Flexible Composite Electrolyte Membranes with Fast Ion Transport Channels for Solid-State Lithium Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
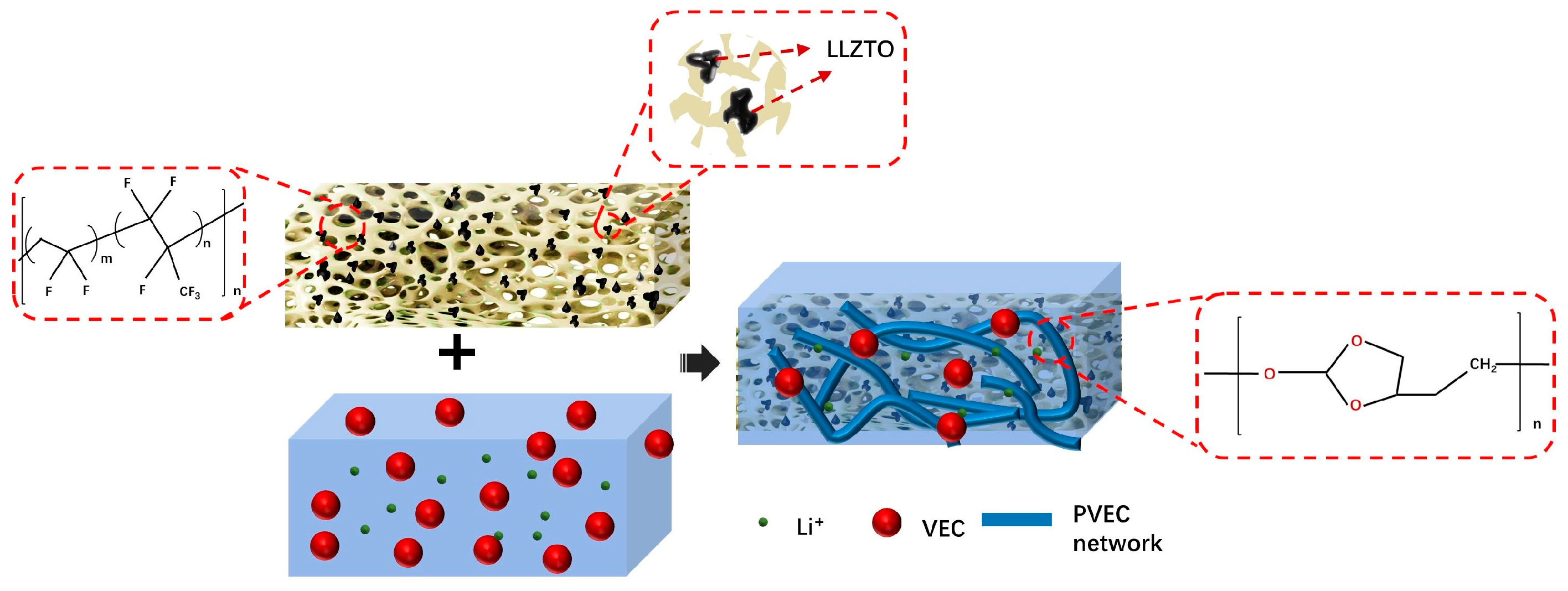
2.2. Materials Synthesis
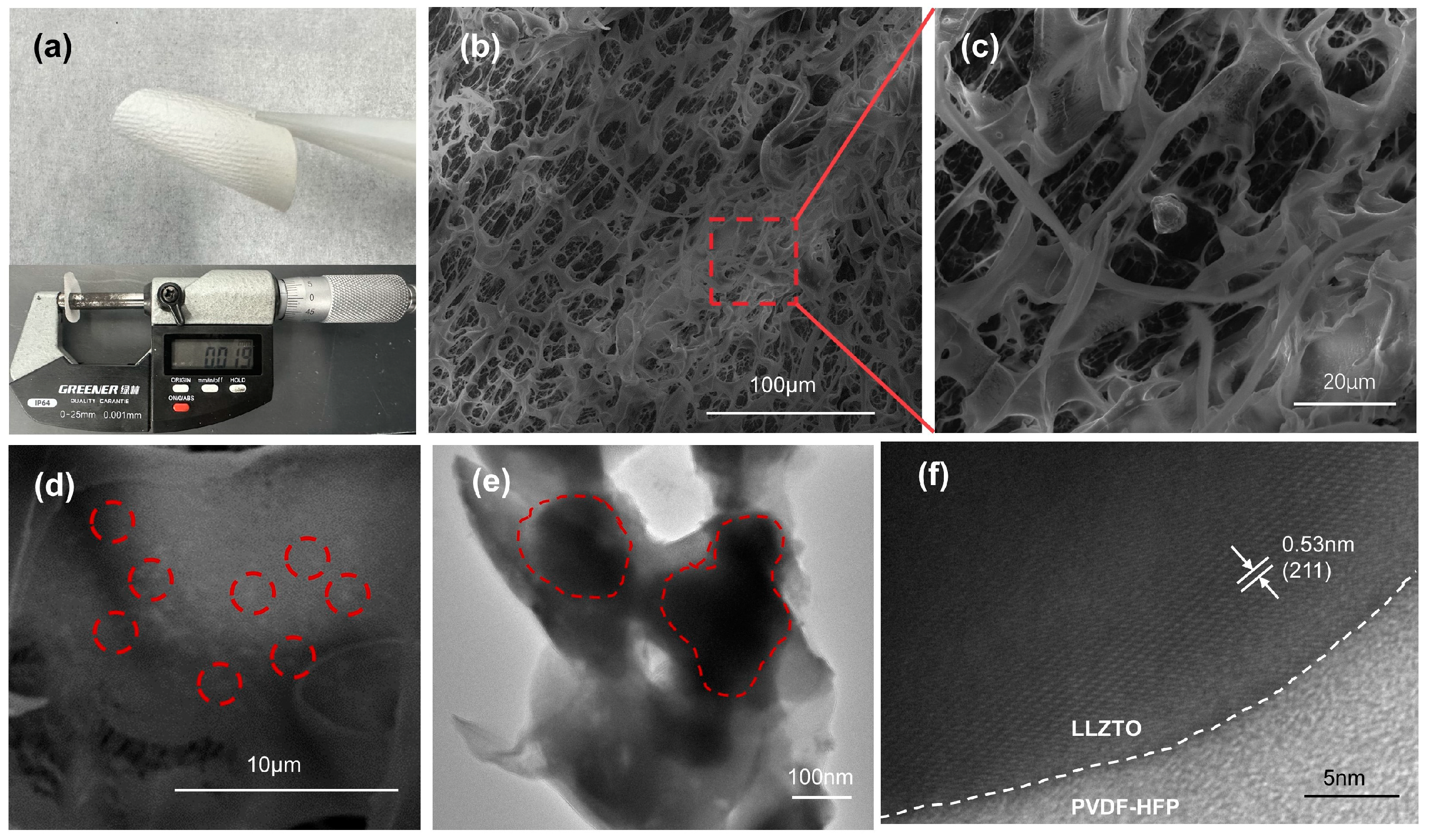
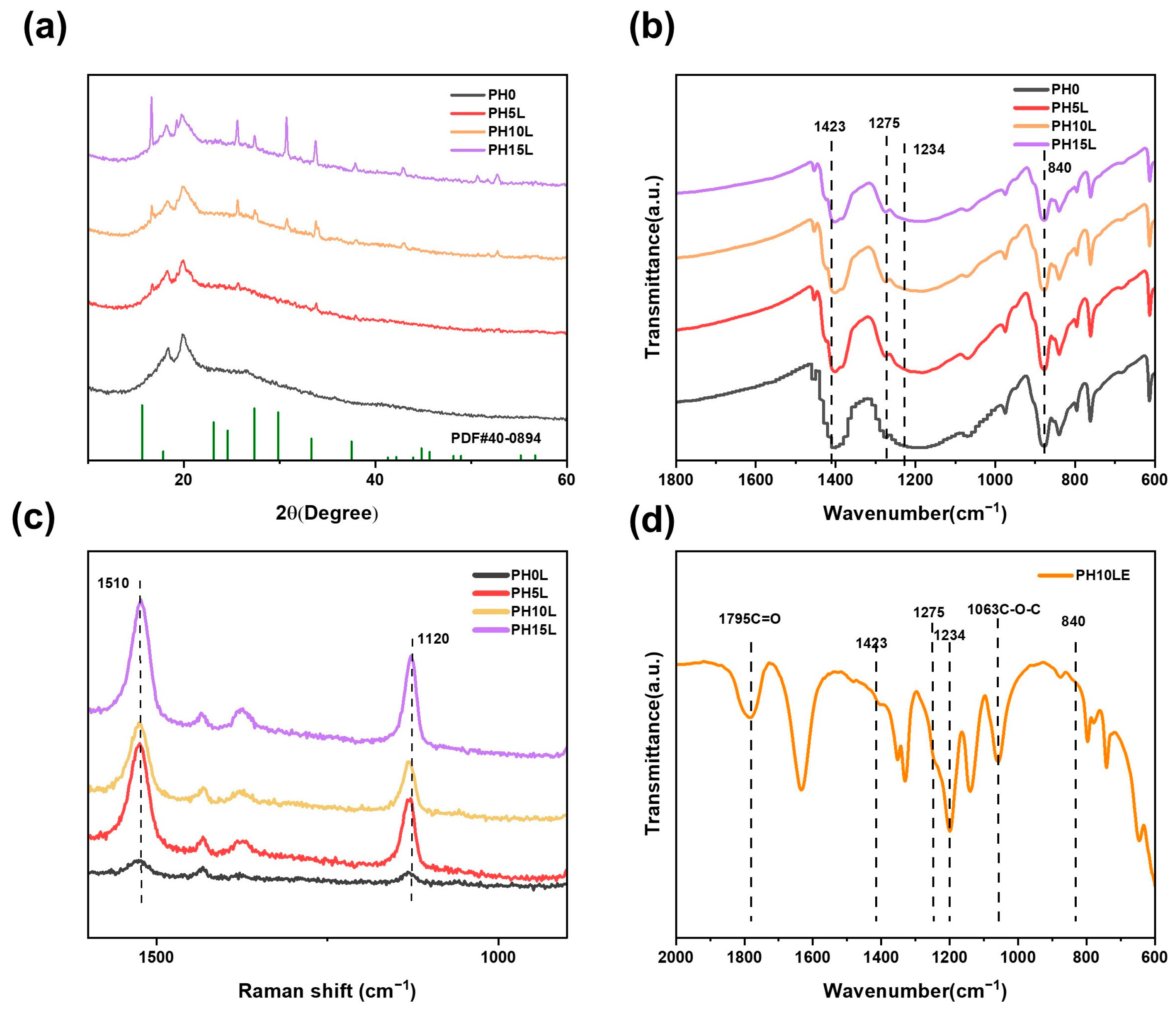
2.3. Characterization
2.4. Electrochemical Characterization
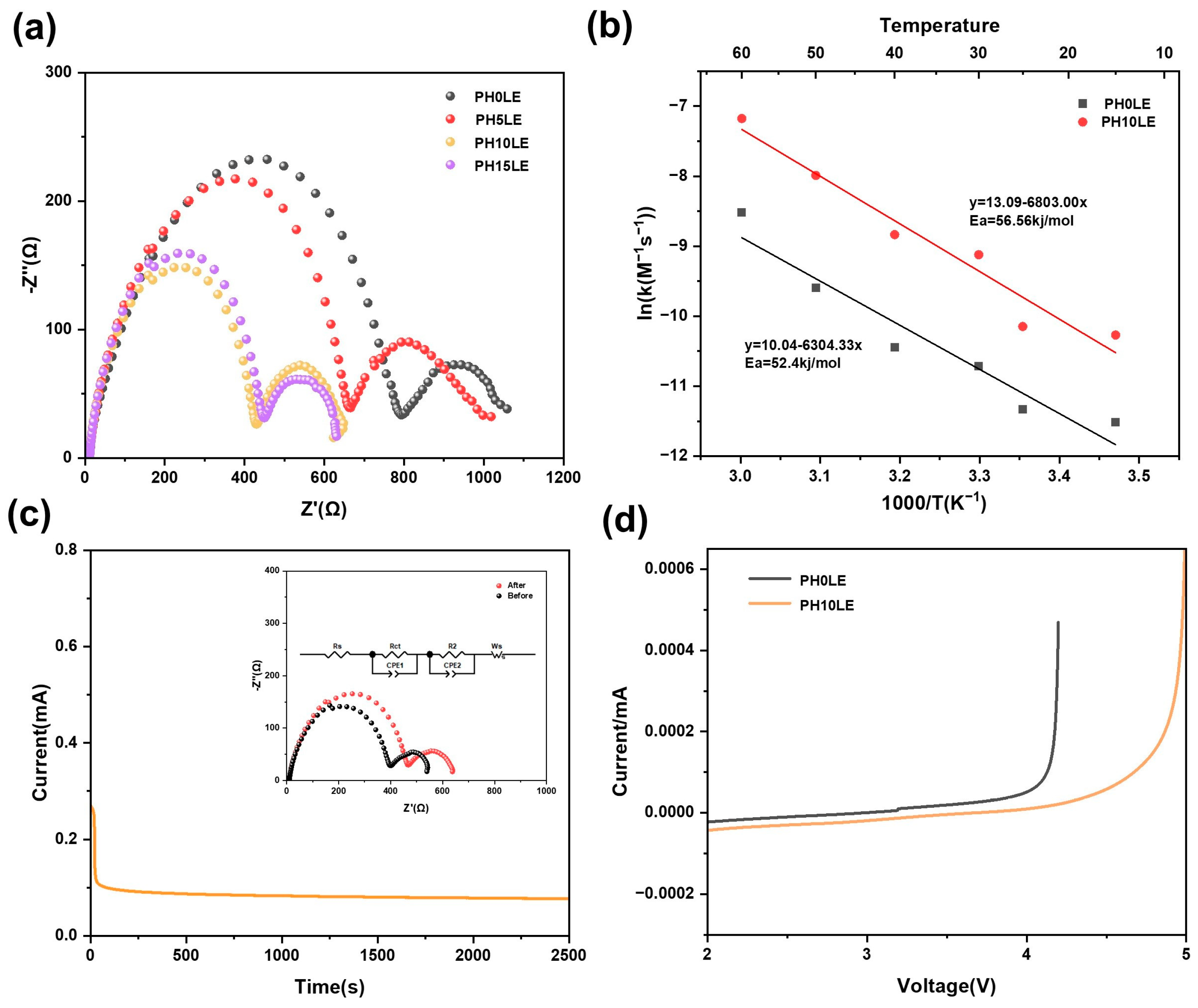
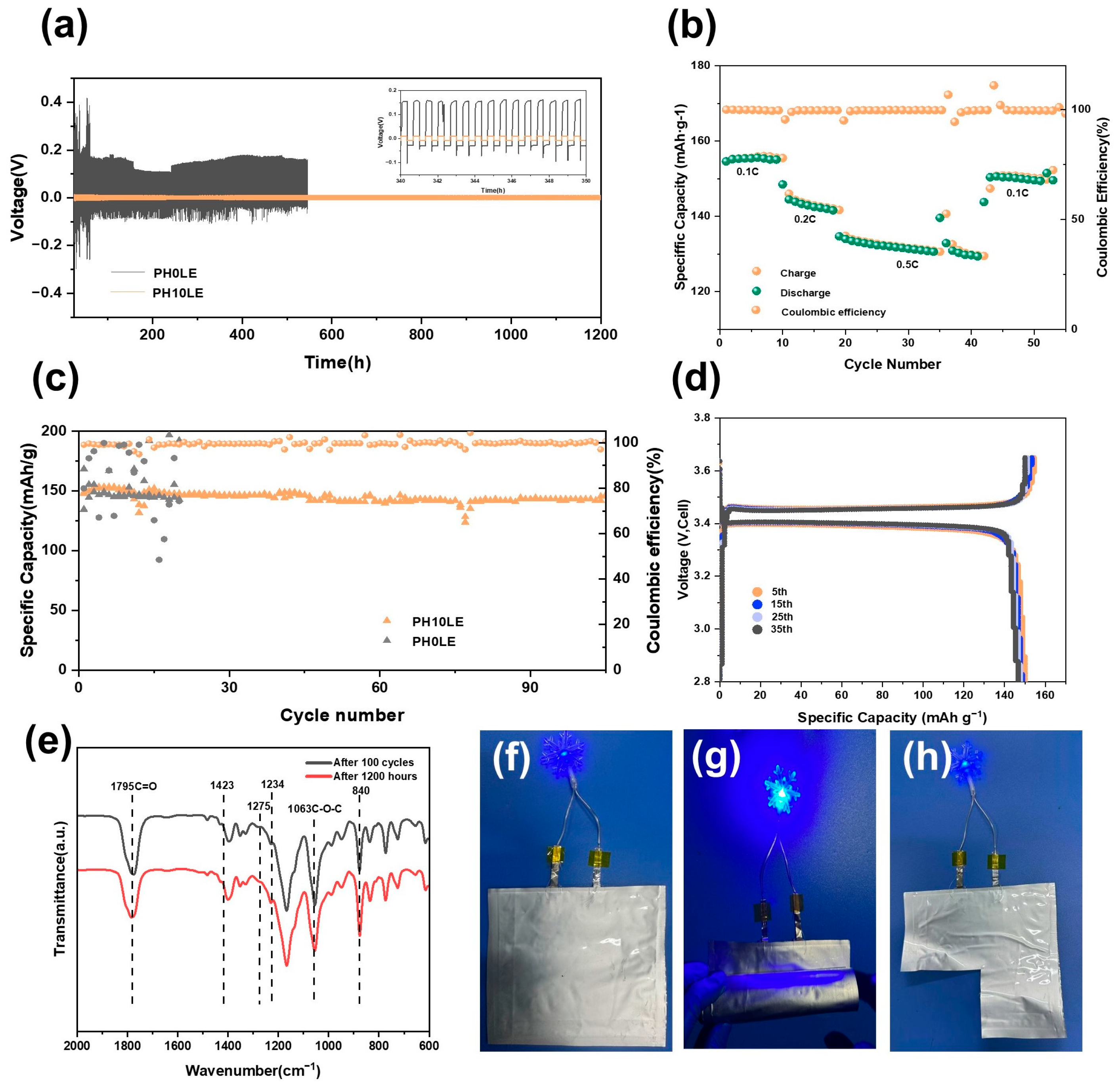
3. Results and Discussion
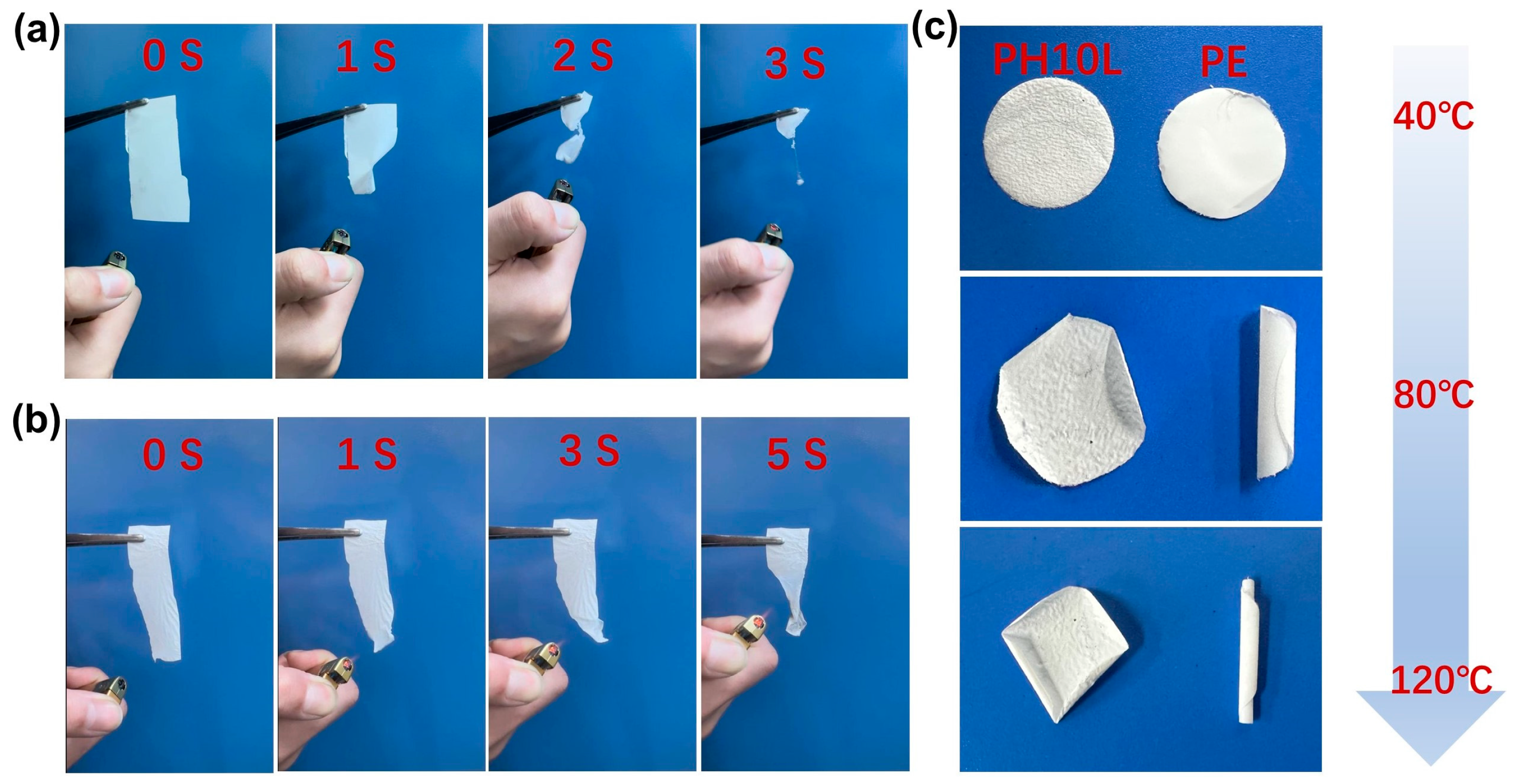
Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Nie, L.; Hu, X.; Zhang, Y.; Zhang, Y.; Yu, Y.; Liu, W. Ultrafast Sintering for Ceramic-Based All-Solid-State Lithium-Metal Batteries. Adv. Mater. 2022, 34, 2200430. [Google Scholar] [CrossRef]
- Du, F.; Zhao, N.; Li, Y.; Chen, C.; Liu, Z.; Guo, X. All solid state lithium batteries based on lamellar garnet-type ceramic electrolytes. J. Power Sources 2015, 300, 24–28. [Google Scholar] [CrossRef]
- Feng, W.; Lai, Z.; Dong, X.; Li, P.; Wang, Y.; Xia, Y. Garnet-Based All-Ceramic Lithium Battery Enabled by Li(2.985)B(0.005)OCl Solder. iScience 2020, 23, 101071. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yang, P.; Dong, X.; Xia, Y. A Low Temperature Soldered All Ceramic Lithium Battery. ACS Appl. Mater. Interfaces 2022, 14, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ju, J.; Yu, M.; Chen, S.; Cui, G. In-situ Polymerization Integrating 3D Ceramic Framework in All Solid-state Lithium Battery. J. Inorg. Mater. 2020, 35, 1357–1364. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Chen, C.; Zhao, N.; Fan, X.; Ma, Z.; Gao, T.; Wang, F.; Guo, X.; Wang, C. Interphase Engineering Enabled All-Ceramic Lithium Battery. Joule 2018, 2, 497–508. [Google Scholar] [CrossRef]
