Synergistic Self-Healing Enhancement in Multifunctional Silicone Elastomers and Their Application in Smart Materials
Abstract
1. Introduction
2. General Characteristics of Self-Healing Mechanisms in Silicone Elastomers
2.1. Extrinsic S-H of Silicone Elastomers
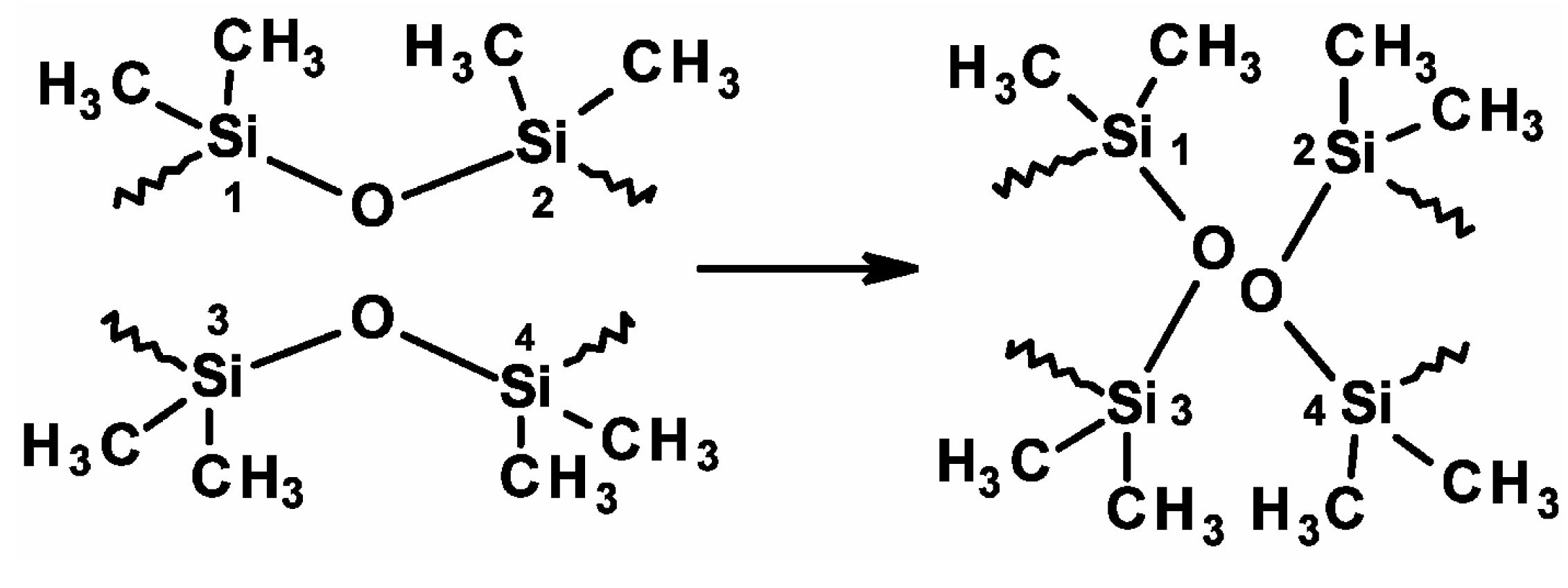
2.2. Intrinsic S-H of Polysiloxane Materials
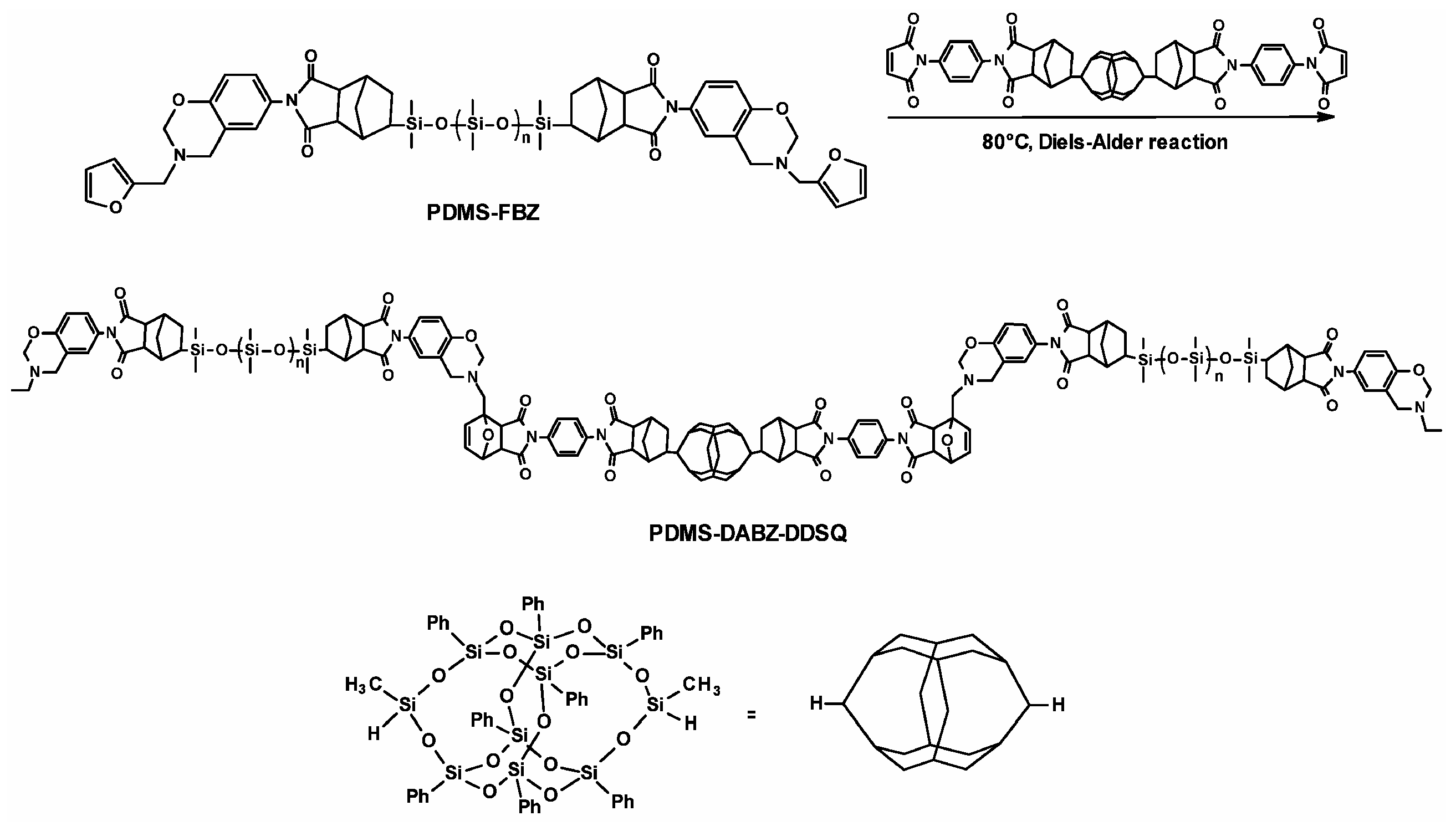
2.2.1. Polysiloxanes Self-Healed through Reactions of Diels–Alder Cycloaddition
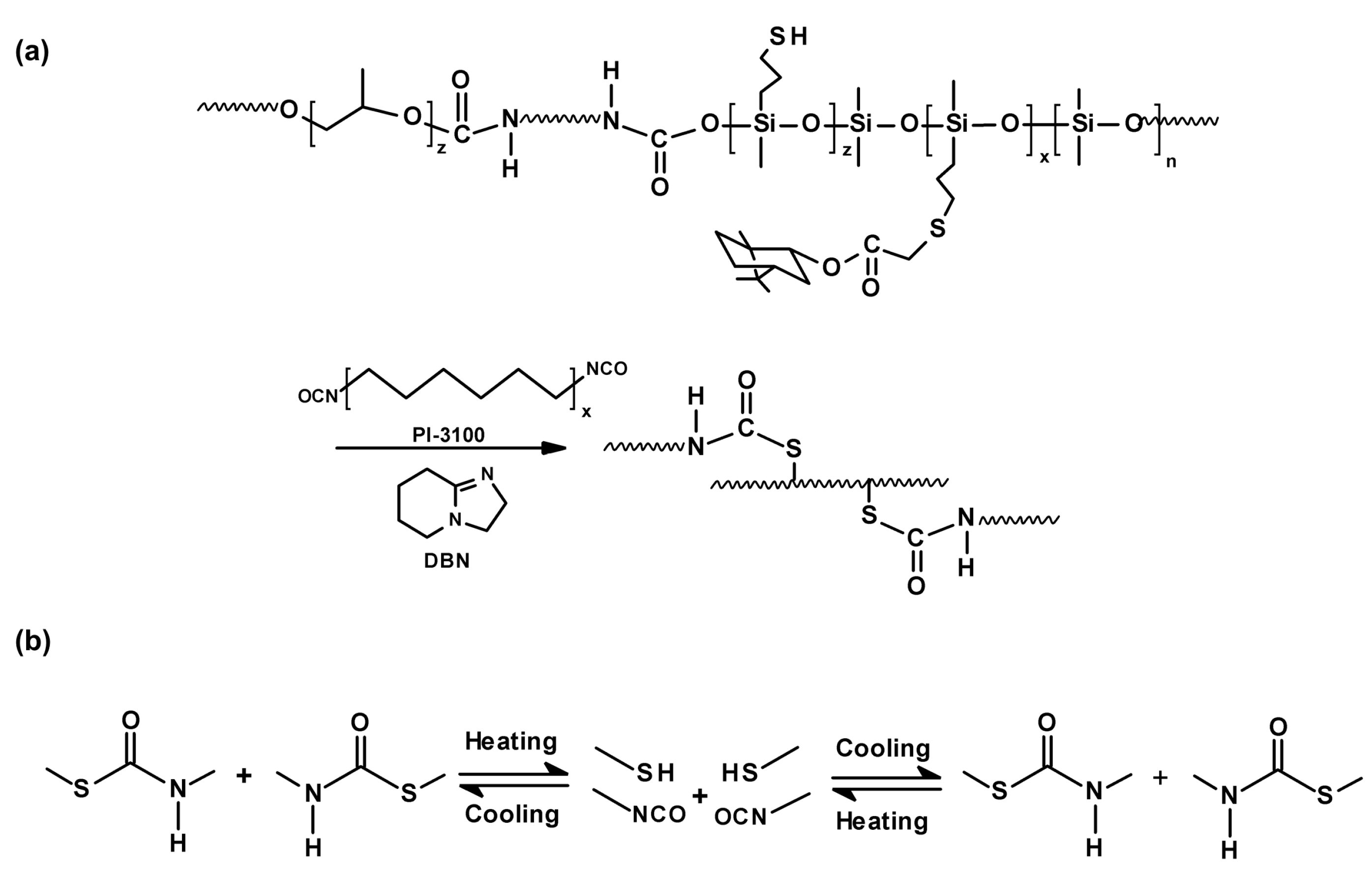
2.2.2. Silicone Self-Healing via Reversible Thiol Coupling Reactions
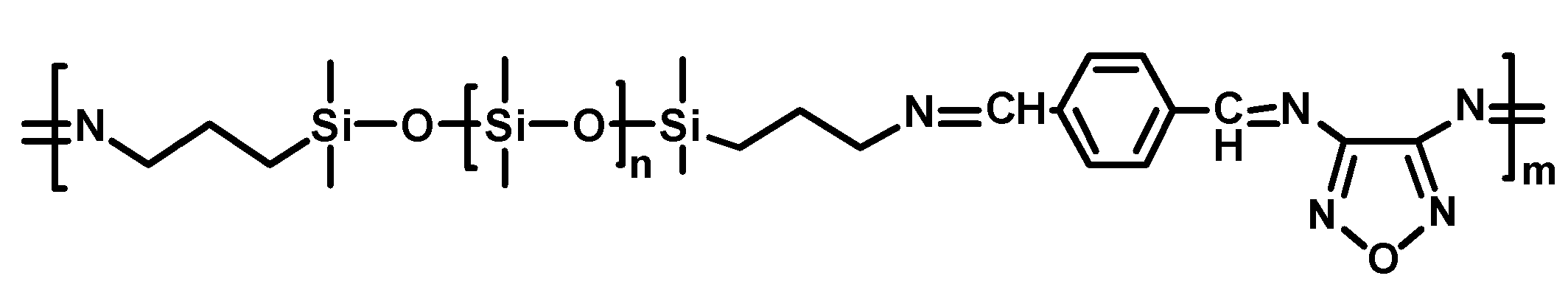
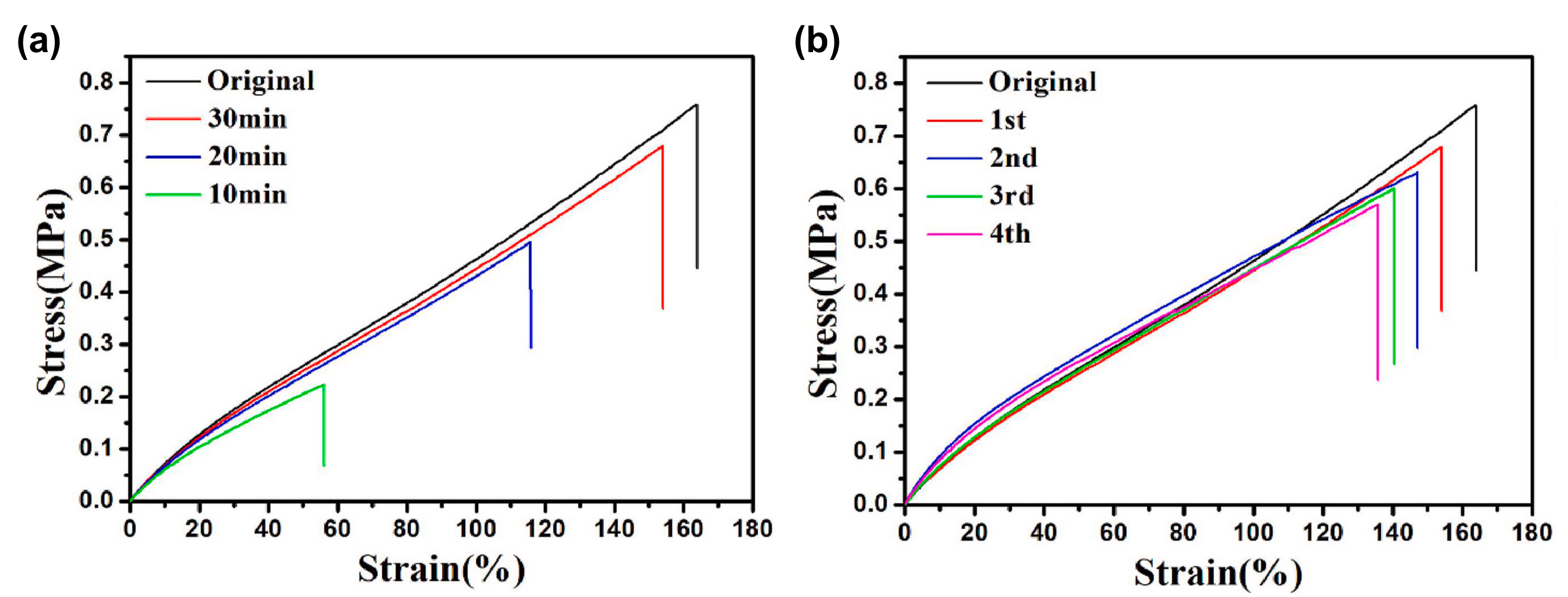
2.2.3. Self-Healing of Silicones Using Schiff Base Chemistry
2.2.4. Silicone Elastomers’ Autoregeneration through Acid–Base Ionic Interactions
2.2.5. Silicone Elastomers Self-Healed by Means of Hydrogen Bonding
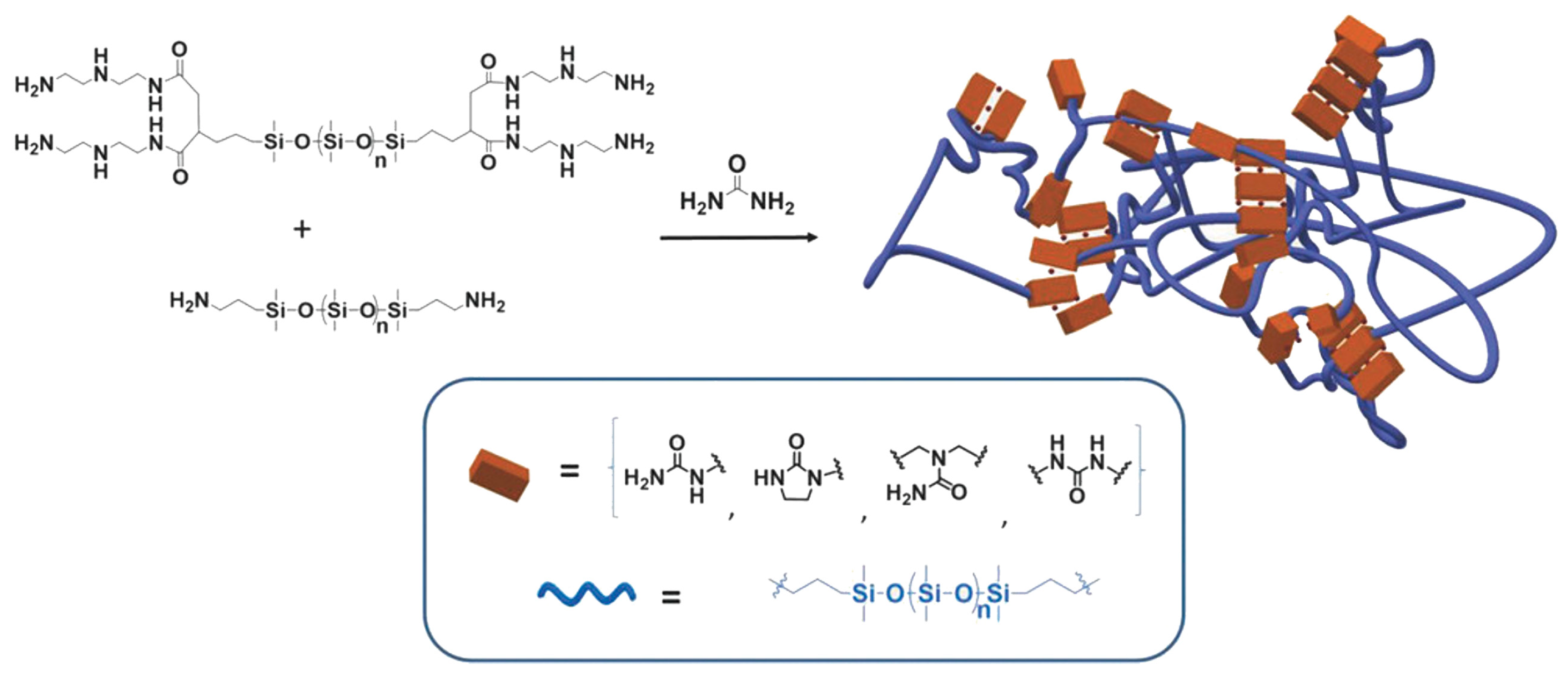
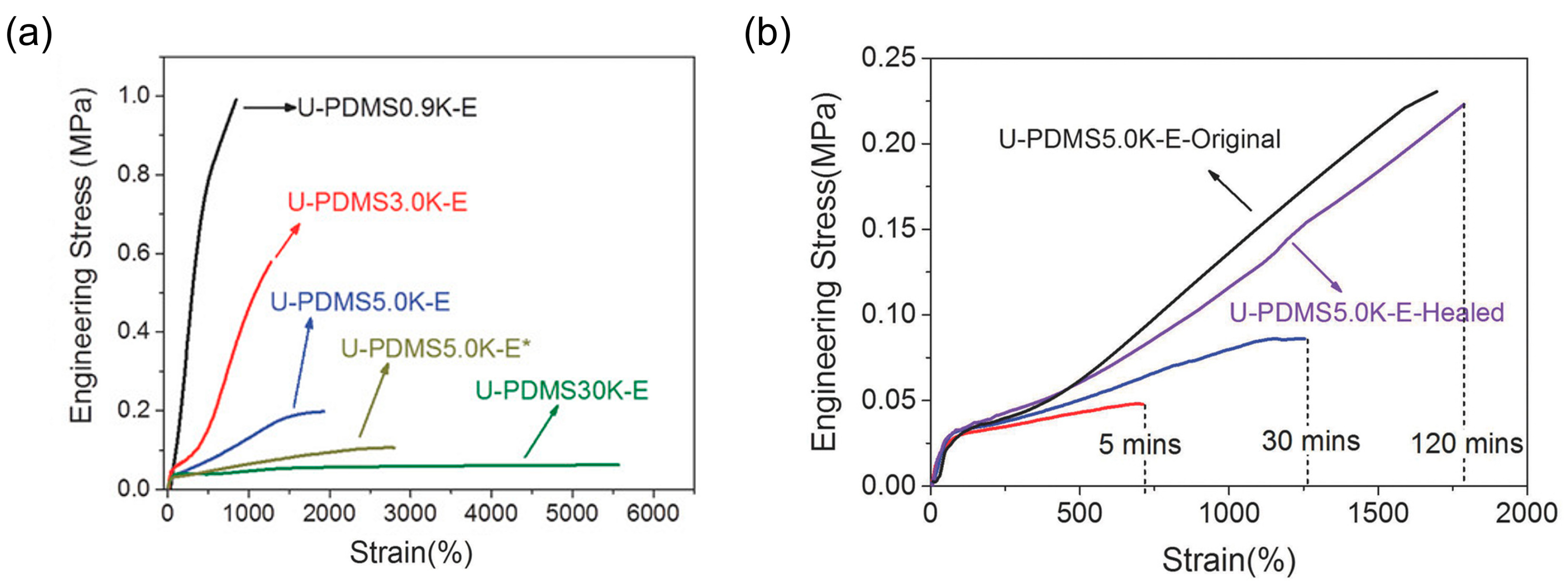
- Silicones Crosslinked with Multiple Paired Hydrogen Bonds
3. Silicone Elastomers Acting through Multiple Synergistic Self-Healing Mechanisms
3.1. Multifunctional Elastomers with Urea Motifs Incorporated as Hydrogen Bonding Sites
3.1.1. Silicone-Urea Self-Healing by Combined Action of Various Hydrogen Bonds
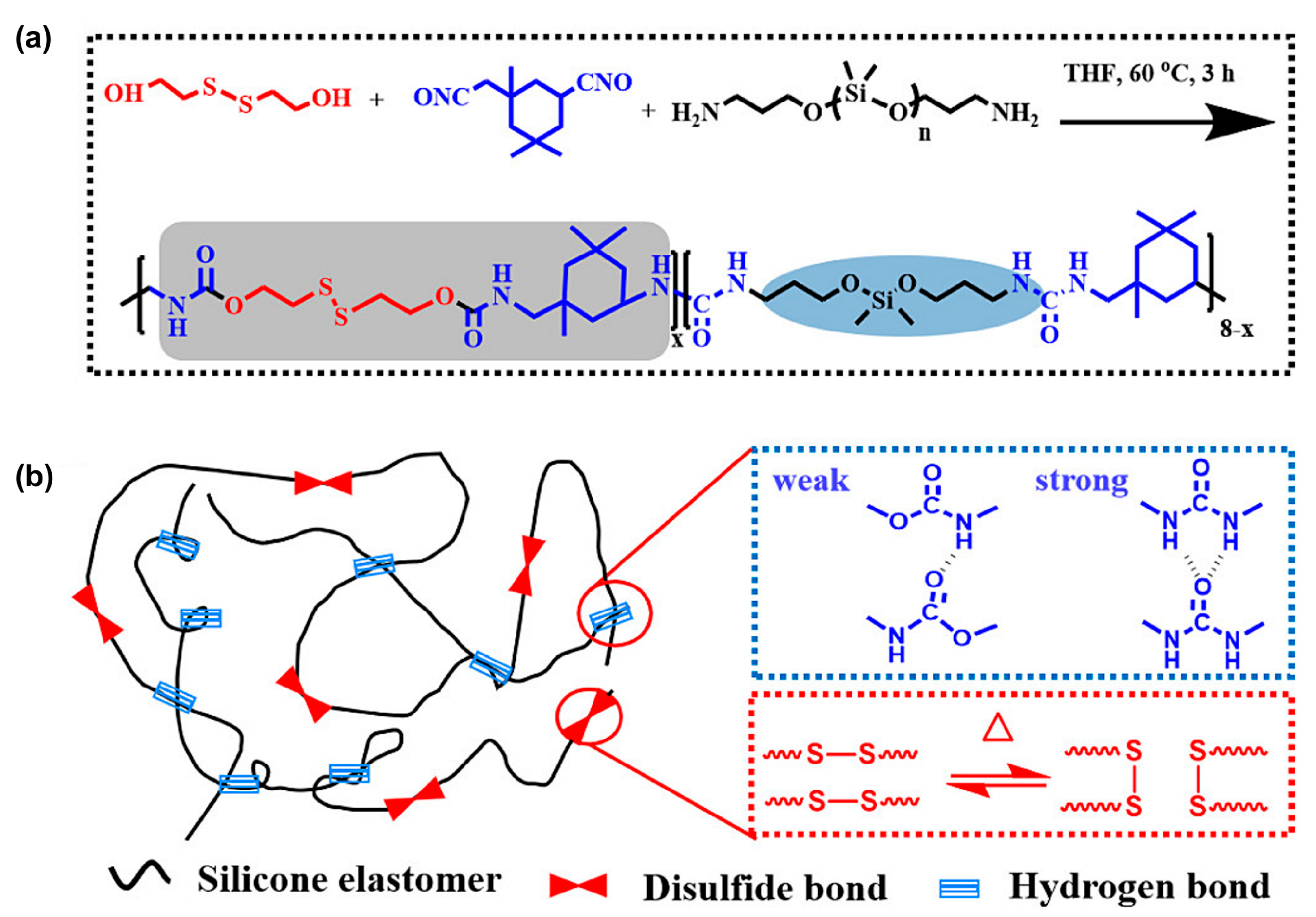
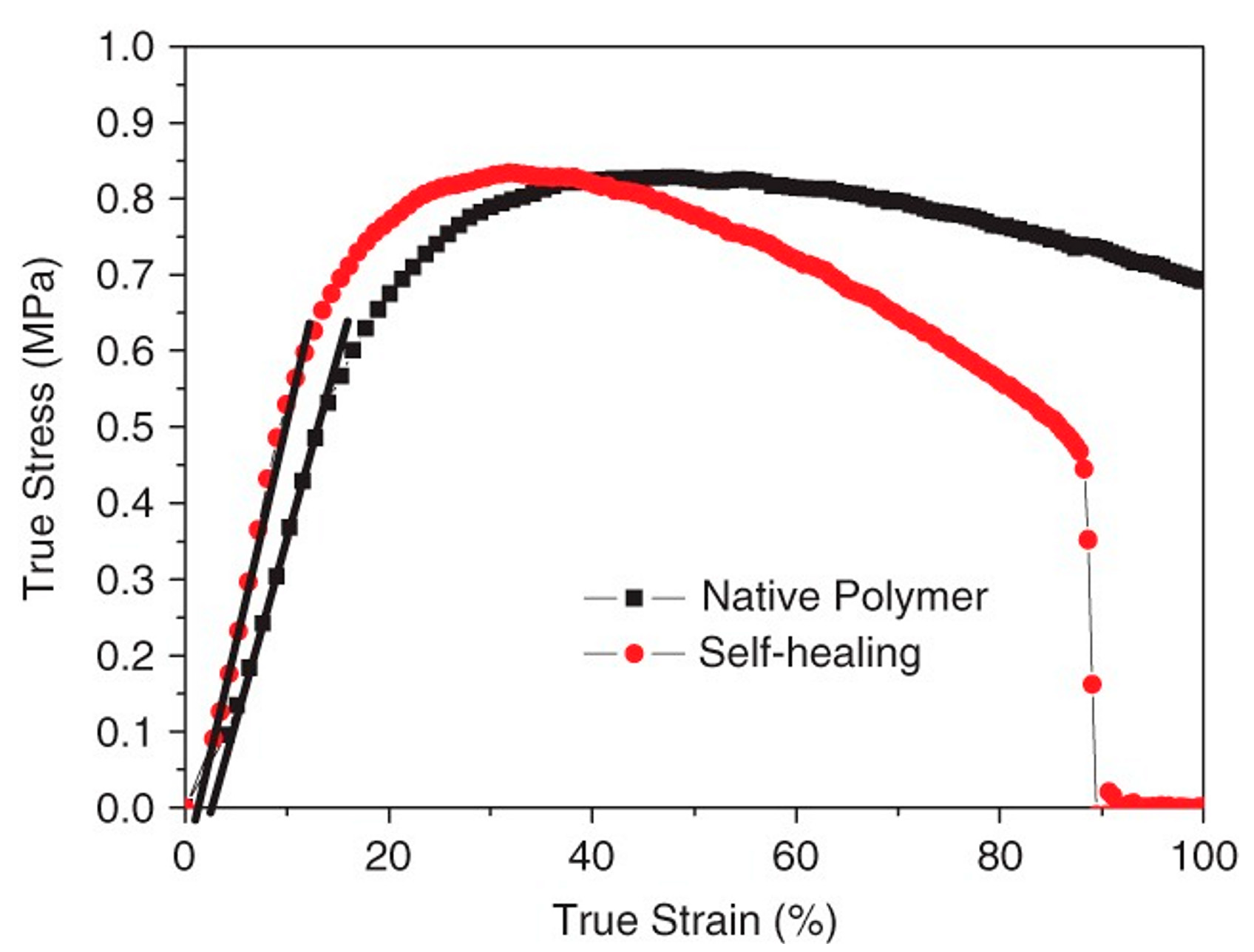
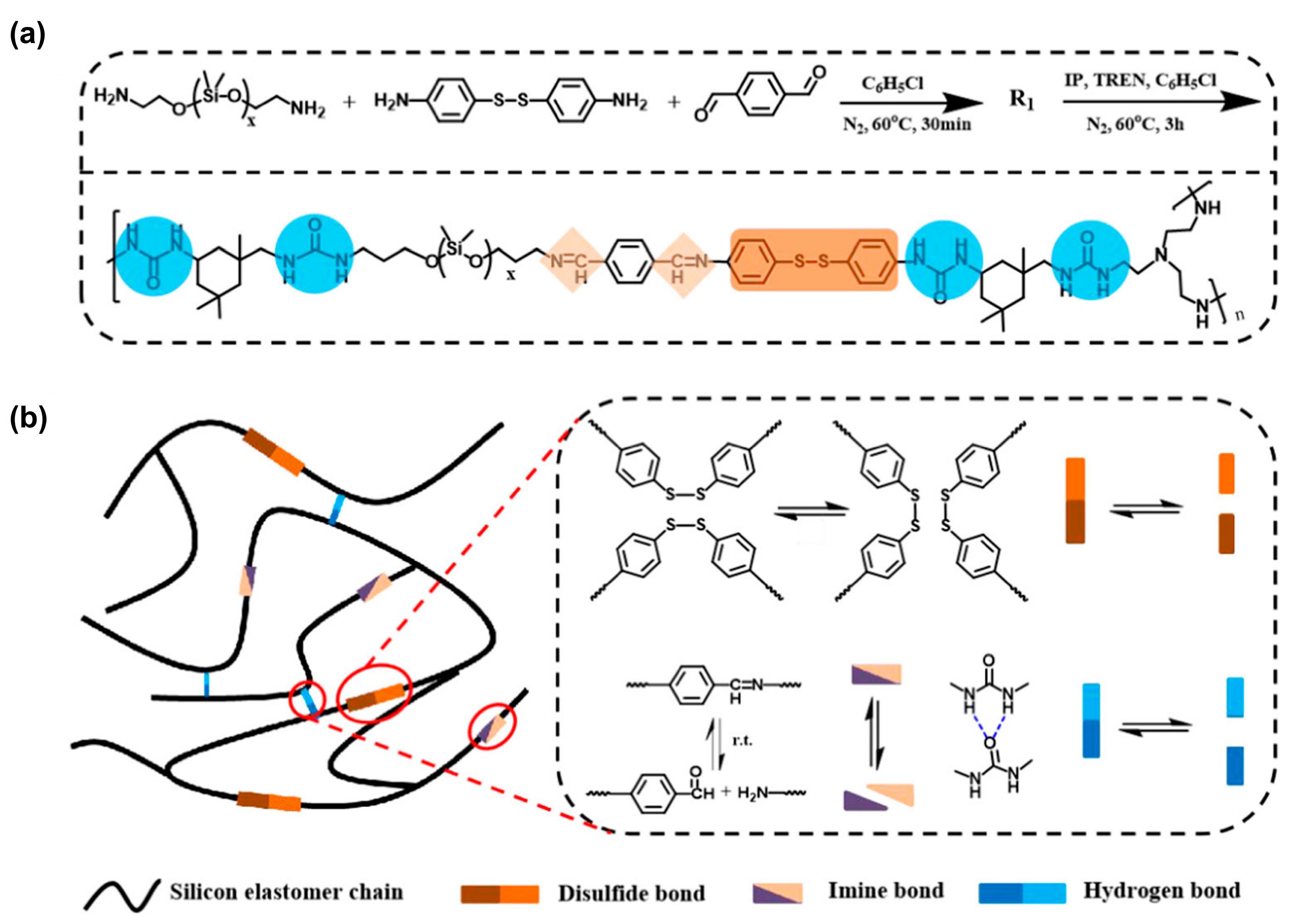
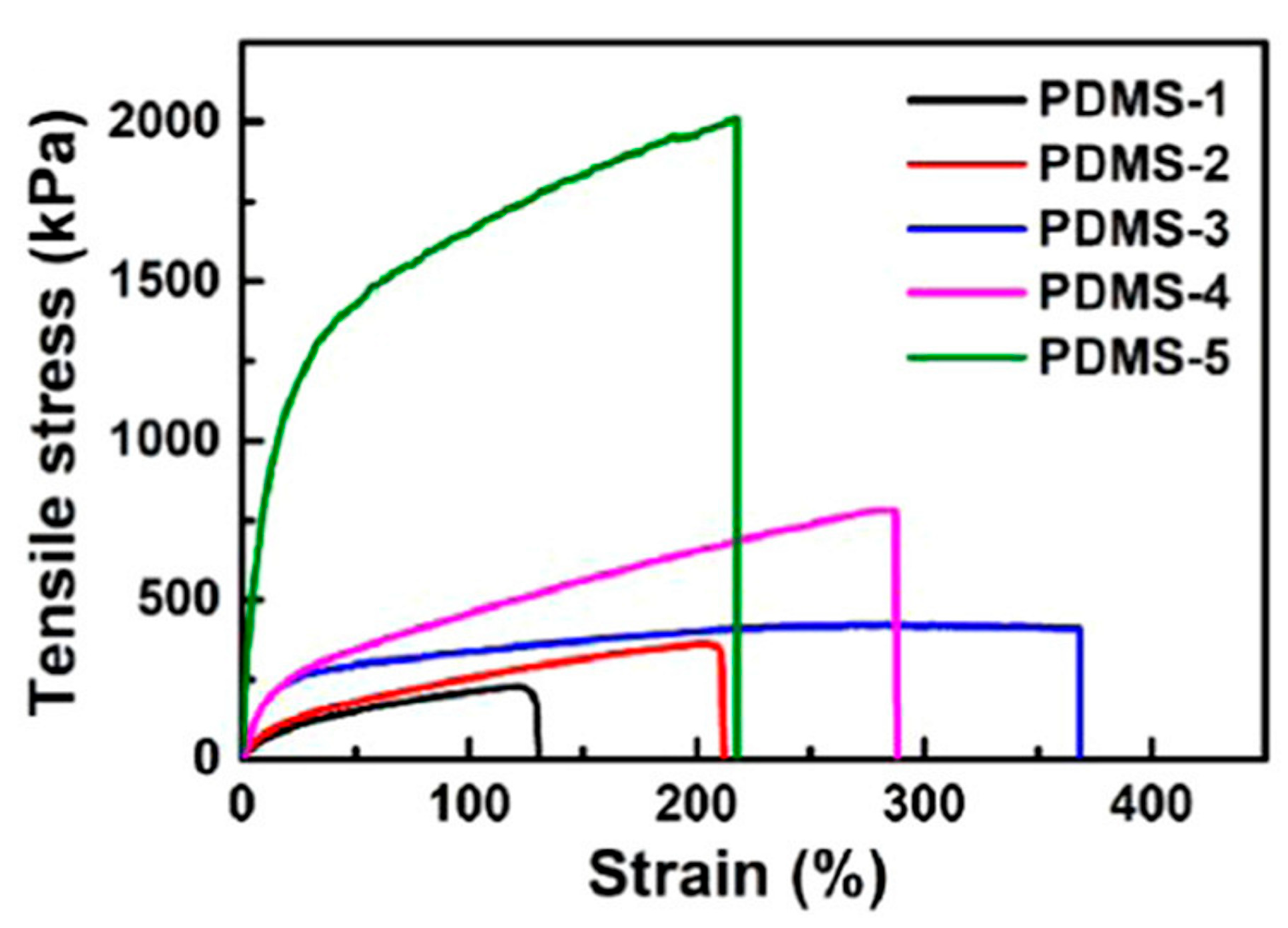
3.1.2. Polymeric Materials Self-Healing by Combined Action of H-Bonded Urea Groups and Dynamic Disulfide Bonds
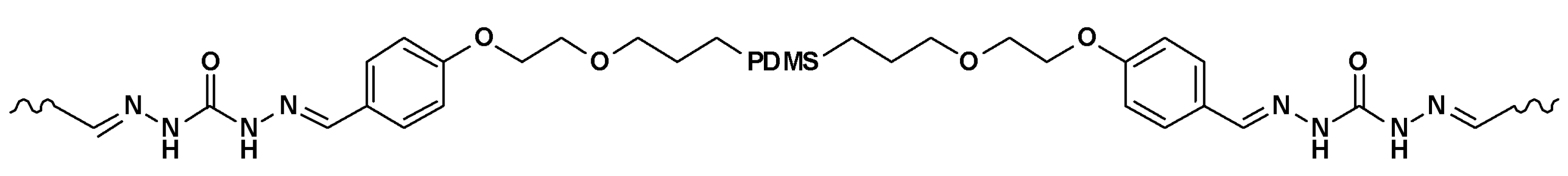
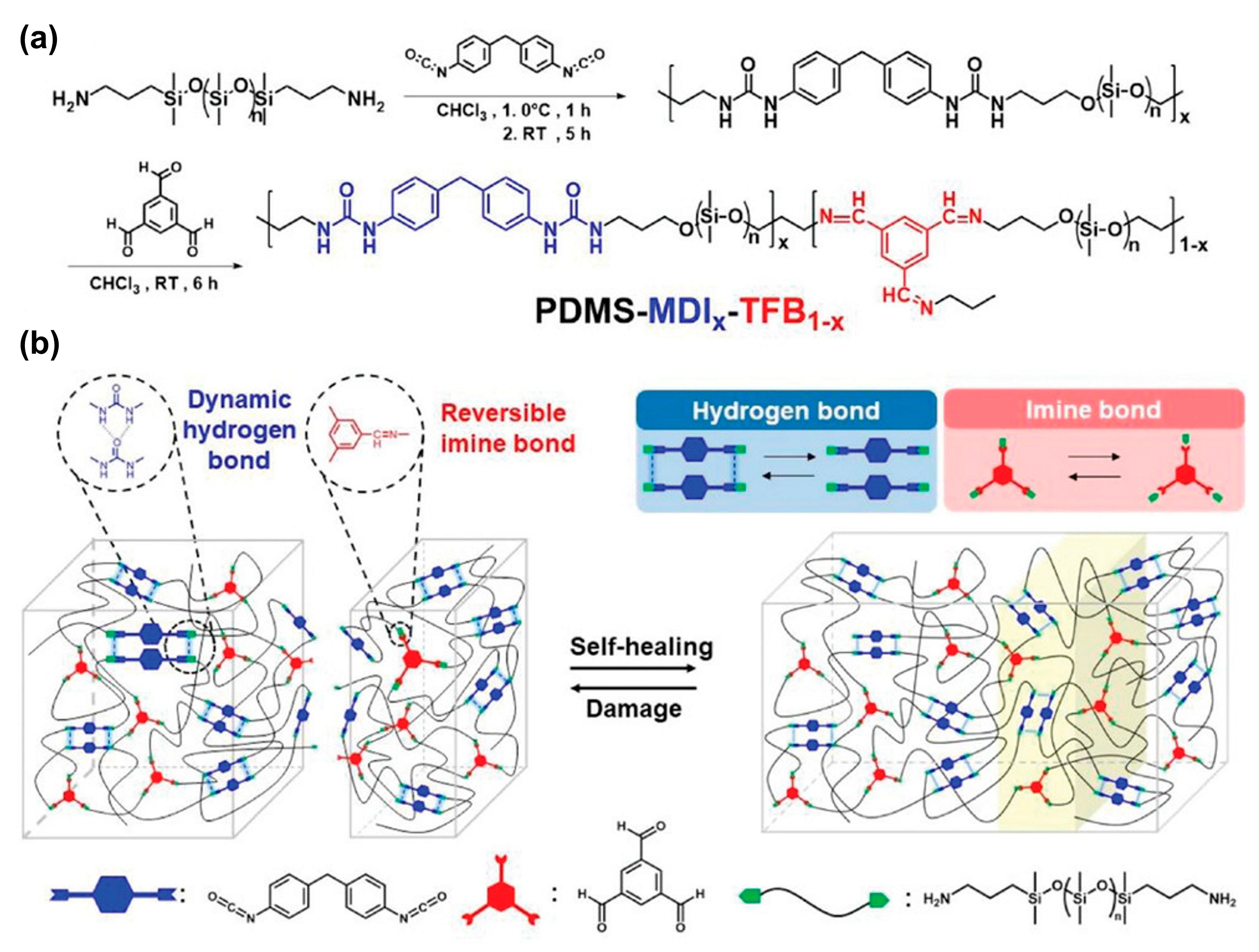
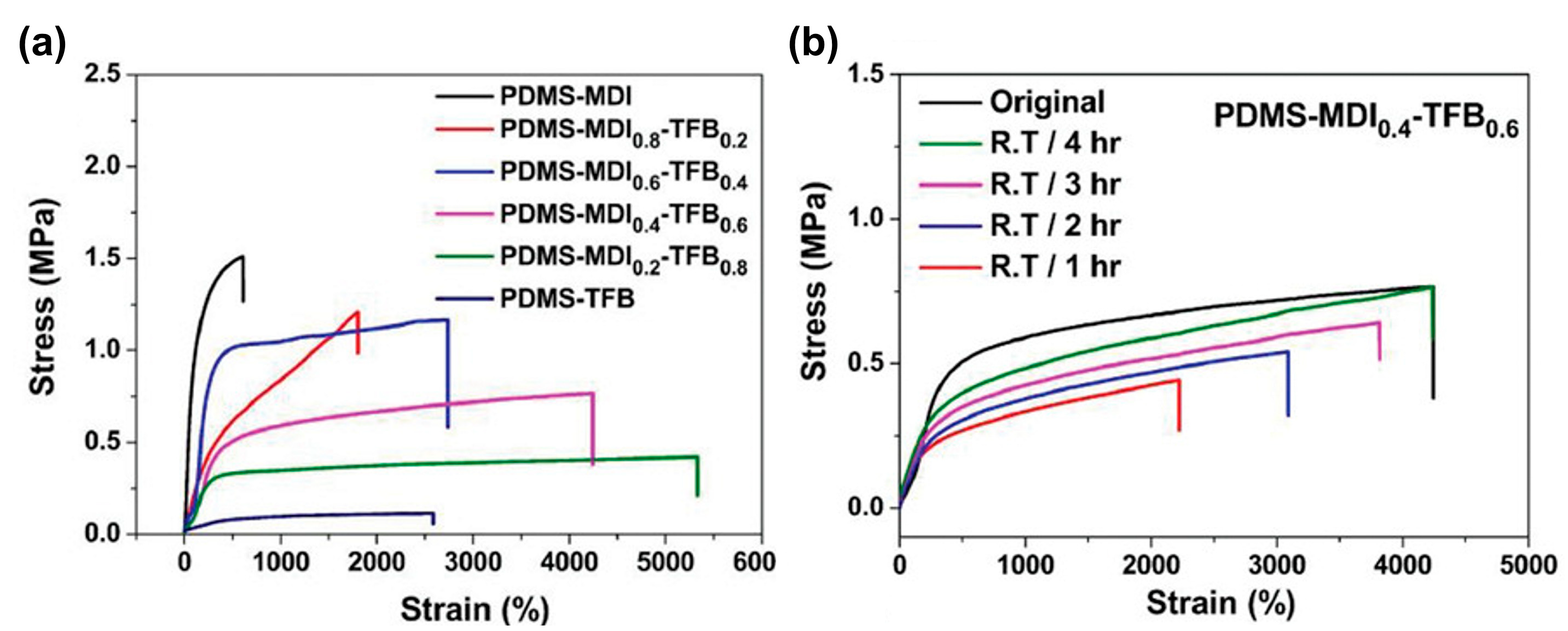
3.1.3. Silicone Elastomers Regeneration by Synergistic Action of H-Bonding between Urea Groups and Dynamic Imine Bonds
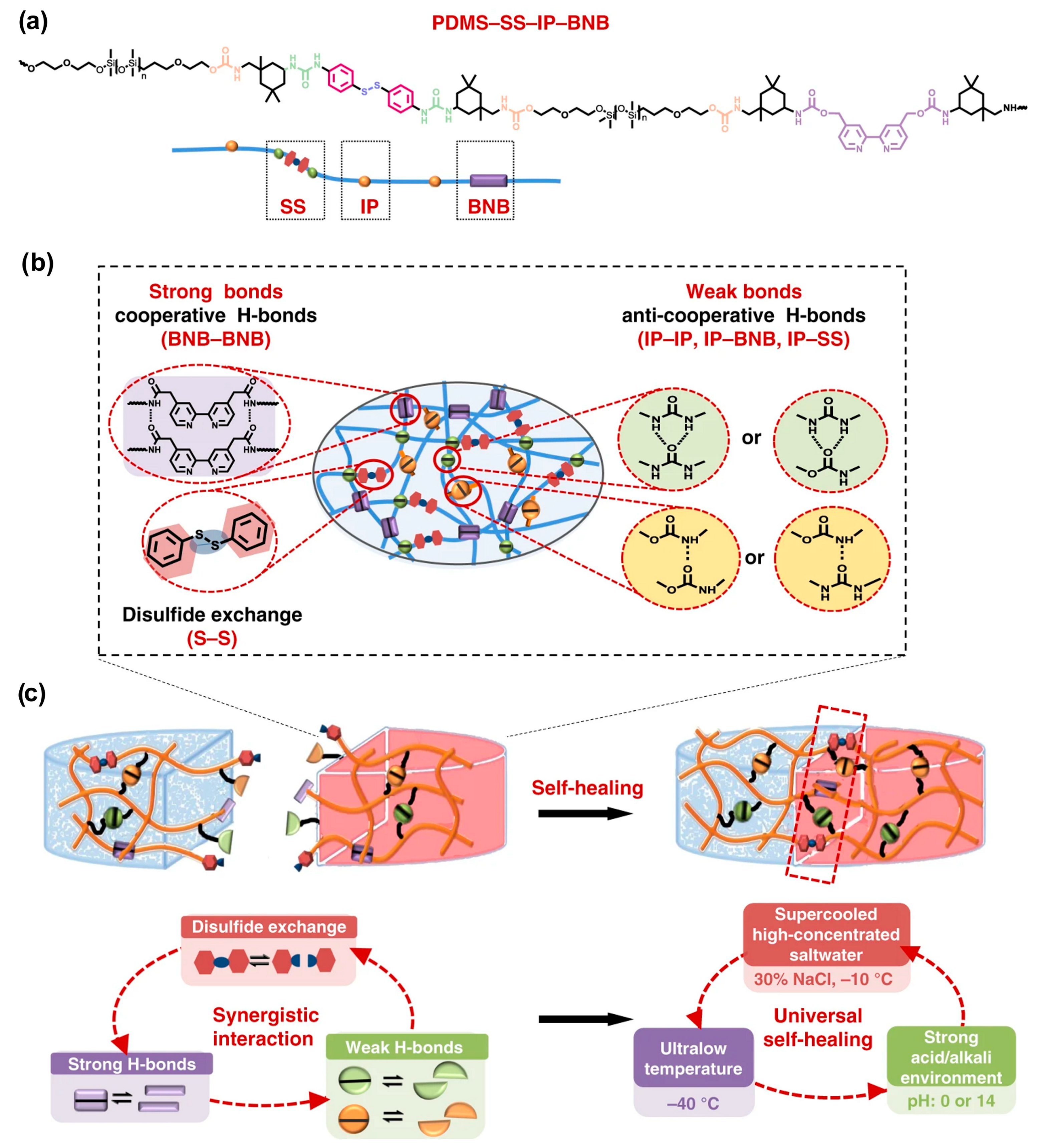
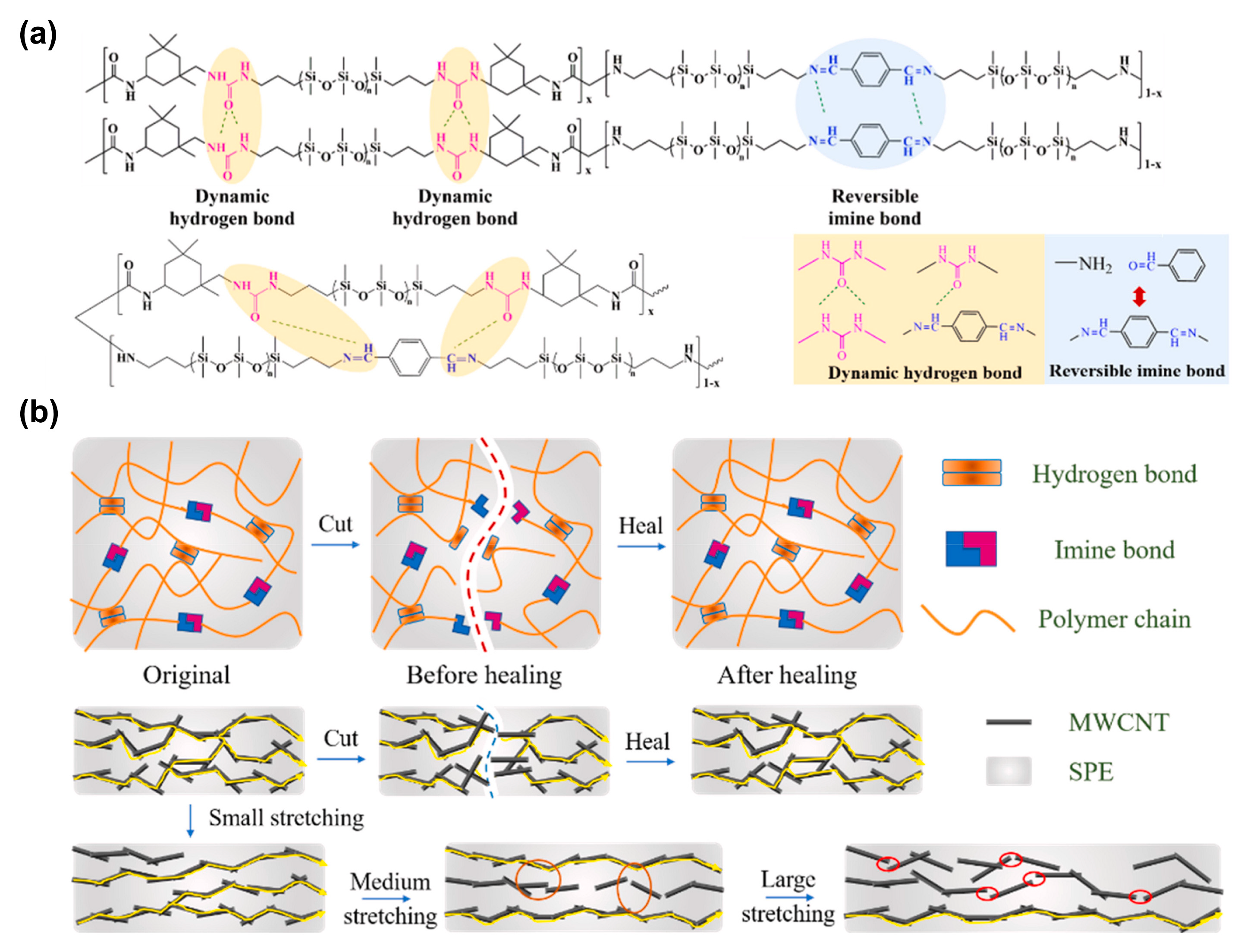
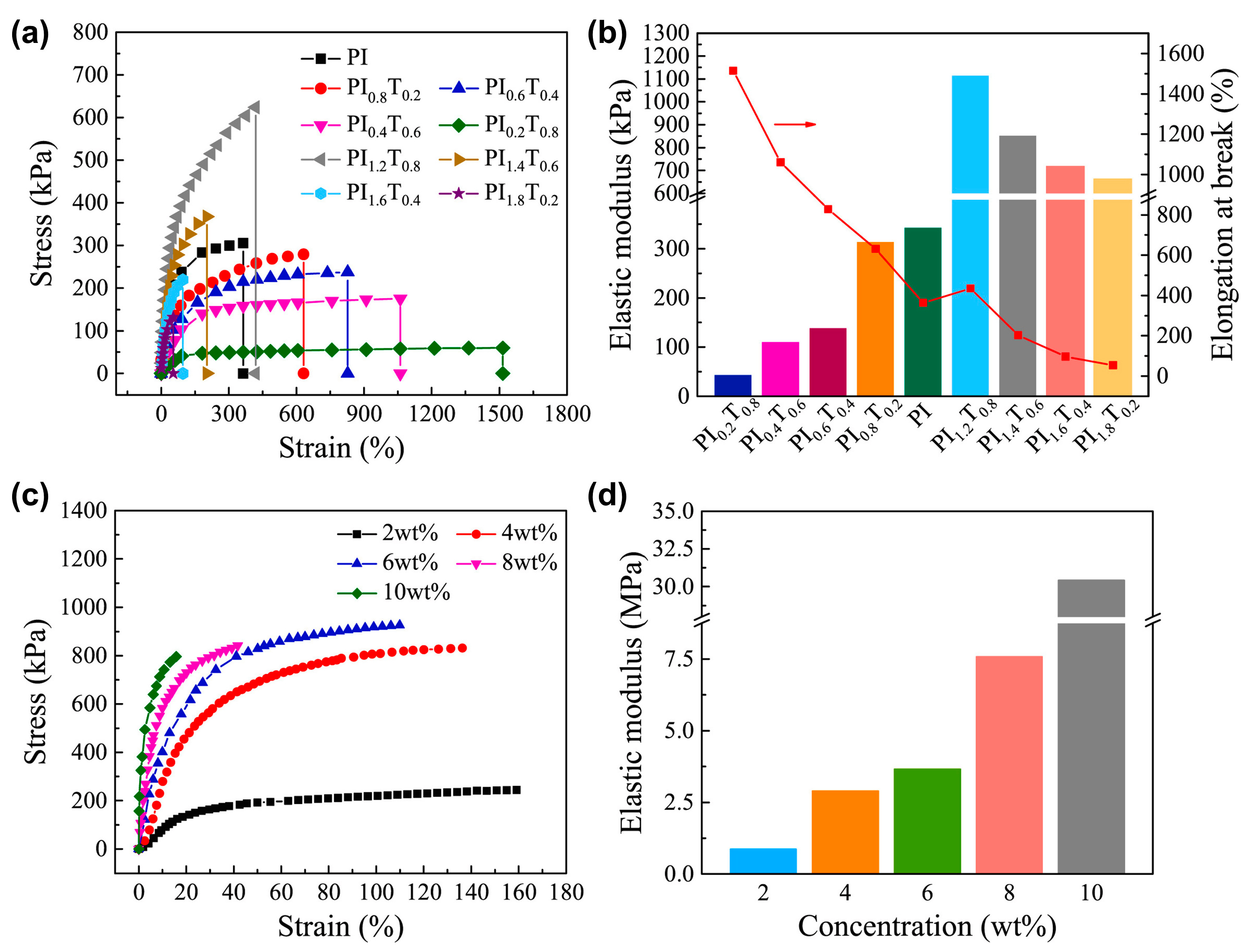
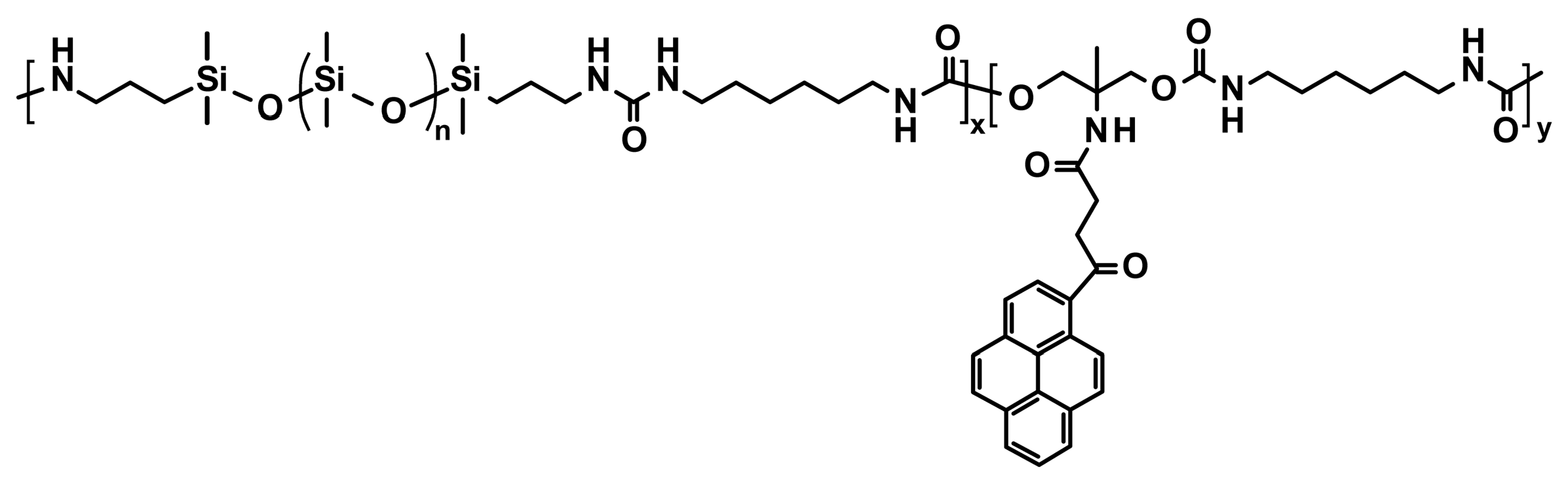
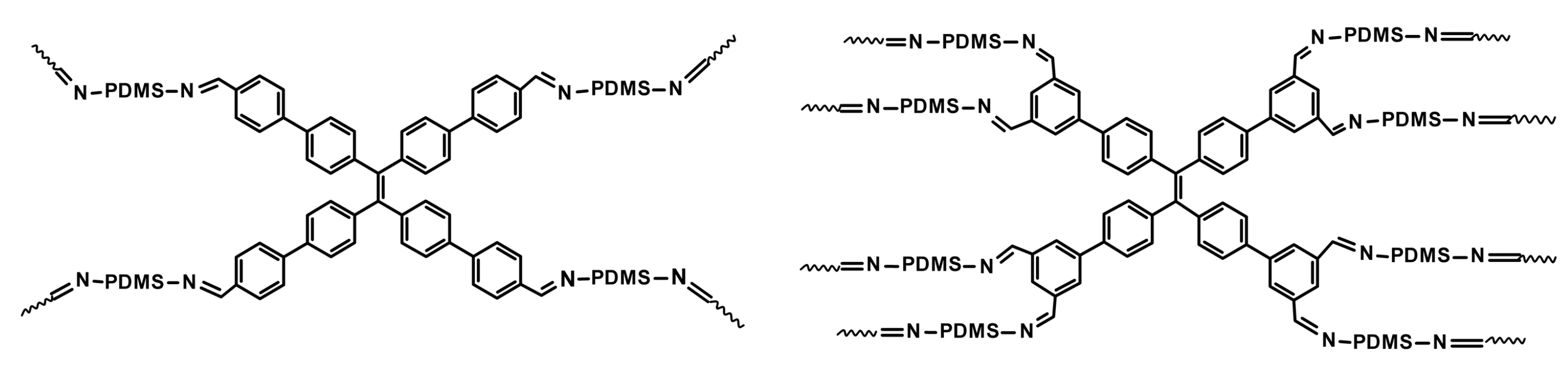
3.1.4. Silicones S-H by Synergistic Effect of H-Bonding and Other Supramolecular Interactions
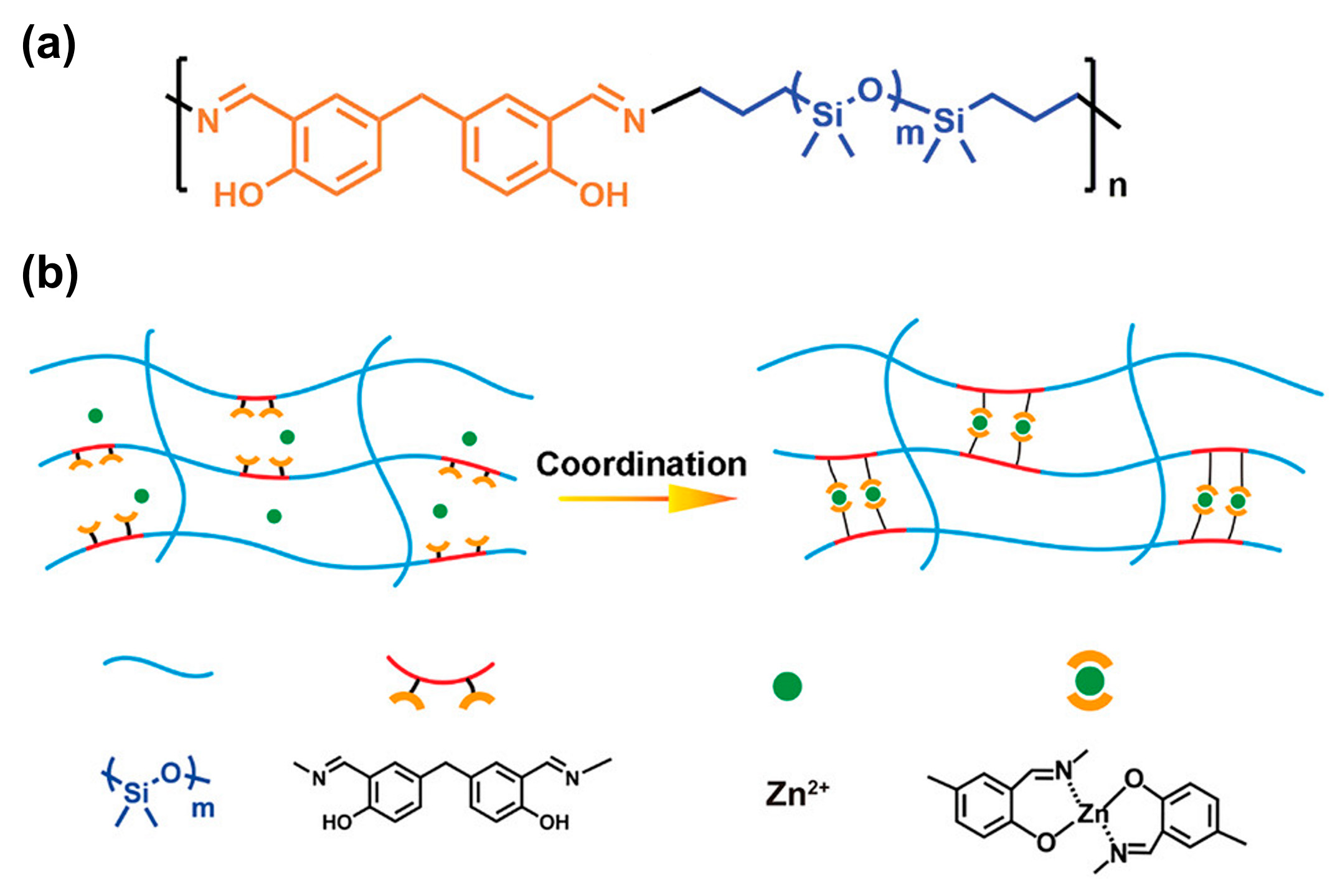
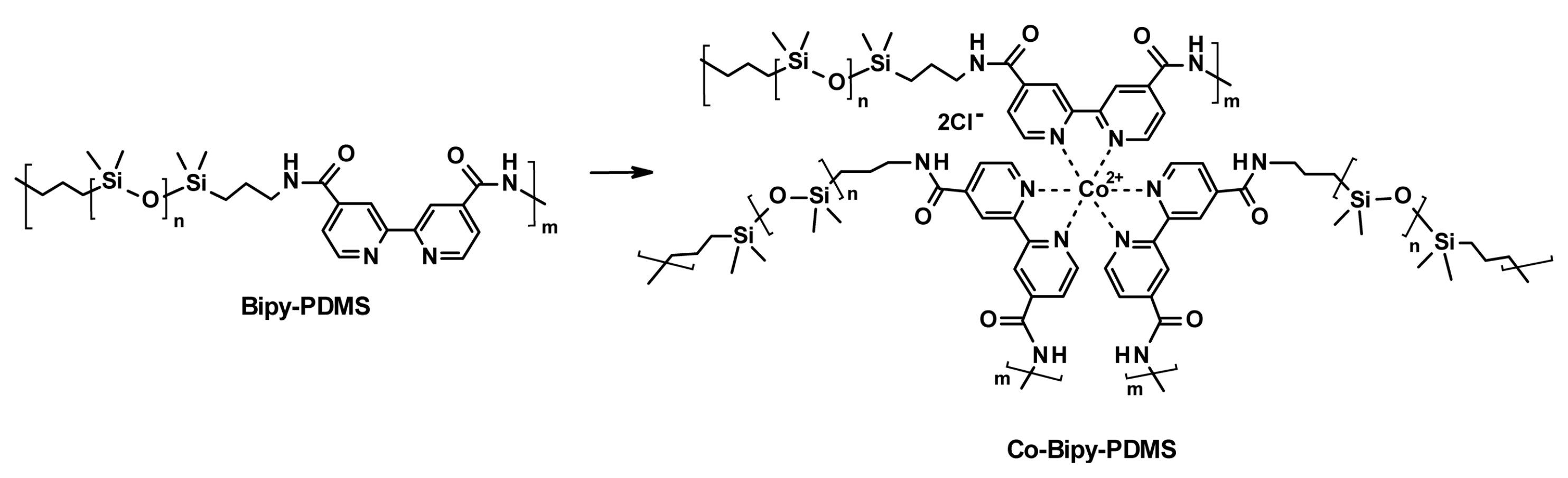
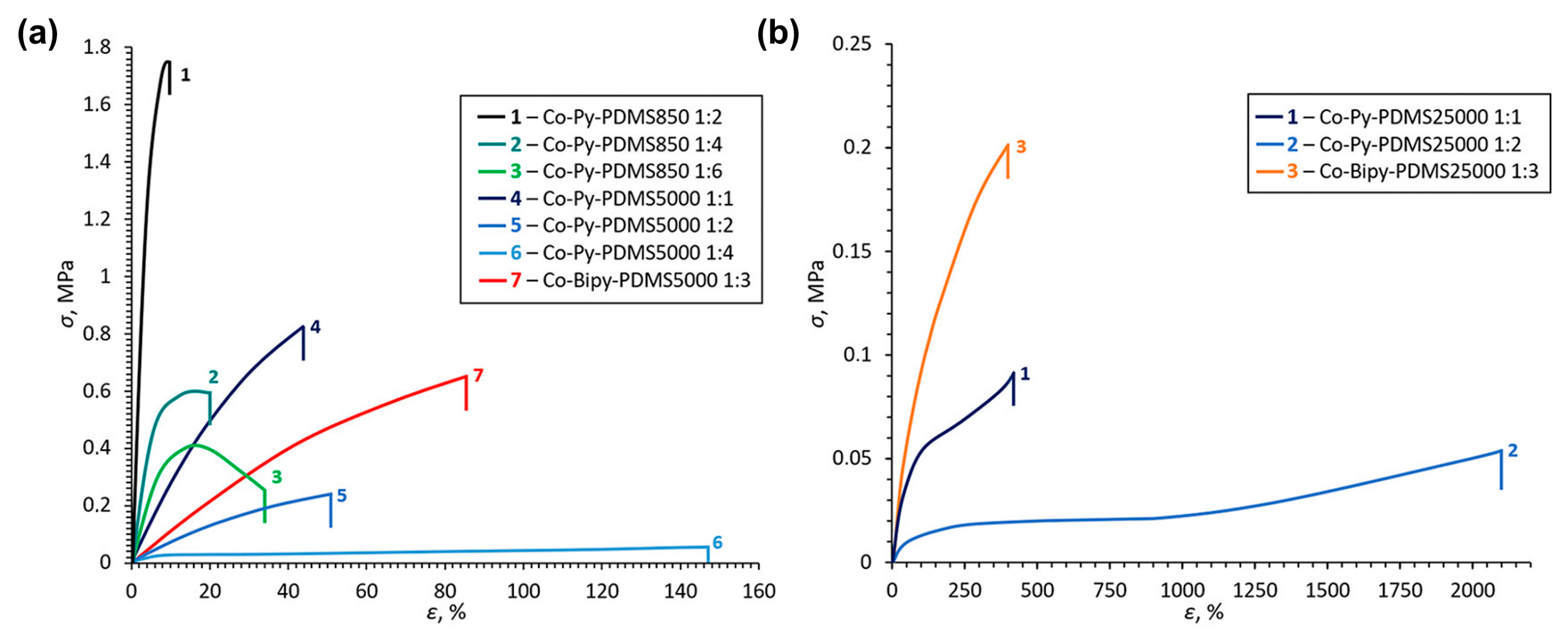
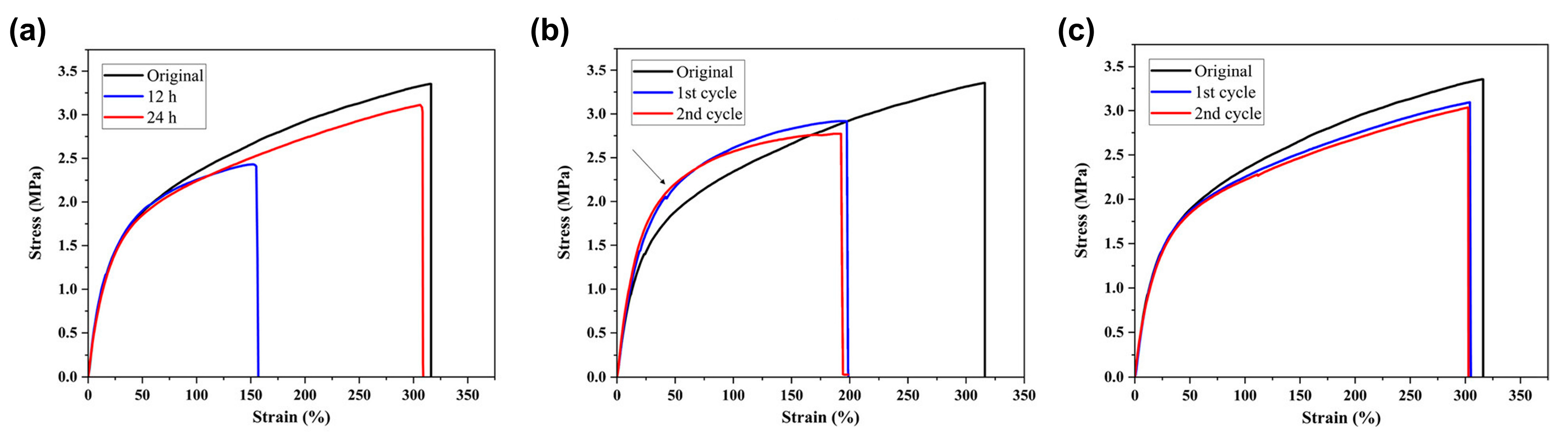
3.2. Self-Healing of Polysiloxanes Based on H-Bonding and Metal Cations’ Coordination
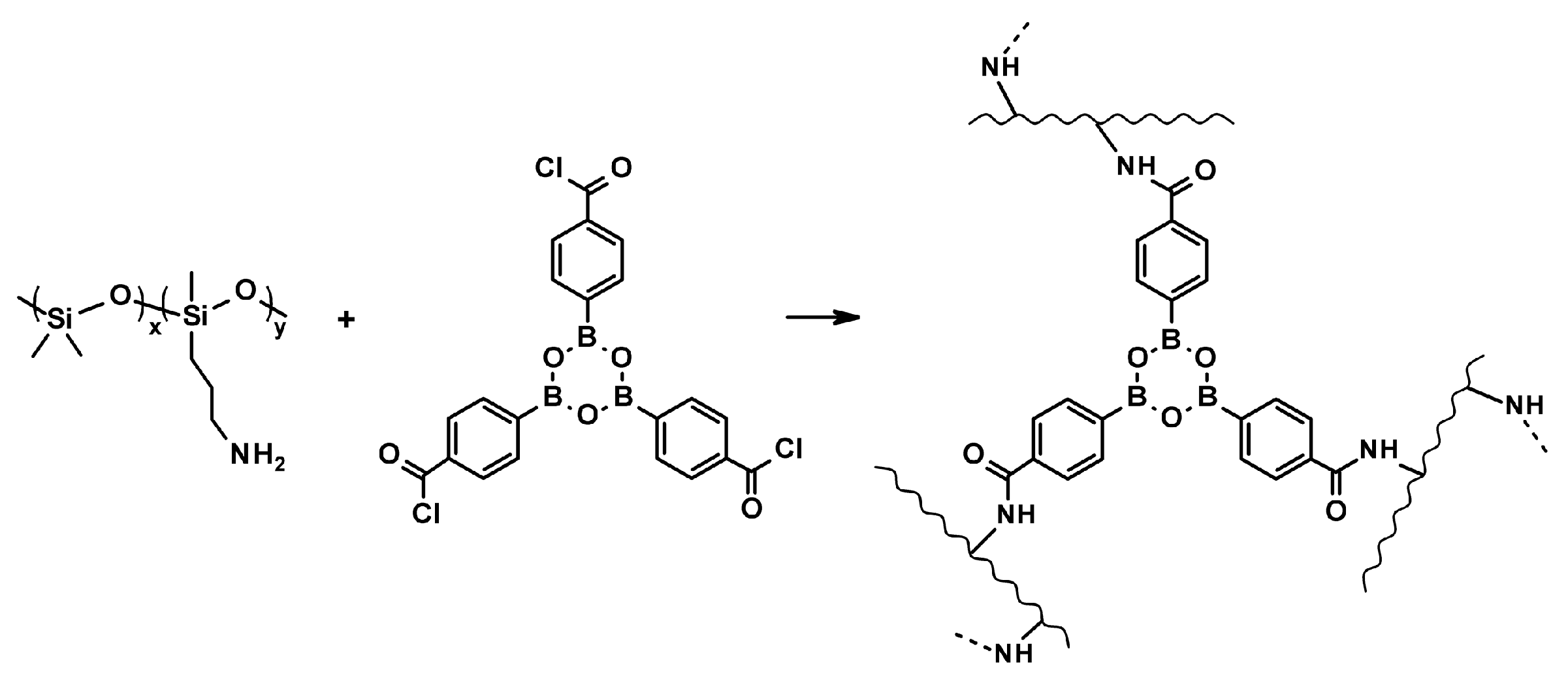
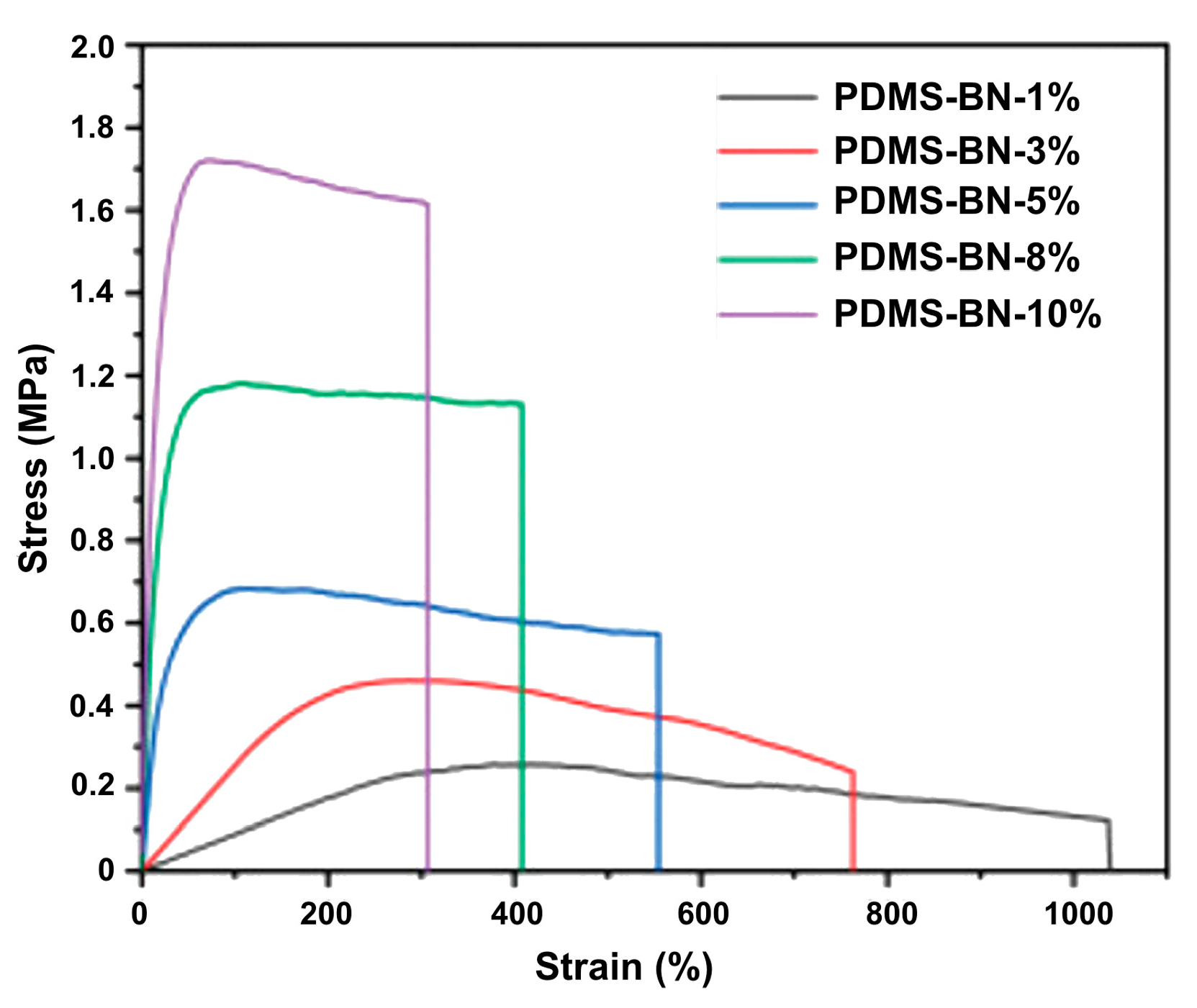
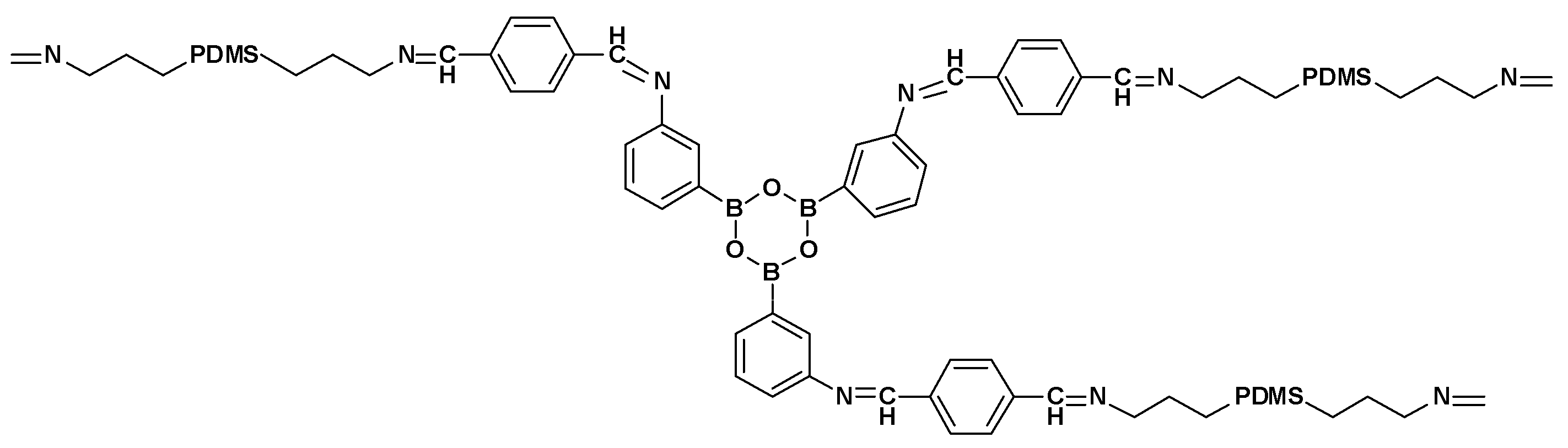
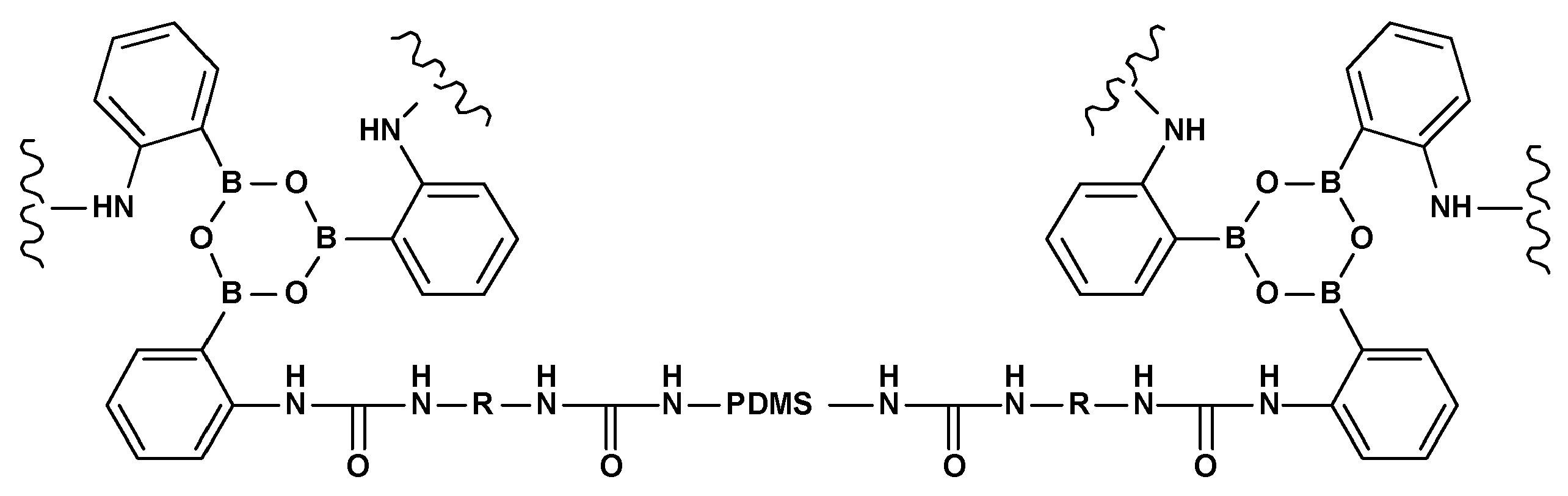
3.3. Self-Regeneration of Multifunctional Silicone Elastomers Involving Boroxine Chemistry
4. Smart Applications of Self-Healing Polysiloxanes
4.1. Polysiloxanes for Self-Healable Electronic Skin Applications
4.2. Flexible Strain Sensors
4.3. Coatings
4.4. Self-Healing of Multifunctional Silicone Elastomers with Antimicrobial Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chruściel, J.J. Silicon-Based Polymers and Materials; De Gruyter: Berlin, Germany, 2022. [Google Scholar]
- Cazacu, M.; Dascalu, M.; Stiubianu, G.-T.; Bele, A.; Tugui, C.; Racles, C. From passive to emerging smart silicones. Rev. Chem. Eng. 2023, 39, 941–1003. [Google Scholar] [CrossRef]
- Cheng, L.; Yu, D.; You, J.; Long, T.; Chen, S.; Zhou, C. Silicone Self-Healing Materials. Prog. Chem. 2018, 30, 1852–1862. [Google Scholar] [CrossRef]
