Study of the Chemical Activities of Carbon Monoxide, Carbon Dioxide, and Oxygen Traces as Critical Inhibitors of Polypropylene Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Details
Assessment of the Global and Local Reactivity Descriptors of CO2, CO, and O2
2.2. Experimental Stage
2.2.1. Materials and Reactive Species
Propylene Polymerization
3. Results
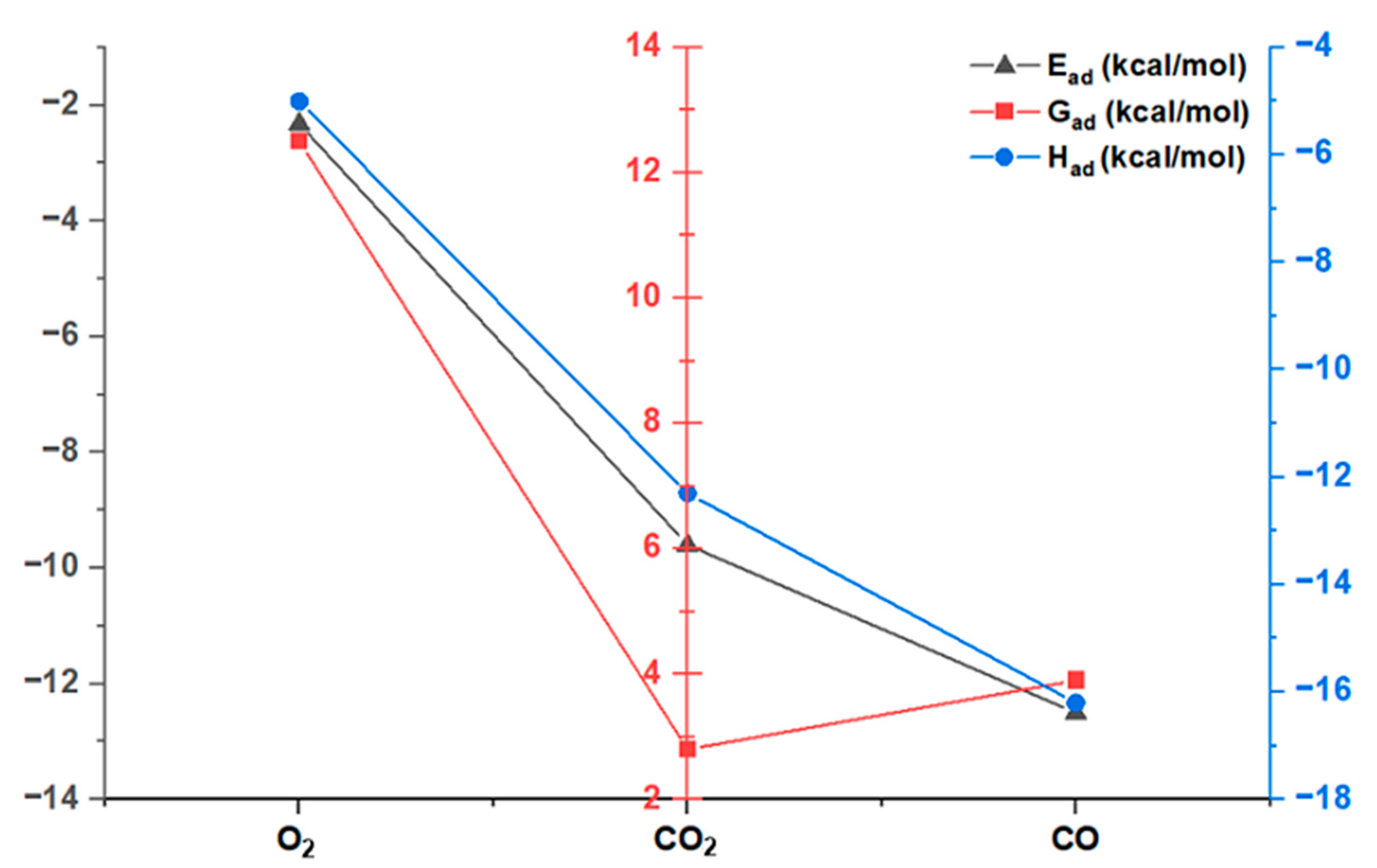
3.1. Interaction of Inhibitors CO2, CO, and O2 with the Active Center of Ti in ZN (Ti-Poisoning Interaction)
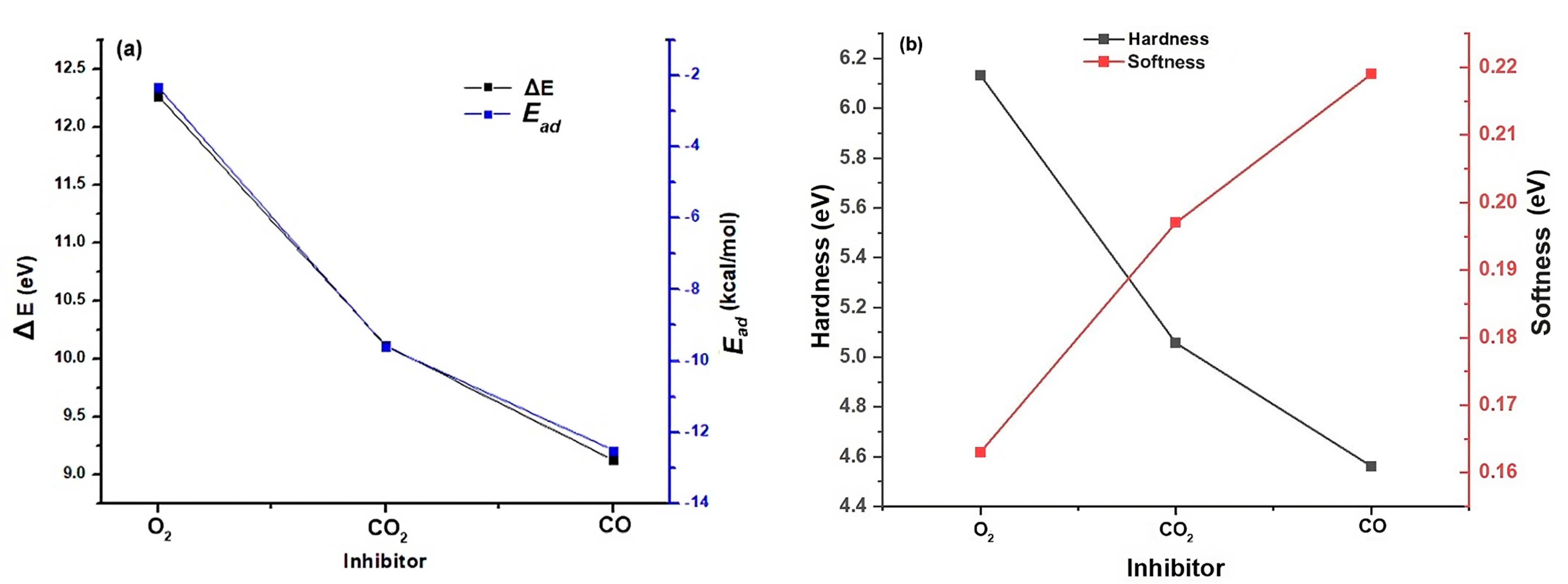
3.1.1. Validation of Theoretical Calculations of Interaction of Inhibitors CO2, CO, and O2 with ZN
3.1.2. Local Descriptors of CO2, CO, and O2
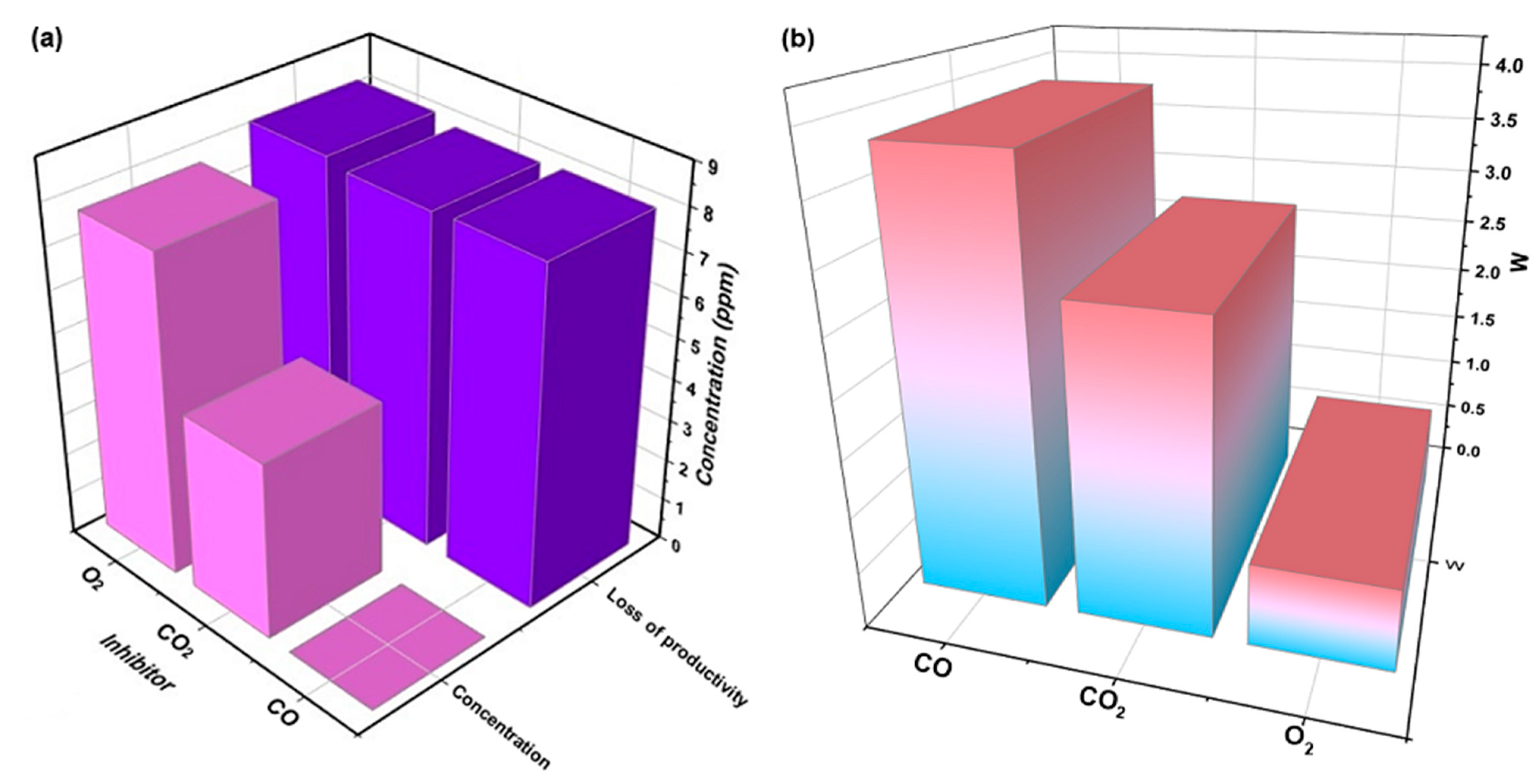
3.2. Experimental Analysis
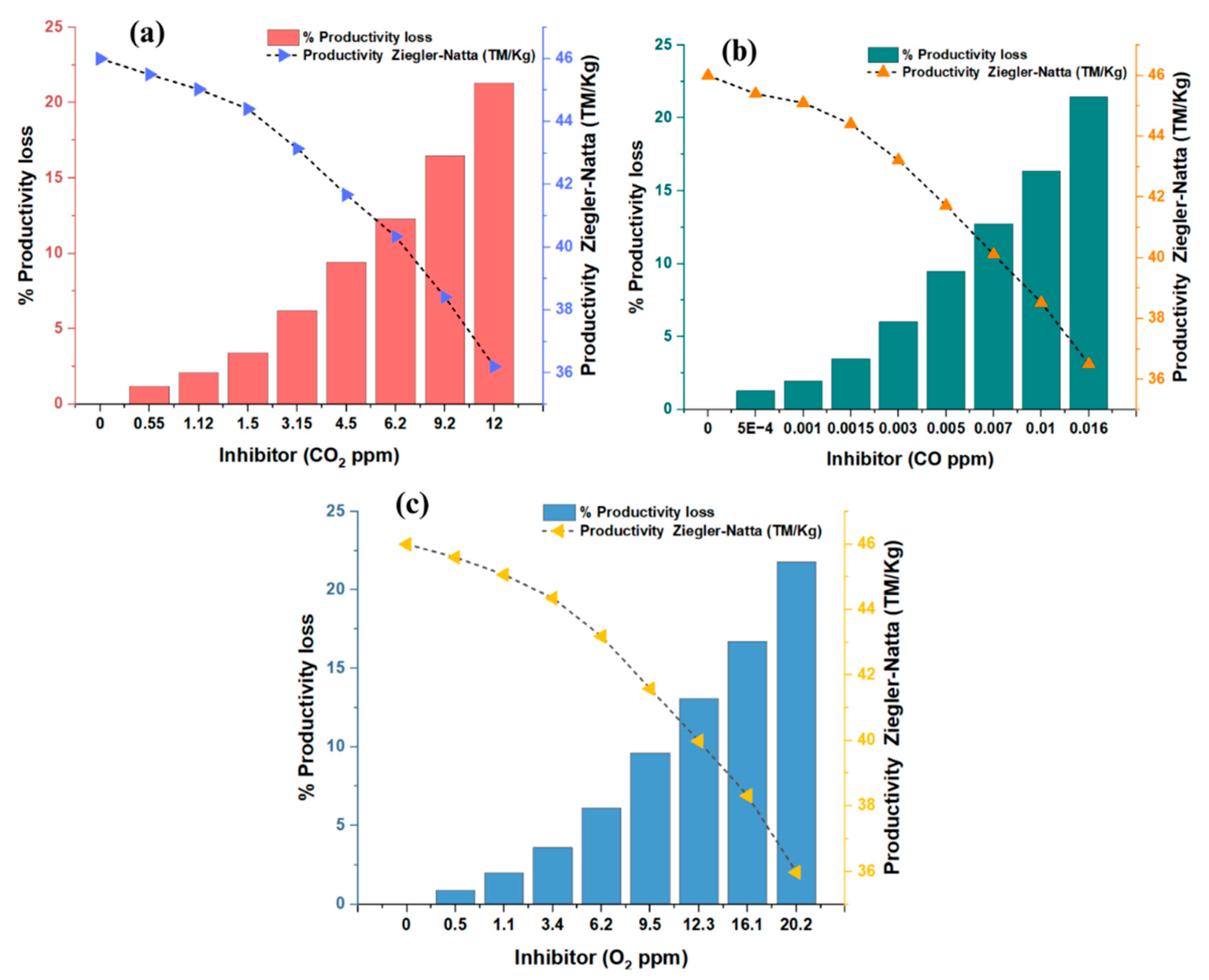
3.2.1. Effect of Inhibitors on the Polymerization of Propylene
3.2.2. Ziegler–Natta Catalyst Poisoning with Carbon Monoxide
3.2.3. Poisoning of the Ziegler–Natta Catalyst with Carbon Dioxide
3.2.4. Ziegler–Natta Catalyst Poisoning with Oxygen
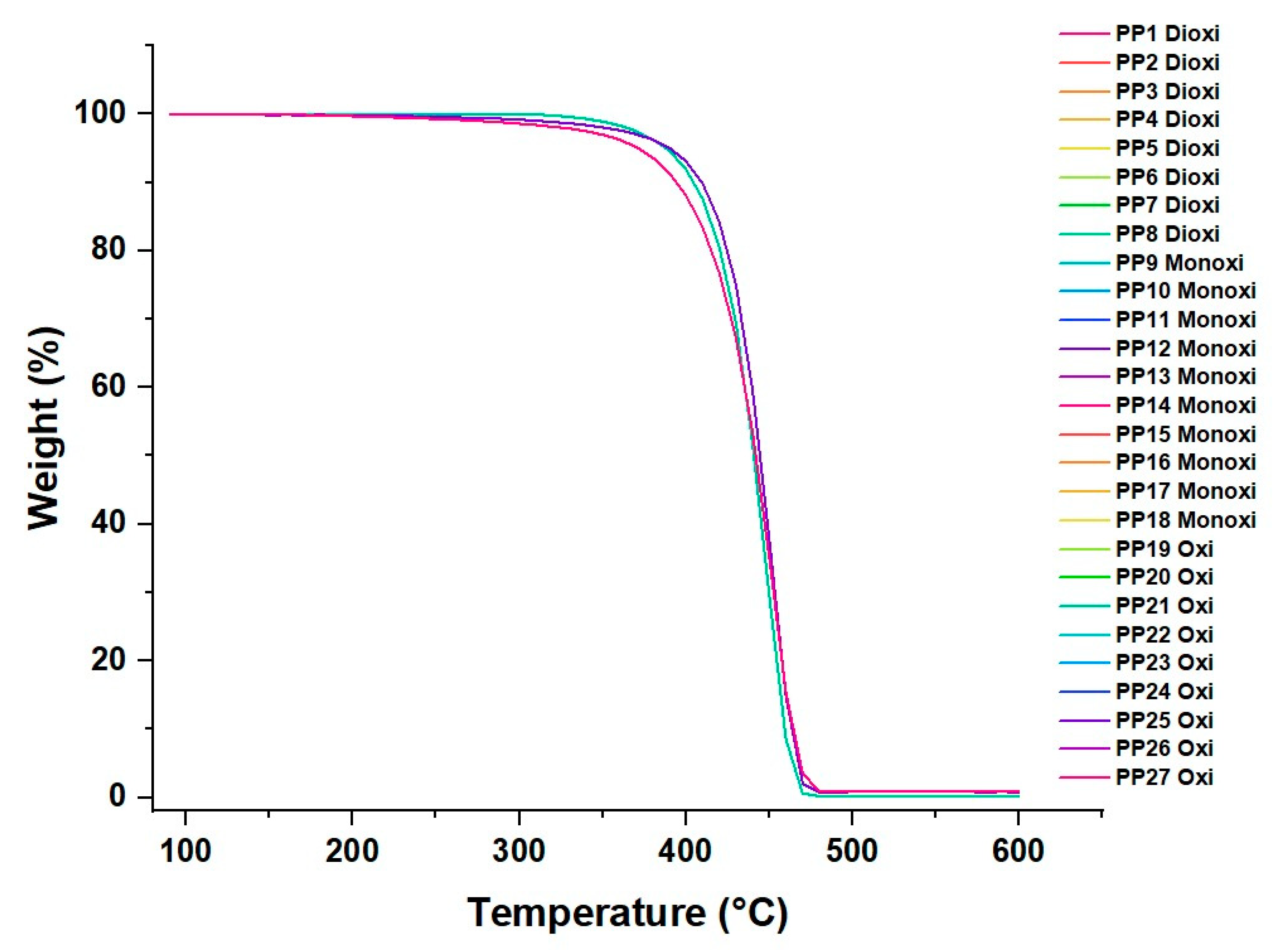
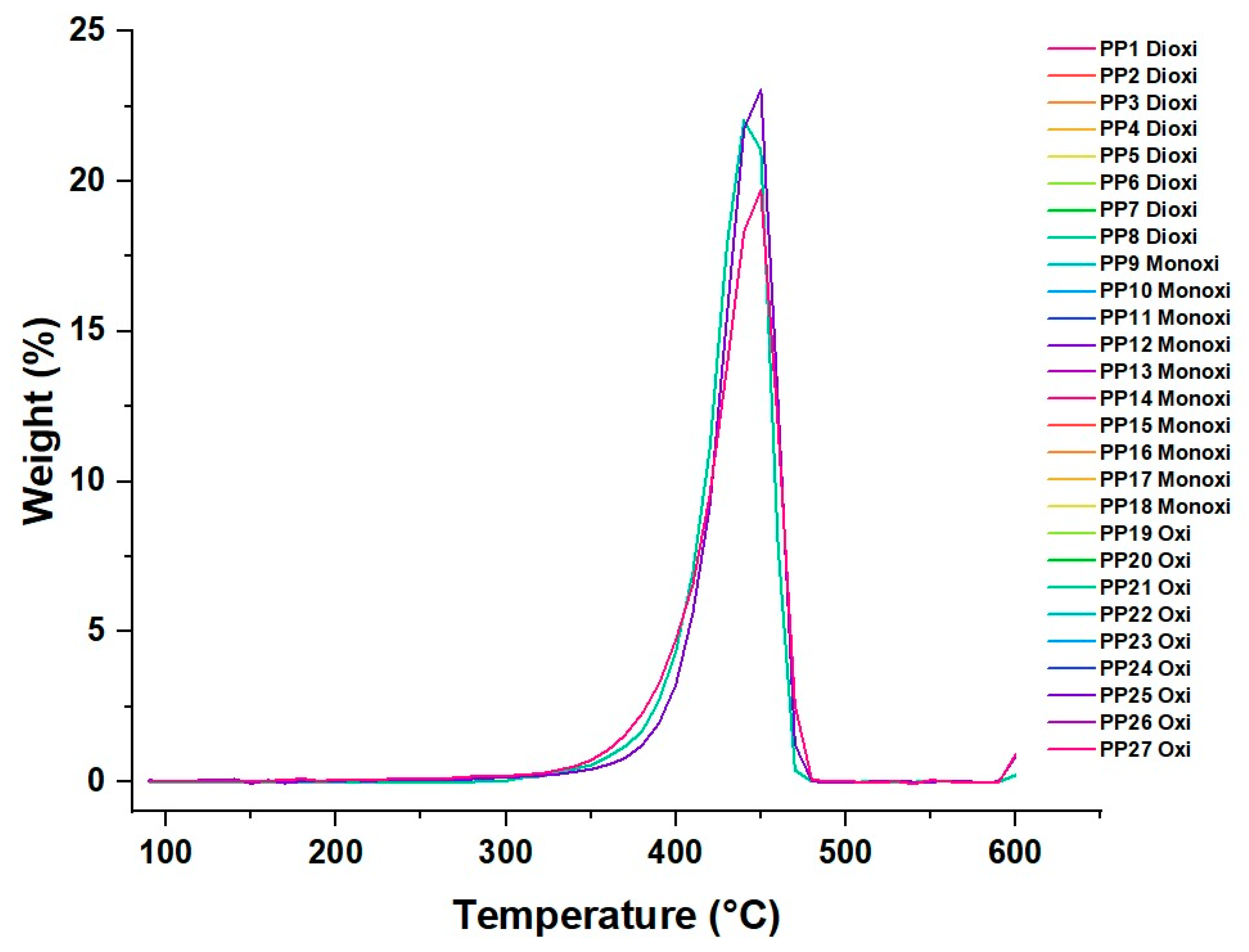
3.3. PP Thermal Analysis
Thermal Degradation of the Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aizenshtein, E.M.; Efremov, V.N. Production and use of polypropylene fibres and yarn. Fibre Chem. 2006, 38, 345–350. [Google Scholar] [CrossRef]
- Bora, R.R.; Wang, R.; You, F. Waste Polypropylene Plastic Recycling toward Climate Change Mitigation and Circular Economy: Energy, Environmental, and Technoeconomic Perspectives. ACS Sustain. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Pernusch, D.C.; Spiegel, G.; Paulik, C.; Hofer, W. Influence of Poisons Originating from Chemically Recycled Plastic Waste on the Performance of Ziegler–Natta Catalysts. Macromol. React. Eng. 2021, 16, 2100020. