Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.2.1. Preparation of Polymer Solutions
2.2.2. Calcite Crystal Incubation with Polyacrylamides
2.2.3. Atomic Force Microscopy (AFM)
2.2.4. MD Simulation Methods
3. Results and Discussion
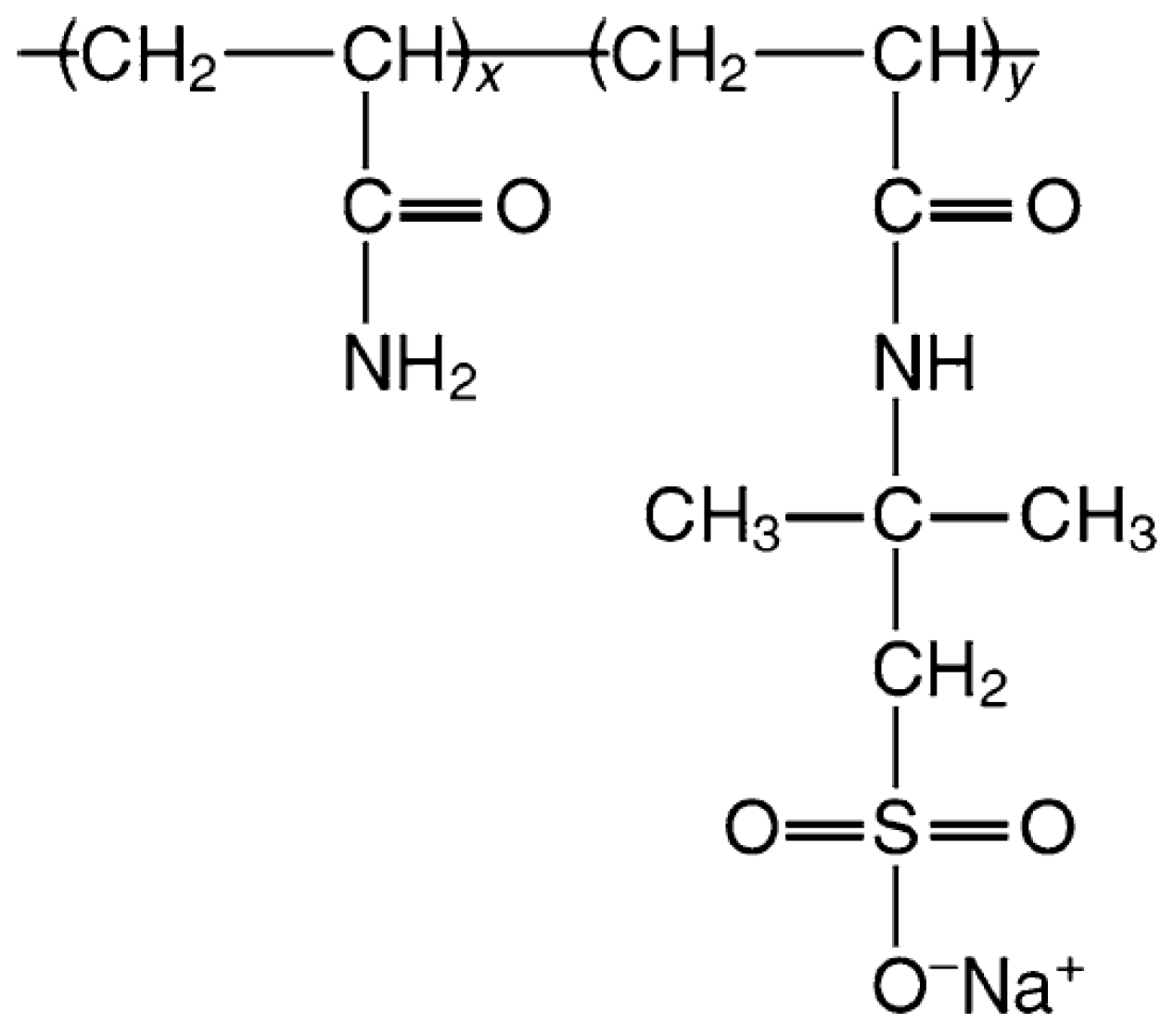
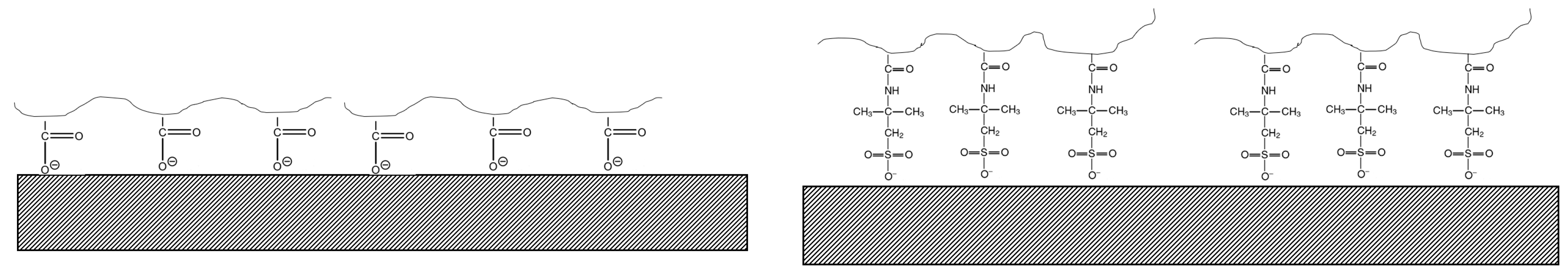
3.1. Effect of Functional Group
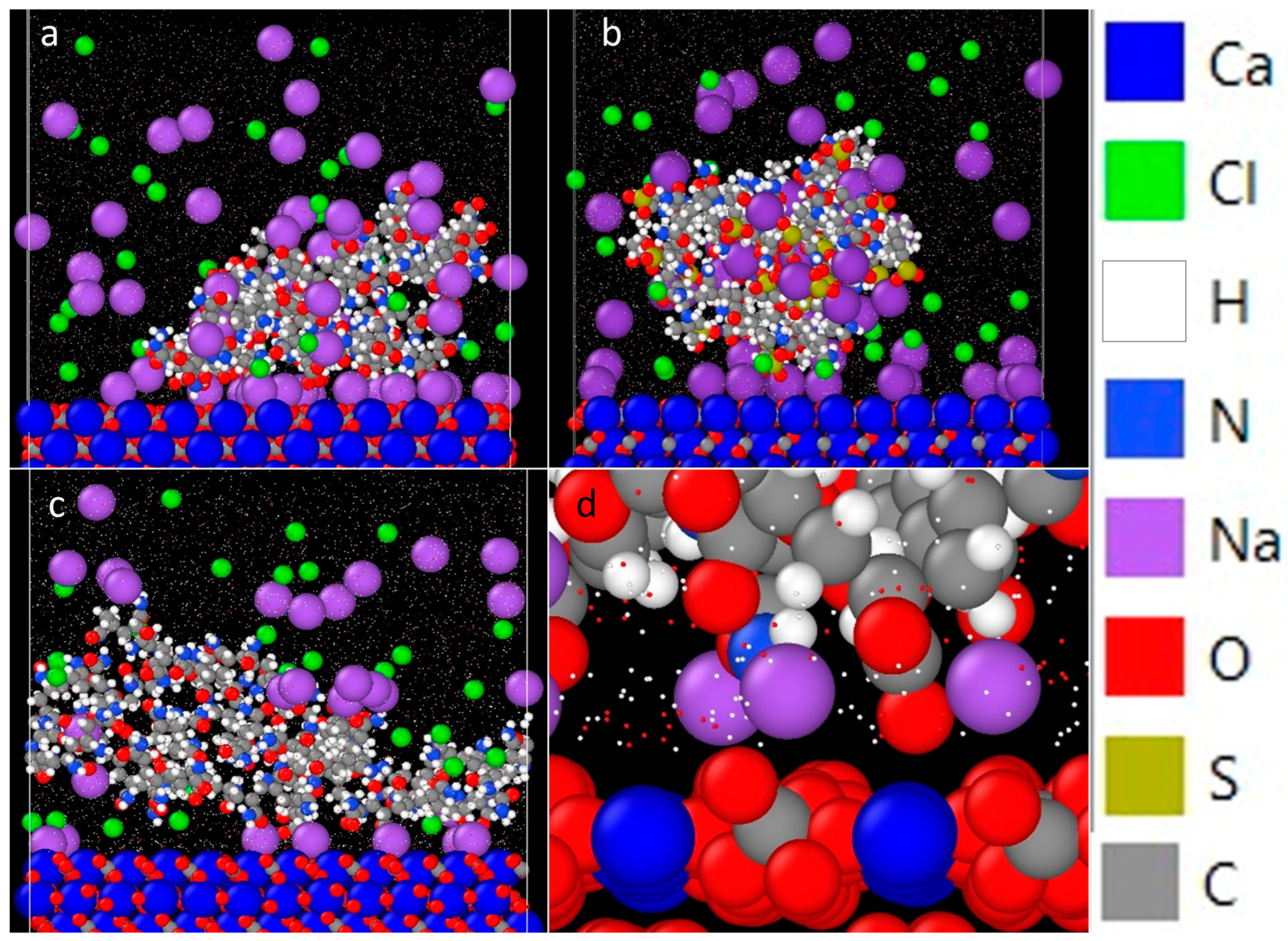
3.2. Effect of Charge Density
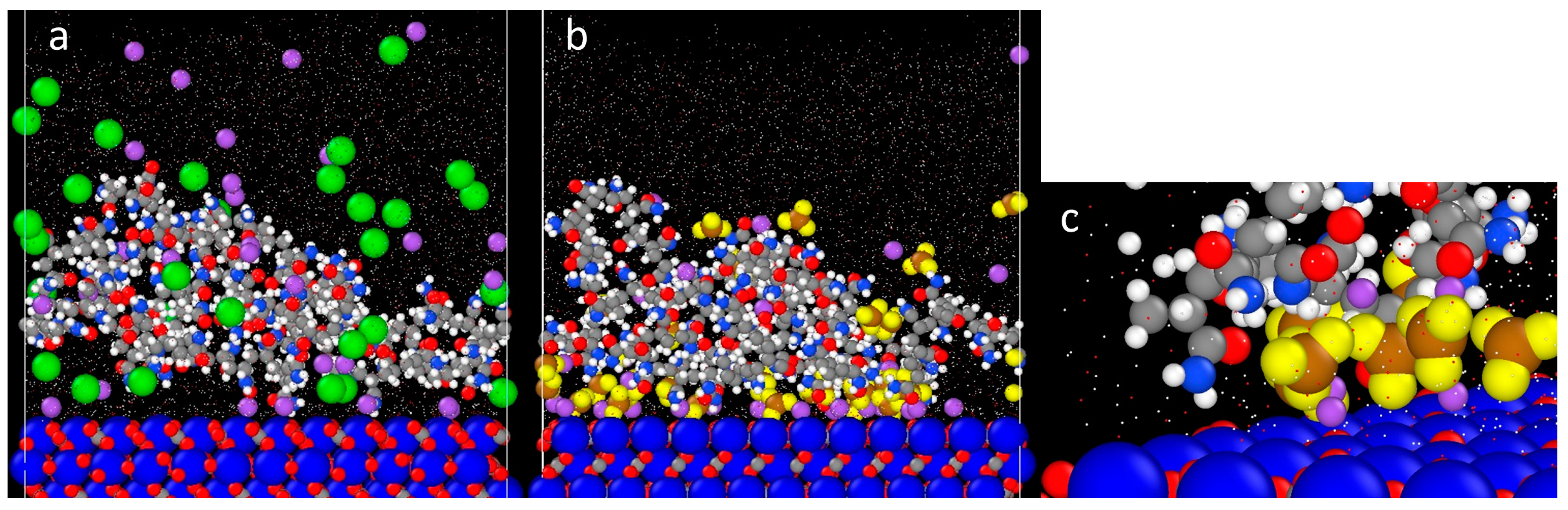
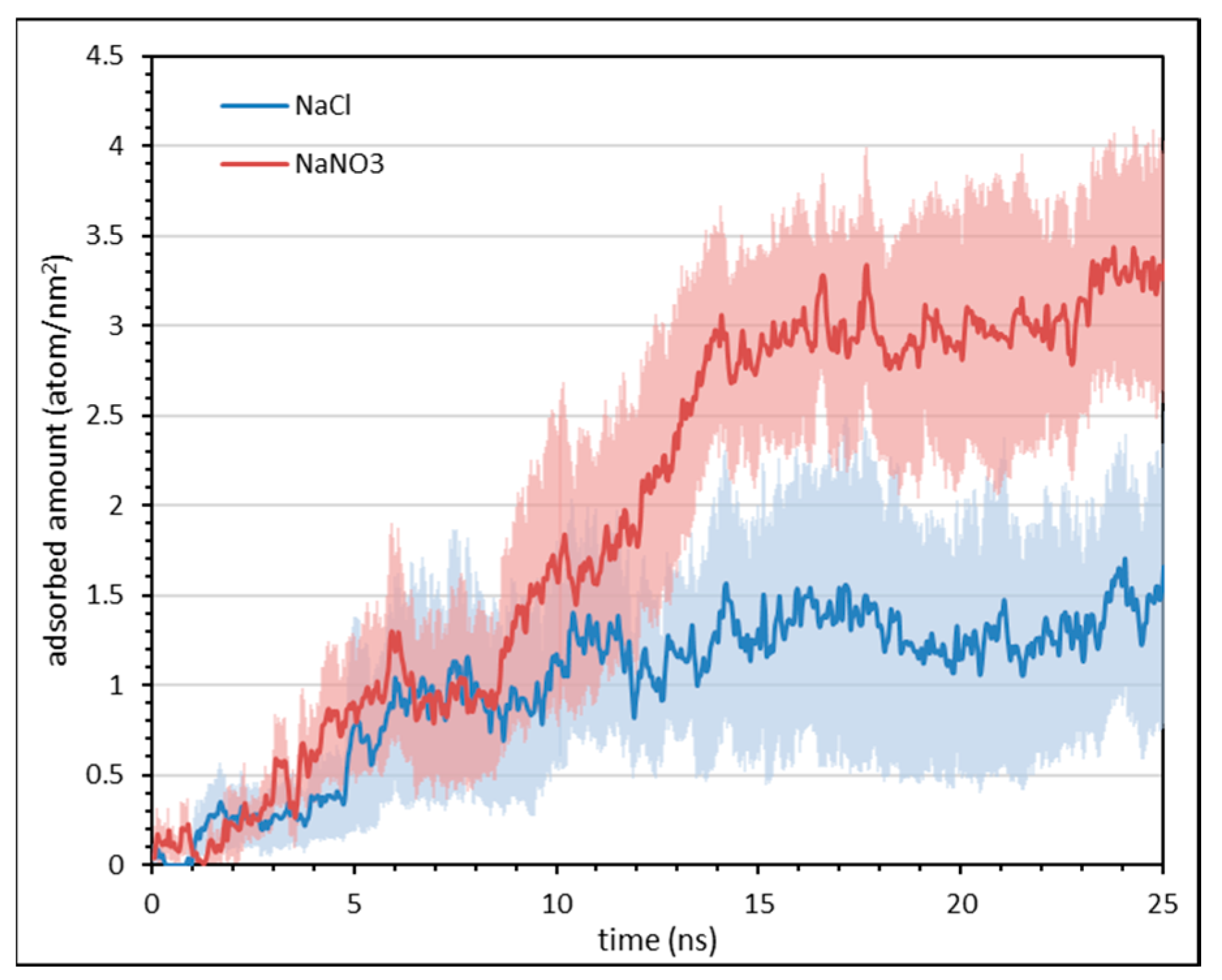
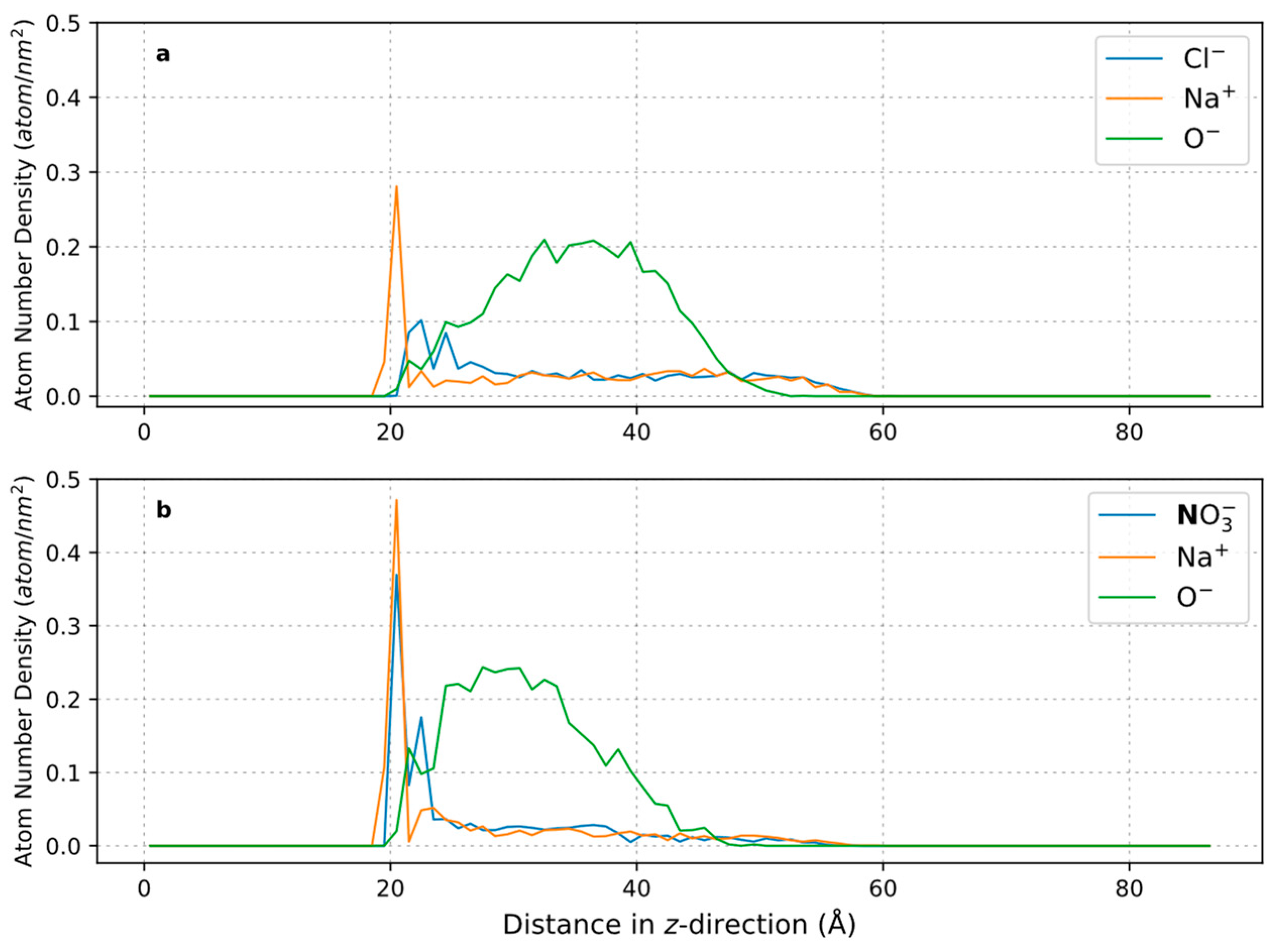
3.3. Effect of Salt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Li, G.; Li, Y.; Guo, J.; Zhou, S.; Yong, S.; Pan, B.; Bai, B. Adsorption of new hydrophobic polyacrylamide on the calcite surface. J. Appl. Polym. Sci. 2017, 134, 45314–45321. [Google Scholar] [CrossRef]
- Mahmood, A.; Vissapragada, B.; Alghamdi, A.H.; Allen, D.; Herron, M.; Carnegie, A.; Dutta, D.; Olesen, J.-R.; Chourasiya, R.D.; Logan, D.; et al. A Snapshot of Carbonate Reservoir Evaluation. Oilfield Rev. 2000, 12, 20–41. [Google Scholar]
- Nouri, A.; Vaziri, H.; Belhaj, H.; Islam, R. Effect of Volumetric Failure on Sand Production in Oil-Wellbores. SPE 80448. In Proceedings of the SPE-Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 9–11 September 2003; pp. 86–93. [Google Scholar]
- Talaghat, M.R.; Esmaeilzadeh, F.; Mowla, D. Sand production control by chemical consolidation. J. Pet. Sci. Eng. 2009, 67, 34–40. [Google Scholar] [CrossRef]
- Mowar, S.; Zaman, M.; Stearns, D.W.; Roegiers, J.C. Micro-mechanisms of pore collapse in limestone. J. Pet. Sci. Eng. 1996, 15, 221–235. [Google Scholar] [CrossRef]
- Soares, A.C.; Altoe, J.E.; Bedrikovetsky, P.; Ferreira, F.H. Formation Damage Due To Pore Collapse During Pressure Depletion-SPE 82254. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 13–14 May 2003. [Google Scholar]
- Zaman, M.; Roegiers, J.C.; Abdulraheem, A.; Azeemuddin, M. Pore Collapse in Weakly Cemented and Porous Rocks. J. Energy Resour. Technol. 1994, 116, 97–103. [Google Scholar] [CrossRef]
- Lee, R.Y. Development of Sand Agglomeration Formulation for Oil & Gas Well Applications to Reduce the Production of Fine Particulates. Ph.D. Thesis, Imperial College London, London, UK, 2019. [Google Scholar]