- Yi, B.; Wang, S.; Hou, C.; Huang, X.; Cui, J.; Yao, X. Dynamic siloxane materials: From molecular engineering to emerging applications. Chem. Eng. J. 2021, 405, 127023. [Google Scholar] [CrossRef]
- Kamand, F.Z.; Mehmood, B.; Ghunem, R.; Hassan, M.K.; El-Hag, A.; Al-Sulaiti, L.; Abdala, A. Self-Healing Silicones for Outdoor High Voltage Insulation: Mechanism, Applications and Measurements. Energies 2022, 15, 1677. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Filippova, S.S.; Islamova, R.M. Self-Healing Silicone Materials: Looking Back and Moving Forward. Biomimetics 2023, 8, 286. [Google Scholar] [CrossRef]
- Kowalewska, A.; Majewska-Smolarek, K. Self-Healing Antimicrobial Silicones—Mechanisms and Applications. Polymers 2023, 15, 3945. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Wen, N.; Song, T.; Ji, Z.; Jiang, D.; Wu, Z.; Wang, Y.; Guo, Z. Recent advancements in self-healing materials: Mechanicals, performances and features. React. Funct. Polym. 2021, 168, 105041. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Han, Z.; Li, Q.; He, J.; Wang, Q. Self-Healing Polymers for Electronics and Energy Devices. Chem. Rev. 2023, 123, 558–612. [Google Scholar] [CrossRef]
- Kim, C.; Yoshie, N. Polymers healed autonomously and with the assistance of ubiquitous stimuli: How can we combine mechanical strength and a healing ability in polymers? Polym. J. 2018, 50, 919–929. [Google Scholar] [CrossRef]
- Liu, M.; Liu, P.; Lu, G.; Xu, Z.; Yao, X. Multiphase-Assembly of Siloxane Oligomers with Improved Mechanical Strength and Water-Enhanced Healing. Angew. Chem. 2018, 57, 11242–11246. [Google Scholar] [CrossRef]
- Neal, J.A.; Mozhdehi, D.; Guan, Z. Enhancing Mechanical Performance of a Covalent Self-Healing Material by Sacrificial Noncovalent Bonds. J. Am. Chem. Soc. 2015, 137, 4846–4850. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Huang, J.; Yang, S. Room temperature readily self-healing polymer via rationally designing molecular chain and crosslinking bond for flexible electrical sensor. J. Coll. Interf. Sci. J. Colloid Interface Sci. 2019, 559, 152–161. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. A Surprise from 1954: Siloxane Equilibration Is a Simple, Robust, and Obvious Polymer Self-Healing Mechanism. J. Am. Chem. Soc. 2012, 134, 2024–2027. [Google Scholar] [CrossRef]
- Strąkowska, A.; Kosmalska, A.; Masłowski, M.; Szmechtyk, T.; Strzelec, K.; Zaborski, M. POSS as promoters of self-healing process in silicone composites. Polym. Bull. 2019, 76, 3387–3402. [Google Scholar] [CrossRef]
- Kowalewska, A.; Fortuniak, W.; Chojnowski, J.; Pawlak, A.; Gadzinowska, K.; Zaród, M. Polymer Nano-Materials Through Self-Assembly of Polymeric POSS Systems. Silicon 2012, 4, 95–107. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A. Self-Healing Silsesquioxane-Based Materials. Polymers 2022, 14, 1869. [Google Scholar] [CrossRef] [PubMed]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Suzuki, M.; Hayashi, T.; Hikino, T.; Kishi, M.; Matsuno, T.; Wada, H.; Kuroda, K.; Shimojima, A. Integrated Extrinsic and Intrinsic Self-Healing of Polysiloxane Materials by Cleavable Molecular Cages Encapsulating Fluoride Ions. Adv. Sci. 2023, 10, 2303655. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.W.; White, S.R.; Sottos, N.R. A Self-Healing Poly(Dimethyl Siloxane) Elastomer. Adv. Funct. Mater. 2007, 17, 2399–2404. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Zhang, C.; Feng, S. Polysiloxane-Based Autonomic Self-Healing Elastomers Obtained through Dynamic Boronic Ester Bonds Prepared by Thiol–Ene “Click” Chemistry. Macromol. Rapid Commun. 2016, 37, 1052–1059. [Google Scholar] [CrossRef]