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental impacts of polypropylene (PP) production and prospects of its recycling in the GCC region. Mater. Today Proc. 2021, 56, 2245–2251. [Google Scholar] [CrossRef]
- Pernusch, D.C.; Paulik, C.; Mastalir, M.; Hofer, W. Assessing the Downstream Contamination of Chemically Recycled Ethylene Feed Streams on the Kinetic Behavior of Ziegler-Natta Catalysts and Microstructural Properties of HDPE and LLDPE. Macromol. React. Eng. 2022, 16, 42. [Google Scholar] [CrossRef]
- Arjmand, S.; Shakeri, A.; Arabi, H. Effect of water and carbonyl sulfide toxins in gas propylene feed in polymerization process on physical properties of polypropylene. J. Polym. Res. 2019, 26, 195. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial Polypropylene Waste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Development and validation of a methodology for quantifying parts-per-billion levels of arsine and phosphine in nitrogen, hydrogen and liquefied petroleum gas using a variable pressure sampler coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2021, 1637, 461833. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; Hernández-Fernández, J.; López-Martínez, J. Comparative characterization of gum rosins for their use as sustainable additives in polymeric matrices. J. Appl. Polym. Sci. 2021, 139, 51734. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Environ. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Chacon, H.; Cano, H.; Fernández, J.H.; Guerra, Y.; Puello-Polo, E.; Ríos-Rojas, J.F.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef]