- Kurawle, I.B.; Mahalle, N.A.; Kaul, M.; Nair, A.V.; Kulkarni, N.J. Silanol Resin consolidation system for deepwater completions and production optimisation. In Proceedings of the 8th European Formation Damage Conference, Scheveningen, The Netherlands, 27–29 May 2009. [Google Scholar]
- Aggour, M.A.; Abu Khamsin, S.A.; Osman, E.S.A. A New method of Sand Control: The Process and Its First Field Implementation. In Proceedings of the SPE/IADC Middle East Drilling and Technology Conference, Cairo, Egypt, 22–24 October 2007. [Google Scholar]
- Alakbari, F.S.; Mohyaldinn, M.E.; Muhsan, A.S.; Hasan, N.; Ganat, T. Chemical sand consolidation: From polymers to nanoparticles. Polymers 2020, 12, 1069. [Google Scholar] [CrossRef]
- Nguyen, P.; Sanders, M. Chapter 6-Sand control completion using in-situ resin consolidation. In Flow Assurance; Wang, Q., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2022; Volume 1, pp. 443–501. [Google Scholar]
- Al-Hashmi, A.R.; Luckham, P.F.; Heng, J.Y.Y.; Al-Maamari, R.S.; Zaitoun, A.; Al-Sharji, H.H.; Al-Wehaibi, T.K. Adsorption of High-Molecular-Weight EOR Polymers on Glass Surfaces Using AFM and QCM-D. Energy Fuels 2013, 27, 2437–2444. [Google Scholar] [CrossRef]
- Parsa, S.; Santanach-Carreras, E.; Xiao, L.; Weitz, D.A. Origin of anomalous polymer-induced fluid displacement in porous media. Phys. Rev. Fluids 2020, 5, 022001. [Google Scholar] [CrossRef]
- Moulin, P.; Roques, H. Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 2003, 261, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Tweheyo, M.T.; Sjøblom, J.; Øye, G. Surface characterization of model, outcrop, and reservoir samples in low salinity aqueous solutions. J. Dispers. Sci. Technol. 2011, 32, 519–531. [Google Scholar] [CrossRef]
- Sohal, M.A.; Thyne, G.; Søgaard, E.G. Review of recovery mechanisms of ionically modified waterflood in carbonate reservoirs. Energy Fuels 2016, 30, 1904–1914. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Somasundaran, O.; Agar, G.E. The Zero Point of Charge of Calcite. J. Colloid Interface Sci. 1967, 24, 433–440. [Google Scholar] [CrossRef]
