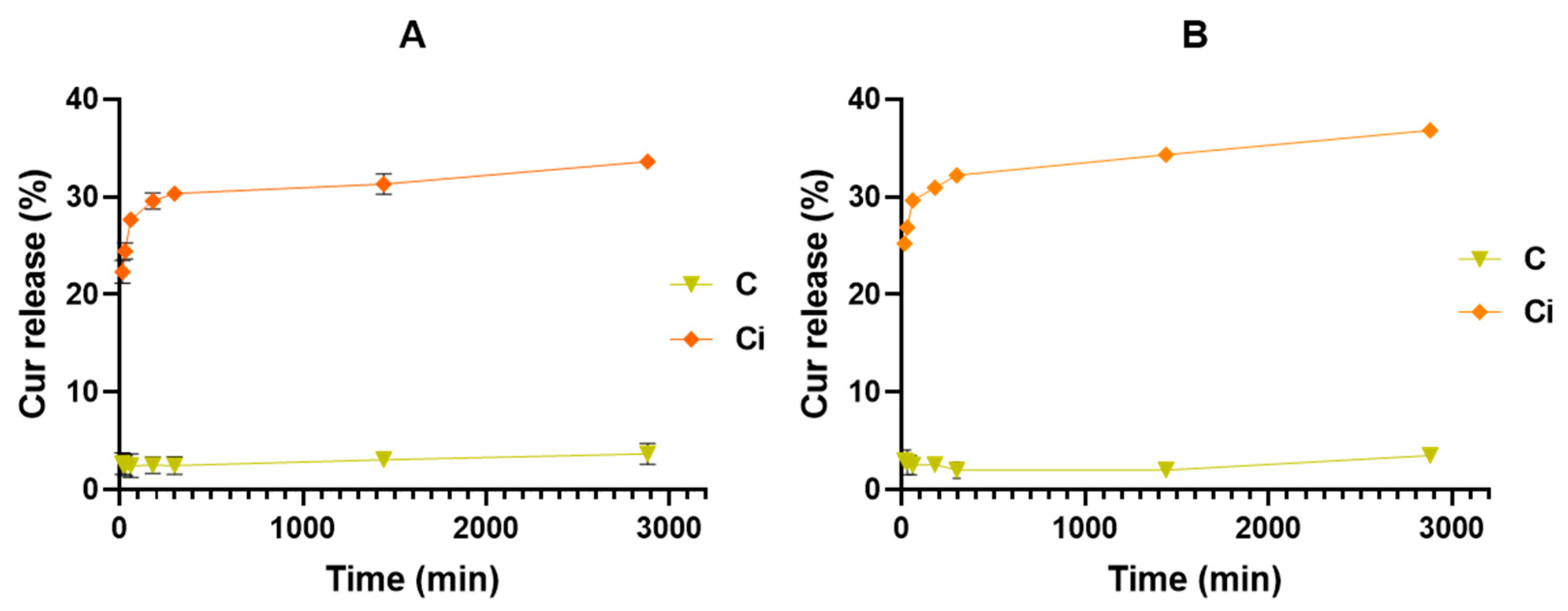
2.2.3. Curcumin Release from Capsules Conditions Assessment
2.2.4. CHT/VCO-Based Emulsion Preparation
2.2.5. Physico-Chemical Characterization
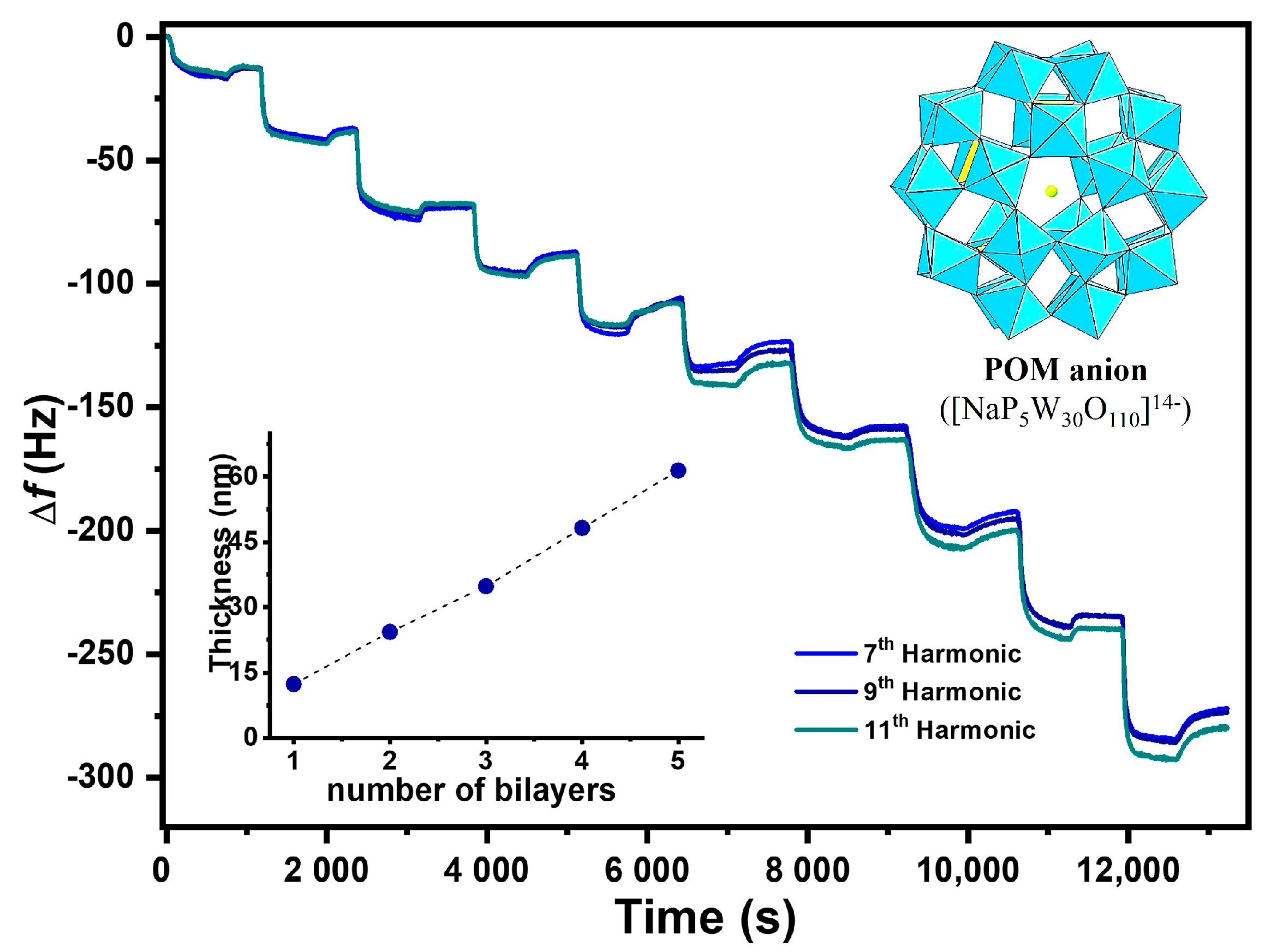
Structural Features (QCM-D)
Thermal Analysis–Differential Scanning Calorimetry (DSC)
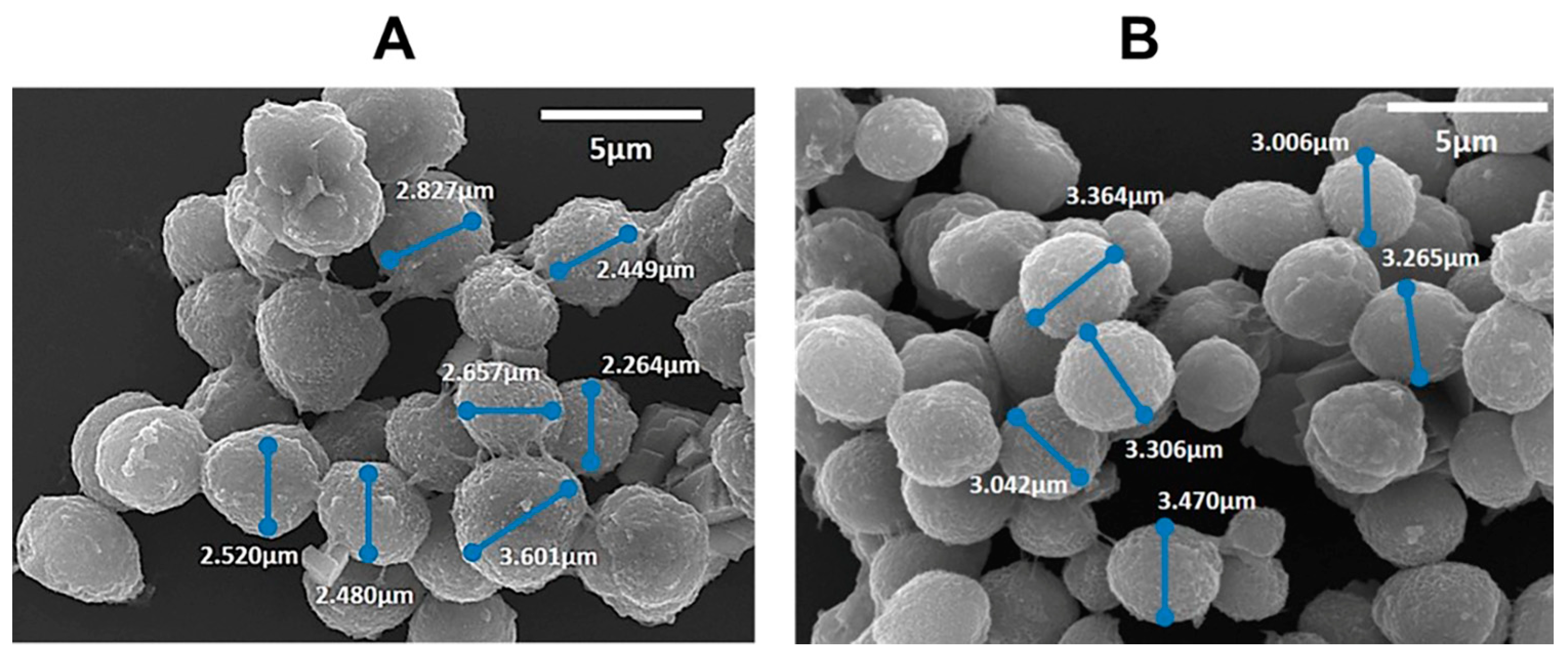
Morphology Assessment (SEM)
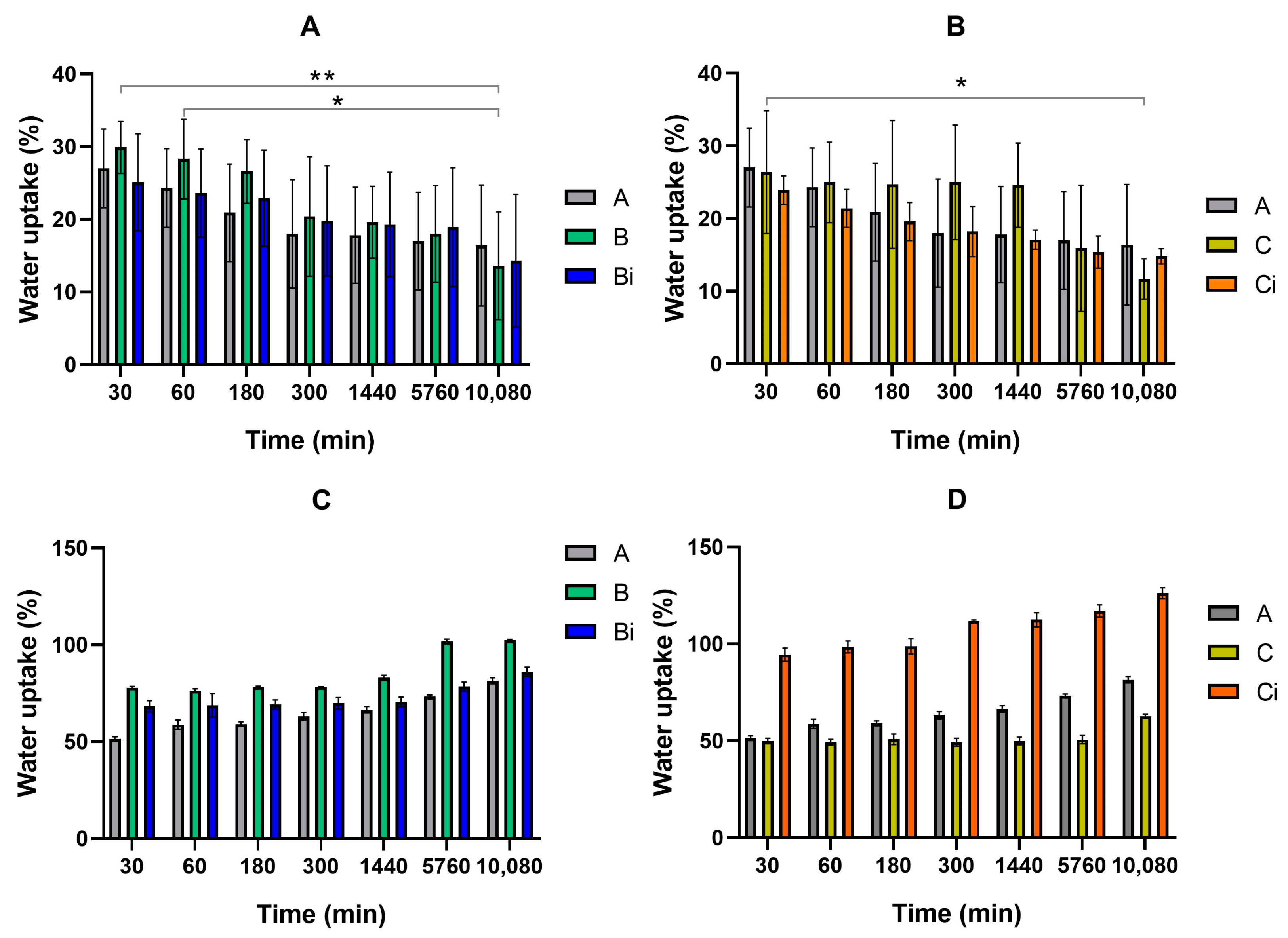
Swelling and Release Assays
Antioxidant Activity Assay
2.2.6. Statistical Data Treatment
3. Results and Discussion
Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulchar, R.J.; Singh, R.; Ding, S.; Alexander, E.; Leong, K.W.; Daniell, H. Delivery of biologics: Topical administration. Biomaterials 2023, 302, 122312. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Thakur, V.K.; Gupta, M. New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective. ACS Omega 2023, 8, 19145–19167. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Tapfumaneyi, P.; Imran, M.; Mohammed, Y.; Roberts, M.S. Recent advances and future prospective of topical and transdermal delivery systems. Front. Drug Deliv. 2022, 2, 957732. [Google Scholar] [CrossRef]
- Costa, R.R.; Castro, E.; Arias, F.J.; Rodríguez-Cabello, J.C.; Mano, J.F. Multifunctional Compartmentalized Capsules with a Hierarchical Organization from the Nano to the Macro Scales. Biomacromolecules 2013, 14, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Alvarez-Lorenzo, C.; Mano, J.F. Design Advances in Particulate Systems for Biomedical Applications. Adv. Healthc. Mater. 2016, 5, 1687–1723. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Borges, J.; Costa, A.M.S.; Gaspar, V.M.; Bermudez, V.D.Z.; Mano, J.F. Preparation of Well-Dispersed Chitosan/Alginate Hollow Multilayered Microcapsules for Enhanced Cellular Internalization. Molecules 2018, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.S.; Li, T.; Akinade, T.; Zhu, Y.; Leong, K.W. Drug delivery carriers with therapeutic functions. Adv. Drug Deliv. Rev. 2021, 176, 113884. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Kushwaha, P.; Shukla, B.; Ahmad, M. Design and characterization of dual-pronged liposome-embedded gel for enhanced dermal delivery. J. Surfactants Deterg. 2023, 26, 867–879. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zeng, J.; Groll, J.; Matsusaki, M. Layer-by-layer assembly methods and their biomedical applications. Biomater. Sci. 2022, 10, 4077–4094. [Google Scholar] [CrossRef] [PubMed]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach—A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Čolović, B.M.; Lacković, M.; Lalatović, J.; Mougharbel, S.A.; Kortz, U.; Krstić, Z.D. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef]
- Farzana, R.; Iqra, P.; Hunaiza, T. Antioxidant and antimicrobial effects of polyoxometalates. Microbiol. Curr. Res. 2018, 2, 3. [Google Scholar]
- Rodrigues, L.C.; Custódio, C.A.; Reis, R.L.; Mano, J.F. Light responsive multilayer surfaces with controlled spatial extinction capability. J. Mater. Chem. B 2016, 4, 1398–1404. [Google Scholar] [CrossRef]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef]
- Szczęsna, W.; Tsirigotis-Maniecka, M.; Lamch, Ł.; Szyk-Warszyńska, L.; Zboińska, E.; Warszyński, P.; Wilk, K.A. Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function. Molecules 2022, 27, 1415. [Google Scholar] [CrossRef]
- Sideek, S.A.; El-Nassan, H.B.; Fares, A.R.; ElMeshad, A.N.; Elkasabgy, N.A. Different Curcumin-Loaded Delivery Systems for Wound Healing Applications: A Comprehensive Review. Pharmaceutics 2023, 15, 38. [Google Scholar] [CrossRef]
