In Situ Study and Improvement of the Temperature Increase and Isothermal Retention Stages in the Polyacrylonitrile (PAN) Fiber Pre-Oxidation Process
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Information
2.2. Thermal Analysis
2.3. Synchrotron Wide-Angle X-ray Diffraction (WAXD)
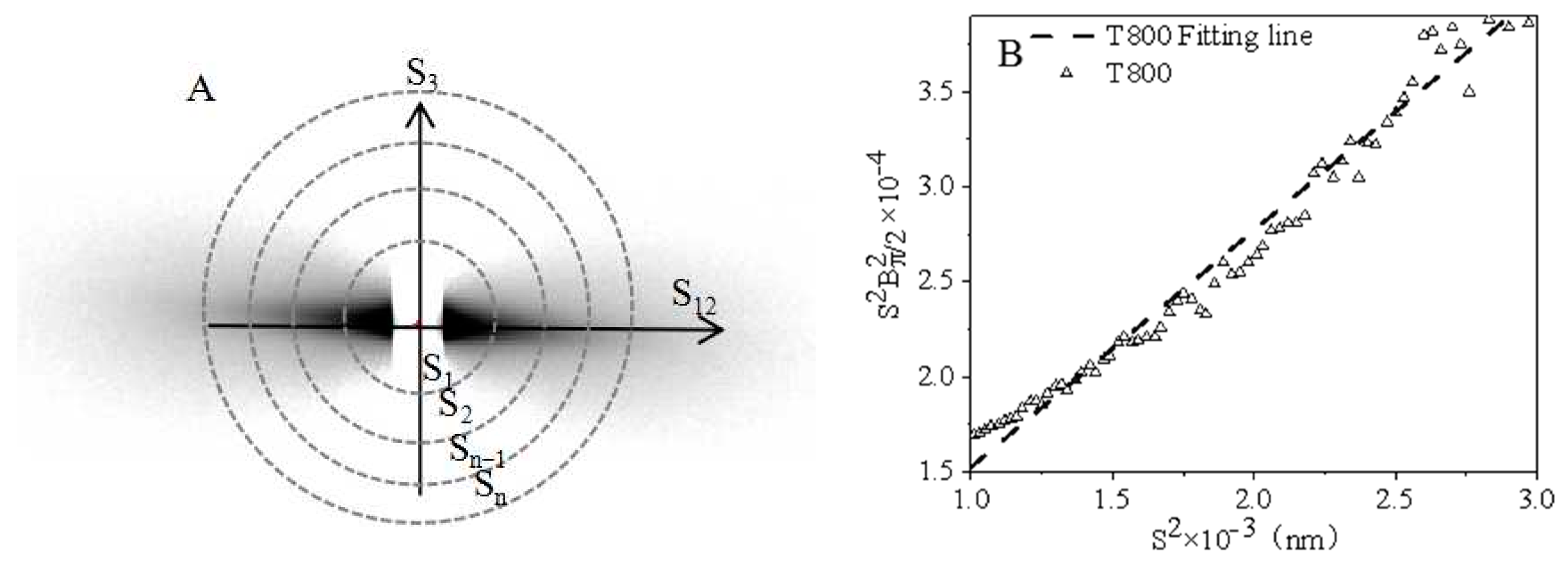
2.4. Synchrotron Small-Angle X-ray Scattering (SAXS)
3. Results and Discussion
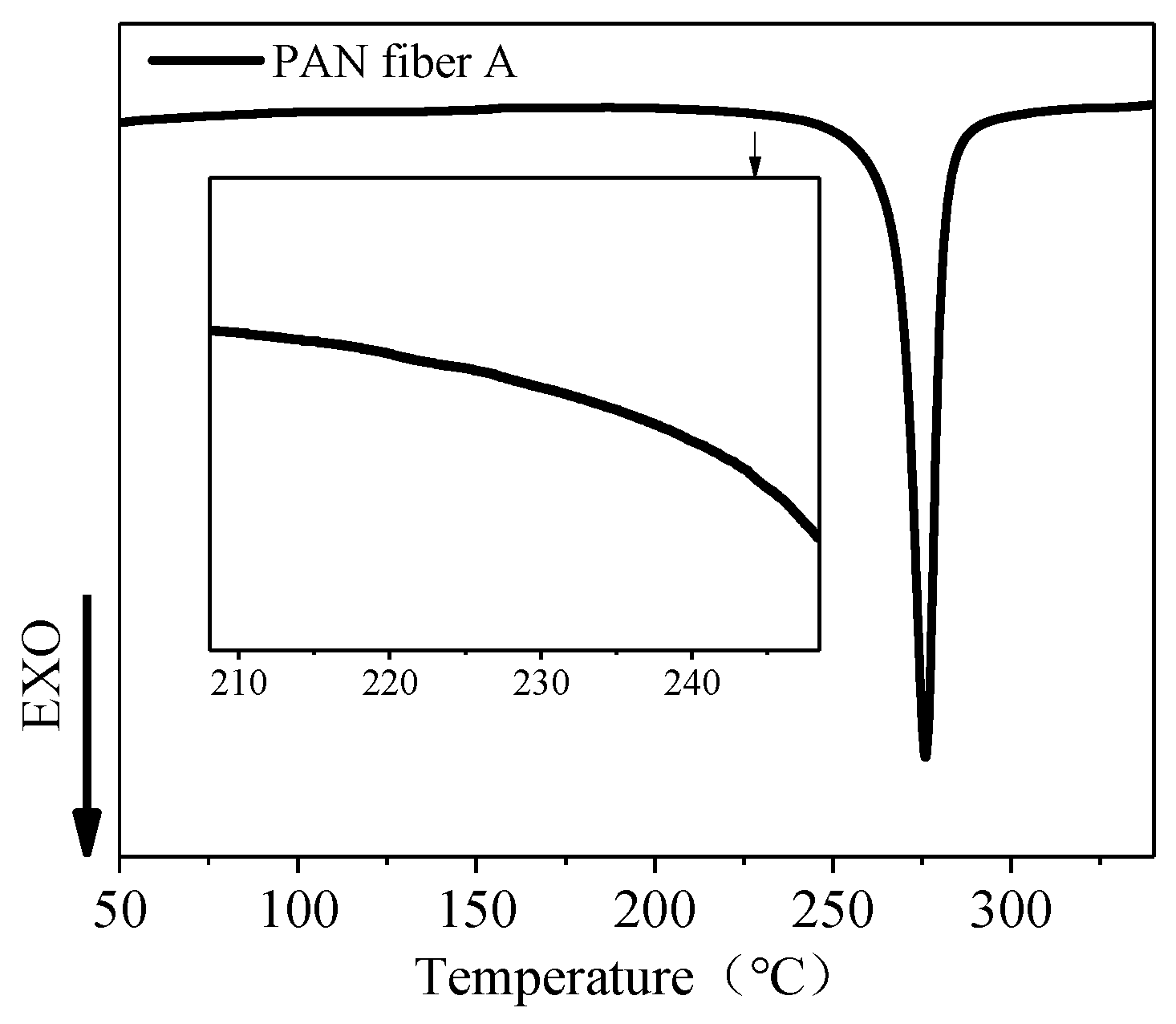
3.1. Thermal Analysis of PAN Fiber
3.2. Data Analysis
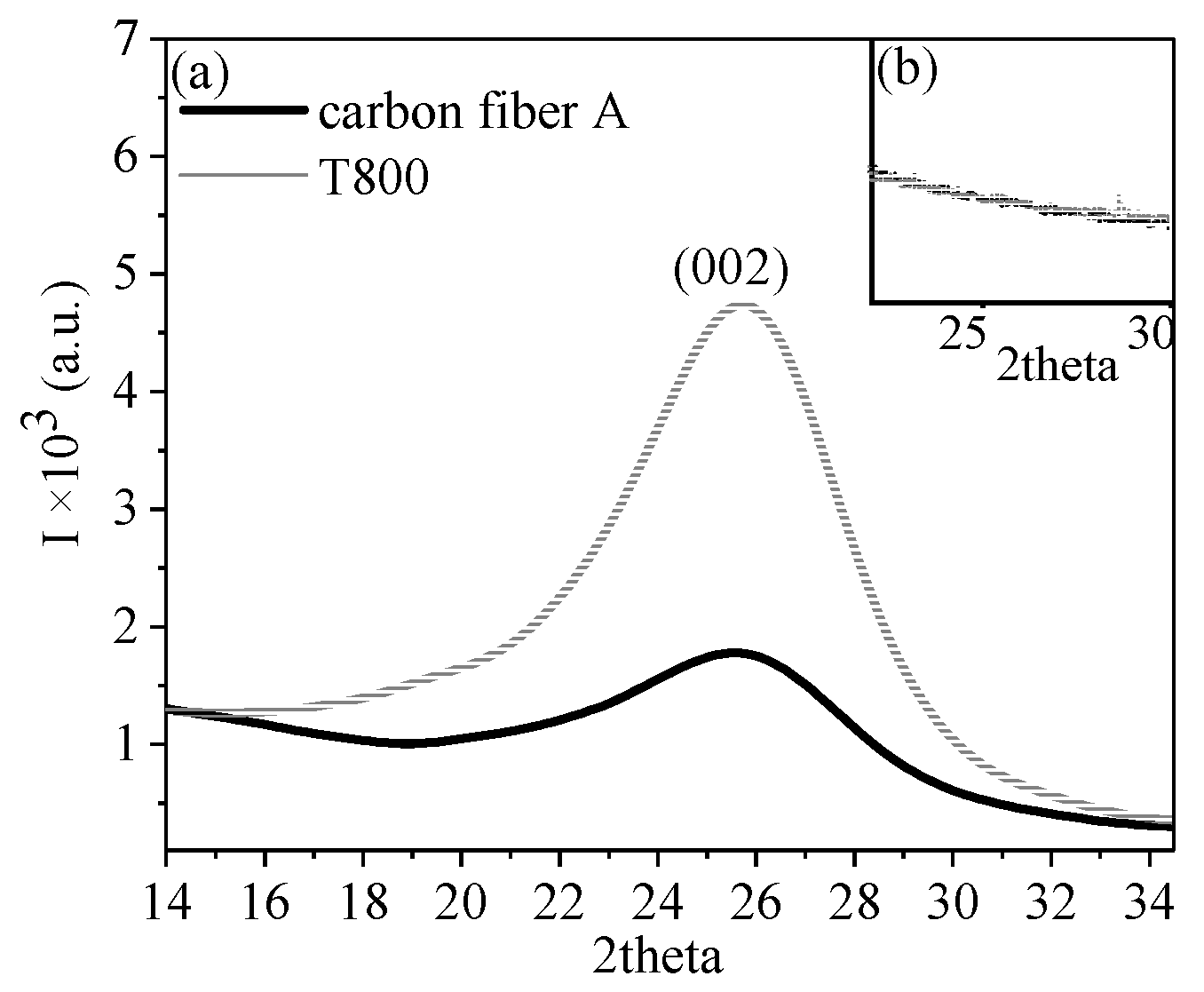
3.2.1. WAXD Analysis
3.2.2. Research on the Crystal Structure of Carbon Fiber
3.2.3. SAXS Analysis
3.2.4. Research on the Microporous Structure of Carbon Fiber
3.3. In Situ Study of the Pre-Oxidation Process
3.3.1. Research on the Crystal Structure of PAN Fiber
3.3.2. Research on the Microporous Structure of PAN Fiber
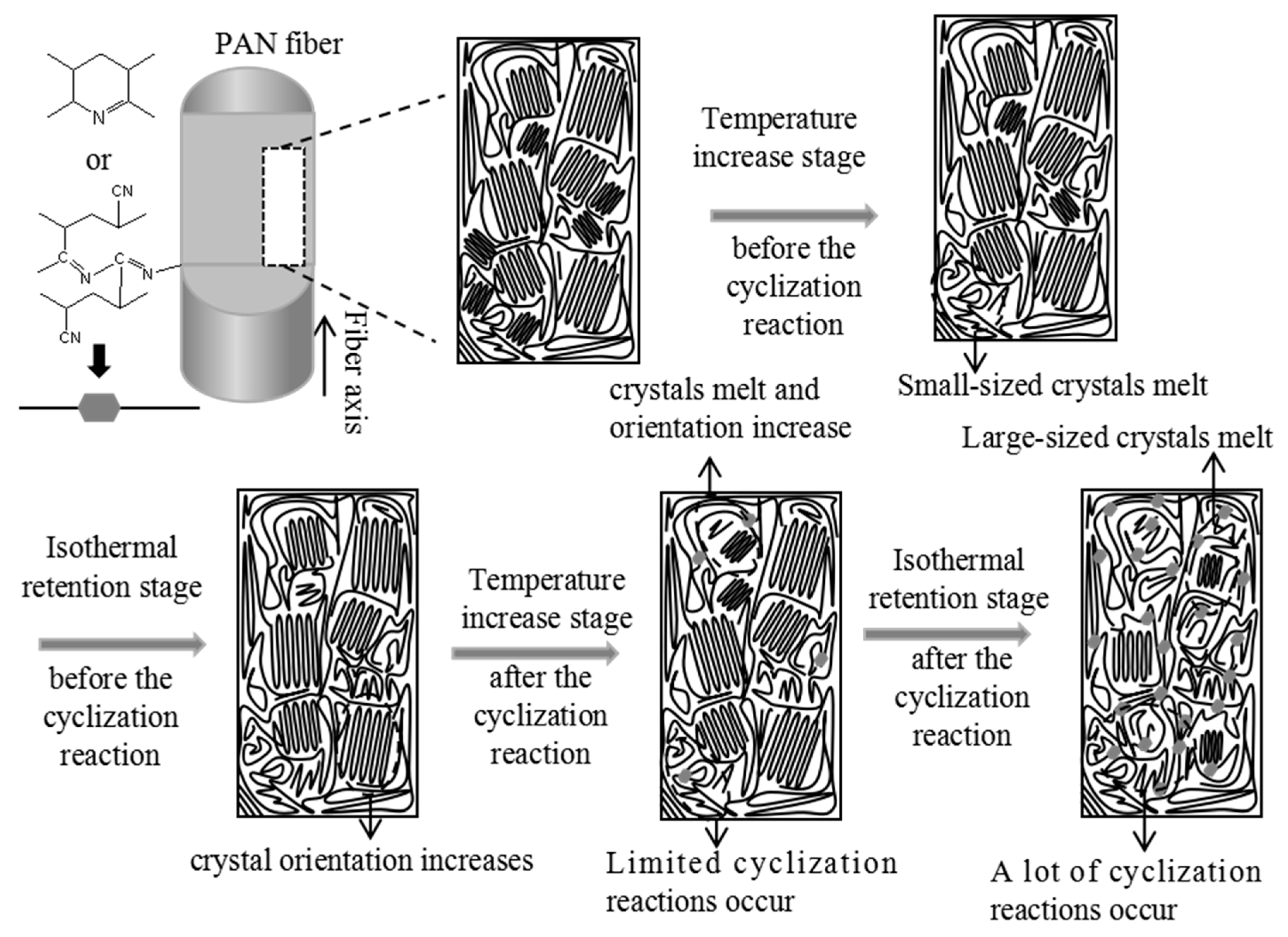
- Temperature increase stage before the cyclization reaction: The melting of crystals expands the volume of PAN fiber molecular chains, resulting in the shrinkage of micropores. Simultaneously, this melting process increases the molecular chain mobility of PAN fibers, leading to an enhanced orientation of micropores under fixed tension.
- Isothermal retention stage before the cyclization reaction: The crystal structure is well maintained during this stage, leading to minimal evolution of micropores. Moreover, no cyclization reaction occurs in the PAN fibers, allowing the PAN chains to maintain a relatively high mobility. Under the influence of fixed tension, the orientation of micropores increases.
- Temperature increase stage after the cyclization reaction: The average chord length (Lp), the orientation angle of the micropores (Beq), and the relative micropore volume (Vrel) decrease during this stage. This phenomenon can be attributed to the melting of crystals, which expands the PAN fiber molecular chains’ volume, reducing the average chord length and the relative micropore volume. Furthermore, only a few PAN molecular chains occur in the cyclization reactions, allowing the PAN molecular chains to maintain a high degree of mobility. Consequently, the orientation angle decreases, increasing the micropore orientation under fixed tension.