- Bahri-Laleh, N. Interaction of different poisons with MgCl2/TiCl4 based Ziegler-Natta catalysts. Appl. Surf. Sci. 2016, 379, 395–401. [Google Scholar] [CrossRef]
- Trivedi, P.M.; Gupta, V.K. Progress in MgCl2 supported Ziegler-Natta catalyzed polyolefin products and applications. J. Polym. Res. 2021, 28, 45. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction. Polymers 2023, 15, 3619. [Google Scholar] [CrossRef]
- Yadav, P.; Ismail, N.; Essalhi, M.; Tysklind, M.; Athanassiadis, D.; Tavajohi, N. Assessment of the environmental impact of polymeric membrane production. J. Membr. Sci. 2021, 622, 118987. [Google Scholar] [CrossRef]
- Schwarz, J.F.; Holtrichter-Rößmann, T.; Liedtke, C.G.; Diddens, D.; Paulik, C. Modified Magnesium Alkyls for Ziegler–Natta Catalysts. Catalysts 2022, 12, 973. [Google Scholar] [CrossRef]
- Boukabcha, N.; Benmohammed, A.; Belhachemi, M.H.M.; Goudjil, M.; Yahiaoui, S.; Megrouss, Y.; Djafri, A.; Khelloul, N.; Benyehlou, Z.D.; Djafri, A.; et al. Spectral investigation, TD-DFT study, Hirshfeld surface analysis, NCI-RDG, HOMO-LUMO, chemical reactivity and NLO properties of 1-(4-fluorobenzyl)-5-bromolindolin-2,3-dione. J. Mol. Struct. 2023, 1285, 135492. [Google Scholar] [CrossRef]
- Ahmed, M.S.M.; Mekky, A.E.; Sanad, S.M. Regioselective [3 + 2] cycloaddition synthesis and theoretical calculations of new chromene-pyrazole hybrids: A DFT-based Parr Function, Fukui Function, local reactivity indexes, and MEP analysis. J. Mol. Struct. 2022, 1267, 133583. [Google Scholar] [CrossRef]
- Zülfikaroğlu, A.; Batı, H.; Dege, N. A theoretical and experimental study on isonitrosoacetophenone nicotinoyl hydrazone: Crystal structure, spectroscopic properties, NBO, NPA and NLMO analyses and the investigation of interaction with some transition metals. J. Mol. Struct. 2018, 1162, 125–139. [Google Scholar] [CrossRef]
