Synthesis, Characterization, and Catalytic Behaviors in Isoprene Polymerization of Pyridine–Oxazoline-Ligated Cobalt Complexes
Abstract
1. Introduction
2. Experimental Section
2.1. General Conditions
2.2. General Procedure for the Synthesis of Ligands
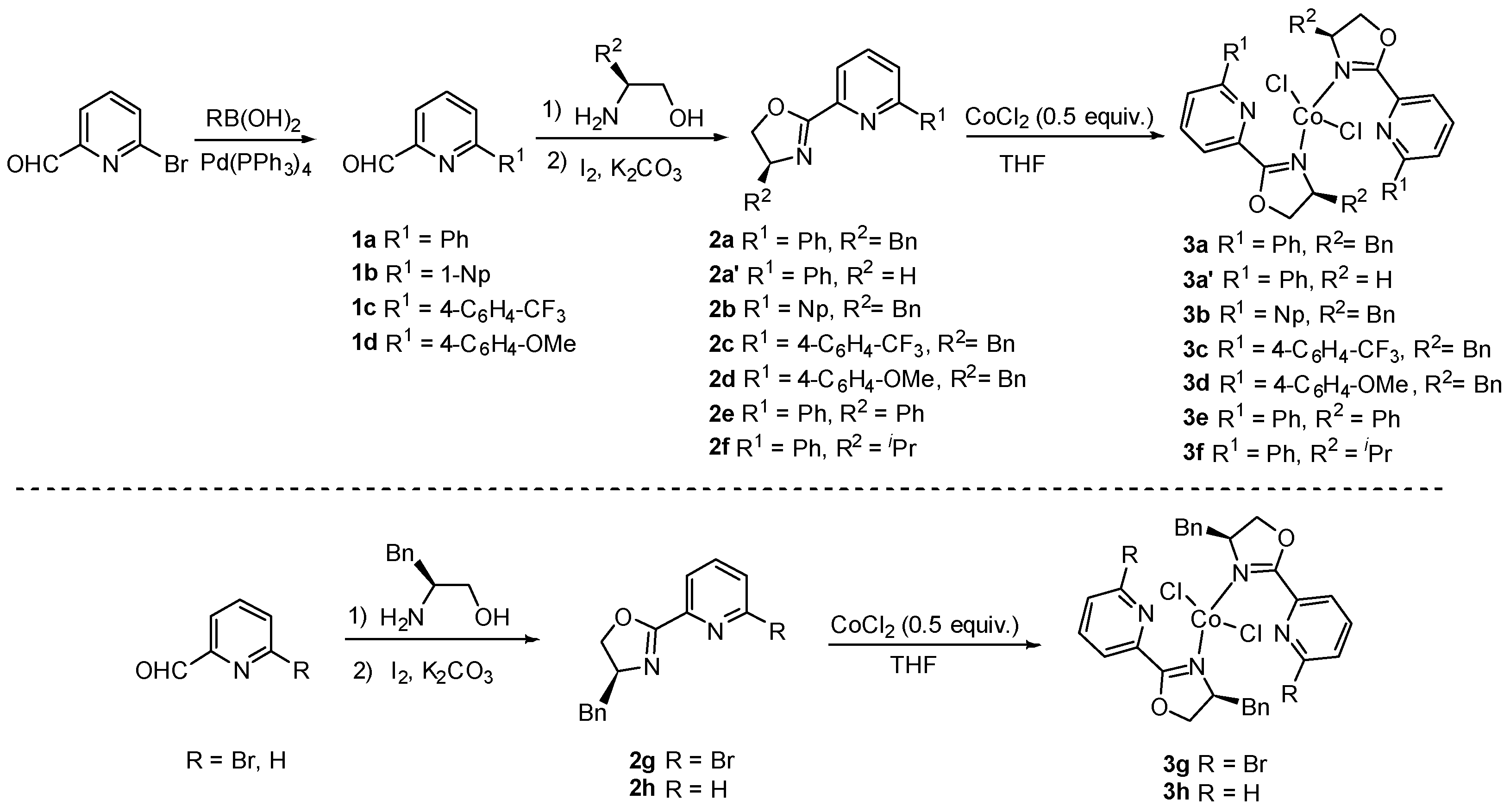
2.3. General Procedure for the Synthesis of Cobalt Complexes
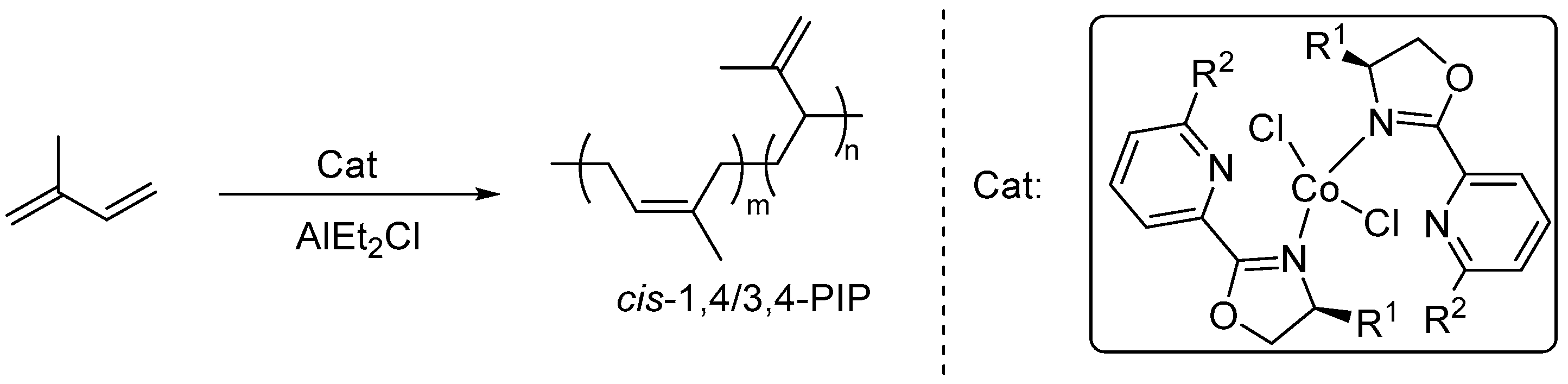
2.4. General Procedure for Isoprene Polymerization
3. Results and Discussion
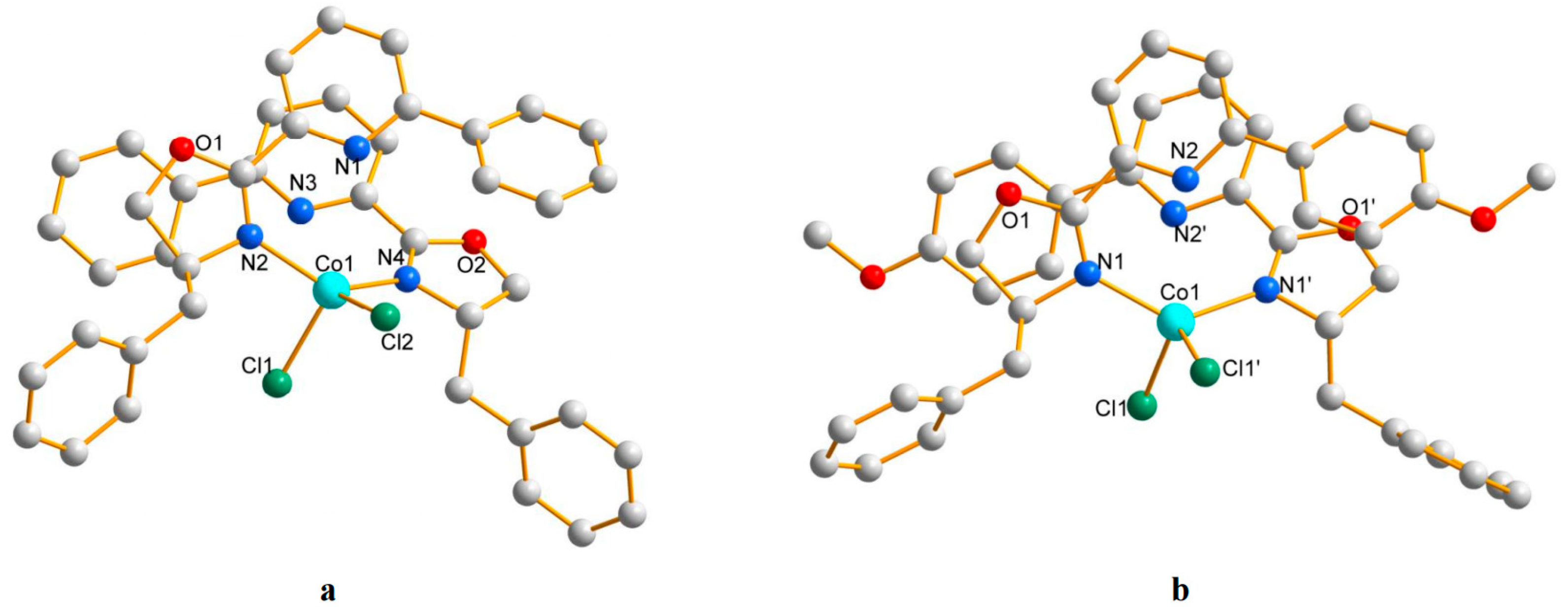
3.1. Characterization of Pyridine–Oxazoline-Ligated Cobalt Catalysts
3.2. Isoprene Polymerization Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.-H.; Cui, D.-M.; Li, D.-F.; Hou, Z.-M. Highly 3,4-Selective Polymerization of Isoprene with NPN Ligand Stabilized Rare-Earth Metal Bis(alkyl)s. Structures and Performances. Organometallics 2009, 28, 4814–4822. [Google Scholar] [CrossRef]
- Wang, X.-X.; Fan, L.-L.; Huang, C.-B.; Tong, T.-L.; Guo, C.-Y.; Sun, W.-H. Highly Cis-1,4 Selective Polymerization of Isoprene Promoted by α-Diimine Cobalt(II) Chlorides. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3609–3615. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Chen, H.-F.; Li, W.-X.; Jia, X.-Y.; Zhang, X.-Q.; Gong, D.-R. Polymerization of Isoprene Promoted by Aminophosphine(ory)-Fused Bipyridine Cobalt Complexes: Precise Control of Molecular Weight and Cis-1,4-alt-3,4 Sequence. Inorg. Chem. 2018, 57, 4088–4097. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes polymerization: Where we are and what lies ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Song, J.S.; Huang, B.C.; Yu, D.S. Progress of synthesis and application of trans-1,4-polyisoprene. J. App. Polym. Sci. 2001, 82, 81–89. [Google Scholar] [CrossRef]
- Thiele, S.K.-H.; Wilson, D.R. Alternate Transition Metal Complex Based Diene Polymerization. J. Macromol. Sci. Polym. Rev. 2003, 43, 581–628. [Google Scholar] [CrossRef]