- Lei, F.; Li, P.; Chen, T.; Wang, Q.; Wang, C.; Liu, Y.; Deng, Y.; Zhang, Z.; Xu, M.; Tian, J.; et al. Recent advances in curcumin-loaded biomimetic nanomedicines for targeted therapies. J. Drug Deliv. Sci. Technol. 2023, 80, 104200. [Google Scholar] [CrossRef]
- Mitra, S.; Mateti, T.; Ramakrishna, S.; Laha, A. A Review on Curcumin-Loaded Electrospun Nanofibers and their Application in Modern Medicine. JOM 2022, 74, 3392–3407. [Google Scholar] [CrossRef]
- Perez-Pacheco, C.G.; Fernandes, N.A.R.; Camilli, A.C.; Ferrarezi, D.P.; Silva, A.F.; Zunareli, M.C.; Amantino, C.F.; Primo, F.L.; Guimarães-Stabilli, M.R.; Junior, C.R. Local administration of curcumin-loaded nanoparticles enhances periodontal repair in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 311–321. [Google Scholar] [CrossRef]
- Sood, A.; Dev, A.; Das, S.S.; Kim, H.J.; Kumar, A.; Thakur, V.K.; Han, S.S. Curcumin-loaded alginate hydrogels for cancer therapy and wound healing applications: A review. Int. J. Biol. Macromol. 2023, 232, 123283. [Google Scholar] [CrossRef]
- Torbat, N.A.; Akbarzadeh, I.; Rezaei, N.; Moghaddam, Z.S.; Bazzazan, S.; Mostafavi, E. Curcumin-Incorporated Biomaterials: In silico and in vitro evaluation of biological potentials. Coord. Chem. Rev. 2023, 492, 215233. [Google Scholar] [CrossRef]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Fu, S.-Z.; Meng, X.-H.; Fan, J.; Yang, L.-L.; Wen, Q.-L.; Ye, S.-J.; Lin, S.; Wang, B.-Q.; Chen, L.-L.; Wu, J.-B.; et al. Acceleration of dermal wound healing by using electrospun curcumin-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) fibrous mats. J. Biomed. Mater. Res. 2014, 102, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Gomes, J.M.; Vilas-Boas, Â.; Pirraco, R.P.; Reis, R.L. Approach on chitosan/virgin coconut oil-based emulsion matrices as a platform to design superabsorbent materials. Carbohydr. Polym. 