- Isothermal retention stage after the cyclization reaction: The average chord length, the relative micropore volume, and the orientation angle of the micropores increase. This is attributed to the prominent cyclization reaction that occurs in this stage, leading to intermolecular or intramolecular cross-linking of the molecular chains in PAN fibers. As a result, the distance between PAN fiber molecular chains decreases [46], leading to an increase in the average chord length of the micropores. Additionally, the generation of new, small molecular gases after the cyclization reaction contributes to the increase in the relative micropore volume. Furthermore, the degree of mobility of PAN fiber molecular chains decreases after the cyclization reaction, weakening the influence of fixed tension on micropores and thus reducing the overall orientation of the micropores.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, C.; Zhong, G.; Yue, Z.; Chen, G.; Zhang, L.; Vakili, A.; Wang, Y.; Zhu, L.; Liu, J.; Fong, H. Investigation of post-spinning stretching process on morphological, structural, and mechanical properties of electrospun polyacrylonitrile copolymer nanofibers. Polymer 2011, 52, 519–528. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.; Avilés, M.; del Río, J.; Ginés, J.; Pascual, J.; Pérez-Rodríguez, J. Thermal study of the effect of several solvents on polymerization of acrylonitrile and their subsequent pyrolysis. J. Anal. Appl. Pyrolysis 2001, 58, 155–172. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Zhao, G. Study on the structure evolution and temperature zone regulation mechanism of polyacrylonitrile fibers during pre-oxidation process. Iran. Polym. J. 2023, 32, 1511–1522. [Google Scholar] [CrossRef]
