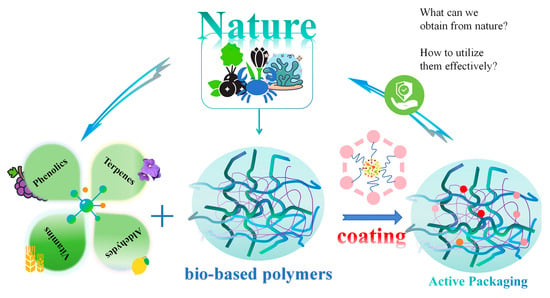
Natural Extracts and Their Applications in Polymer-Based Active Packaging: A Review
Abstract
1. Introduction
2. Antibacterial and Antioxidant Properties of Natural Extracts
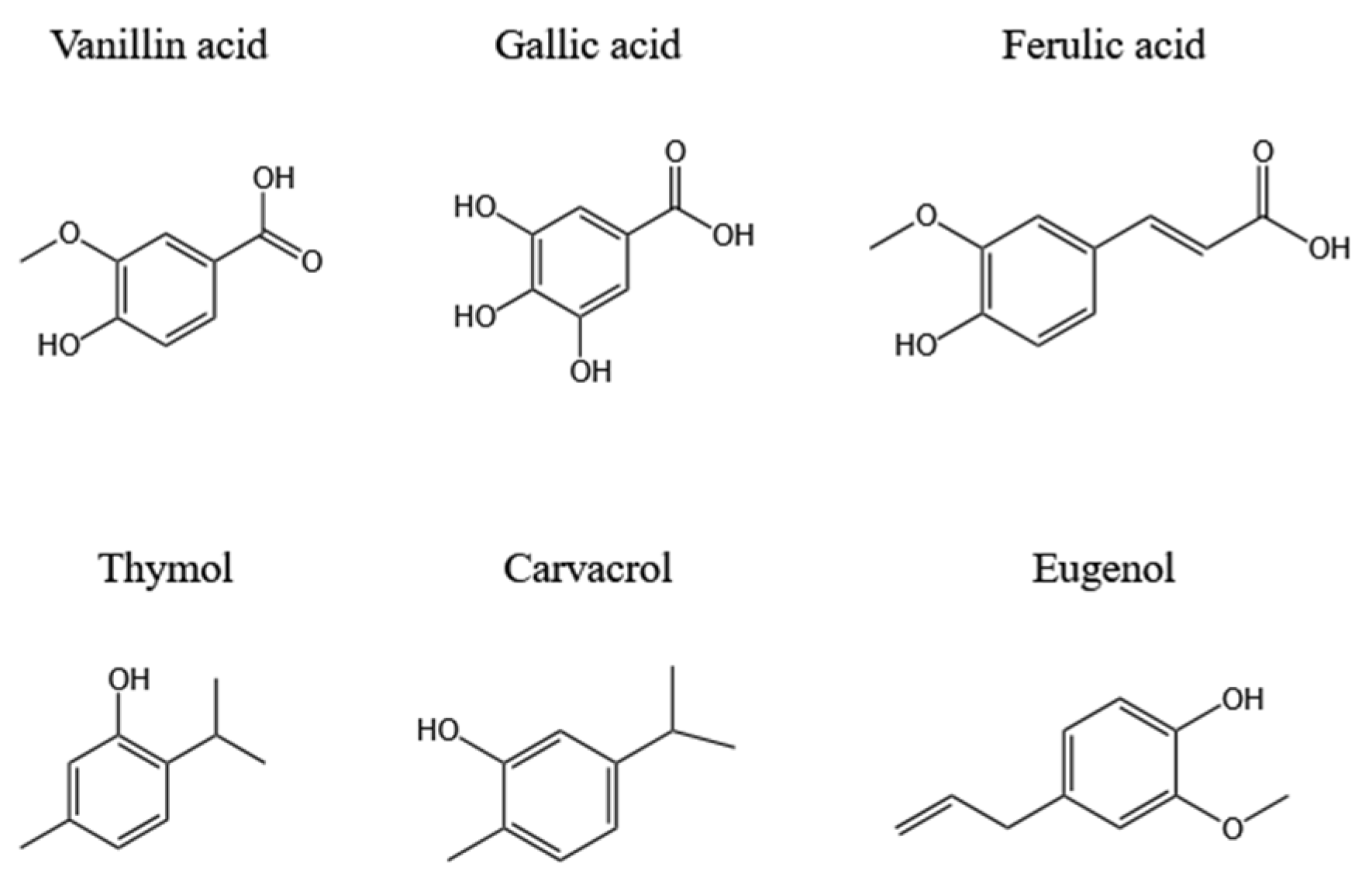
2.1. Phenolics
2.2. Terpenes
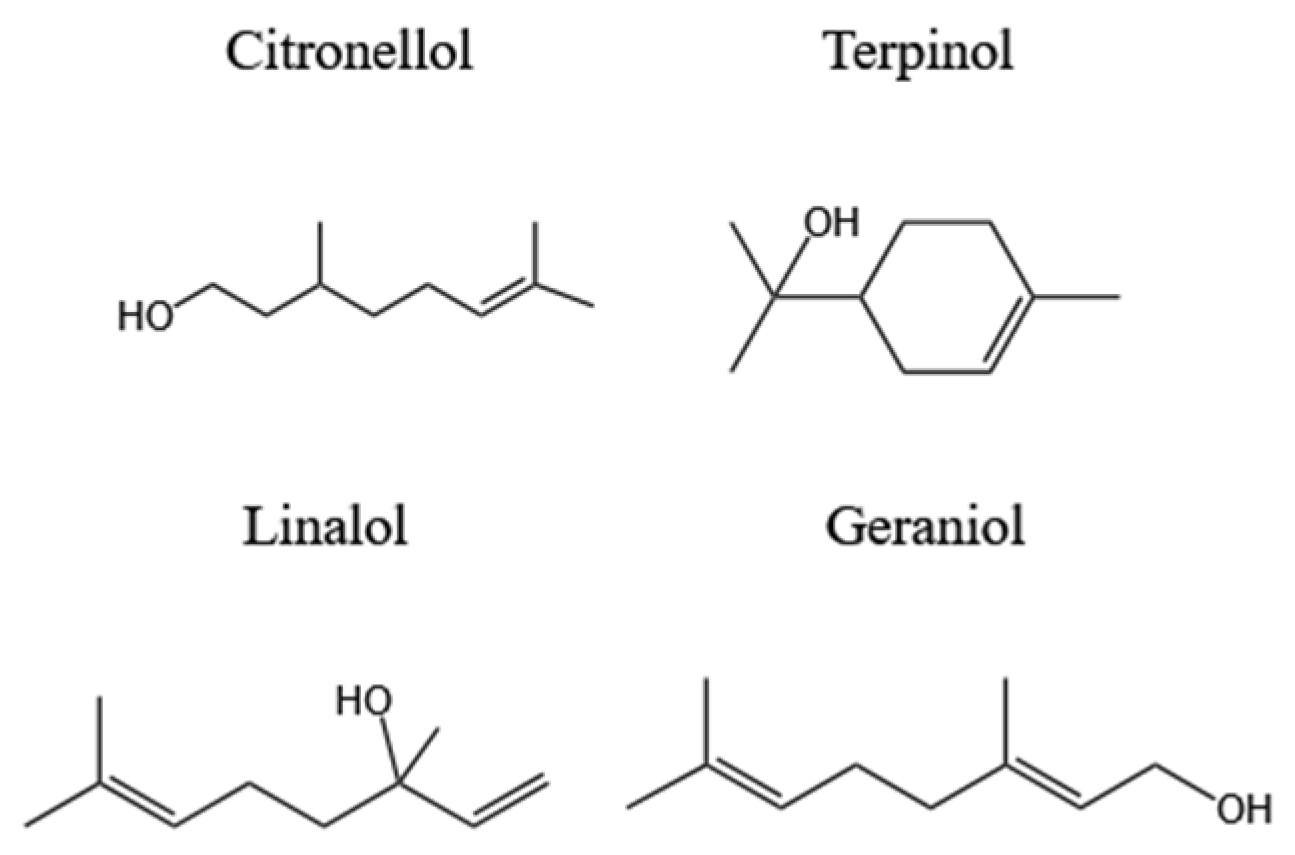
2.2.1. Monoterpene Alcohol
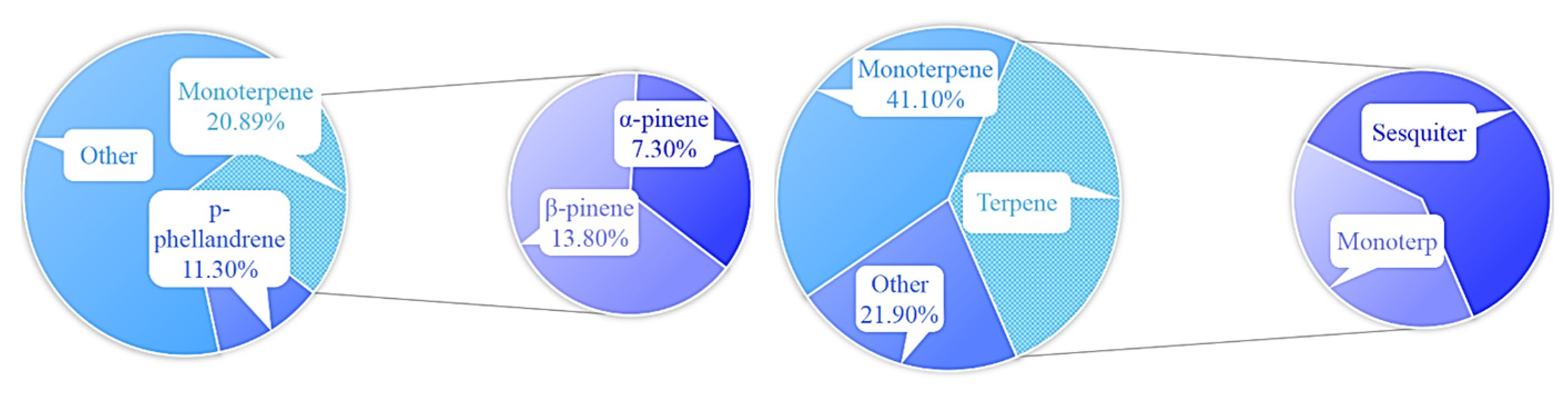
2.2.2. Monoterpene
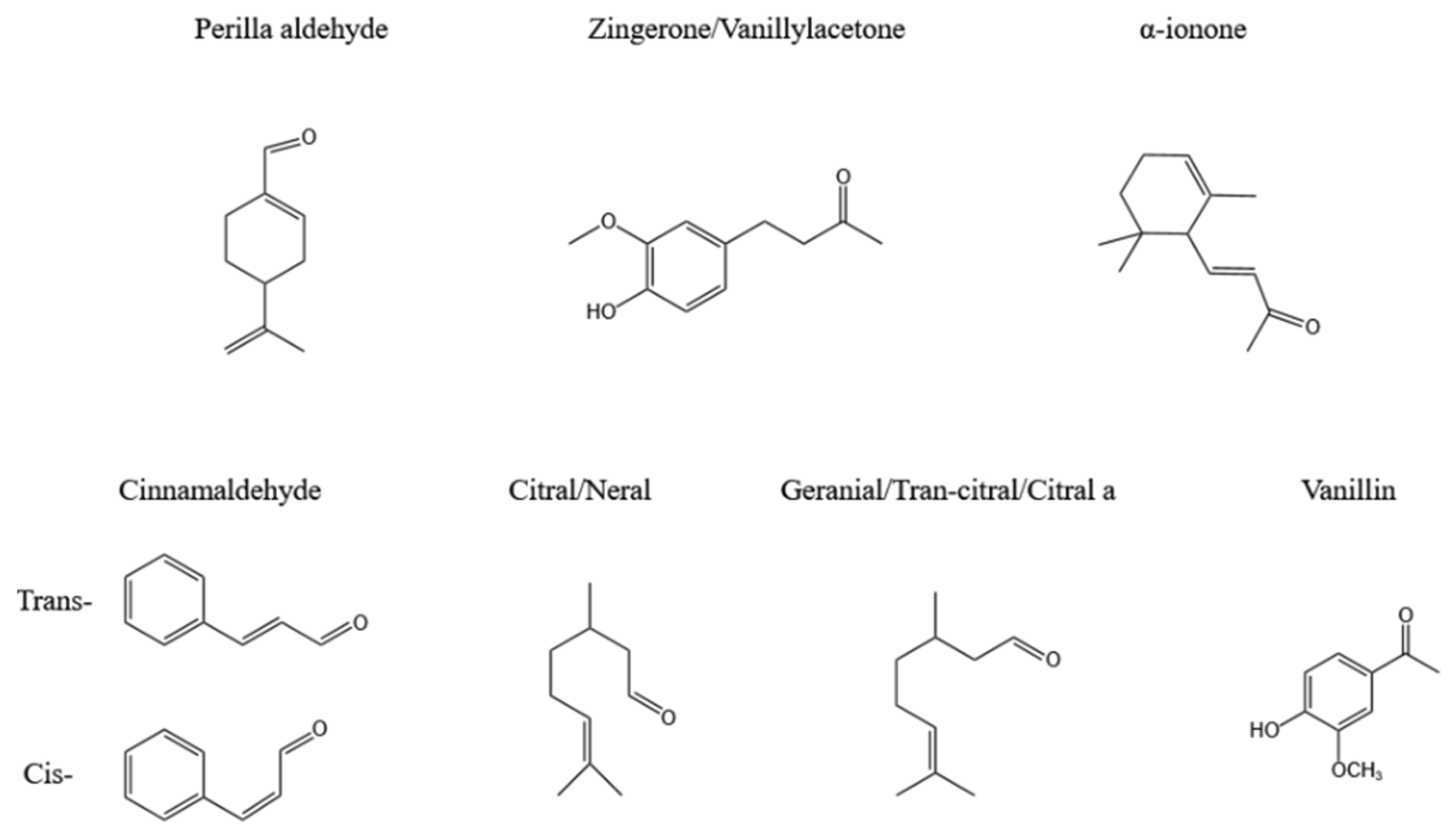
2.3. Ketones and Aldehydes
2.4. The Preservative Properties of Mixtures of Natural Extracts
3. Preservation Materials Containing a Single Active Compound
4. Preservation Materials Containing Mixed Extracts
4.1. Essential Oil
4.2. Non Essential Oils
5. Food Safety of Natural Antibacterial Extracts
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dequeker, S.; van Hensbergen, M.; den Heijer, C.D.J.; Dhaeze, W.; Raven, S.F.H.; Ewalts-Hakkoer, H.; Tolsma, P.; Willemsen, I.; van Drunen-Kamp, K.J.; van der Slikke-verstraten, K.; et al. Cross-border differences in the prevalence and risk factors for carriage of antimicrobial resistance in children attending daycare centers: A point prevalence study in the Netherlands and Belgium. BMC Infect. Dis. 2024, 24, 131. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Dong, X.L.; Zang, J.X.; Zhao, X.P.; Jiang, F.; Jiang, L.F.; Xiong, C.L.; Wang, N.; Fu, C.W. Antibiotic residues of drinking-water and its human exposure risk assessment in rural Eastern China. Water Res. 2023, 236, 119940. [Google Scholar] [CrossRef]
- Guo, D.S.; Chen, G.H.; Tong, M.Z.; Wu, C.Q.; Fang, R.; Yi, L.X. Determination of Five Preservatives in Food by Capillary Electrophoresis with Quantum Dot Indirect Laser Induced Fluorescence. Chin. J. Anal. Chem. 2012, 40, 1379–1384. [Google Scholar]
- Mir, S.A.; Wani, S.M.; Naseem, Z.; Rizwan, D. Application of sodium diacetate, potassium lactate and calcium lactate as a microbial decontaminant during processing and storage of the traditional meatballs (rista). Food Prod. Process. Nutr. 2023, 5, 1. [Google Scholar] [CrossRef]
- Ma, J.; Huang, G.X.; Li, J.S.; Yan, L.J.; Zhang, Q. A Visual Colorimetric Method for Hydrogen Peroxide Detection Based on the Peroxidase-Like Properties of Cu (II). Spectrosc. Spect. Anal. 2022, 42, 2795–2799. [Google Scholar]
- Ban, Z.; Chen, F.; Liu, L.; Zhang, S.; Wang, L.; Wang, H.; Wang, L.; Zhu, Y. Gliadin nanoparticles stabilized by sodium carboxymethyl cellulose as carriers for improved dispersibility, stability and bacteriostatic activity of Natamycin. Food Biosci. 2023, 53, 102575. [Google Scholar] [CrossRef]