- Pierre, A.; Lamarche, J.M.; Mercier, R.; Foissy, A.; Persello, J. Calcium as Potential Determining Ion in Aqueous Calcite Suspensions. J. Dispers. Sci. Technol. 1990, 11, 611–635. [Google Scholar] [CrossRef]
- Fimbel, P.; Siffert, B. Interaction of calcium carbonate (calcite) with cellulose fibres in aqueous medium. Colloids Surf. 1986, 20, 1–16. [Google Scholar] [CrossRef]
- Renpu, W. Chapter 1-Basis of Well Completion Engineering. In Advanced Well Completion Engineering, 3rd ed.; Renpu, W., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2011; pp. 1–74. [Google Scholar]
- Laguecir, A.; Ulrich, S.; Labille, J.; Fatin-Rouge, N.; Stoll, S.; Buffle, J. Size and pH effect on electrical and conformational behavior of poly(acrylic acid): Simulation and experiment. Eur. Polym. J. 2006, 42, 1135–1144. [Google Scholar] [CrossRef]
- Rasteiro, M.G.; Pinheiro, I.; Ahmadloo, H.; Hunkeler, D.; Garcia, F.A.P.; Ferreia, P.; Wandrey, C. Correlation between flocculation and adsorption of cationic polyacrylamides on precipitated calcium carbonate. Chem. Eng. Res. Des. 2015, 95, 298–306. [Google Scholar] [CrossRef]
- Rice, S.A.; Nagasawa, M. Polyelectrolyte Solutions, a Theoretical Introduction; Academic Press: London, UK, 1961. [Google Scholar]
- Zhou, Y.; Yu, H.; Wanless, E.; Jameson, G.; Franks, G.V. Influence of polymer charge on the shear yield stress of silica aggregated with adsorbed cationic polymers. J. Colloid Interface Sci. 2009, 336, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.H.; Matar, O.K.; Müller, E.A.; Maung, M.T.M.; Luckham, P.F. Adsorption of Hydrolysed Polyacrylamide onto Calcium Carbonate. Polymers 2022, 14, 405. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Yin, G.; Han, M.; Xu, Z. Anions bonded on the supramolecular hydrogel surface as the growth center of biominerals. Colloid Surf. B 2008, 66, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Mahmood, S.; Tan, I.; Bharadwaj, A.; Hematpur, H. Experimental investigation of the effect of different process variables on the viscosity of sulfonated polyacrylamide copolymers. J. Pet. Explor. Prod. Technol. 2016, 7, 87–101. [Google Scholar] [CrossRef]