- Halasa, A.F.; Hsu, W.L. Synthesis of High Vinyl Elastomers via Mixed Organolithium and Sodium Alkoxide in the Presence of Polar Modifier. Polymer 2002, 43, 7111–7118. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Lv, K.; Gao, W.; Cui, D.-M.; Chen, X.-S.; Jing, X.-B. Pyrrolide-Supported Lanthanide Alkyl Complexes. Influence of Ligands on Molecular Structure and Catalytic Activity toward Isoprene Polymerization. Organometallics 2007, 26, 4575–4584. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Luo, Y.; Hou, Z.-M. Unprecedented Isospecific 3,4-Polymerization of Isoprene by Cationic Rare Earth Metal Alkyl Species Resulting from a Binuclear Precursor. J. Am. Chem. Soc. 2005, 127, 14562–14563. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cui, D.; Lv, K. Highly 3,4-selective living polymerization of isoprene with rare earth metal fluorenyl N-heterocyclic carbene precursors. Macromolecules 2008, 41, 1983–1988. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Liu, Z.; Lin, Z.; Du, G.; Zhang, S.; Li, X. Quasi-living trans-1,4-polymerization of isoprene by cationic rare earth metal alkyl species bearing a chiral (S,S)-bis(oxazolinylphenyl)amido ligand. Macromolecules 2013, 46, 3257–3265. [Google Scholar] [CrossRef]
- Dai, Q.-Q.; Jia, X.-Y.; Yang, F.; Bai, C.-X.; Hu, Y.-M.; Zhang, X.-Q. Iminopyridine-Based Cobalt(II) and Nickel(II) Complexes: Synthesis, Characterization, and Their Catalytic Behaviors for 1,3-Butadiene Polymerization. Polymers 2016, 8, 12. [Google Scholar] [CrossRef]
- Xiao, T.P.-F.; Zhang, S.; Kehr, G.; Hao, X.; Erker, G.; Sun, W.-H. Bidentate Iron(II) Dichloride Complexes Bearing Substituted 8-(Benzimidazol-2-yl)quinolines: Synthesis, Characterization, and Ethylene Polymerization Behavior. Organometallics 2011, 30, 3658–3665. [Google Scholar] [CrossRef]
- Xiao, T.P.-F.; Zhang, S.; Li, B.-X.; Hao, X.; Redshaw, C.; Li, Y.-S.; Sun, W.-H. Ferrous and Cobaltous Chloride Complexes Bearing 2-(1-(Arylimino)methyl)-8-(1H-benzimidazol-2-yl)quinolines: Synthesis, Characterization and Catalytic Behavior in Ethylene Polymerization. Polymer 2011, 52, 5803–5810. [Google Scholar] [CrossRef]
- Ashitaka, H.; Ishikawa, H.; Ueno, H.; Nagasaka, A. Syndiotactic 1,2-Polybutadiene with Co-CS2 Catalyst system. I. Preparation, Properties, and Application of Highly Crystalline Syndiotactic 1,2-Polybutadiene. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 1853–1860. [Google Scholar] [CrossRef]