- Jing, M.; Wang, C.-G.; Wang, Q.; Bai, Y.-J.; Zhu, B. Chemical structure evolution and mechanism during pre-carbonization of PAN-based stabilized fiber in the temperature range of 350–600 °C. Polym. Degrad. Stab. 2007, 92, 1737–1742. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Cheng, L.; Wu, G. Radiation assisted pre-oxidation of polyacrylonitrile fiber: Graphite formation and lower crystal size revealed by 2D WAXD at a synchrotron facility. Polym. Degrad. Stab. 2020, 179, 109264. [Google Scholar] [CrossRef]
- Ge, Y.; Fu, Z.; Deng, Y.; Zhang, M.; Zhang, H. The effects of chemical reaction on the microstructure and mechanical properties of polyacrylonitrile (PAN) precursor fibers. J. Mater. Sci. 2019, 54, 12592–12604. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, G.; Liu, J.; Xiang, Y.; Guo, S. Interaction between thermal stabilization temperature program, oxidation reaction, and mechanical properties of polyacrylonitrile (PAN) based carbon fibers. Diam. Relat. Mater. 2024, 141, 110588. [Google Scholar] [CrossRef]
- Sun, X.; Song, J.; Zhang, J.; Liu, J.; Ke, H.; Wei, Q.; Cai, Y. Effects of chemical pre-treatment on structure and property of polyacrylonitrile based pre-oxidized fibers. J. Eng. Fibers Fabr. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Zhang, W.; Liu, W.; Yang, C.; Shen, R.; Wu, G. Significantly reduced pre-oxidation period of PAN fibers by continuous electron beam irradiation: Optimization by monitoring radical variation. Polym. Degrad. Stab. 2018, 158, 72–82. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Chen, J.; Li, X.; Zhao, Z.; Wang, X. New insights into orientation differences between skin and core regions of polyacrylonitrile fibers during pre-stabilization with the stretching process. Polymer 2020, 210, 123043. [Google Scholar] [CrossRef]
- Wu, S.; Gao, A.; Xu, L. Effect of In Situ Thermal Stretching during Oxidative Stabilization on the Orientation of Cyclized Ladder Structure and Its Carbon Fiber. Fibers Polym. 2018, 19, 1184–1193. [Google Scholar] [CrossRef]
- Jing, M.; Wang, C.-G.; Bai, Y.-J.; Zhu, B.; Wang, Y.-X. Effect of temperatures in the rearmost stabilization zone on structure and properties of PAN-based oxidized fibers. Polym. Bull. 2006, 58, 541–551. [Google Scholar] [CrossRef]