- Mascetti, J. Metal Coordination of CO2. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Whiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Cook, J.E.; Hagerty, R.O.; Jacob, F.W. Reactor System for Rapid Kill Gas Injection to Gas Phase Polymerization Reactors. U.S. Patent 4,834,947, 30 May 1989. [Google Scholar]
- Craddock, R.E., III; Jenkins, J.M., III; Tighe, M.T. Method and Apparatus for Stopping Reaction in a Gas Phase Polymerization Reactor System. U.S. Patent 5,399,320, 14 December 1993. [Google Scholar]
- El Ashry, H.E.; El Nemr, A.; Esawy, S.A.; Ragab, S. Corrosion Inhibitors: Part II: Quantum Chemical Studies on the Corrosion Inhibitions of Steel in Acidic Medium by Some Triazole, Oxadiazole and Thiadiazole Derivatives. Electrochim. Acta 2006, 51, 3957. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Adsorption Characteristics and Corrosion Inhibitive Properties of Clotrimazole for Aluminium Corrosion in Hydrochloric Acid. Int. J. Electchem. Sci. 2009, 4, 863. [Google Scholar] [CrossRef]
- Fleming, I. Molecular Orbitals and Organic Chemical Reactions; John Wiley and Sons: New York, NY, USA, 2009. [Google Scholar]
- Ebenso, E.E.; Isabirye, D.A.; Eddy, N.O. Adsorption and Quantum Chemical Studies on the Inhibition Potentials of Some Thiosemicarbazides for the Corrosion of Mild Steel in Acidic Medium. Int. J. Mol. Sci. 2010, 11, 2473. [Google Scholar] [CrossRef]
- Hasanov, R.; Sadikglu, M.; Bilgic, S. Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution. Appl. Surf. Sci. 2007, 253, 3913. [Google Scholar] [CrossRef]
- Kläui, W. The Coordination Chemistry and Organometallic Chemistry of Tridentate Oxygen Ligands withπ-Donor Properties. Angew. Chem. Int. Ed. 1990, 29, 627–637. [Google Scholar] [CrossRef]