- Ashitaka, H.; Inaishi, K.; Ueno, H. Syndiotactic 1,2-Polybutadiene with Co-CS2 Catalyst System. III.1H- and 13C-NMR Study of Highly Syndiotactic 1,2-Polybutadiene. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 1973–1988. [Google Scholar] [CrossRef]
- Racanelli, P.; Porri, L. Cis-1,4-polybutadiene by Cobalt Catalysts. Some Features of the Catalysts Prepared from Alkyl Aluminium Compounds Containing Al-O-Al Bonds. Eur. Polym. J. 1970, 6, 751–761. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Porri, L. Polymerization of Butadiene to 1,2-Syndiotactic Polymer with (η3-C8H13)(C4H6)Co. Some Observations on the Factors That Determine the Stereospecificity. Polym. Commun. 1988, 29, 305–307. [Google Scholar]
- Appukuttan, V.; Zhang, L.; Ha, C.-S.; Kim, I. Highly Active and Stereospecific Polymerizations of 1,3-Butadiene by Using Bis(benzimidazolyl)amine Ligands Derived Co(II) Complexes in Combination with Ethylaluminum Sesquichloride. Polymer 2009, 50, 1150–1158. [Google Scholar] [CrossRef]
- Cai, Z.-G.; Shinzawa, M.; Nakayama, Y.; Shiono, T. Synthesis of Regioblock Polybutadiene with CoCl2-Based Catalyst via Reversible Coordination of Lewis Base. Macromolecules 2009, 42, 7642–7643. [Google Scholar] [CrossRef]
- Gong, D.-R.; Wang, B.-L.; Bai, C.-X.; Bi, J.-F.; Wang, F.; Dong, W.-M.; Zhang, X.-Q.; Jiang, L.-S. Metal Dependent Control of Cis-/trans-1,4 Regioselectivity in 1,3-Butadiene Polymerization Catalyzed by Transition Metal Complexes Supported by 2,6-Bis[1-(iminophenyl)ethyl]pyridine. Polymer 2009, 50, 6259–6264. [Google Scholar] [CrossRef]
- Gong, D.-R.; Wang, B.-L.; Cai, H.-G.; Zhang, X.-Q.; Jiang, L.-S. Synthesis, Characterization and Butadiene Polymerization Studies of Cobalt(II) Complexes Bearing Bisiminopyridine Ligand. J. Organomet. Chem. 2011, 696, 1584–1590. [Google Scholar] [CrossRef]
- Gong, D.-R.; Wang, B.-L.; Jia, X.-Y.; Zhang, X.-Q. The Enhanced Catalytic Performance of Cobalt Catalysts towards Butadiene Polymerization by Introducing a Labile Donor in a Salen Ligand. Dalton Trans. 2014, 43, 4169–4178. [Google Scholar] [CrossRef]