- Carrión, M.G.; Corripio, M.A.R.; Contreras, J.V.H.; Marrón, M.R.; Olán, G.M.; Cázares, A.S.H. Optimization and characterization of taro starch, nisin, and sodium alginate-based biodegradable films: Antimicrobial effect in chicken meat. Poult. Sci. 2023, 102, 103100. [Google Scholar] [CrossRef]
- Kimani, B.G.; Tako, M.; Veres, C.; Krisch, J.; Papp, T.; Kerekes, E.B.; Vagvoelgyi, C. Activity of Binary Combinations of Natural Phenolics and Synthetic Food Preservatives against Food Spoilage Yeasts. Foods 2023, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, L.Q.; Jiang, D.M.; Wang, M.; Liu, H.M.; Yu, H.; Yao, W.R. Transcriptomic analysis of inhibition by eugenol of ochratoxin A biosynthesis and growth of Aspergillus carbonarius. Food Control 2022, 135, 108788. [Google Scholar] [CrossRef]
- Kumar Pradhan, S.; Vivek, S. Polyphenols in different plant parts of Inula grandiflora collected from two habitats of Uttarakhand Himalayas. J. Herbs Spices Med. Plants 2023, 29, 199–212. [Google Scholar] [CrossRef]
- Silva, D.; Diniz-Neto, H.; Cordeiro, L.; Silva-Neta, M.; Silva, S.; Andrade-Júnior, F.; Leite, M.; Nóbrega, J.; Morais, M.; Souza, J.; et al. (R)-(+)-β-Citronellol and (S)-(−)-β-Citronellol in Combination with Amphotericin B against Candida Spp. Int. J. Mol. Sci. 2020, 21, 1785. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.Y.; Zhao, S.H.; Zhang, F.; Meng, X.H.; Liu, B.J. Highly stable nanostructured lipid carriers containing candelilla wax for D-limonene encapsulation: Preparation, characterization and antifungal activity. Food Hydrocoll. 2023, 145, 109101. [Google Scholar] [CrossRef]
- Huong, L.; Huong, T.T.; Huong, N.T.T.; Hung, N.H.; Dat, P.T.T.; Luong, N.X.; Ogunwande, I.A. Chemical composition and larvicidal activity of essential oils from (J. Koenig) Link ex. A. Dietr. against three mosquito vectors. Bol. Latinoam. Caribe Plantas Med. Aromát. 2020, 19, 569–579. [Google Scholar] [CrossRef]
- Akbari, S.; Didar, Z.; Vazifedoost, M.; Hajirostamloo, B.; Mohtashami, M. Antibiofilm Activity of Ginger (Zingiber officinale) Extracts In Vitro and Food Model. J. Food Process. Preserv. 2023, 2023, 5134332. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.Z.; Lu, Z.Q.; Sun, C.H.; Zhang, M.; Zhu, A.H.; Peng, X. Perillaldehyde, a Promising Antifungal Agent Used in Food Preservation, Triggers Apoptosis through a Metacaspase-Dependent Pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404–7413. [Google Scholar] [CrossRef] [PubMed]