- Konstantopoulos, G.; Soulis, S.; Dragatogiannis, D.; Charitidis, C. Introduction of a methodology to enhance the stabilization process of PAN fibers by modeling and advanced characterization. Materials 2020, 13, 2749. [Google Scholar] [CrossRef]
- Soulis, S.; Dragatogiannis, D.A.; Charitidis, C.A. A novel methodology for designing thermal processes in order to optimize stabilization of polyacrylonitrile (PAN) fibers. Polym. Adv. Technol. 2020, 31, 1403–1413. [Google Scholar] [CrossRef]
- Dang, W.; Liu, J.; Wang, X.; Yan, K.; Zhang, A.; Yang, J.; Chen, L.; Liang, J. Structural transformation of polyacrylonitrile (PAN) fibers during rapid thermal pretreatment in nitrogen atmosphere. Polymers 2020, 12, 63. [Google Scholar] [CrossRef]
- Wang, G.; Lu, C.; Sun, T.; Li, Y. Accelerating the stabilization of polyacrylonitrile fibers by nitrogen pretreatment. J. Appl. Polym. Sci. 2022, 139, 52129. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Xu, L.; Guo, S. Study on structure evolution and reaction mechanism in microwave pre-oxidation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3562–3571. [Google Scholar] [CrossRef]
- Li, X.-Y.; Tian, F.; Gao, X.-P.; Bian, F.-G.; Li, X.-H.; Wang, J. WAXD/SAXS study and 2D fitting (SAXS) of the microstructural evolution of PAN-based carbon fibers during the pre-oxidation and carbonization process. New Carbon Mater. 2017, 32, 130–136. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Yu, X.-L.; Liu, X.-F.; Mao, Y.-Z.; Liu, R.-G.; Zhao, N.; Zhang, X.-L.; Xu, J. 2D SAXS/WAXD analysis of pan carbon fiber microstructure in organic/inorganic transformation. Chin. J. Polym. Sci. 2013, 31, 823–832. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Y.; Kai, Y.; Jin, R.-G. Structural evolution and kinetic study of high isotacticity poly(acrylonitrile) during isothermal pre-oxidation. Carbon Lett. 2011, 12, 229–235. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.-Z.; Song, L.; Wang, Y.; Li, S.; Shi, Y. Investigating the Effect of Fixation Tension on the Thermal Analysis Process and Crystal Structure of Polyacrylonitrile (PAN) Fibers. J. Macromol. Sci. Part B 2023, 1–18. [Google Scholar] [CrossRef]