- Jie, S.-Y.; Ai, P.-F.; Li, B.-G. Highly Active and Stereospecific Polymerization of 1,3-Butadiene Catalyzed by Dinuclear Cobalt(II) Complexes Bearing 3-Aryliminomethyl-2-hydroxybenzaldehydes. Dalton Trans. 2011, 40, 10975–10982. [Google Scholar] [CrossRef]
- Nobbs, J.D.; Tomov, A.K.; Cariou, R.; Gibson, V.C.; White, A.J.; Britovsek, G.J. Thio-Pybox and Thio-Phebox Complexes of Chromium, Iron, Cobalt and Nickel and Their Application in Ethylene and Butadiene Polymerisation Catalysis. Dalton Trans. 2012, 41, 5949–5964. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Bertini, F.; Boccia, A.C.; Zetta, L. Synthesis and Characterization of Isotactic 1,2-Poly(E-3-methyl-1,3-pentadiene). Some Remarks about the Influence of Monomer Structure on Polymerization Stereoselectivity. Macromolecules 2009, 42, 3048–3056. [Google Scholar] [CrossRef]
- Chen, X.-M.; Huang, L.-C.; Gao, W. One-pot synthesis of cobalt complexes with 2,6-bis(arylimino)phenoxyl/phenthioxyl ligands and catalysis on isoprene polymerization. Dalton Trans. 2021, 50, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- You, J.-Y.; Chen, B.-H.; Gong, D.-R. Polymerization of isoprene, myrcene, and butadiene catalyzed by cobalt complexes supported with 2-acetyl-6-iminopyridine ligand. Appl. Organomet. Chem. 2023, 37, e7258. [Google Scholar] [CrossRef]
- Du, Y.-X.; Gao, S.; Ma, H.; Lu, S.-Q.; Zhang, Z.-H.; Zhao, M.-M. Catalytic Behavior of Cobalt Complexes Bearing Pyridine–Oxime Ligands in Isoprene Polymerization. Polymers 2023, 15, 4660. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-H.; Jing, X.-Y.; Xiong, S.-Y.; Liu, W.-J.; Liu, Y.-L.; Liu, Z.; Chen, C.-L. Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2016, 8, 389. [Google Scholar] [CrossRef]
- Zhu, G.-Q.; Zhang, X.-H.; Zhao, M.-M.; Wang, L.; Jing, C.-Y.; Wang, P.; Wang, X.-W.; Wang, Q.-G. Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2018, 10, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Zhu, G.-Q.; Mahmood, Q.; Zhao, M.-M.; Wang, L.; Jing, C.-Y.; Wang, X.-W.; Wang, Q.-G. Iminoimidazole-based Co(II) and Fe(II) Complexes: Syntheses, Characterization, and Catalytic Behaviors for Isoprene Polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 767–775. [Google Scholar] [CrossRef]