- Mugahi, S.M.; Aberoumand, A.; Ziaei-nejad, S. Effects of Turmeric, Cinnamon, and Lemon Extracts on Shelf Life, Nutrients, and Preservation of Carp Fish in Cold Storage. J. Food Qual. 2022, 2022, 3502464. [Google Scholar] [CrossRef]
- Byun, S.; Chen, C.H.; Yin, H.B.; Patel, J. Antimicrobial effect of natural fruit extracts against on whole and fresh-cut cucumbers. J. Food Process. Preserv. 2022, 46, 16437. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.Q.; Gao, Y.C.; Wang, Q. Antibacterial Activity and Mechanism of Polygonum orientale L. Essential Oil against Pectobacterium carotovorum subsp. carotovorum. Foods 2022, 11, 1585. [Google Scholar] [CrossRef]
- Zejli, H.; EL Amrani, B.; Metouekel, A.; Bousseraf, F.Z.; Fitat, A.; Taleb, M.; Abdellaoui, A. Comparative assessment of total phenolics content and in vitro antioxidant capacity variations of leaf extracts of Origanum grossii and Thymus pallidus. Moroc. J. Chem. 2024, 12, 361–375. [Google Scholar]
- Mohammed, B.S.; Sanadelaslam, E.; Salwa, I.A.E.; Ahmed, S.J. HPLC-PDA-MS Identification of Phenolic Profile and in Vitro Antioxidant Activity of Adansonia digitata L. Leaves from Sudan. Moroc. J. Chem. 2024, 12, 221–232. [Google Scholar]
- Silva, M.L.; Rita, K.; Bernardo, M.A.; de Mesquita, M.F.; Pintao, A.M.; Moncada, M. Adansonia digitata L. (Baobab) Bioactive Compounds, Biological Activities, and the Potential Effect on Glycemia: A Narrative Review. Nutrients 2023, 15, 2170. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Guan, C.X.; Sun, L.R.; Zhang, Q.L.; Pan, S.H.; Chen, H. Improvement of the UV-resistance capability of fish gelatin-oxidized starch film via inserting mycosporine-like amino acids. J. Sci. Food Agric. 2023, 103, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Korge, K.; Seme, H.; Bajic, M.; Likozar, B.; Novak, U. Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese. Foods 2020, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Pascale, C.; Geaman, J.; Mendoza, C.; Gao, F.; Kaminski, A.; Cuevas-Nunez, M.; Darvishan, B.; Mitchell, J.C.; Carrilho, M.R.; Sigar, I. In vitro assessment of antimicrobial potential of low molecular weight chitosan and its ability to mechanically reinforce and control endogenous proteolytic activity of dentine. Int. Endod. J. 2023, 56, 1337–1349. [Google Scholar] [CrossRef]