- Hao, F.; Liang, Y.; Zhang, Y.; Chen, Z.; Zhang, J.; Ai, Q.; Guo, H.; Fan, Z.; Lou, J.; Yao, Y. High-Energy All-Solid-State Organic–Lithium Batteries Based on Ceramic Electrolytes. ACS Energy Lett. 2020, 6, 201–207. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, C.; Zong, X.; Li, D.; Fu, M.; Tan, S.; Xiong, Y.; Wei, J. Interface engineering of Li1.3Al0.3Ti1.7(PO4)3 ceramic electrolyte via multifunctional interfacial layer for all-solid-state lithium batteries. J. Power Sources 2020, 460, 228125. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Long Cycle Life, All-Solid-State Lithium Battery with a Ceramic–Polymer Composite Electrolyte. ACS Appl. Energy Mater. 2020, 3, 2916–2924. [Google Scholar] [CrossRef]
- Huo, H.; Chen, Y.; Luo, J.; Yang, X.; Guo, X.; Sun, X. Rational Design of Hierarchical “Ceramic-in-Polymer” and “Polymer-in-Ceramic” Electrolytes for Dendrite-Free Solid-State Batteries. Adv. Energy Mater. 2019, 9, 1804004. [Google Scholar] [CrossRef]
- Jiang, T.; He, P.; Wang, G.; Shen, Y.; Nan, C.W.; Fan, L.Z. Solvent-Free Synthesis of Thin, Flexible, Nonflammable Garnet-Based Composite Solid Electrolyte for All-Solid-State Lithium Batteries. Adv. Energy Mater. 2020, 10, 1903376. [Google Scholar] [CrossRef]
- Kang, Q.; Zhuang, Z.; Liu, Y.; Liu, Z.; Li, Y.; Sun, B.; Pei, F.; Zhu, H.; Li, H.; Li, P.; et al. Engineering the Structural Uniformity of Gel Polymer Electrolytes via Pattern-guided Alignment for Durable, Safe Solid-state Lithium Metal Batteries. Adv. Mater. 2023, 35, 2303460. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Han, J.; Lee, K.; Lee, Y.J.; Kim, B.G.; Jung, K.-N.; Kim, B.J.; Lee, S.W. Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature 2022, 601, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Yang, T.; Luo, D.; Liu, Y.; Ma, Q.; Yang, L.; Yao, Y.; Huang, R.; Li, Z.; Akinoglu, E.M.; et al. Tailoring Vertically Aligned Inorganic-Polymer Nanocomposites with Abundant Lewis Acid Sites for Ultra-Stable Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2023, 13, 2204218. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Gong, Y.; Shi, C.; Liang, J.; Li, Y.; He, C.; Zhang, Q.; Ren, X. Insight into the integration way of ceramic solid-state electrolyte fillers in the composite electrolyte for high performance solid-state lithium metal battery. Energy Storage Mater. 2022, 51, 130–138. [Google Scholar] [CrossRef]
- Shan, X.; Zhao, S.; Ma, M.; Pan, Y.; Xiao, Z.; Li, B.; Sokolov, A.P.; Tian, M.; Yang, H.; Cao, P.-F. Single-Ion Conducting Polymeric Protective Interlayer for Stable Solid Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 56110–56119. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J.; et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Liu, C.; Liu, G.; Yu, J.; Zou, Q. Solid-state lithium battery with garnet Li7La3Zr2O12 nanofibers composite polymer electrolytes. Solid State Ion. 2022, 378, 115897. [Google Scholar] [CrossRef]
- Wang, Y.; Zanelotti, C.J.; Wang, X.; Kerr, R.; Jin, L.; Kan, W.H.; Dingemans, T.J.; Forsyth, M.; Madsen, L.A. Solid-state rigid-rod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 2021, 20, 1255–1263. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, B.; Jing, M.; Shen, X.; Wang, L.; Xu, H.; Yan, X.; He, X. In Situ Catalytic Polymerization of a Highly Homogeneous PDOL Composite Electrolyte for Long-Cycle High-Voltage Solid-State Lithium Batteries. Adv. Energy Mater. 2022, 12, 2201762. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Feng, Y.; Tan, R.; Zuo, Y.; Gao, R.; Zhao, Y.; Han, L.; Wang, Z.; Pan, F. Flexible Composite Solid Electrolyte Facilitating Highly Stable “Soft Contacting” Li-Electrolyte Interface for Solid State Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701437. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shen, X.; Luo, L.; Chen, H.; Cao, R.; Feng, X.; Chen, W.; Fang, Y.; Cao, Y. Correlating the Solvating Power of Solvents with the Strength of Ion-Dipole Interaction in Electrolytes of Lithium-ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202312373. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, X.; Du, C.; Luo, Y.; Chen, J.; Yan, J.; Zhu, D.; Geng, L.; Liu, S.; Zhao, J.; et al. An All-Solid-State Battery Based on Sulfide and PEO Composite Electrolyte. Small 2022, 18, 2202069. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-W.; Lee, J.-M.; Kim, T.Y.; Song, M.-S.; Park, Y. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 2013, 249, 197–206. [Google Scholar] [CrossRef]
- Chen, W.-P.; Duan, H.; Shi, J.-L.; Qian, Y.; Wan, J.; Zhang, X.-D.; Sheng, H.; Guan, B.; Wen, R.; Yin, Y.-X.; et al. Bridging Interparticle Li+ Conduction in a Soft Ceramic Oxide Electrolyte. J. Am. Chem. Soc. 2021, 143, 5717–5726. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Li, K.; Sun, S.; Sun, S.; Chen, J.; Pan, L.; Sun, Z. Plasma optimized Li7La3Zr2O12 with vertically aligned ion diffusion pathways in composite polymer electrolyte for stable solid-state lithium metal batteries. Chem. Eng. J. 2021, 430, 132874. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, J.; Zhang, W.; Lu, Y. Recent Advances of Composite Solid-State Electrolytes for Lithium-Based Batteries. Energy Fuels 2021, 35, 11118–11140. [Google Scholar] [CrossRef]