- Zhao, M.-M.; Ma, Y.; Zhang, X.-H.; Wang, L.; Zhu, G.-Q.; Wang, Q.-G. Synthesis, Characterization and Catalytic Property Studies for Isoprene Polymerization of Iron Complexes Bearing Unionized Pyridine-Oxime Ligands. Polymers 2022, 14, 3612. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhao, W.-P.; Han, C.; Zhang, C.-Y.; Liu, H.; Hu, Y.-M.; Zhang, X.-Q. 1,3-Butadiene Polymerizations Catalyzed by Cobalt and Iron Dichloride Complexes Bearing Pyrazolylimine Ligands. Chin. J. Polym. Sci. 2019, 37, 462–470. [Google Scholar] [CrossRef]
- Yousuf, N.; Ma, Y.-P.; Mahmood, Q.; Zhang, W.-J.; Liu, M.; Yuan, R.-Y.; Sun, W.-H. Structurally Rigid (8-(Arylimino)-5,6,7-trihydroquinolin-2-yl)-methyl Acetate Cobalt Complex Catalysts for Isoprene Polymerization with High Activity and cis-1,4 Selectivity. Catalysts 2023, 13, 1120. [Google Scholar] [CrossRef]
- Desimoni, G.; Faita, G.; Quadrelli, P. Pyridine-2,6-bis(oxazolines), Helpful Ligands for Asymmetric Catalysts. Chem. Rev. 2003, 103, 3119–3154. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, H.; Kondo, M.; Nakamura, T.; Itoh, K. Highly Enantioselective Hydrosilylation of Ketones with Chiral and C2-symmetrical Bis(oxazolinyl)pyridine-rhodium Catalysts. Organometallics 1991, 10, 500–508. [Google Scholar] [CrossRef]
- Gao, R.; Xiao, L.-W.; Hao, X.; Sun, W.-H.; Wang, F.-S. Synthesis of Benzoxazolylpyridine Nickel Complexes and Their Efficient Dimerization of Ethylene to Alpha-butene. Dalton Trans. 2008, 7, 5645–5651. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Bi, J.-F.; Zhang, C.-Y.; Zhang, H.-X.; Bai, C.-X.; Hu, Y.-M.; Zhang, X.-Q. Pyridine-oxazoline and Quinoline-oxazoline Ligated Cobalt Complexes: Synthesis, Characterization, and 1,3-Butadiene Polymerization Behaviors. Inorganica Chim. Acta 2015, 435, 305–312. [Google Scholar] [CrossRef]
- Ochędzan-Siodłak, W.; Bihun-Kisiel, A.; Siodłak, D.; Poliwoda, A.; Dziuk, B. Titanium and Vanadium Catalysts with Oxazoline Ligands for Ethylene-Norbornene (co)Polymerization. Eur. Polym. J. 2018, 106, 148–155. [Google Scholar] [CrossRef]