- Carli, C.d.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomas, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcao, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.Y.; Sun, H.G.; Zhou, Y.; Tao, Y.; Zhang, C. Enhancement of Antioxidant Property of N-Carboxymethyl Chitosan and Its Application in Strawberry Preservation. Molecules 2022, 27, 8496. [Google Scholar] [CrossRef]
- Liu, W.J.; Xie, J.; Li, L.; Xue, B.; Li, X.H.; Gan, J.H.; Shao, Z.H.; Writing, T.S. Properties of phenolic acid-chitosan composite films and preservative effect on Penaeus vannamei. J. Mol. Struct. 2021, 1239, 130531. [Google Scholar] [CrossRef]
- Orsuwan, A.; Kwon, S.; Bumbudsanpharoke, N.; Ko, S. Novel LDPE-riboflavin composite film with dual function of broad-spectrum light barrier and antimicrobial activity. Food Control 2019, 100, 176–182. [Google Scholar] [CrossRef]
- Wang, L.; Fogliano, V.; Heising, J.; Meulenbroeks, E.; Dekker, M. Volatile antimicrobial absorption in food gel depends on the food matrix characteristics. Food Hydrocoll. 2020, 107, 105933. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.J.; Chen, Y.Z.; Ma, X.J.; Xia, M.Y. Chitosan and procyanidin composite films with high antioxidant activity and pH responsivity for cheese packaging. Food Chem. 2021, 338, 128013. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xie, Y.R.; Li, C.; Xue, F. Comparative studies on physicochemical properties of gluten- And glutenin-based films functionalized by polyphenols. Cereal Chem. 2022, 99, 640–651. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT-Food Sci. Technol. 2020, 118, 108851. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, R.P.; Gao, T.; Hu, Y.; Zhou, J.H.; Li, C.H.; Wang, P.P.; Yang, H.Y.; Xing, W.J.; Dong, L.; et al. Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats. Nutrients 2023, 15, 2480. [Google Scholar] [CrossRef] [PubMed]
- Wakui, N.; Togawa, C.; Ichikawa, K.; Matsuoka, R.; Watanabe, M.; Okami, A.; Shirozu, S.; Yamamura, M.; Machida, Y. Relieving psychological stress and improving sleep quality by bergamot essential oil use before bedtime and upon awakening: A randomized crossover trial. Complement. Ther. Med. 2023, 77, 102976. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, K. Effect of Inhalation of Aromatherapy Oil on Patients with Perennial Allergic Rhinitis: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2016, 2016, 7896081. [Google Scholar]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Ismail, L.C.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef]
- GRAS Notice Inventory. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 31 October 2023).