- Rashidi, M.; Blokhus, A.M.; Skauge, A. Viscosity and retention of sulfonated polyacrylamide polymers at high temperature. J. Appl. Polym. Sci. 2011, 119, 3623–3629. [Google Scholar] [CrossRef]
- Rashidi, M.; Sandvik, S.; Blokhus, A.M.; Skauge, A. Static and Dynamic Adsorption of Salt Tolerant Polymers. In Proceedings of the IOR 2009-15th European Symposium on Improved Oil Recovery, Paris, France, 27–29 April 2009. [Google Scholar]
- Sorbie, K.S. Polymer retention in porous media. In Polymer-Improved Oil Recovery; Springer: Dordrecht, TheNetherlands, 1991. [Google Scholar]
- Thomas, M.M.; Clouse, J.A.; Longo, J.M. Adsorption of organic compounds on carbonate minerals 1. Model compounds and their influence on mineral wettability. Chem. Geol. 1993, 109, 201–213. [Google Scholar] [CrossRef]
- Salehi, M.B.; Moghadam, A.M.; Marandi, S.Z. Polyacrylamide hydrogel application in sand control with compressive strength testing. Pet. Sci. 2019, 16, 94–104. [Google Scholar] [CrossRef]
- Sodeifian, G.; Daroughegi, R.; Aalaie, J. Study of adsorptive behavior of sulfonated polyacrylamide onto carbonate rock particles to enhance oil recovery. Korean J. Chem. Eng. 2015, 32, 2484–2491. [Google Scholar] [CrossRef]
- Samoshina, Y.; Diaz, A.; Becker, Y.; Nylander, T.; Lindman, B. Adsorption of cationic, anionic and hydrophobically modified polyacrylamides on silica surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2003, 231, 195–205. [Google Scholar] [CrossRef]
- Shubin, V.; Linse, P. Effect of Electrolytes on Adsorption of Cationic Polyacrylamide on Silica: Ellipsometric Study and Theoretical Modeling. J. Phys. Chem. 1995, 99, 1285–1291. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Adsorption behavior of poly(N,N-dimethylacrylamide-co-na 2-acrylamido-2-methylpropanesulfonate) on sand surface. J. Appl. Polym. Sci. 2004, 91, 2482–2490. [Google Scholar] [CrossRef]