- Betley, T.A.; Surendranath, Y.; Childress, M.V.; Alliger, G.E.; Fu, R.; Cummins, C.C.; Nocera, D.G. A ligand field chemistry of oxygen generation by the oxygen-evolving complex and synthetic active sites. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 1293–1303. [Google Scholar] [CrossRef]
- Oláh, J.; Van Alsenoy, C.; Sannigrahi, A.B. Condensed Fukui Functions Derived from Stockholder Charges: Assessment of Their Performance as Local Reactivity Descriptors. J. Phys. Chem. A 2002, 106, 3885–3890. [Google Scholar] [CrossRef]
- Cavallo, L.; Guerra, G.; Corradini, P. Mechanisms of Propagation and Termination Reactions in Classical Heterogeneous Ziegler−Natta Catalytic Systems: A Nonlocal Density Functional Study. J. Am. Chem. Soc. 1998, 120, 2428–2436. [Google Scholar] [CrossRef]
- Piovano, A.; Zarupski, J.; Groppo, E. Disclosing the Interaction between Carbon Monoxide and Alkylated Ti3+ Species: A Direct Insight into Ziegler–Natta Catalysis. J. Phys. Chem. Lett. 2020, 11, 5632–5637. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Liu, Z.; Somsook, E.; Landis, C.R. A 2H-Labeling Scheme for Active-Site Counts in Metallocene-Catalyzed Alkene Polymerization. J. Am. Chem. Soc. 2001, 123, 2915–2916. [Google Scholar] [CrossRef]
- Hong, D.S.; Jeong, D.W.; Cho, H.Y.; Cui, L.; Tarte, N.H.; Woo, S.I. Poisoning effect of CO on ethylene polymerization with Ni(II)–diimine/MAO. Polymer 2006, 47, 184–192. [Google Scholar] [CrossRef]
- Gibson, D.H. Carbon dioxide coordination chemistry: Metal complexes and surface-bound species. What relationships? Coord. Chem. Rev. 1999, 185–186, 335–355. [Google Scholar] [CrossRef]