- Nunna, S.; Maghe, M.; Fakhrhoseini, S.M.; Polisetti, B.; Naebe, M. A Pathway to Reduce Energy Consumption in the Thermal Stabilization Process of Carbon Fiber Production. Energies 2018, 11, 1145. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, T.; Wu, S.; Tong, Y.-J.; Gao, A.-J.; Xu, L.-H. Stretching Deformation Mechanism of Polyacrylonitrile-based Carbon Fiber Structure at High Temperatures. Fibers Polym. 2018, 19, 751–759. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Gao, Q.; Wang, Y.; Zhao, S.; Cui, B.; Yue, Y. Study on the relationship between chemical structure transformation and morphological change of polyacrylonitrile based preoxidized fibers. Eur. Polym. J. 2021, 159, 110742–110751. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R. New aspects in the oxidative stabilization of PAN-based carbon fibers: II. Carbon 1997, 35, 809–818. [Google Scholar] [CrossRef]
- Sun, T.; Hou, Y.; Wang, H. Mass DSC/TG and IR ascertained structure and color change of polyacrylonitrile fibers in air/nitrogen during thermal stabilization. J. Appl. Polym. Sci. 2010, 118, 462–468. [Google Scholar] [CrossRef]
- Thünemann, A.F.; Ruland, W. Microvoids in Polyacrylonitrile Fibers: A Small-Angle X-ray Scattering Study. Macromolecules 2000, 33, 1848–1852. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I. New aspects in the oxidative stabilization of PAN-based carbon fibers. Carbon 1996, 34, 1427–1445. [Google Scholar] [CrossRef]
- He, B.B. Introduction to two-dimensional X-ray diffraction. Powder Diffr. 2003, 18, 71–85. [Google Scholar] [CrossRef]
- He, B.B. Two-Dimensional X-ray Diffraction; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–469. [Google Scholar] [CrossRef]
- Kong, L.; Liu, H.; Cao, W.; Xu, L. PAN fiber diameter effect on the structure of PAN-based carbon fibers. Fibers Polym. 2014, 15, 2480–2488. [Google Scholar] [CrossRef]
- Tang, H.; Meng, F.; Liu, Y.; Jin, S.; Wang, X.; Gao, Z.; Che, X. Investigation of Voids in Polyacrylonitrile Fibers by USAXS and SAXS. Chem. Res. Chin. Univ. 2019, 35, 1070–1075. [Google Scholar] [CrossRef]