- Vorob’ev, P.D.; Krut’ko, N.P.; Vorob’eva, E.V.; Strnadova, N. Successive adsorption of polyacrylamide compounds from electrolyte solutions on the surface of kaolinitic clay particles. Colloid J. 2008, 70, 148–151. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Koleini, M.M.; Ayatollahi, S. Molecular dynamics simulation investigation of hexanoic acid adsorption onto calcite (101¯4)surface. Fluid Phase Equilibria 2015, 387, 24–31. [Google Scholar] [CrossRef]
- Gurina, D.; Surov, O.; Voronova, M.; Zakharov, A. Molecular Dynamics Simulation of Polyacrylamide Adsorption on Cellulose Nanocrystals. Nanomaterials 2020, 10, 1256. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, S.; Wang, X.; Guo, M.; Wang, Y.; Wang, D. Molecular dynamics simulation of oil detachment from calcite surface in aqueous surfactant solution. Comput. Theor. Chem. 2016, 1092, 82–89. [Google Scholar] [CrossRef]
- Krishna, S.; Sreedhar, I.; Patel, C.M. Molecular dynamics simulation of polyamide-based materials—A review. Comput. Mater. Sci. 2021, 200, 110853. [Google Scholar] [CrossRef]
- Willemsen, J.A.R.; Myneni, S.C.B.; Bourg, I.C. Molecular Dynamics Simulations of the Adsorption of Phthalate Esters on Smectite Clay Surfaces. J. Phys. Chem. C 2019, 123, 13624–13636. [Google Scholar] [CrossRef]
- Carnal, F.; Stoll, S. Adsorption of weak polyelectrolytes on charged nanoparticles. Impact of salt valency, pH, and nanoparticle charge density. Monte Carlo simulations. J. Phys. Chem. B 2011, 115, 12007–12018. [Google Scholar] [CrossRef]
- Carrillo, J.-M.Y.; Dobrynin, A.V. Molecular Dynamics Simulations of Polyelectrolyte Adsorption. Langmuir 2007, 23, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Polyacrylamide adsorption on (1 0 1) quartz surfaces in saltwater for a range of pH values by molecular dynamics simulations. Miner. Eng. 2021, 162, 106741. [Google Scholar] [CrossRef]
- Ali, A.; Le, T.T.B.; Striolo, A.; Cole, D.R. Salt Effects on the Structure and Dynamics of Interfacial Water on Calcite Probed by Equilibrium Molecular Dynamics Simulations. J. Phys. Chem. C 2020, 124, 24822–24836. [Google Scholar] [CrossRef]