- Chen, F.; Yang, D.; Zha, W.; Zhu, B.; Zhang, Y.; Li, J.; Gu, Y.; Shen, Q.; Zhang, L.; Sadoway, D.R. Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries. Electrochim. Acta 2017, 258, 1106–1114. [Google Scholar] [CrossRef]
- Liang, Y.F.; Deng, S.J.; Xia, Y.; Wang, X.L.; Xia, X.H.; Wu, J.B.; Gu, C.D.; Tu, J.P. A superior composite gel polymer electrolyte of Li7La3Zr2O12- poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) for rechargeable solid-state lithium ion batteries. Mater. Res. Bull. 2018, 102, 412–417. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, K.; Xiong, C.; Wu, M.; Zhao, T. A composite solid electrolyte with an asymmetric ceramic framework for dendrite-free all-solid-state Li metal batteries. J. Mater. Chem. A 2021, 9, 9665–9674. [Google Scholar] [CrossRef]
- Pearse, A.J.; Schmitt, T.E.; Fuller, E.J.; El-Gabaly, F.; Lin, C.-F.; Gerasopoulos, K.; Kozen, A.C.; Talin, A.A.; Rubloff, G.; Gregorczyk, K.E. Nanoscale Solid State Batteries Enabled by Thermal Atomic Layer Deposition of a Lithium Polyphosphazene Solid State Electrolyte. Chem. Mater. 2017, 29, 3740–3753. [Google Scholar] [CrossRef]
- Thomas-Alyea, K.E. Design of Porous Solid Electrolytes for Rechargeable Metal Batteries. J. Electrochem. Soc. 2018, 165, A1523–A1528. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, H.; Wang, S.; Song, X.; Yao, X.; Wang, H. Perovskite Membranes with Vertically Aligned Microchannels for All-Solid-State Lithium Batteries. Adv. Energy Mater. 2018, 8, 1801433. [Google Scholar] [CrossRef]
- Hu, J.; He, P.; Zhang, B.; Wang, B.; Fan, L.-Z. Porous film host-derived 3D composite polymer electrolyte for high-voltage solid state Lithium batteries. Energy Storage Mater. 2020, 26, 283–289. [Google Scholar] [CrossRef]