2020, 249, 116839. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; da Costa, D.S.; Villalva, D.G.; Loh, W.; Reis, R.L. Chitosan/Virgin-Coconut-Oil-Based System Enriched with Cubosomes: A 3D Drug-Delivery Approach. Mar. Drugs 2023, 21, 394. [Google Scholar] [CrossRef] [PubMed]
- Arifin, H.R.; Utaminingsih, F.; Djali, M.; Nurhadi, B.; Lembong, E.; Marta, H. The Role of Virgin Coconut Oil in Corn Starch/NCC-Based Nanocomposite Film Matrix: Physical, Mechanical, and Water Vapor Transmission Characteristics. Polymers 2023, 15, 3239. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Mohamad, S.F.; Ibrahim, M.A.; Amin, K.A.M. Evaluation of Gellan Gum Film Containing Virgin Coconut Oil for Transparent Dressing Materials. Adv. Biomater. 2014, 2014, 351248. [Google Scholar] [CrossRef]
- Signini, R.; Filho, S.P.C. Características e propriedades de quitosanas purificadas nas formas neutra, acetato e cloridrato. Polímeros 2001, 11, 58–64. [Google Scholar] [CrossRef]
- Creaser, I.; Heckel, M.C.; Neitz, R.J.; Pope, M.T. Rigid nonlabile polyoxometalate cryptates [ZP5W30O110](15-n)—That exhibit unprecedented selectivity for certain lanthanide and other multivalent cations. Inorg. Chem. 1993, 32, 1573–1578. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.L.; Ramirez, A.; McEnnis, K. Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery. Pharmaceutics 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Hu, B.; Guo, Y.; Li, H.; Liu, X.; Fu, Y.; Ding, F. Recent advances in chitosan-based layer-by-layer biomaterials and their biomedical applications. Carbohydr. Polym. 2021, 271, 118427. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Al-Amin, M.; Rashid, T.; Sultan, Z.; Ashaduzzaman, M.; Sarker, M.; Shamsuddin, S. Preparation, Characterization and Performance Evaluation of Chitosan as an Adsorbent for Remazol Red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Nabila, N.; Hassan, S.R.; Larasati, G.P.; Yohan, B.; Sasmono, R.; Adi, A.C.; Iskandar, F.; Rachmawati, H. The Influence of Surface Charge on the Antiviral Effect of Curcumin Loaded in Nanocarrier System. Pharm. Nanotechnol. 2021, 9, 210–216. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin- and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Escobar, A.P.; Andris, M.N.; Boardman, B.M.; Peters, G.M. Understanding the Molecular-Level Interactions of Glucosamine-Glycerol Assemblies: A Model System for Chitosan Plasticization. ACS Omega 2021, 6, 25227–25234. [Google Scholar] [CrossRef] [PubMed]
- Derwin, R.; Patton, D.; Strapp, H.; Moore, Z. Wound pH and temperature as predictors of healing: An observational study. J. Wound Care 2023, 32, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Amra, K.; Momin, M.; Desai, N.; Khan, F. Therapeutic benefits of natural oils along with permeation enhancing activity. Int. J. Dermatol. 2022, 61, 484–507. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, J.M.; Cowley, A.; du Preez, J.; Gerber, M.; du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef]
- Hashim, A.F.; Hamed, S.F.; Hamid, H.A.A.; Abd-Elsalam, K.A.; Golonka, I.; Musiał, W.; El-Sherbiny, I.M. Antioxidant and antibacterial activities of omega-3 rich oils/curcumin nanoemulsions loaded in chitosan and alginate-based microbeads. Int. J. Biol. Macromol. 2019, 140, 682–696. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.; Hwa, P.C.H.; Ja’Afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]


| Abbreviation | Sample Composition |
|---|---|