- Chen, H.; Panagiotopoulos, A.Z.; Giannelis, E.P. Atomistic molecular dynamics simulations of carbohydrate-calcite interactions in concentrated brine. Langmuir 2015, 31, 2407–2413. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, H.; Tang, T. Enhanced Adsorption of Anionic Polymer on Montmorillonite by Divalent Cations and the Effect of Salinity. J. Phys. Chem. A 2021, 125, 1025–1035. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, R.I.; Fawell, P.D.; Toledo, P.G. Use of molecular dynamics to study the conformation of an anionic polyelectrolyte in saline medium and its adsorption on a quartz surface. Miner. Eng. 2018, 129, 102–105. [Google Scholar] [CrossRef]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Molecular Dynamics Simulations of Quartz (101)–Water and Corundum (001)–Water Interfaces: Effect of Surface Charge and Ions on Cation Adsorption, Water Orientation, and Surface Charge Reversal. J. Phys. Chem. C 2017, 121, 25271–25282. [Google Scholar] [CrossRef]
- Quezada, G.R.; Toro, N.; Saavedra, J.; Robles, P.; Salazar, I.; Navarra, A.; Jeldres, R.I. Molecular Dynamics Study of the Conformation, Ion Adsorption, Diffusion, and Water Structure of Soluble Polymers in Saline Solutions. Polymers 2021, 13, 3550. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, X.; Ungerer, P.; Leblanc, B.; Saxe, P.; Wimmer, E. Automatic and Systematic Atomistic Simulations in the MedeA® Software Environment: Application to EU-REACH. Oil Gas Sci. Technol.-Rev. D’ifp Energ. Nouv. 2014, 70, 405–417. [Google Scholar] [CrossRef]
- Lew, J.H.; Matar, O.K.; Müller, E.A.; Luckham, P.F.; Sousa Santos, A.; Myo Thant, M.M. Atomic Force Microscopy of Hydrolysed Polyacrylamide Adsorption onto Calcium Carbonate. Polymers 2023, 15, 4037. [Google Scholar] [CrossRef] [PubMed]
- Denys, K.F.J. Flow of Polymer Solutions through Porous Media. Ph.D. Thesis, Delft University, Delft, The Netherland, 2003. [Google Scholar]
- Ekanem, E.M.; Rücker, M.; Yesufu-Rufai, S.; Spurin, C.; Ooi, N.; Georgiadis, A.; Berg, S.; Luckham, P.F. Novel adsorption mechanisms identified for polymer retention in carbonate rocks. JCIS Open 2021, 4, 100026. [Google Scholar] [CrossRef]