- Meek, N.; Penumadu, D. Nonlinear elastic response of pan based carbon fiber to tensile loading and relations to microstructure. Carbon 2021, 178, 133–143. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R.; Lahijani, J. Small-angle X-ray scattering in carbon fibers. J. Appl. Crystallogr. 1994, 27, 627–636. [Google Scholar] [CrossRef]
- Schaper, A.; Zenke, D.; Schulz, E.; Hirte, R.; Taege, M. Structure–property relationships of high-performance polyethylene fibres. Phys. Status Solidi A 1989, 116, 179–195. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Liu, J.; Liang, J.; Wang, X. Structure and tensile properties of carbon fibers based on electron-beam irradiated polyacrylonitrile fibers. J. Mater. Sci. 2020, 55, 4962–4969. [Google Scholar] [CrossRef]
- Sun, L.; Shang, L.; Xiao, L.; Zhang, M.; Ao, Y.; Li, M. The influence of stabilization efficiency on skin–core structure and properties of polyacrylonitrile fibers. J. Mater. Sci. 2020, 55, 3408–3418. [Google Scholar] [CrossRef]
- Yang, F.; Liu, W.; Yi, M.; Ran, L.; Ge, Y.; Peng, K. Effect of high temperature treatment on the microstructure and elastoplastic properties of polyacrylonitrile-based carbon fibers. Carbon 2020, 158, 783–794. [Google Scholar] [CrossRef]
- Chen, L.; Hao, L.; Liu, S.; Ding, G.; Sun, X.; Zhang, W.; Li, F.; Jiao, W.; Yang, F.; Xu, Z.; et al. Modulus distribution in polyacrylonitrile-based carbon fiber monofilaments. Carbon 2019, 157, 47–54. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Song, L.; Wang, Y.; Li, S.; Shi, Y. Research on Improving the Pre-Oxidation Process of the Excellent Mechanical Strength Carbon Fiber. Macromol. Chem. Phys. 2023, 224, 2300114. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Cao, W. Effect of stretching on the orientation structure and reaction behavior of PAN fiber during the thermal stabilization. Mater. Res. Express 2021, 8, 085603. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, B.; Liu, Y.; Li, B.; Zhang, H. Detailed cyclization pathways identification of polyacrylonitrile and poly (acrylonitrile-co-itaconic acid) by in situ FTIR and two-dimensional correlation analysis. Ind. Eng. Chem. Res. 2018, 57, 8348–8359. [Google Scholar] [CrossRef]