- Guo, W.; Shen, F.; Liu, J.; Zhang, Q.; Guo, H.; Yin, Y.; Gao, J.; Sun, Z.; Han, X.; Hu, Y. In-situ optical observation of Li growth in garnet-type solid state electrolyte. Energy Storage Mater. 2021, 41, 791–797. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Xiaokaiti, P.; Yoshida, A.; Abudula, A.; Guan, G. A sandwich-type composite polymer electrolyte for all-solid-state lithium metal batteries with high areal capacity and cycling stability. J. Membr. Sci. 2020, 596, 117739. [Google Scholar] [CrossRef]
- Gao, L.; Li, J.; Ju, J.; Cheng, B.; Kang, W.; Deng, N. Polyvinylidene fluoride nanofibers with embedded Li6.4La3Zr1.4Ta0.6O12 fillers modified polymer electrolytes for high-capacity and long-life all-solid-state lithium metal batteries. Compos. Sci. Technol. 2020, 200, 108408. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2019, 29, 361–366. [Google Scholar] [CrossRef]
- Li, X.; Cong, L.; Ma, S.; Shi, S.; Li, Y.; Li, S.; Chen, S.; Zheng, C.; Sun, L.; Liu, Y.; et al. Low Resistance and High Stable Solid–Liquid Electrolyte Interphases Enable High-Voltage Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2010611. [Google Scholar] [CrossRef]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-state batteries: The critical role of mechanics. Science 2023, 381, eabg5998. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Feng, W.; Han, G. Strippable and flexible solid electrolyte membrane by coupling Li6.4La3Zr1.4Ta0.6O12 and insulating polyvinylidene fluoride for solid state lithium ion battery. Ionics 2021, 27, 3339–3346. [Google Scholar] [CrossRef]