- Materials, D. MedeA 3.5 (Materials Exploration and Design Analysis); Materials Design Inc.: San Diego, CA, USA, 2022. [Google Scholar]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Hue, K.Y.; Lew, J.H.; Myo Thant, M.M.; Matar, O.K.; Luckham, P.F.; Müller, E.A. Molecular Dynamics Simulation of Polyacrylamide Adsorption on Calcite. Molecules 2023, 28, 6367. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Impact of Molecular Structure on Zeta Potential and Adsorbed Conformation of .ALPHA.Allyl.OMEGA.-Methoxypolyethylene Glycol-Maleic Anhydride Superplasticizers. J. Adv. Concr. Technol. 2006, 4, 233–239. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, W.; Jia, Z.; Zhou, C.; Guo, M.; Wang, Y. Conformation and adsorption behavior of associative polymer for enhanced oil recovery using single molecule force spectroscopy. J. Polym. Res. 2014, 21, 523. [Google Scholar] [CrossRef]
- Sun, W.; Long, J.; Xu, Z.; Masliyah, J.H. Study of Al(OH)3−Polyacrylamide-Induced Pelleting Flocculation by Single Molecule Force Spectroscopy. Langmuir 2008, 24, 14015–14021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gan, Y.; Wanless, E.J.; Jameson, G.J.; Franks, G.V. Interaction Forces between Silica Surfaces in Aqueous Solutions of Cationic Polymeric Flocculants: Effect of Polymer Charge. Langmuir 2008, 24, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashmi, A.R.; Luckham, P.F. Characterization of the adsorption of high molecular weight non-ionic and cationic polyacrylamide on glass from aqueous solutions using modified atomic force microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 142–148. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Jeldres, M.; Gálvez, E.; Robles, P.; Alvarado, O.; Toledo, P.G.; Jeldres, R.I. Estimating the Shear Resistance of Flocculated Kaolin Aggregates: Effect of Flocculation Time, Flocculant Dose, and Water Quality. Polymers 2022, 14, 1381. [Google Scholar] [CrossRef]
- Al-Hashmi, A.R.; Luckham, P.F.; Al-Maamari, R.S.; Zaitoun, A.; Al-Sharji, H.H. The role of hydration degree of cations and anions on the adsorption of high-molecular-weight nonionic polyacrylamide on glass surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 91–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |



| Polymers | Steric Repulsion Distance (nm) | Batch | Adhesion (pN) | Interaction Energy (aJ) | ||
|---|---|---|---|---|---|---|