- Martin, S.; Liggat, J.; Snape, C. In situ NMR investigation into the thermal degradation and stabilisation of PAN. Polym. Degrad. Stab. 2001, 74, 407–412. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, C.; Zhang, A.; Yang, P. Structural characteristics of polyacrylonitrile (PAN) fibers during oxidative stabilization. Compos. Sci. Technol. 1987, 29, 33–44. [Google Scholar] [CrossRef]
- Demirel, T.; Rahman, M.; Tunçel, K.; Karacan, I. A Study on Structural Characterization of Thermally Stabilized PAN Precursor Fibers Impregnated with Ammonium Bromide before Carbonization Stage. Fibers Polym. 2022, 23, 3046–3057. [Google Scholar] [CrossRef]
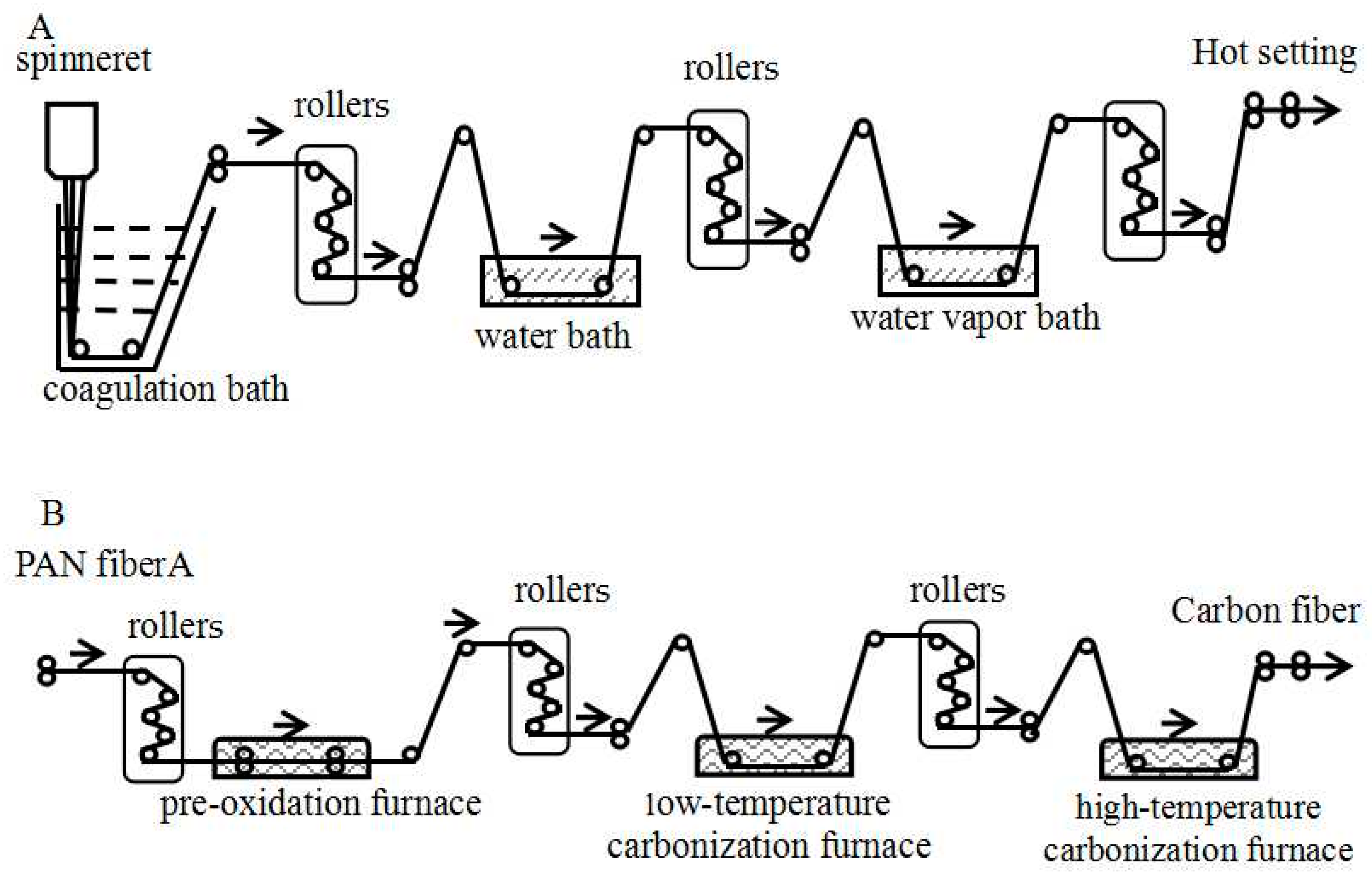










| Sample | Coagulation Draft Ratio | Water Bath Draft Ratio | Vapor Bath Draft Ratio |
|---|---|---|---|
| PAN fiber A | 2 | 2 | 2.2 |
| Carbon Fiber A | Toray T800 | Retention Time (min) | Atmosphere | |
|---|---|---|---|---|
| Pre-oxidation temperature (°C) | 180–270 | - | 70 | Air |
| low-temperature carbonization (°C) | 900 | - | 1 | N2 |
| high-temperature carbonization (°C) | 1500 | 1 | N2 | |
| Tensile strength/GPa | 4.9 GPa | 5.9 GPa | - | - |
| Tensile modulus/GPa | 230 GPa | 294 GPa | - | - |
| Sample | Pre-Oxidation Temperature (°C) | |||
|---|---|---|---|---|
| PAN fiber A | 180 | 220 | 240 | 270 |
| Sample | 2θ (°) | Hermans Orientation | Crystal Size (nm) | L (nm) | Beq (°) | Lp(nm) | L/Lp | Vrel |
|---|---|---|---|---|---|---|---|---|
| T800 | 25.7 | 0.84 | 1.64 | 191.92 | 20.24 | 2.49 | 76.94 | 7.74 |
| carbon fiber A | 25.7 | 0.78 | 1.60 | 96.96 | 13.05 | 4.35 | 22.31 | 4.90 |
| Sample | Crystal Size (nm) | Hermans Orientation | Temperature (°C) | Retention Time a (min) |
|---|---|---|---|---|
| PAN fiber A | 8.97 | 0.59 | 25 | - |
| PAN fiber A | 12.77 | 0.64 | 180 | - |
| PAN fiber A | 13.18 | 0.72 | 180 | 15 |
| PAN fiber A | 14.74 | 0.65 | 220 | - |