- Nurain, A.; Noriham, A.; Aishah, B.; Mohd Noor, Z.; Abdul Aziz, A.; Rohaya, A. Phytochemicals of ethanolic extract and essential oil of Persicaria hydropiper and their potential as antibacterial agents for food packaging polylactic acid film. J. Food Saf. 2021, 41, e12864. [Google Scholar]
- Shi, C.; Zhou, A.Y.; Fang, D.L.; Lu, T.; Wang, J.Y.; Song, Y.X.; Lyu, L.; Wu, W.L.; Huang, C.B.; Li, W.L. Oregano essential oil/β-cyclodextrin inclusion compound polylactic acid/polycaprolactone electrospun nanofibers for active food packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Dogan, N.; Dogan, C.; Eticha, A.K.; Gungor, M.; Akgul, Y. Centrifugally spun micro-nanofibers based on lemon peel oil/gelatin as novel edible active food packaging: Fabrication, characterization, and application to prevent foodborne pathogens E. coli and S. aureus in cheese. Food Control 2022, 139, 109081. [Google Scholar] [CrossRef]
- Li, L.H.; Zhao, Z.L.; Wei, S.Y.; Xu, K.; Xia, J.F.; Wu, Q.S.; Lue, X.; Wang, L. Development and application of multifunctional films based on modified chitosan/gelatin polyelectrolyte complex for preservation and monitoring. Food Hydrocoll. 2024, 147, 109336. [Google Scholar] [CrossRef]
- Yi, G.H.; Yin, C.X.; Lao, Y.L.; Shi, Z.F.; He, X.W.; Wu, J.Y.; Jiang, Y.M.; Gong, L. Antibacterial and antitumor activities of chitosan/polyvinyl alcohol films containing microemulsion of papaya seed essential oil. Mater. Today Commun. 2022, 31, 103475. [Google Scholar] [CrossRef]
- Fan, Y.T.; Luo, D.X.; Yi, J. Resveratrol-loaded α-lactalbumin-chitosan nanoparticle-encapsulated high internal phase Pickering emulsion for curcumin protection and its in vitro digestion profile. Food Chem X 2022, 15, 100433. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Ran, Z.; Weiliang, G.; Xiaomin, Z.; Minjun, L.; Luyun, C. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT-Food Sci. Technol. 2022, 153, 112506. [Google Scholar]
- Hao, R.Y.; Shah, B.R.; Sternisa, M.; Mozina, S.S.; Mráz, J. Development of essential oil-emulsion based coating and its preservative effects on common carp. LWT-Food Sci. Technol. 2022, 154, 112582. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.M.; Warner, R.D.; Fang, Z.X. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, B.; Zhu, L.B.; Wang, P.; Xu, F.; Zhang, Y.J. Octenyl Succinic Acid Starch-Stabilized Vanilla Essential Oil Pickering Emulsion: Preparation, Characterization, Antioxidant Activity, and Storage Stability. Foods 2022, 11, 987. [Google Scholar] [CrossRef]
- Arellano, S.; Zhu, L.B.; Kumar, G.D.; Law, B.; Friedman, M.; Ravishankar, S. Essential Oil Microemulsions Inactivate Antibiotic-Resistant Bacteria on Iceberg Lettuce during 28-Day Storage at 4 °C. Molecules 2022, 27, 6699. [Google Scholar] [CrossRef] [PubMed]
- Madivala, B.; Vandebril, S.; Fransaer, J.; Vermant, J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717–1727. [Google Scholar] [CrossRef]
- Katepalli, H.; John, V.T.; Tripathi, A.; Bose, A. Microstructure and rheology of particle stabilized emulsions: Effects of particle shape and inter-particle interactions. J. Colloid. Interf. Sci. 2017, 485, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, L.; Feng, X.; Cui, F.; Wu, R.; Kong, Q.; Sun, K.; Gao, J.; Guo, J. Development of functional, sustainable pullulan-sodium alginate-based films by incorporating essential oil microemulsion for chilled pork preservation. Int. J. Biol. Macromol. 2023, 253, 127257. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Xin, J.; Jiali, H.; Ling, L.; Kitazawa, H.; Xiangyou, W.; Yanyin, G.; Xinru, D.; Haipeng, L. Properties of an active film based on glutenin/tamarind gum and loaded with binary microemulsion of melatonin/pummelo essential oil and its preservation for Agaricus bisporus. Food Chem 2023, 429, 136901. [Google Scholar]
- Tavakoli, S.; Liang, S.J.; Tan, Y.Q.; Liu, Y.Y.; Gao, S.; Zhao, Y.; Hong, H.; Luo, Y.K. The potential application of a novel biodegradable film activated with co-encapsulated epsilon-poly-l-lysine and Spirulina platensis extract for fish fillets preservation. Food Packag. Shelf Life 2023, 39, 101158. [Google Scholar] [CrossRef]