- Walther, D.; Ruben, M.; Rau, S. Carbon dioxide and metal centres: From reactions inspired by nature to reactions in compressed carbon dioxide as solvent. Coord. Chem. Rev. 1999, 182, 67–100. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Trivalent Titanium Salen Complex: Thermally Robust and Highly Active Catalyst for Copolymerization of CO2 and Cyclohexene Oxide. ACS Catal. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Dodson, L.G.; MThompson, C.; Weber, J.M. Titanium Insertion into CO Bonds in Anionic Ti-CO2 Complexes. J. Phys. Chem. A 2018, 122, 2983–2991. [Google Scholar] [CrossRef]
- Tang, S.Y.; Rijs, N.J.; Li, J.; Schlangen, M.; Schwarz, H. Ligand-Controlled CO2 Activation Mediated by Cationic Titanium Hydride Complexes, [LTiH]+ (L=Cp2, O). Chem. Eur. J. 2015, 21, 8483–8490. [Google Scholar] [CrossRef]
- Chen, S.; Yang, F.; Gao, H.; Wang, J.; Chen, X.; Zhang, X.; Li, J.; Li, A. Construction of dual ligand Ti-based MOFs with enhanced photocatalytic CO2 reduction performance. J. CO2 Util. 2021, 48, 101528. [Google Scholar] [CrossRef]
- Byun, Y.M.; Lee, J.M.; Ryu, J.Y.; Go, M.J.; Na, K.S.; Kim, Y.; Lee, J. Titanium complexes containing tridentate [ONO] type Schiff base ligands for the cycloaddition reaction of CO2 to propylene oxide. Polyhedron 2018, 141, 191–197. [Google Scholar] [CrossRef]
- Hatcher, L.Q.; Karlin, K.D. Oxidant Types in Copper—Dioxygen Chemistry: The Ligand Coordination Defines the Cun-O2 Structure and Subsequent Reactivity. J. Biol. Inorg. Chem. 2004, 9, 669–683. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Vivas-Reyes, R.; Toloza, C.A.T. Experimental Study of the Impact of Trace Amounts of Acetylene and Methylacetylene on the Synthesis, Mechanical and Thermal Properties of Polypropylene. Int. J. Mol. Sci. 2022, 23, 12148. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Cano, H.; Aldas, M. Impact of Traces of Hydrogen Sulfide on the Efficiency of Ziegler–Natta Catalyst on the Final Properties of Polypropylene. Polymers 2022, 14, 3910. [Google Scholar] [CrossRef] [PubMed]