| Average | Peak | Average | Peak | |||
| AN125 | 30–70 | 1 | 312 ± 130 | 253 ± 40 | 24 ± 14 | 14 ± 3 |
| 2 | 157 ± 102 | 83 ± 20 | 12 ± 9 | 7 ± 2 | ||
| Polymers | Steric Repulsion Distance (nm) | Batch | Adhesion (pN) | Interaction Energy (aJ) | ||
|---|---|---|---|---|---|---|
| Average | Peak | Average | Peak | |||
| AN910 | 40–90 | 1 | 41 ± 30 | 22 ± 4 | 2.8 ± 2.4 | 0.09 ± 0.05 |
| 2 | 55 ± 33 | 41 ± 4 | 1.7 ± 1.5 | 0.12 ± 0.1 | ||
| Polymers | Steric Repulsion Distance (nm) | Batch | Adhesion (pN) | Interaction Energy (aJ) | ||
|---|---|---|---|---|---|---|
| Average | Peak | Average | Peak | |||
| AN125 | 50–90 | 1 | 169 ± 78 | 103 ± 20 | 17 ± 8.1 | 12 ± 2 |
| 2 | 71 ± 40 | 23 ± 10 | 4 ± 3.7 | 0.42 ± 0.3 | ||
| AN910 | 20–30 | 1 | 119 ± 49 | 89 ± 10 | 12 ± 6 | 10 ± 2 |
| 2 | 120 ± 66 | 55 ± 10 | 6 ± 3 | 3.6 ± 1 | ||
| Salt Type | Batch | Adhesion (pN) | Interaction Energy (aJ) | ||
|---|---|---|---|---|---|
| Average | Peak | Average | Peak | ||
| NaCl | 1 | 119 ± 49 | 89 ± 10 | 12 ± 6 | 10 ± 2 |
| 2 | 120 ± 66 | 55 ± 10 | 6 ± 3 | 3.6 ± 1 | |
| NaNO3 | 1 | 212 ± 113 | 183 ± 20 | 20 ± 11 | 11 ± 2 |
| 2 | 167 ± 84 | 157 ± 20 | 13 ± 8.7 | 7.4 ± 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lew, J.H.; Hue, K.Y.; Matar, O.K.; Müller, E.A.; Luckham, P.F.; Santos, A.S.; Myo Thant, M.M. Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate. Polymers 2024, 16, 494. https://doi.org/10.3390/polym16040494
Lew JH, Hue KY, Matar OK, Müller EA, Luckham PF, Santos AS, Myo Thant MM. Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate. Polymers. 2024; 16(4):494. https://doi.org/10.3390/polym16040494
Chicago/Turabian StyleLew, Jin Hau, Keat Yung Hue, Omar K. Matar, Erich A. Müller, Paul F. Luckham, Adrielle Sousa Santos, and Maung Maung Myo Thant. 2024. "Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate" Polymers 16, no. 4: 494. https://doi.org/10.3390/polym16040494
APA StyleLew, J. H., Hue, K. Y., Matar, O. K., Müller, E. A., Luckham, P. F., Santos, A. S., & Myo Thant, M. M. (2024). Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate. Polymers, 16(4), 494. https://doi.org/10.3390/polym16040494