- Karolina, P.; Wiktoria, G.; Nikola, N.; Leslaw, J.; Agnieszka, K.; Michal, S.; Tomasz, M.; Anna, B.-W.; Anna, K.-M.; Ewelina, J. Application possibilities of triple-layer furcellaran film with hazelnut oil microemulsion for packing cod liver oil. Food Hydrocoll. 2024, 147 Pt B, 109428. [Google Scholar]
- Yeyen, L.; Nathdanai, H. Ginger oil and lime peel oil loaded PBAT/PLA via cast-extrusion as shrimp active packaging: Microbial and melanosis inhibition. Food Packag. Shelf Life 2023, 38, 101116. [Google Scholar]
- Ali, A.; Basit, A.; Hussain, A.; Sammi, S.; Wali, A.; Goksen, G.; Muhammad, A.; Faiz, F.; Trif, M.; Rusu, A.; et al. Starch-based environment friendly, edible and antimicrobial films reinforced with medicinal plants. Front. Nutr. 2023, 9, 1066337. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.K.S.; Sharaby, M.R.; Abdelrahim, D.S. Novel active edible food packaging films based entirely on citrus peel wastes. Food Hydrocoll. 2023, 134, 107961. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Z. Xanthoceras sorbifolium Bunge leaf extract activated chia seeds mucilage/chitosan composite film: Structure, performance, bioactivity, and molecular dynamics perspectives. Food Hydrocoll. 2023, 144, 109050. [Google Scholar]
- Joana, M.; Bruno, M.; Vitor, D.A.; Margarida, M.-M.; Fernanda, M.A.M.; Celestino, S.-B.; Lillian, B.; Sandra Cabo, V. Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples. Foods 2023, 12, 1926. [Google Scholar]
- Andrade, M.A.; Rodrigues, P.V.; Barros, C.; Cruz, V.; Machado, A.V.; Barbosa, C.H.; Coelho, A.; Furtado, R.; Correia, C.B.; Saraiva, M.; et al. Extending High Fatty Foods Shelf-Life Protecting from Lipid Oxidation and Microbiological Contamination: An Approach Using Active Packaging with Pomegranate Extract. Coatings 2023, 13, 93. [Google Scholar] [CrossRef]
- Fan, X.J.; Zhang, B.; Zhang, X.; Ma, Z.Q.; Feng, X.C. Incorporating Portulaca oleracea extract endows the chitosan-starch film with antioxidant capacity for chilled meat preservation. Food Chem. X 2023, 18, 100662. [Google Scholar] [CrossRef]
- Stoll, L.; Maillard, M.N.; Roux, E.l.; Flores, S.H.; Nachtigall, S.M.B.; Rios, A.; Domenek, S. Bixin, a performing natural antioxidant in active food packaging for the protection of oxidation sensitive food. LWT-Food Sci. Technol. 2023, 180, 114730. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative determination of volatile organic compounds formed during Polylactide processing by MHS-SPME. Polym. Degrad. Stabil. 2017, 136, 80–88. [Google Scholar] [CrossRef]
- Seyedeh Fatemeh, M.; Domenico, Z.; Gabriella, S.; Giosafatto, C.V.L. Cardoon seed oil cake proteins as substrate for microbial transglutaminase: Their application as matrix for bio-based packaging to extend the shelf-life of peanuts. Food Hydrocoll. 2023, 147 Pt A, 109339. [Google Scholar]
- Shi, X.L.; Davis, J.P.; Xia, Z.T.; Sandeep, K.P.; Sanders, T.H.; Dean, L.O. Characterization of peanuts after dry roasting, oil roasting, and blister frying. LWT-Food Sci. Technol. 2017, 75, 520–528. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Helguera, M.; Briones, M.; Dávalos-Saucedo, C.A.; Di Pierro, P. Edible Coating from Enzymatically Reticulated Whey Protein-Pectin to Improve Shelf Life of Roasted Peanuts. Coatings 2021, 11, 329. [Google Scholar] [CrossRef]
- Karkar, B.; Patir, I.; Eyüboglu, S.; Sahin, S. Development of an edible active chitosan film loaded with Nigella sativa L. extract to extend the shelf life of grapes. Biocatal. Agric. Biotechnol. 2023, 50, 102708. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gómez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Functional Properties of Bioplastics Made from Wheat Gliadins Modified with Cinnamaldehyde. J. Agric. Food Chem. 2011, 59, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Z.; Khoshbakht, R.; Javadi, B.; Firoozi, E.; Shahbazi, N. The Effect of Sodium Alginate Coating Containing Citrus (Citrus aurantium) and Lemon (Citrus lemon) Extracts on Quality Properties of Chicken Meat. J. Food Qual. 2022, 2022, 6036113. [Google Scholar] [CrossRef]