- Yoshida, D.; Park, J.; Ikura, R.; Yamashita, N.; Yamaguchi, H.; Takashima, Y. Self-healable Poly(dimethyl siloxane) Elastomers Based on Host-guest Complexation between Methylated β-Cyclodextrin and Adamantane. Chem. Lett. 2023, 52, 93–96. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Der Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Nasresfahania, A.; Zelisko, P.M. Synthesis of a self-healing siloxane-based elastomer cross-linked via a furan-modified polyhedral oligomeric silsesquioxane: Investigation of a thermally reversible silicon-based cross-link. Polym. Chem. 2017, 8, 2942–2952. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, W.-C.; Mohamed, M.G.; Chen, Z.-Y.; Kuo, S.-W. Highly Thermally Stable, Reversible, and Flexible Main Chain Type Benzoxazine Hybrid Incorporating Both Polydimethylsiloxane and Double-Decker Shaped Polyhedral Silsesquioxane Units through Diels–Alder Reaction. Macromol. Rapid Commun. 2023, 44, 2200910. [Google Scholar] [CrossRef]
- Kang, S.H.; Seo, J.Y.; Oh, H.J.; Lee, J.-H.; Lee, A.S.; Baek, K.-Y. Durable and thermally switchable polysilsesquioxane adhesives via dynamic covalent bonds: Effect of a crosslinker on reversible chemistry. Mater. Chem. Front. 2023, 7, 679–688. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, R.; Luo, G.; Wu, J.; Xia, H. A self-healing, re-moldable and biocompatible crosslinked polysiloxane elastomer. J. Mater. Chem. B 2016, 4, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Kickelbick, G. Self-healing polymer nanocomposites based on Diels-Alder-reactions with silica nanoparticles: The role of the polymer matrix. Polymer 2015, 69, 357–368. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Lee, A.S.; Baek, K.-Y.; Lee, H.; Hwang, S.S. Thermally reversible self-healing polysilsesquioxane structure-property relationships based on Diels-Alder chemistry. Polymer 2017, 108, 58–65. [Google Scholar] [CrossRef]
- Namin, P.A.; Booth, P.; Silva, J.T.; Voigt, L.J.; Zelisko, P.M. Transparent and Thermoplastic Silicone Materials Based on Room-Temperature Diels–Alder Reactions. Macromolecules 2023, 56, 2038–2051. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Li, L.; Feng, S. Novel clickable and fluorescent poly(siloxane amine)s for reusable adhesives and reprocessable elastomers. Polym. Chem. 2020, 11, 4780–4786. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, X.; Yin, Y.; Jiang, B.; Huang, Y. A self-healing silicone/BN composite with efficient healing property and improved thermal conductivities. Compos. Sci. Technol. 2020, 186, 107919. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Wang, D.; Feng, S.; Zhou, C. Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction. Polymers 2021, 13, 3729. [Google Scholar] [CrossRef]