- Fu, L.-R.; Wang, Y.-B.; Jiang, H.; Hao, X.-Q.; Song, M.-P. Applications of Cobalt Complexes in Olefin Polymerization. Chin. J. Org. Chem. 2022, 42, 3530–3548. [Google Scholar]
- Hou, S.-Y.; Hao, X.-G.; Jiang, H.; Hao, X.-Q.; Song, M.-P. Progress in the Polymerization of 1,3-Diene Catalyzed by Fe and Co Metal Complexes. Acta Polym. Sin. 2023, 54, 186–205. [Google Scholar]
- Albrecht, M.; Lindner, M.M. Cleavage of Unreactive Bonds with Pincer Metal Complexes. Dalton Trans. 2011, 40, 8733–8744. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hao, X.-Q.; Huang, J.-J.; Wang, K.; Gong, J.-F.; Song, M.-P. Chiral CNN Pincer Palladium(II) Complexes with 2-Aryl-6-(oxazolinyl)pyridine Ligands: Synthesis, Characterization, and Application to Enantioselective Allylation of Isatins and Suzuki-Miyaura Coupling Reaction. Organometallics 2014, 33, 194–205. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Wang, L.; Wang, L.; Zhu, G.; Wang, Q. Pyridine-oxazoline ligated iron complexes: Synthesis, characterization, and catalytic activity for isoprene polymerization. Appl. Organomet. Chem. 2022, 36, e6848. [Google Scholar] [CrossRef]



| Entry | Co-Catalyst | Al/Co | Conv/% | Activity b | Microstructure c | Mn d | Ð d | |
|---|---|---|---|---|---|---|---|---|
| cis-1,4 | 3,4 | |||||||
| 1 | MAO | 500 | trace | - | - | - | - | - |
| 2 | AlMe3 | 500 | - | - | - | - | - | - |
| 3 | AlEt3 | 500 | - | - | - | - | - | - |
| 4 | AliBu3 | 500 | - | - | - | - | - | - |
| 5 | AlEt2Cl | 500 | 99 | 1.36 | 67 | 33 | 1.33 | 2.81 |
| 6 | AlEt2Cl | 200 | 99 | 1.36 | 65 | 35 | 5.74 | 2.45 |
| 7 | AlEt2Cl | 80 | 99 | 1.36 | 64 | 36 | 12.5 | 2.05 |
| 8 | AlEt2Cl | 50 | 97 | 1.33 | 67 | 33 | 17.6 | 2.35 |
| 9 | AlEt2Cl | 20 | 84 | 1.15 | 62 | 38 | 12.2 | 2.03 |
| 10 | AlEt2Cl | 5 | 56 | 0.76 | 65 | 35 | 3.56 | 2.56 |
| 11 e | AlEt2Cl | 80 | 94 | 0.64 | 63 | 37 | 10.5 | 2.82 |
| 12 f | AlEt2Cl | 80 | 59 | 1.61 | 63 | 37 | 11.5 | 1.95 |
| Entry | T/°C | t/min | Conv/% | Activity b | Microstructure c | Mn d | Ð d | |
|---|---|---|---|---|---|---|---|---|
| cis-1,4 | 3,4 | |||||||
| 1 | 25 | 120 | 99 | 1.36 | 64 | 36 | 12.5 | 2.05 |