- Praveenkumar, P.; Ajeet, S.; Preetham, E. Inhibition of melanosis and quality changes on Indian white prawn treated with lemon and pomelo peel extracts conjugated with copper sulfide nanoparticles during chilled storage. J. Aquat. Food Prod. Technol. 2022, 31, 497–507. [Google Scholar]
- Yaghoubi, M.; Alirezalu, K.; Hesari, J.; Peighambardoust, S.H.; Marcinkowska-Lesiak, M.; Barzegar, Y.; Hoseinian-Khosrowshahi, S.R.; Marsza, K.; Khaneghah, A.M. Application of oleaster leaves (Elaeagnus angustifolia L.) essential oil and natural nanoparticle preservatives in frankfurter-type sausages: An assessment of quality attributes and stability during refrigerated storage. Meat Sci. 2023, 198, 109097. [Google Scholar] [CrossRef] [PubMed]

| Source | Chemical Compounds | Antioxidant Experiments | Ref. | ||
|---|---|---|---|---|---|
| Experiment | Results | ||||
| Inula grandiflora | 10 phenols (vanillic acid, vanillin, ferulic acid, etc.) and 5 flavonoids | DPPH/ (µg/mL) | IC50 = 55.13 ± 1.84 − 442.8 ± 12.13 | [10] | |
| origanum grossii and Thymus pallidus | Naringin, Hesperidin, licoflavone C | TAC/ (mg AAE/g DW) | 945.43 ± 7.98 (oregano) | 928.407 ± 4.41 (thyme) | [19] |
| Adansonia digitata L. | Rutin (31.9), quercetin-3-β-d-glucoside (8.86), caffeic acid (5.33), etc. | DPPH/ (mg/mL) | IC50 = 0.23 ± 0.01 | [20] | |
| Citrus | - | Salmonella sterilization on cucumbers | Reduced by 1.8 (10 °C) and 2.5 (22 °C) logCFU/cm2 | [17] | |
| Source | Chemical Compounds | Microbial Species | Index | Mechanism | Ref. |
|---|---|---|---|---|---|
| - | Vanillin and cinnamic acid | 4 food spoilage yeasts | MIC ≤ 0.125 mg/mL | The adherence on abiotic surface decreased. | [8] |
| - | Eugenol | A. Carbonarius | MIC = 0.8 μL/mL | The clustered genes for OTA biosynthesis were significantly reduced. | [9] |
| - | Isomers of β-citronellol | C. albicans C. tropicalis | MIC50% = 64 µg/mL MIC50% = 256 µg/mL | Both substances displayed aneffect on the fungal membrane but not on the fungal cell wall. | [11] |
| Ginger | 6-gingerol, 6-shogaol, zingerone | B. Subtilis P. aeruginosabacterium | Biofilm activity: 50% subcritical water extract = 0.5% peracetic acid | Curcumene, 6-shogaol, and zingerone in ginger’s subcritical water extract, which destroyed biofilms. | [14] |
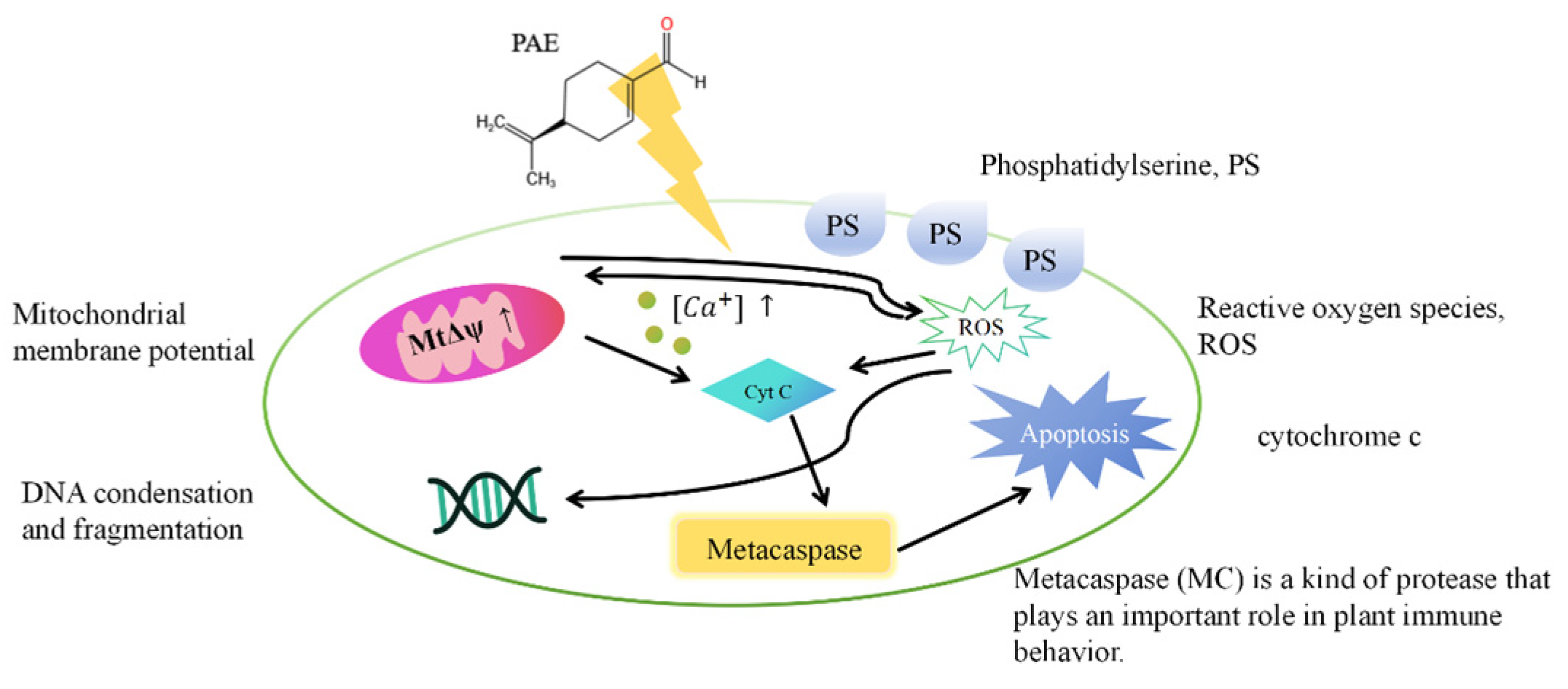
| - | PAE | A. flavus | The percentage of early apoptotic cells: (1) 27.4%(0.25 µg/mL PAE) (2) 48.7%(0.5 µg/mL PAE) | PAE induces fungal apoptosis through a caspase-dependent mitochondrial pathway. | [15] |
| POEO | Phytol, phytone, n-pentacosane, 1-octen-3-ol, and β-ionone | Pcc | MIC = 0.625 mg/mL | POEO destroyed cell morphology. | [18] |
| Nanoemulsion | Microemulsion | Pickering Emulsion | |
|---|---|---|---|
| Composition | Water, oil, emulsifier | Water, oil, surfactant, cosurfactant | Water, oil, solid particles |
| Particle size | 0.1–1 μm; monodispersed system | 10–100 nm; monodisperse system, sphericity | <500 nm; nonsphericity [50,51] or sphericity |
| Optical property | Transparent or semi transparent | Transparent or semi transparent | Opaque |
| Stability | Dynamic stability | Thermodynamic stability | Dynamic stability |
| Spontaneous formation | No | Yes | Yes |
| Mesophilic Bacteria | Filamentous Fungi | Coliforms | |
|---|---|---|---|
| Recommended limits | 6 | 2.7 | 4 |
| PLA with EXT | 3.75 | 3.3 | not detected |
| OPP with EXT | 5.2 | 5.25 | not detected |
| Matrix Materials | Antibacterial Composition | Food Category | Storage Conditions | Index | Ref. |
|---|---|---|---|---|---|
| Pea protein isolate, candelilla wax | d-limonene | Tomato | Soaking treatment, 8 days | MIC = 12.5 mg/mL | [12] |
| Fish gelatin, oxidized starch | MAAs from dried Pyropia haitanensis | Grease and winter dates | Natural light, 2 days |
| [22] |
| N-CMCS | GA | Strawberry | 20 ± 2 °C, RH 50%, 4 days |
| [26] |
| Sodium alginate | Citral nanoemulsion | Fresh-cut pineapple | 37 °C, 12 days | 0.5% citral nanoemulsion coated pineapple caused reduction of artificially inoculated food-borne pathogens and were sensory accepted. | [32] |