- Zhang, T.; Su, H.; Shi, X.; Li, C. Self-healing siloxane elastomers constructed by hierarchical covalent crosslinked networks and reversible dynamic bonds for flexible electronics. J. Mater. Sci. 2022, 57, 10444–10456. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, Y.; Jiang, B.; Guo, Z.; Qu, C.; Huang, Y. Fast room-temperature self-healing siloxane elastomer for healable stretchable electronics. J. Colloid Interface Sci. 2020, 573, 105–114. [Google Scholar] [CrossRef]
- Juyal, V.K.; Pathak, A.; Panwar, M.; Thakuri, S.C.; Prakash, O.; Agrwal, A.; Nand, V. Schiff base metal complexes as a versatile catalyst: A review. J. Organomet. Chem. 2023, 999, 122825. [Google Scholar] [CrossRef]
- Wang, N.; Feng, H.-W.; Hao, X.; Cao, Y.; Xu, X.-D.; Feng, S. Dynamic covalent bond and metal coordination bond-cross-linked silicone elastomers with excellent mechanical and aggregation-induced emission properties. Polym. Chem. 2023, 14, 1396–1403. [Google Scholar] [CrossRef]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.; Qi, H.J.; Zhang, W. Heat- or Water-Driven Malleability in a Highly Recyclable Covalent Network Polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, P.; Zhang, H.; Yan, C.; Zheng, Z.; Wu, B.; Yu, Y. A Transparent, Highly Stretchable, Autonomous Self-Healing Poly(dimethyl siloxane) Elastomer. Macromol. Rapid Commun. 2017, 38, 1700110. [Google Scholar] [CrossRef] [PubMed]
- Ciubotaru, B.-I.; Dascalu, M.; Zaltariov, M.-F.; Macsim, A.-M.; Damoc, M.; Bele, A.; Tugui, C.; Varganici, C.-D.; Cazacu, M. Catalyst-free crosslinked sustainable functional silicones by supramolecular interactions. React. Funct. Polym. 2022, 181, 105419. [Google Scholar] [CrossRef]
- Suriano, R.; Boumezgane, O.; Tonelli, C.; Turri, S. Viscoelastic properties and self-healing behavior in a family of supramolecular ionic blends from silicone functional oligomers. Polym. Adv. Technol. 2020, 31, 3247–3257. [Google Scholar] [CrossRef]
- Yılgör, E.; Yılgör, I.; Yurtsever, E. Hydrogen bonding and polyurethane morphology. I. Quantum mechanical calculations of hydrogen bond energies and vibrational spectroscopy of model compounds. Polymer 2002, 43, 6551–6559. [Google Scholar] [CrossRef]
- Fauvre, L.; Fleury, E.; Ganachaud, F.; Portinha, D. Extra-Soft Self-Healable Supramolecular Silicone Elastomers from Bis-Amide-PDMS Multiblock Copolymers Prepared by Aza-Michael Reaction. ACS Appl. Polym. Mater. 2023, 5, 1229–1240. [Google Scholar] [CrossRef]
- Wang, D.-P.; Zhao, Z.-H.; Li, C.-H. Universal Self-Healing Poly(dimethylsiloxane) Polymer Crosslinked Predominantly by Physical Entanglements. ACS Appl. Mater. Interfaces 2021, 13, 31129–31139. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Håkonsen, V.; Li, T.; Wang, F.; He, J.; Zhang, Z. Ultrafast self-healing and highly transparent coating with mechanically durable icephobicity. Appl. Mater. Today 2020, 19, 100542. [Google Scholar] [CrossRef]
- Wu, L.; Wang, W.; Lu, J.; Sun, R.; Wong, C.-P. Study of the interfacial adhesion properties of a novel Self-healable siloxane polymer material via molecular dynamics simulation. Appl. Surf. Sci. 2022, 583, 152471. [Google Scholar] [CrossRef]
- Cao, P.-F.; Li, B.; Hong, T.; Townsend, J.; Qiang, Z.; Xing, K.; Vogiatzis, K.D.; Wang, Y.; Mays, J.W.; Sokolov, A.P.; et al. Superstretchable, Self-Healing Polymeric Elastomers with Tunable Properties. Adv. Funct. Mater. 2018, 28, 1800741. [Google Scholar] [CrossRef]