| A | CHT/VCO |
| B | CHT/VCO/empty caps |
| Bi | CHT/VCO/empty caps after irradiation |
| C | CHT/VCO/curcumin loaded caps |
| Ci | CHT/VCO/curcumin loaded caps after irradiation |
| Composition | Mass Loss (%) | Thickness Variation (%) | ||
|---|---|---|---|---|
| PBS | pH 5 Buffer | PBS | pH 5 Buffer | |
| A | (37.4 ± 4.2)% | (19.9 ± 6.8)% | (54.8 ± 8.6)% | (15.1 ± 5.0)% |
| B | (37.4 ± 1.4)% | (18.2 ± 2.7)% | (54.8 ± 9.9)% | (28.6 ± 5.9)% |
| Bi | (39.7 ± 5.5)% | (18.9 ± 6.1)% | (86.9 ± 8.8)% | (35.6 ± 8.8)% |
| C | (39.9 ± 2.3)% | (39.5 ± 4.7)% | (63.1 ± 4.4)% | (25.3 ± 3.6)% |
| Ci | (53.1 ± 2.6)% | (62.5 ± 5.9)% | (131.0 ± 5.1)% | (68.1 ± 6.5)% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.C.; Ribeiro, A.P.; Silva, S.S.; Reis, R.L. Chitosan/Virgin Coconut Oil-Based Emulsions Doped with Photosensitive Curcumin Loaded Capsules: A Functional Carrier to Topical Treatment. Polymers 2024, 16, 641. https://doi.org/10.3390/polym16050641
Rodrigues LC, Ribeiro AP, Silva SS, Reis RL. Chitosan/Virgin Coconut Oil-Based Emulsions Doped with Photosensitive Curcumin Loaded Capsules: A Functional Carrier to Topical Treatment. Polymers. 2024; 16(5):641. https://doi.org/10.3390/polym16050641
Chicago/Turabian StyleRodrigues, Luísa C., Adriana P. Ribeiro, Simone S. Silva, and Rui L. Reis. 2024. "Chitosan/Virgin Coconut Oil-Based Emulsions Doped with Photosensitive Curcumin Loaded Capsules: A Functional Carrier to Topical Treatment" Polymers 16, no. 5: 641. https://doi.org/10.3390/polym16050641
APA StyleRodrigues, L. C., Ribeiro, A. P., Silva, S. S., & Reis, R. L. (2024). Chitosan/Virgin Coconut Oil-Based Emulsions Doped with Photosensitive Curcumin Loaded Capsules: A Functional Carrier to Topical Treatment. Polymers, 16(5), 641. https://doi.org/10.3390/polym16050641