| PAL and PCL | OEO | Blackberry | Dark, 4 ± 1 °C, 90% RH, 4 days |
| [39] |
| Chitosan and gelatin | Garlic essential oil and anthocyanins from purple cabbage | Cherry tomato/fish | room temperature for 9 days/4 °C for 3 days. |
| [41] |
| Grapefruit pectin (GFPec) | MD-LPE, GFPec, and GFPE | Cherry tomato | Chilled, 6 days | Growth of E. coli O157:H7 inhibited by similar to 1.6 log units. | [58] |
| PLA and OPP | Olive pomace extracts | Apple | 4 °C, 12 days |
| [60] |
| Chitosan | Nigella sativa | Grape | 1 week |
| [68] |
| LDPE | Vitamin B2 | EVOO | Ultraviolet and short-visible light |
| [28] |
| A plant-based emulsifier | Oregano oil or lemon grass oil or cinnamon oil | Iceberg lettuce | 4 °C 28 days |
| [49] |
| Glutenin and tamarind gum | Melatonin/ pommelo essential oil | White mushroom | 3 ± 1 °C, 12 days |
| [53] |
| Furcellaran | Hazelnut oil Microemulsion | Cod liver oil | 22 ± 1 °C, 3 months |
| [55] |
| PLA | Wort, grape, Pomegranate | Almond Beef |
|
| [61] |
| PLA | Bixin | Sunflower oil | 40 °C, light or dark, 15 days |
| [63] |
| Cps | mTGase | Peanut | 33 ± 2.5 °C, the RH 65 ± 5%, 28 days |
| [65] |
| Gelatin | Lemon peel oil | Cheese | 4 °C, 28 days | Microbial counts decreased 2.3 (S. aureus) and 2.04 (E. coli) logs | [40] |
| Chitosan | C-PEO Pickering nanoemulsions | Red sea bream fillets | Chilled, 14 days |
| [45] |
| Sodium alginate | Thyme, oregano, and pimento essential oil emulsion | Chilled carp fillets | 10 days |
| [46] |
| Pectin | OEO and RES | Pork loin | 4 °C, 20 days |
| [47] |
| Pullulan and sodium alginate | Thyme essential oil microemulsion | Pork | 4 °C, 10 days |
| [52] |
| Soybean polysaccharide and bovine skin gelatin | S. platensis | Grass carp fillets | 4 °C, 10 days | Overall acceptability: 3.82–5.12 | [54] |
| PBAT/PLA | GO and LPO | Shrimp | 4 °C, 6 days | TVC = 7 Log CFU/g | [56] |
| Chitosan and starch | Portulaca oleracea extract | Meat | Chilled, 16 days |
| [62] |
| Sodium alginate | Citrus and lemon extracts | Chicken | 4 °C, 16 days | The peroxide value (mEq/kg), the TBA value (mg MDA/kg), and the TVC (log10 CFU/g) all below the control during 16 days storage. | [70] |
| CuSNPs | LPE and/or PPE | Indian white shrimp | Chilled, 15 days |
| [71] |
| ε-PL and nisin nanoparticles | Oleaster leaf essential oil | Emulsion-type Sausages | Vacuum PE bag, 4 °C, 45 days | Total viable bacteria values all decreased (1.28 Log CFU/g): (1) 1.43 Log CFU/g for Clostridium perfringens; (2) 0.24 Log CFU/g for E. coli, (3) 0.63 Log CFU/g for S. aureus, (4) 0.86 Log CFU/g for molds and yeasts. | [72] |
| Matrix Materials | Antibacterial Composition | Food Category | Storage Conditions | Index | Ref. |
|---|---|---|---|---|---|
| Chia seed, chitosan | Xanthoceras sorbifolium leaf | 4 food simulants | Normal, 60 min |
| [59] |
| PLA | Persicaria hydropiper | S. aureus 6538P | 37 ± 2 °C, 24 h |
| [38] |
| Chitosan and PVA | CPEO | E. coli and S. aureus | 37 °C, 24 h |
| [42] |
| OSA-starch Pickering emulsion | Vanilla essential oil (30.54% vanillin) | - | 25 ± 0.5 °C, 14 days | DPPH and ABTS+: better | [48] |
| Corn starch | Acontium heterophyllum, Artemisia annua, and Thymus serpyllum | S.aureus and Salmonella | 37 °C, 24 h |
| [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, H.; Weng, Y. Natural Extracts and Their Applications in Polymer-Based Active Packaging: A Review. Polymers 2024, 16, 625. https://doi.org/10.3390/polym16050625
Li J, Sun H, Weng Y. Natural Extracts and Their Applications in Polymer-Based Active Packaging: A Review. Polymers. 2024; 16(5):625. https://doi.org/10.3390/polym16050625
Chicago/Turabian StyleLi, Jiawei, Hui Sun, and Yunxuan Weng. 2024. "Natural Extracts and Their Applications in Polymer-Based Active Packaging: A Review" Polymers 16, no. 5: 625. https://doi.org/10.3390/polym16050625
APA StyleLi, J., Sun, H., & Weng, Y. (2024). Natural Extracts and Their Applications in Polymer-Based Active Packaging: A Review. Polymers, 16(5), 625. https://doi.org/10.3390/polym16050625