- Qian, Y.; Dong, F.; Guo, L.; Lu, S.; Xu, X.; Liu, H. Self-Healing and Reprocessable Terpene Polysiloxane-Based Poly(thiourethane-urethane) Material with Reversible Thiourethane Bonds. Biomacromolecules 2023, 24, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.F.; Feng, L.; Feng, S. Preparation of supramolecular silicone elastomers via homo- and hetero-assembly. New J. Chem. 2018, 42, 1973–1978. [Google Scholar] [CrossRef]
- Chen, L.; Peng, H.; Wei, Y.; Wang, X.; Jin, Y.; Liu, H.; Jiang, Y. Self-Healing Properties of PDMS Elastomers via Guanine and Cytosine Base Pairs. Macromol. Chem. Phys. 2019, 220, 1900280. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, F.; Yang, S.B.; Gong, S.-Y.; Luo, Y.-X.; Wei, F.-F.; Ding, F.-C.; Bai, W.-B.; Jian, R.-K. Mechanical Strong, Highly Adhesive, Recyclable and Transparent Supramolecular Silicone Coatings with Easy Water/Oil Sliding. Chin. J. Polym. Sci. 2023, 41, 1796–1804. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, D.; Liu, L.; Wang, L.; Yu, H.; Li, L.; Feng, S. Reprocessable Supramolecular Elastomers of Poly(Siloxane–Urethane) via Self-Complementary Quadruple Hydrogen Bonding. Macromol. Chem. Phys. 2021, 222, 2100116. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Y.; Wang, W.; Zhang, A. The synthesis and characterization of supramolecular elastomers based on linear carboxyl-terminated polydimethylsiloxane oligomers. Polym. Chem. 2014, 5, 153–160. [Google Scholar] [CrossRef]
- Chen, H.; Koh, J.J.; Liu, M.; Li, P.; Fan, X.; Liu, S.; Yeo, J.C.C.; Tan, Y.; Tee, B.C.K.; He, C. Super Tough and Self-Healable Poly(dimethylsiloxane) Elastomer via Hydrogen Bonding Association and Its Applications as Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 31975–31983. [Google Scholar] [CrossRef]
- Xu, J.H.; Chen, P.; Wu, J.W.; Hu, P.; Fu, Y.S.; Jiang, W.; Fu, J.J. Notch-Insensitive, Ultrastretchable, Efficient Self-Healing Supramolecular Polymers Constructed from Multiphase Active Hydrogen Bonds for Electronic Applications. Chem. Mater. 2019, 31, 7951–7961. [Google Scholar] [CrossRef]
- Du, K.; Basuki, J.; Glattauer, V.; Mesnard, C.; Nguyen, A.T.; Alexander, D.J.L.; Hughes, T.C. Digital Light Processing 3D Printing of PDMS-Based Soft and Elastic Materials with Tunable Mechanical Properties. ACS Appl. Polym. Mater. 2021, 3, 3049–3059. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, Z.; Fan, J.; Chen, Y. Self-healable, recyclable, mechanically tough transparent polysiloxane elastomers based on dynamic microphase separation for flexible sensor. Polymer 2021, 237, 124357. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Su, H.; Yang, Y.; Li, W.; Zhang, T.; Chen, W.; Lin, R.; Li, Y.; Liao, I. Mechanically robust and recyclable siloxane elastomers enabled by adjustable dynamic polymer networks for electronic skin. Eur. Polym. J. 2023, 189, 111984. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, X.; Hu, X.; Dong, X.; Jiang, Y.; Cheng, G.; Yuan, N.; Ding, J. Carbon Nanotube Hybrid Yarn with Mechanically Strong Healable Silicone Elastomers for Artificial Muscle. ACS Appl. Nano Mater. 2021, 4, 5123–5130. [Google Scholar] [CrossRef]