| 2 | 25 | 60 | 97 | 2.64 | 63 | 37 | 8.79 | 1.95 |
| 3 | 25 | 30 | 94 | 5.12 | 64 | 36 | 7.67 | 2.79 |
| 4 | 25 | 10 | 83 | 13.6 | 63 | 37 | 4.46 | 1.79 |
| 5 | 25 | 5 | 76 | 25.0 | 63 | 37 | 0.47 | 2.61 |
| 6 | 50 | 120 | 92 | 1.32 | 65 | 35 | 10.9 | 2.21 |
| 7 | 70 | 120 | 82 | 1.19 | 65 | 35 | 10.7 | 2.49 |
| 8 | 90 | 120 | 16 | 0.23 | 66 | 34 | 8.21 | 2.30 |
| Entry | Cat | Conv/% | Activity b | Microstructure c | Mn d | Ð d | |
|---|---|---|---|---|---|---|---|
| cis-1,4 | 3,4 | ||||||
| 1 | 3a | 99 | 1.36 | 64 | 36 | 12.5 | 2.05 |
| 2 | 3a′ | 97 | 1.32 | 64 | 36 | 3.15 | 2.64 |
| 3 | 3b | 99 | 1.36 | 65 | 35 | 14.4 | 2.14 |
| 4 | 3c | 99 | 1.36 | 65 | 35 | 1.92 | 2.75 |
| 5 | 3d | 99 | 1.35 | 65 | 35 | 7.74 | 2.18 |
| 6 | 3e | 98 | 1.32 | 65 | 35 | 4.66 | 2.65 |
| 7 | 3f | 99 | 1.34 | 65 | 35 | 5.78 | 2.60 |
| 8 | 3g | 96 | 1.31 | 62 | 38 | 1.70 | 3.42 |
| 9 | 3h | 99 | 1.36 | 64 | 36 | 10.5 | 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Liu, J.-K.; Zhuo, W.; Zheng, J.; Hao, X.-Q.; Gong, J.-F.; Jiang, H.; Song, M.-P. Synthesis, Characterization, and Catalytic Behaviors in Isoprene Polymerization of Pyridine–Oxazoline-Ligated Cobalt Complexes. Polymers 2024, 16, 578. https://doi.org/10.3390/polym16050578
Hao X, Liu J-K, Zhuo W, Zheng J, Hao X-Q, Gong J-F, Jiang H, Song M-P. Synthesis, Characterization, and Catalytic Behaviors in Isoprene Polymerization of Pyridine–Oxazoline-Ligated Cobalt Complexes. Polymers. 2024; 16(5):578. https://doi.org/10.3390/polym16050578
Chicago/Turabian StyleHao, Xiuge, Jin-Kui Liu, Weize Zhuo, Jiajing Zheng, Xin-Qi Hao, Jun-Fang Gong, Hui Jiang, and Mao-Ping Song. 2024. "Synthesis, Characterization, and Catalytic Behaviors in Isoprene Polymerization of Pyridine–Oxazoline-Ligated Cobalt Complexes" Polymers 16, no. 5: 578. https://doi.org/10.3390/polym16050578
APA StyleHao, X., Liu, J.-K., Zhuo, W., Zheng, J., Hao, X.-Q., Gong, J.-F., Jiang, H., & Song, M.-P. (2024). Synthesis, Characterization, and Catalytic Behaviors in Isoprene Polymerization of Pyridine–Oxazoline-Ligated Cobalt Complexes. Polymers, 16(5), 578. https://doi.org/10.3390/polym16050578