- Peng, L.; Lu, Z.; Zhong, L.; Jian, J.; Rong, Y.; Yang, R.; Xu, Y.; Jin, C. Enhanced ionic conductivity and interface compatibility of PVDF-LLZTO composite solid electrolytes by interfacial maleic acid modification. J. Colloid Interface Sci. 2022, 613, 368–375. [Google Scholar] [CrossRef]
- Shen, F.; Guo, W.; Zeng, D.; Sun, Z.; Gao, J.; Li, J.; Zhao, B.; He, B.; Han, X. A Simple and Highly Efficient Method toward High-Density Garnet-Type LLZTO Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 30313–30319. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, T.; Huang, S.; Mi, J.; Zhang, Y.; Travas-Sejdic, J.; Turner, A.P.; Gao, W.; Cao, P. Constructing a PVDF-based composite solid-state electrolyte with high ionic conductivity Li6.5La3Zr1.5Ta0.1Nb0.4O12 for lithium metal battery. J. Power Sources 2023, 564, 232849. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; Kan, W.H.; Avdeev, M.; Zhu, C.; Zhao, Z.; Chu, X.; Mu, D.; Wu, F. Consolidating the grain boundary of the garnet electrolyte LLZTO with Li3BO3 for high-performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries. J. Mater. Chem. A 2019, 7, 20633–20639. [Google Scholar] [CrossRef]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ion. 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, K.; Zhang, X.; Ma, Y.; Peng, Q.; Gong, Y.; Yi, S.; Guo, H.; Zhang, X.; Sun, X.; et al. Improved Li-Ion Conduction and (Electro)Chemical Stability at Garnet-Polymer Interface through Metal-Nitrogen Bonding. Adv. Energy Mater. 2023, 13, 2204377. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, T.; Cui, J.; Shen, X.; Shi, C.; Cao, S.; Zhao, J. A homogenous solid polymer electrolyte prepared by facile spray drying method is used for room-temperature solid lithium metal batteries. Nano Res. 2021, 16, 5080–5086. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, J.; Li, F.; Wang, Z.L.; Sun, C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.-W.; Shen, Y. Synergistic Coupling between Li6.75La3Zr1.75Ta0.25O12 and Poly(vinylidene fluoride) Induces High Ionic Conductivity, Mechanical Strength, and Thermal Stability of Solid Composite Electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, M.; Peng, Y.; Guan, S. Poly (vinylidene fluoride) binder reinforced poly (propylene carbonate)/3D garnet nanofiber composite polymer electrolyte toward dendrite-free lithium metal batteries. Mater. Today Energy 2022, 24, 100952. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Mao, D.; Xin, W.; Yang, S.; Zhang, H.; Zhang, Y.; Liu, X.; Dong, D.; Ye, Z.; Li, J. Flexible Composite Electrolyte Membranes with Fast Ion Transport Channels for Solid-State Lithium Batteries. Polymers 2024, 16, 565. https://doi.org/10.3390/polym16050565
Ma X, Mao D, Xin W, Yang S, Zhang H, Zhang Y, Liu X, Dong D, Ye Z, Li J. Flexible Composite Electrolyte Membranes with Fast Ion Transport Channels for Solid-State Lithium Batteries. Polymers. 2024; 16(5):565. https://doi.org/10.3390/polym16050565
Chicago/Turabian StyleMa, Xiaojun, Dongxu Mao, Wenkai Xin, Shangyun Yang, Hao Zhang, Yanzhu Zhang, Xundao Liu, Dehua Dong, Zhengmao Ye, and Jiajie Li. 2024. "Flexible Composite Electrolyte Membranes with Fast Ion Transport Channels for Solid-State Lithium Batteries" Polymers 16, no. 5: 565. https://doi.org/10.3390/polym16050565
APA StyleMa, X., Mao, D., Xin, W., Yang, S., Zhang, H., Zhang, Y., Liu, X., Dong, D., Ye, Z., & Li, J. (2024). Flexible Composite Electrolyte Membranes with Fast Ion Transport Channels for Solid-State Lithium Batteries. Polymers, 16(5), 565. https://doi.org/10.3390/polym16050565