- Guo, H.; Han, Y.; Zhao, W.; Yang, J.; Zhang, L. Universally autonomous self-healing elastomer with high stretchability. Nat. Commun. 2020, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Z.; An, R.; Marklund, P.; Björling, M.; Shi, Y. Novel Intrinsic Self-Healing Poly-Silicone-Urea with Super-Low Ice Adhesion Strength. Small 2022, 18, 2200532. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.; Zhang, K.; Guo, K.; Yuan, L.; Wu, Y.; Gao, C. A novel type of self-healing silicone elastomers with reversible cross-linked network based on the disulfide, hydrogen and metal-ligand bonds. Prog. Org. Coat. 2020, 144, 105661. [Google Scholar] [CrossRef]
- Roy, N.; Buhler, E.; Lehn, J.-M. Double dynamic self-healing polymers: Supramolecular and covalent dynamic polymers based on the bis-iminocarbohydrazide motif. Polym. Int. 2014, 63, 1400–1405. [Google Scholar] [CrossRef]
- He, C.; Liang, F.-C.; Veeramuthu, L.; Cho, C.-J.; Benas, J.-S.; Tzeng, Y.-R.; Tseng, Y.-L.; Chen, W.-C.; Rwei, A.; Kuo, C.-C. Super Tough and Spontaneous Water-Assisted Autonomous Self-Healing Elastomer for Underwater Wearable Electronics. Adv. Sci. 2021, 8, 2102275. [Google Scholar] [CrossRef]
- Yu, T.; Lü, X.; Bao, W. High electrical self-healing flexible strain sensor based on MWCNT—Polydimethylsiloxane elastomer with high gauge factor and wide measurement range. Compos. Sci. Technol. 2023, 238, 110049. [Google Scholar] [CrossRef]
- Dai, S.; Li, M.; Yan, H.; Zhu, H.; Hu, H.; Zhang, Y.; Cheng, G.; Yuan, N.; Ding, J. Self-Healing Silicone Elastomer with Stable and High Adhesion in Harsh Environments. Langmuir 2021, 37, 13696–13702. [Google Scholar] [CrossRef]
- Liu, W.-X.; Liu, D.; Xiao, Y.; Zou, M.; Shi, L.-Y.; Yang, K.-K.; Wang, Y.-Z. Healable, Recyclable, and High-Stretchable Polydimethylsiloxane Elastomer Based on Synergistic Effects of Multiple Supramolecular Interactions. Macromol. Mater. Eng. 2022, 307, 2200310. [Google Scholar] [CrossRef]
- Mei, J.-F.; Jia, X.-Y.; Lai, J.-C.; Sun, Y.; Li, C.-H.; Wu, J.-H.; Cao, Y.; You, X.-Z.; Bao, Z. A Highly Stretchable and Autonomous Self-Healing Polymer Based on Combination of Pt···Pt and π–π Interactions. Macromol. Rapid Commun. 2016, 37, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Mohamadhoseini, M.; Mohamadnia, Z. Supramolecular self-healing materials via host-guest strategy between cyclodextrin and specific types of guest molecules. Coord. Chem. Rev. 2021, 432, 213711. [Google Scholar] [CrossRef]
- Du, R.; Xu, Z.; Zhu, C.; Jiang, Y.; Yan, H.; Wu, H.-C.; Vardoulis, O.; Cai, Y.; Zhu, X.; Bao, Z.; et al. A Highly Stretchable and Self-Healing Supramolecular Elastomer Based on Sliding Crosslinks and Hydrogen Bonds. Adv. Funct. Mater. 2020, 30, 1907139. [Google Scholar] [CrossRef]
- Bele, A.; Dascalu, M.; Tugui, C.; Farcas, A. Silicone elastomers with improved electro-mechanical performance using slide-ring polymers. J. Polym. Res. 2022, 29, 202. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xiao, M.; Lv, S.; Cheng, M.; Shi, F. Elastic-Modulus-Dependent Macroscopic Supramolecular Assembly of Poly(dimethylsiloxane) for Understanding Fast Interfacial Adhesion. Langmuir 2021, 37, 4276–4283. [Google Scholar] [CrossRef]