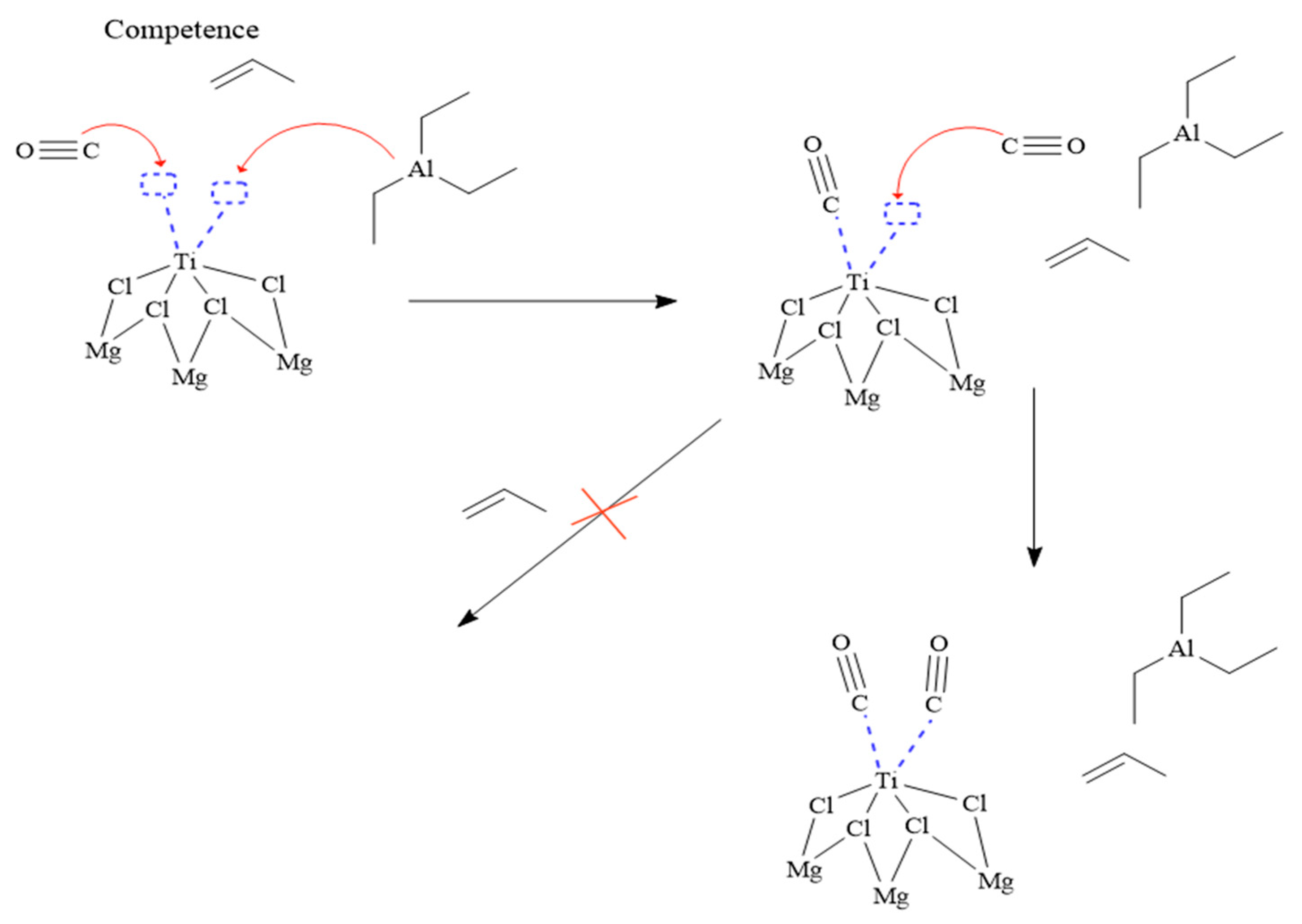
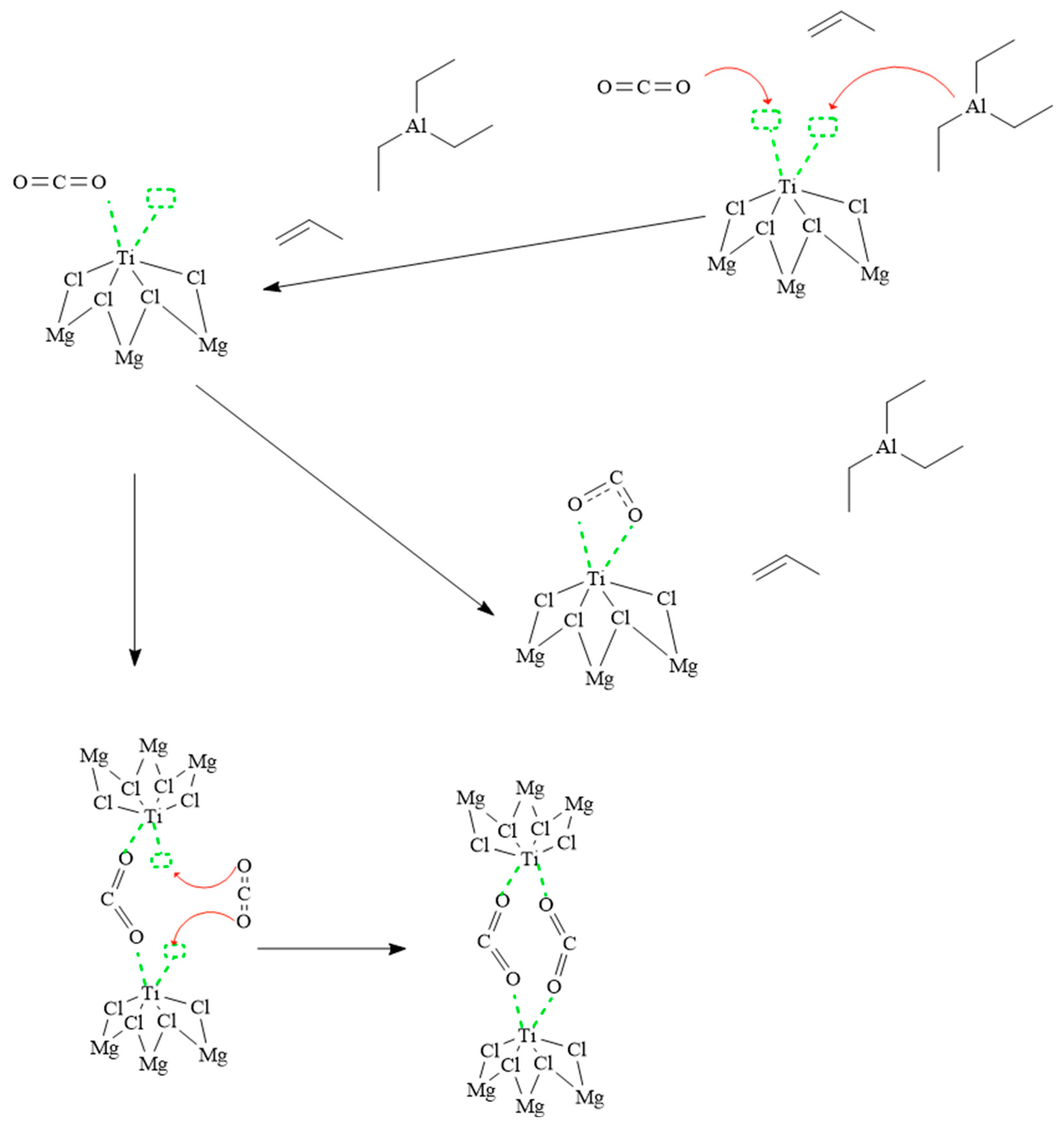
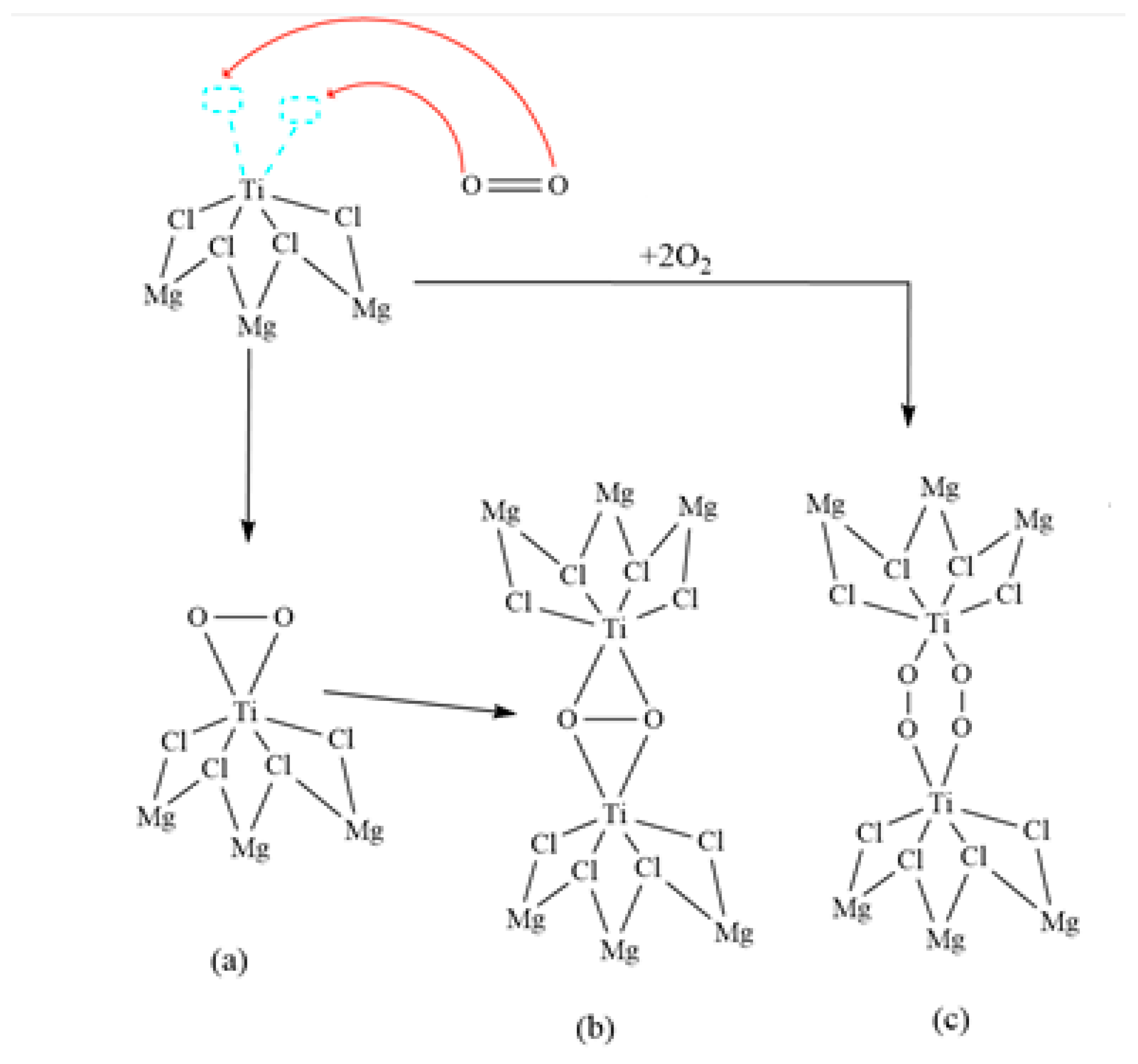
| Materials | Catalyst Kh/h | TEAl Kg/h | CO2 (ppm) | CO (ppm) | O2 (ppm) | T °C | Pressure Bar |
|---|---|---|---|---|---|---|---|
| PP1 | 5 | 0.25 | 0 | 0 | 0 | 70 | 27 |
| PP2 | 5 | 0.25 | 0.55 | 0 | 0 | 70 | 27 |
| PP3 | 5 | 0.25 | 1.12 | 0 | 0 | 70 | 27 |
| PP4 | 5 | 0.25 | 1.5 | 0 | 0 | 70 | 27 |
| PP5 | 5 | 0.25 | 3.15 | 0 | 0 | 70 | 27 |
| PP6 | 5 | 0.25 | 4.5 | 0 | 0 | 70 | 27 |
| PP7 | 5 | 0.25 | 6.2 | 0 | 0 | 70 | 27 |
| PP8 | 5 | 0.25 | 9.2 | 0 | 0 | 70 | 27 |
| PP9 | 5 | 0.25 | 12 | 0 | 0 | 70 | 27 |
| PP10 | 5 | 0.25 | 0 | 0 | 0 | 70 | 27 |
| PP11 | 5 | 0.25 | 0 | 0.0005 | 0 | 70 | 27 |
| PP12 | 5 | 0.25 | 0 | 0.001 | 0 | 70 | 27 |
| PP13 | 5 | 0.25 | 0 | 0.0015 | 0 | 70 | 27 |
| PP14 | 5 | 0.25 | 0 | 0.003 | 0 | 70 | 27 |
| PP15 | 5 | 0.25 | 0 | 0.005 | 0 | 70 | 27 |
| PP16 | 5 | 0.25 | 0 | 0.007 | 0 | 70 | 27 |
| PP17 | 5 | 0.25 | 0 | 0.01 | 0 | 70 | 27 |
| PP18 | 5 | 0.25 | 0 | 0.016 | 0 | 70 | 27 |
| PP19 | 5 | 0.25 | 0 | 0 | 0 | 70 | 27 |
| PP20 | 5 | 0.25 | 0 | 0 | 0.5 | 70 | 27 |
| PP21 | 5 | 0.25 | 0 | 0 | 1.1 | 70 | 27 |
| PP22 | 5 | 0.25 | 0 | 0 | 3.4 | 70 | 27 |
| PP23 | 5 | 0.25 | 0 | 0 | 6.2 | 70 | 27 |
| PP24 | 5 | 0.25 | 0 | 0 | 9.5 | 70 | 27 |
| PP25 | 5 | 0.25 | 0 | 0 | 12.3 | 70 | 27 |
| PP26 | 5 | 0.25 | 0 | 0 | 16.1 | 70 | 27 |
| PP27 | 5 | 0.25 | 0 | 0 | 20.2 | 70 | 27 |
| Type of Poison | Ead | Gad | Had |