| PAN fiber A | 14.18 | 0.68 | 220 | 15 |
| PAN fiber A | 13.94 | 0.64 | 240 | - |
| PAN fiber A | 11.29 | 0.77 | 240 | 15 |
| PAN fiber A | 10.04 | 0.63 | 270 | - |
| PAN fiber A | 5.66 | 0.70 | 270 | 15 |
| Sample | L (nm) | Beq(°) | Lp (nm) | L/Lp | Vrel | Temperature (°C) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| PAN fiber A | 112.51 | 14.47 | 3.94 | 28.54 | 2.99 | 25 | - |
| PAN fiber A | 90.63 | 11.80 | 5.03 | 18.00 | 1.22 | 180 | - |
| PAN fiber A | 88.69 | 10.48 | 5.42 | 16.35 | 1.26 | 180 | 15 |
| PAN fiber A | 87.94 | 9.52 | 5.67 | 15.51 | 1.00 | 220 | - |
| PAN fiber A | 89.65 | 9.77 | 5.25 | 17.07 | 2.50 | 220 | 15 |
| PAN fiber A | 88.81 | 9.36 | 3.63 | 24.44 | 1.52 | 240 | - |
| PAN fiber A | 89.95 | 9.21 | 4.57 | 19.69 | 2.39 | 240 | 15 |
| PAN fiber A | 90.45 | 9.22 | 4.56 | 19.85 | 1.38 | 270 | - |
| PAN fiber A | 94.61 | 9.73 | 4.84 | 19.55 | 2.30 | 270 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Liu, L.; Song, L.; Li, S.; Wang, Y.; Shi, Y.; Wang, Y. In Situ Study and Improvement of the Temperature Increase and Isothermal Retention Stages in the Polyacrylonitrile (PAN) Fiber Pre-Oxidation Process. Polymers 2024, 16, 547. https://doi.org/10.3390/polym16040547
Cui Y, Liu L, Song L, Li S, Wang Y, Shi Y, Wang Y. In Situ Study and Improvement of the Temperature Increase and Isothermal Retention Stages in the Polyacrylonitrile (PAN) Fiber Pre-Oxidation Process. Polymers. 2024; 16(4):547. https://doi.org/10.3390/polym16040547
Chicago/Turabian StyleCui, Ye, Lizhi Liu, Lixin Song, Sanxi Li, Ying Wang, Ying Shi, and Yuanxia Wang. 2024. "In Situ Study and Improvement of the Temperature Increase and Isothermal Retention Stages in the Polyacrylonitrile (PAN) Fiber Pre-Oxidation Process" Polymers 16, no. 4: 547. https://doi.org/10.3390/polym16040547
APA StyleCui, Y., Liu, L., Song, L., Li, S., Wang, Y., Shi, Y., & Wang, Y. (2024). In Situ Study and Improvement of the Temperature Increase and Isothermal Retention Stages in the Polyacrylonitrile (PAN) Fiber Pre-Oxidation Process. Polymers, 16(4), 547. https://doi.org/10.3390/polym16040547