- Yi, B.; Liu, P.; Hou, C.; Cao, C.; Zhang, J.; Sun, H.; Yao, X. Dual-cross-linked supramolecular polysiloxanes for mechanically tunable, damage-healable and oil-repellent polymeric coatings. ACS Appl. Mater. Interfaces 2019, 11, 47382–47389. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Xie, L.; Wang, W.; Wang, L.; Zhang, C.; Li, L.; Feng, S. Mechanically Strong, Autonomous Self-Healing, and Fully Recyclable Silicone Coordination Elastomers with Unique Photoluminescent Properties. Macromol. Rapid Commun. 2021, 42, 2100519. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fan, X.; Guo, J.; Li, L.; Feng, S. Multifunctional Polysiloxane with coordinative ligand for ion recognition, reprocessable elastomer, and reconfigurable shape memory. Polymer 2021, 229, 124021. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Huang, J.; Yang, S. Robust, stretchable, and self-healable supramolecular elastomers synergistically cross-linked by hydrogen bonds and coordination bonds. ACS Appl. Mater. Interfaces 2019, 11, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Deriabin, K.V.; Ignatova, N.A.; Kirichenko, S.O.; Novikov, A.S.; Kryukova, M.A.; Kukushkin, V.Y.; Islamova, R.M. Structural Features of Polymer Ligand Environments Dramatically Affect the Mechanical and Room-Temperature Self-Healing Properties of Cobalt(II)-Incorporating Polysiloxanes. Organometallics 2021, 40, 2750–2760. [Google Scholar] [CrossRef]
- Tian, M.; Zuo, L.; Wang, J.; Ning, N.; Yu, B.; Liqun Zhang, L. A silicone elastomer with optimized and tunable mechanical strength and self-healing ability based on strong and weak coordination bonds. Polym. Chem. 2020, 11, 4047–4057. [Google Scholar] [CrossRef]
- Pignanelli, J.; Qian, Z.; Gu, X.; Ahamed, M.J.; Rondeau-Gagné, S. Modulating the thermomechanical properties and self-healing efficiency of siloxane-based soft polymers through metal–ligand coordination. New J. Chem. 2020, 44, 8977–8985. [Google Scholar] [CrossRef]
- Wang, N.; Feng, L.; Xu, X.-D.; Feng, S. Dynamic Covalent Bond Cross-Linked Luminescent Silicone Elastomer with Self-Healing and Recyclable Properties. Macromol. Rapid Commun. 2022, 43, 2100885. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Huang, Y.; Yuan, Y.; Wang, Y.; Yang, B.; Zhao, X.; Liu, L. Development of a Strong, Recyclable Poly(dimethylsiloxane) Elastomer with Autonomic Self-Healing Capabilities and Fluorescence Response Properties at Room Temperature. Macromol. Mater. Eng. 2021, 306, 2100132. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, Z.; Song, C.; Gao, G.; Wu, Y. A type of self-healable, dissoluble and stretchable organosilicon elastomer for flexible electronic devices. Eur. Polym. J. 2020, 134, 109857. [Google Scholar] [CrossRef]
- Lai, P.; Yuan, Y.; Huang, Y.; Bai, H.; Zhou, Z.; Tang, C.; Wen, J.; Liu, L. Preparation of Robust, Room-Temperature Self-Healable and Recyclable Polysiloxanes Based on Hierarchical Hard Domains. Adv. Eng. Mater. 2023, 25, 2300129. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk Composites, Surface Engineering, and Precision Fabrication. Adv. Sci. 2023, 10, 2304506. [Google Scholar] [CrossRef]
- Benas, J.-S.; Liang, F.-C.; Venkatesan, M.; Yan, Z.-L.; Chen, W.-C.; Han, S.-T.; Zhou, Y.; Kuo, C.-C. Recent development of sustainable self-healable electronic skin applications, a review with insight. Chem. Eng. J. 2023, 466, 142945. [Google Scholar] [CrossRef]
- Guo, Q.; Qiu, X.; Zhang, X. Recent Advances in Electronic Skins with Multiple-Stimuli-Responsive and Self-Healing Abilities. Materials 2022, 15, 1661. [Google Scholar] [CrossRef]