|---|---|---|---|
| O2 | −2.32 | 12.5 | −5.0 |
| CO2 | −9.6 | 2.8 | −12.3 |
| CO | −12.5 | 3.9 | −16.2 |
| Parameter (eV) | O2 | CO2 | CO |
|---|---|---|---|
| 6.1336 | 5.0569 | 4.5620 | |
| S | 0.163 | 0.197 | 0.219 |
| µ | −3.008 | −5.248 | −5.889 |
| 0.737 | 2.723 | 3.800 |
| Inhibitor | Atom | ||||
|---|---|---|---|---|---|
| O2 | O | 0.500 | 0.500 | 0.500 | 0 |
| O | 0.500 | 0.500 | 0.500 | 0 | |
| CO | C | 0.6717 | 0.6977 | 0.6847 | 0.026 |
| O | 0.3283 | 0.3023 | 0.3153 | −0.026 | |
| CO2 | C | 0.0027 | 0.5661 | 0.2844 | 0.5634 |
| O | 0.4986 | 0.2169 | 0.3578 | −0.2817 | |
| O | 0.4986 | 0.2169 | 0.3578 | −0.2817 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fernández, J.; Puello-Polo, E.; Marquez, E. Study of the Chemical Activities of Carbon Monoxide, Carbon Dioxide, and Oxygen Traces as Critical Inhibitors of Polypropylene Synthesis. Polymers 2024, 16, 605. https://doi.org/10.3390/polym16050605
Hernández-Fernández J, Puello-Polo E, Marquez E. Study of the Chemical Activities of Carbon Monoxide, Carbon Dioxide, and Oxygen Traces as Critical Inhibitors of Polypropylene Synthesis. Polymers. 2024; 16(5):605. https://doi.org/10.3390/polym16050605
Chicago/Turabian StyleHernández-Fernández, Joaquín, Esneyder Puello-Polo, and Edgar Marquez. 2024. "Study of the Chemical Activities of Carbon Monoxide, Carbon Dioxide, and Oxygen Traces as Critical Inhibitors of Polypropylene Synthesis" Polymers 16, no. 5: 605. https://doi.org/10.3390/polym16050605
APA StyleHernández-Fernández, J., Puello-Polo, E., & Marquez, E. (2024). Study of the Chemical Activities of Carbon Monoxide, Carbon Dioxide, and Oxygen Traces as Critical Inhibitors of Polypropylene Synthesis. Polymers, 16(5), 605. https://doi.org/10.3390/polym16050605