- Wu, Q.; Xiong, H.; Peng, Y.; Yiang, Y.; Kang, J.; Huang, G.; Ren, X.; Wu, J. Highly Stretchable and Self-Healing “Solid–Liquid” Elastomer with Strain-Rate Sensing Capability. ACS Appl. Mater. Interfaces 2019, 11, 19534–19540. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Li, Z.; Wang, K.; Jiang, Y.; Tian, M.; Qin, Y.; Gong, Y.; Li, Z.; Wu, L. Ultrafast self-healing and self-adhesive polysiloxane towards reconfigurable on-skin electronics. J. Mater. Chem. A 2022, 10, 1750–1759. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Dong, Y.; Li, J. Flexible Self-Repairing Materials for Wearable Sensing Applications: Elastomers and Hydrogels. Macromol. Rapid Commun. 2020, 41, 2000444. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, L.; Cheng, Q.; Zhang, T.; Jiang, B.; Song, Y.; Huang, Y. Self-Healing Polysiloxane Elastomer Based on Integration of Covalent and Reversible Networks. Ind. Eng. Chem. Res. 2019, 58, 21504–21512. [Google Scholar] [CrossRef]
- Mai, D.; Mo, J.; Shan, S.; Lin, Y.; Zhang, A. Self-Healing, Self-Adhesive Strain Sensors Made with Carbon Nanotubes/Polysiloxanes Based on Unsaturated Carboxyl–Amine Ionic Interactions. ACS Appl. Mater. Interface 2021, 13, 49266–49278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Deng, L.; Yang, G. Self-Healable Elastomeric Network with Dynamic Disulfide, Imine, and Hydrogen Bonds for Flexible Strain Sensor. Chem. A Eur. J. 2023, 29, e202203478. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.S.H. (Ed.) Handbook of Smart Coatings for Materials Protection; Woodhead Publishing Ltd.: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Shamshiri, M.; Jafari, R.; Momen, G. Potential use of smart coatings for icephobic applications: A review. Surf. Coat. Technol. 2021, 424, 127656. [Google Scholar] [CrossRef]
- Jiao, Y.; Rong, Z.; Gao, C.; Wu, Y.; Liu, Y. Tannic Acid Crosslinked Self-Healing and Reprocessable Silicone Elastomers with Improved Antibacterial and Flame Retardant Properties. Macromol. Rapid. Commun. 2022, 44, 2200681. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Hölken, I.; Gapeeva, A.; Filiz, V.; Adelung, R.; Baum, M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials 2018, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Fouling resistant silicone coating with self-healing induced by metal coordination. Chem. Eng. J. 2021, 406, 126870. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, Q.; Fu, Q.; Fu, H. Self-healable ZnO@ multiwalled carbon nanotubes (MWCNTs) /DA-PDMS nanocomposite via Diels-Alder chemistry as microwave absorber: A novel multifunctional material. Carbon 2020, 169, 235–247. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, C.; Song, J.; Song, C.; Wang, Z.; Wu, Y.; Liu, Y.; Sun, J. A type of dissoluble organosilicon elastomer with stretchable, self-healable and antibacterial properties. Polym. Test. 2021, 96, 107082. [Google Scholar] [CrossRef]
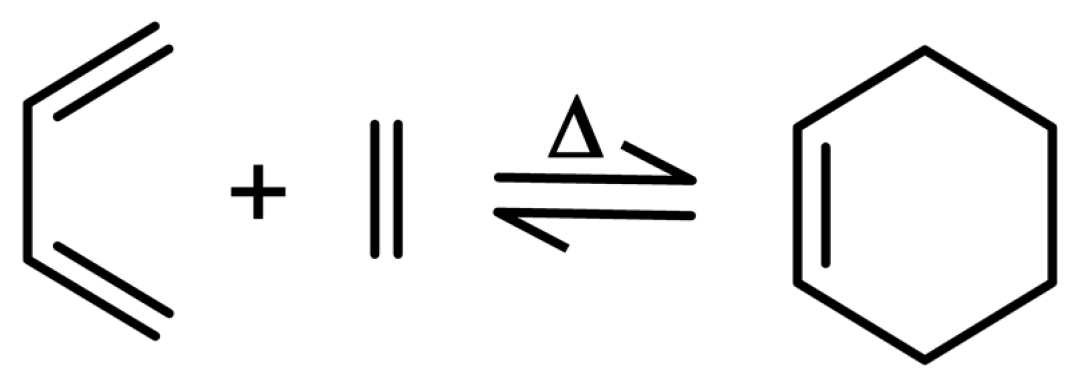

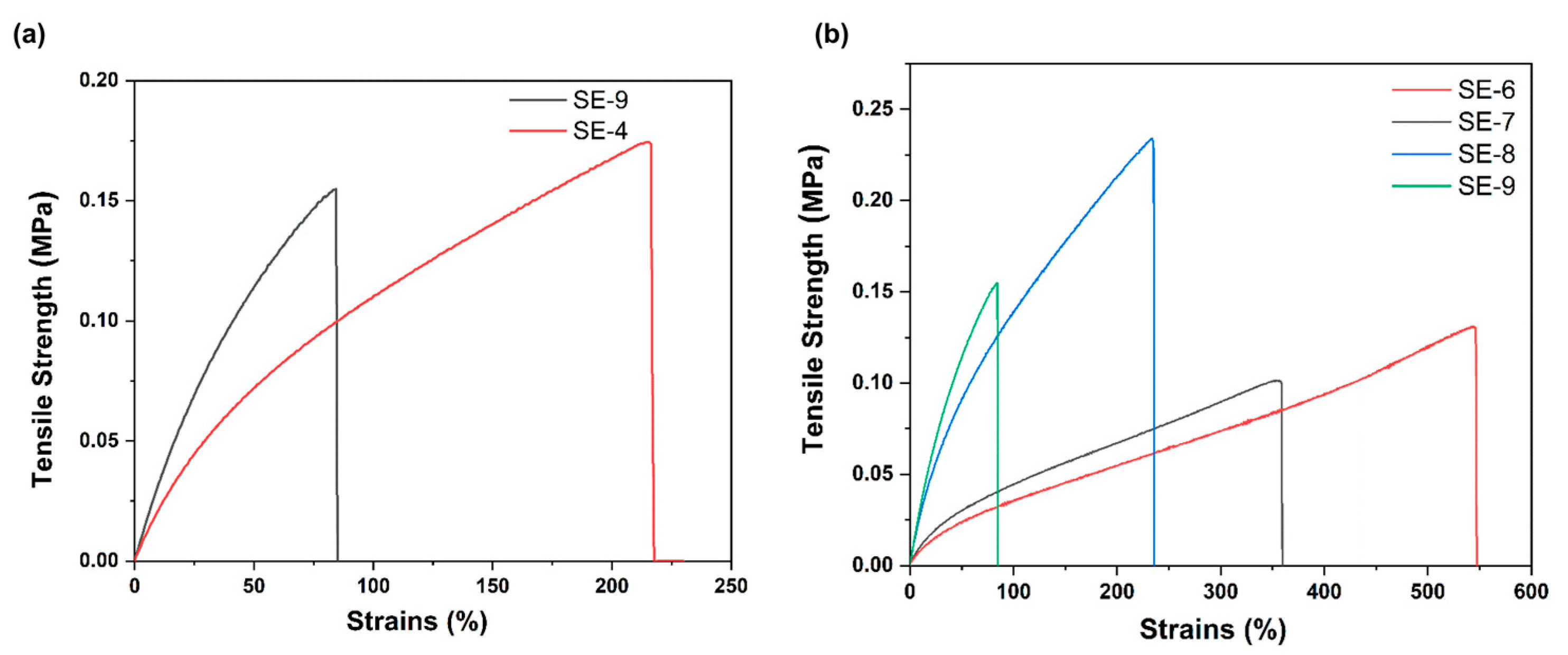
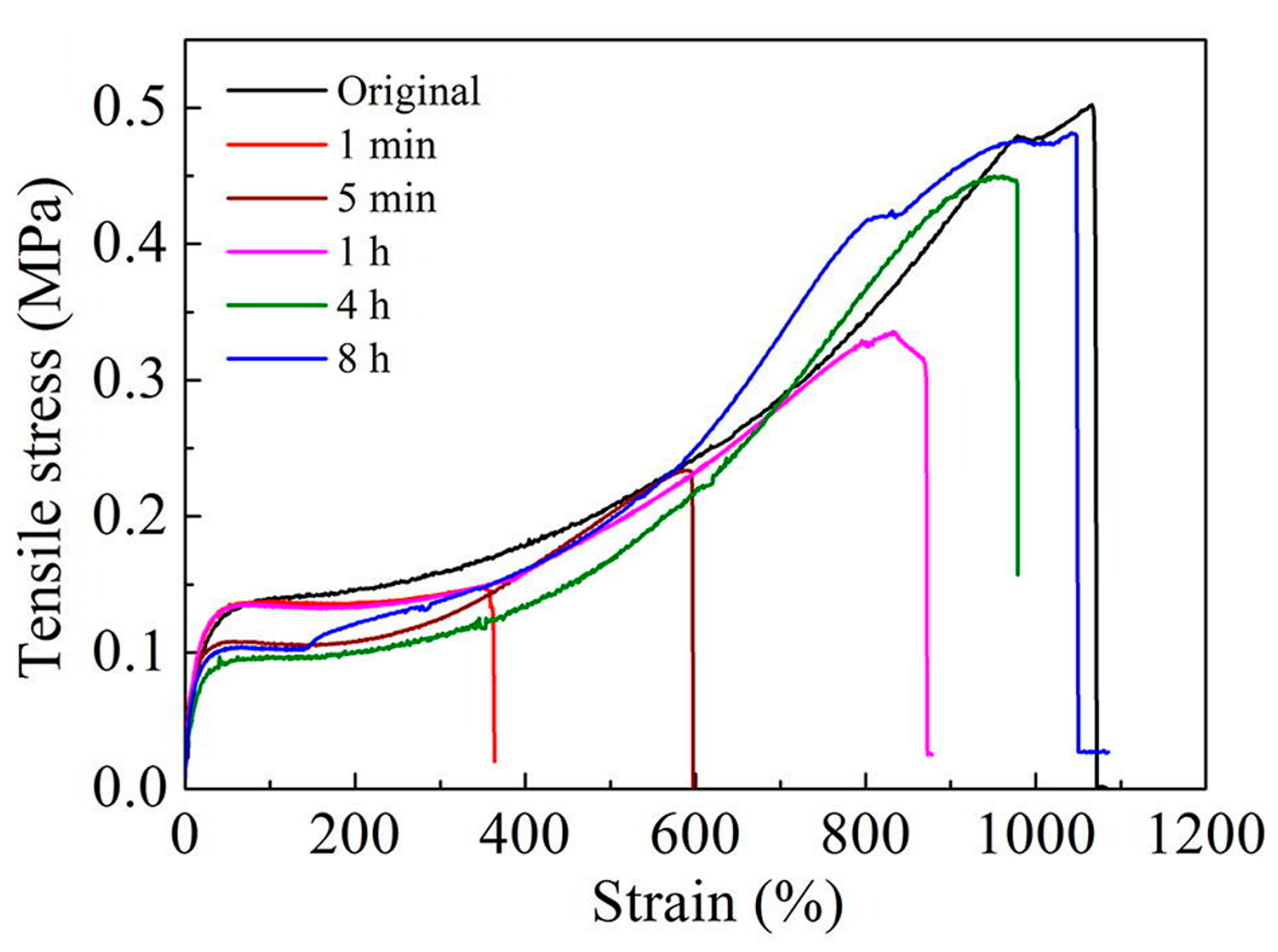
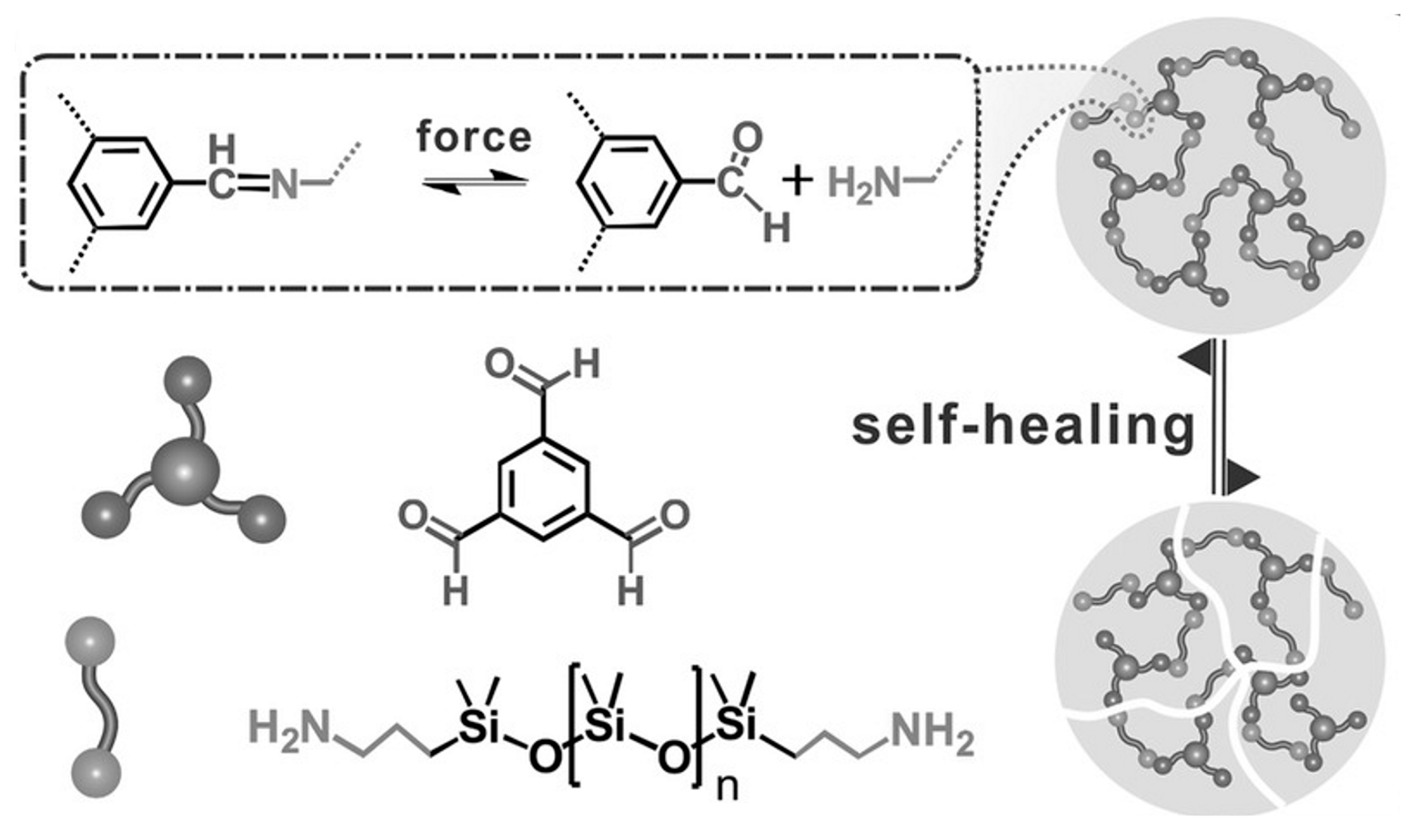
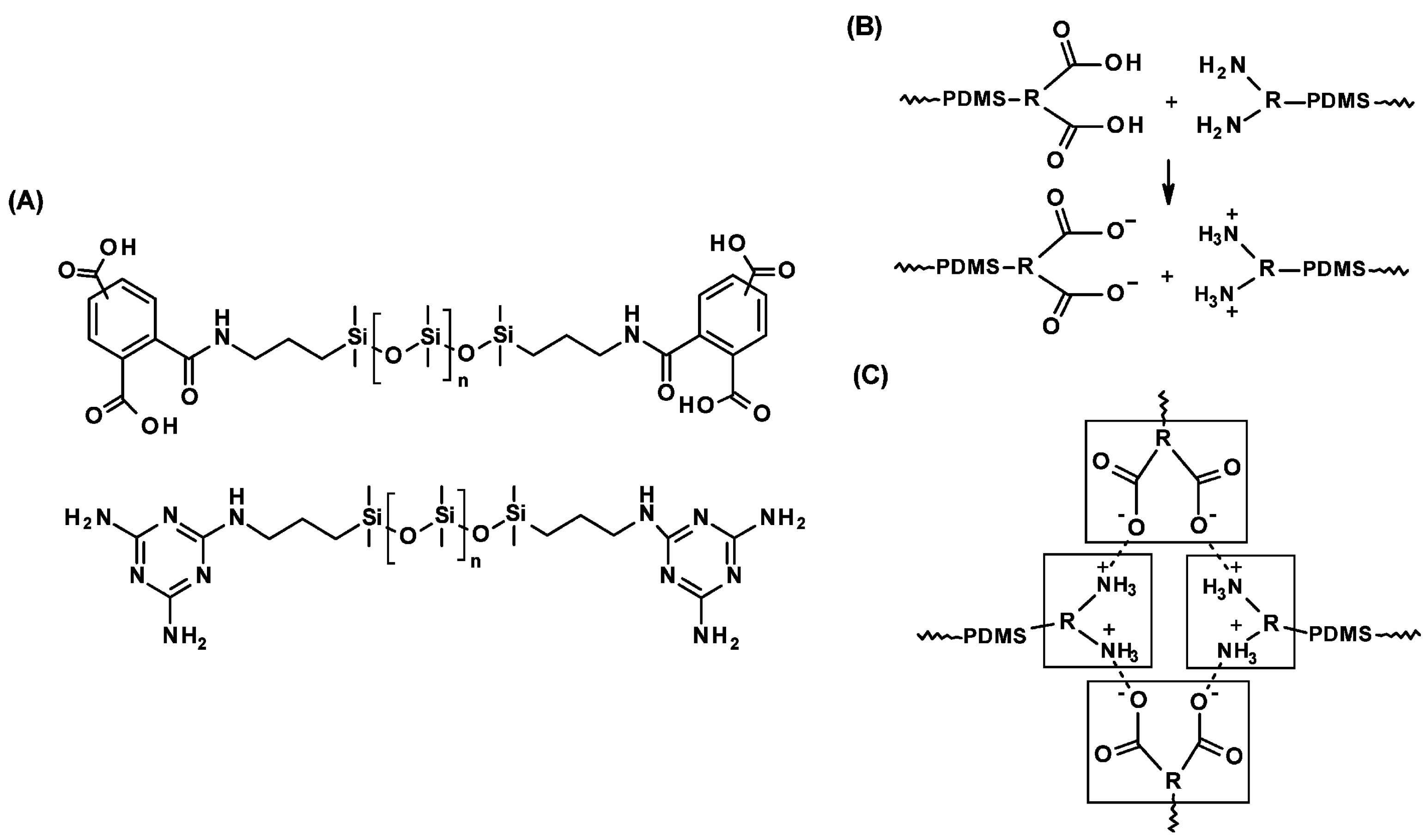
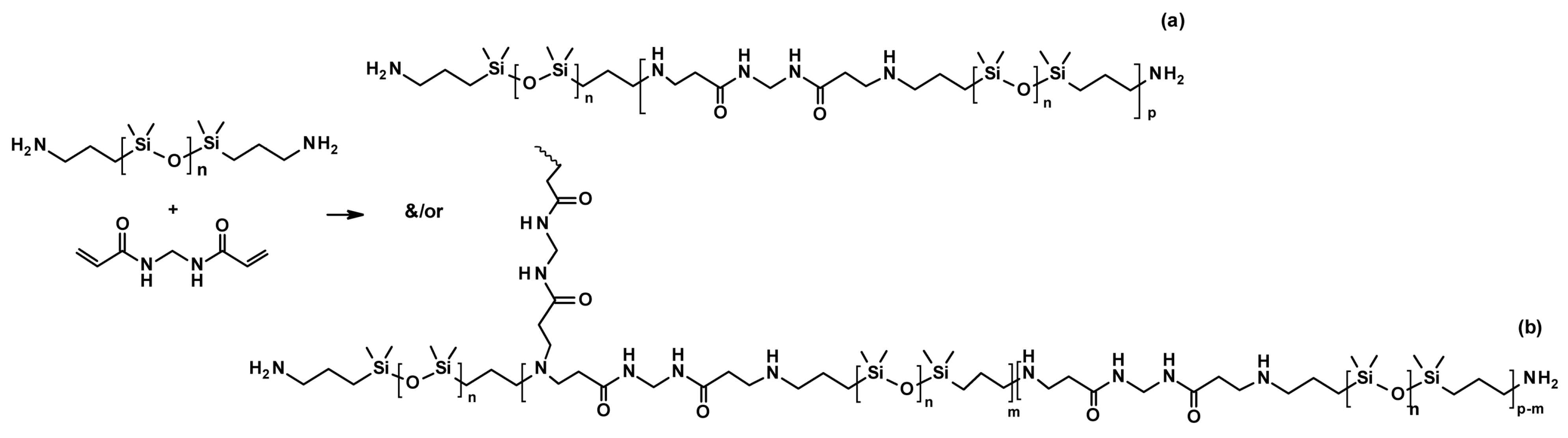

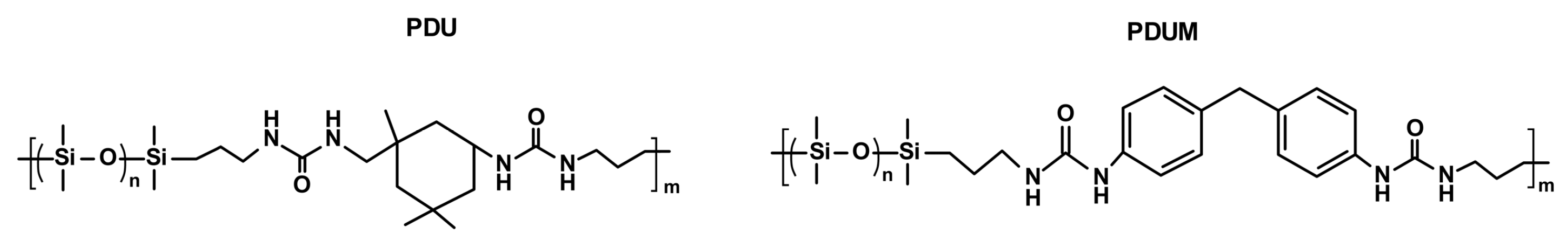

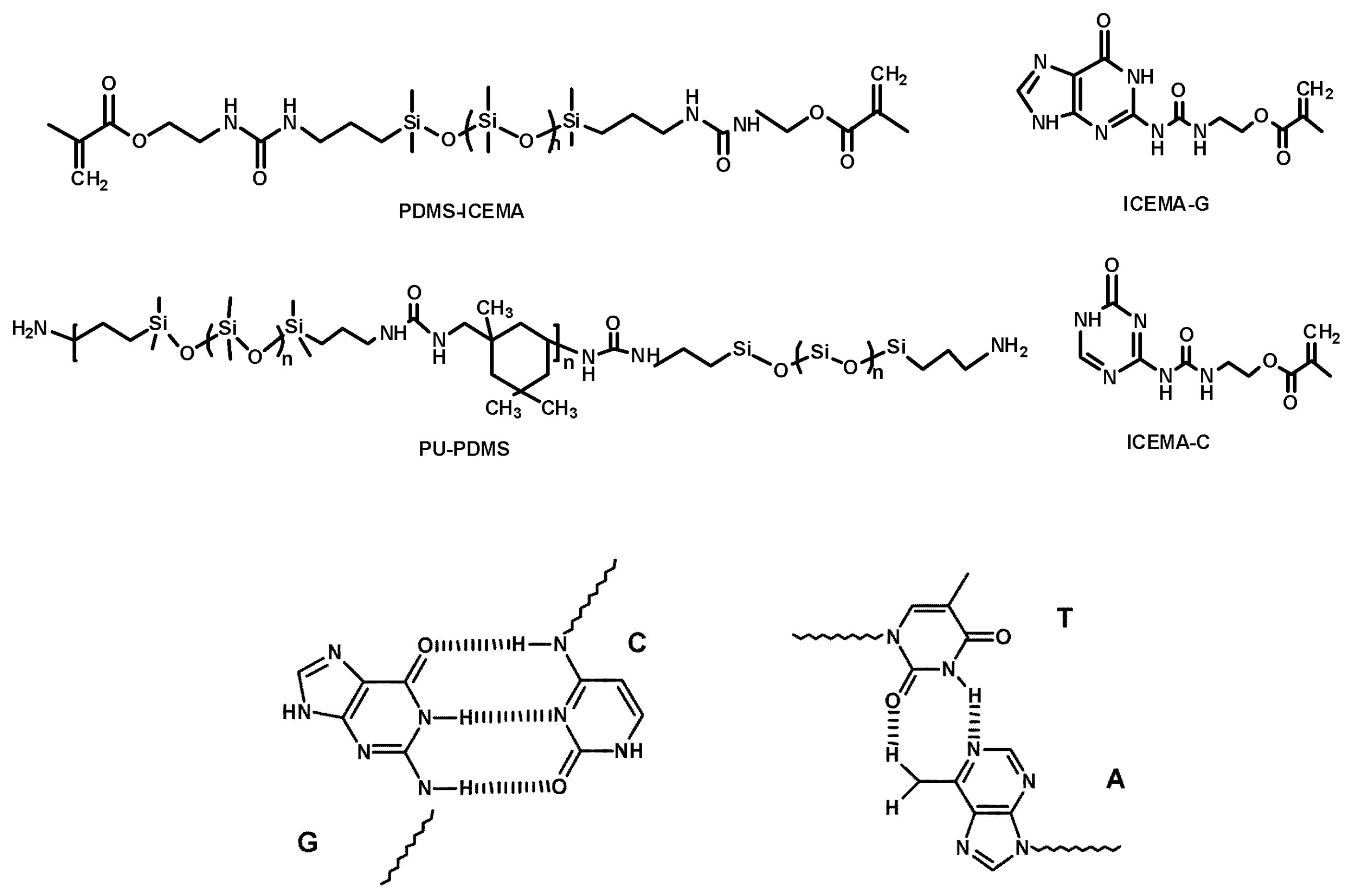

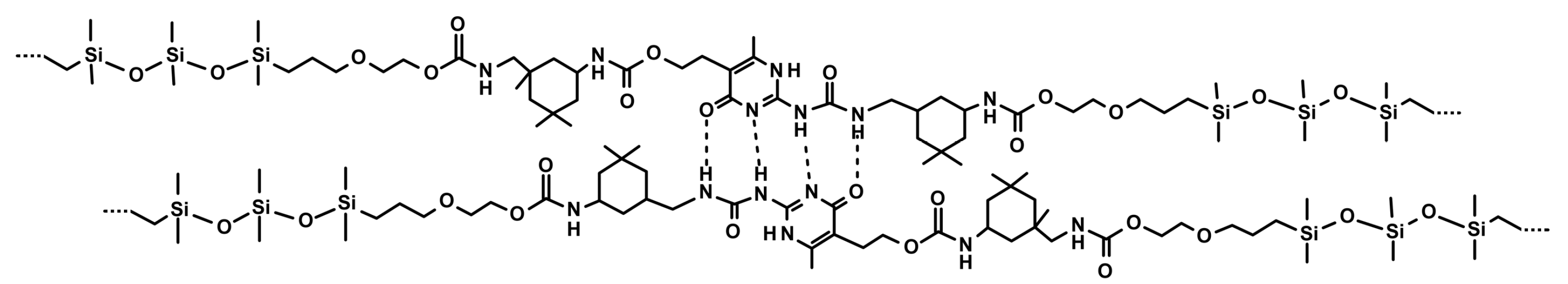
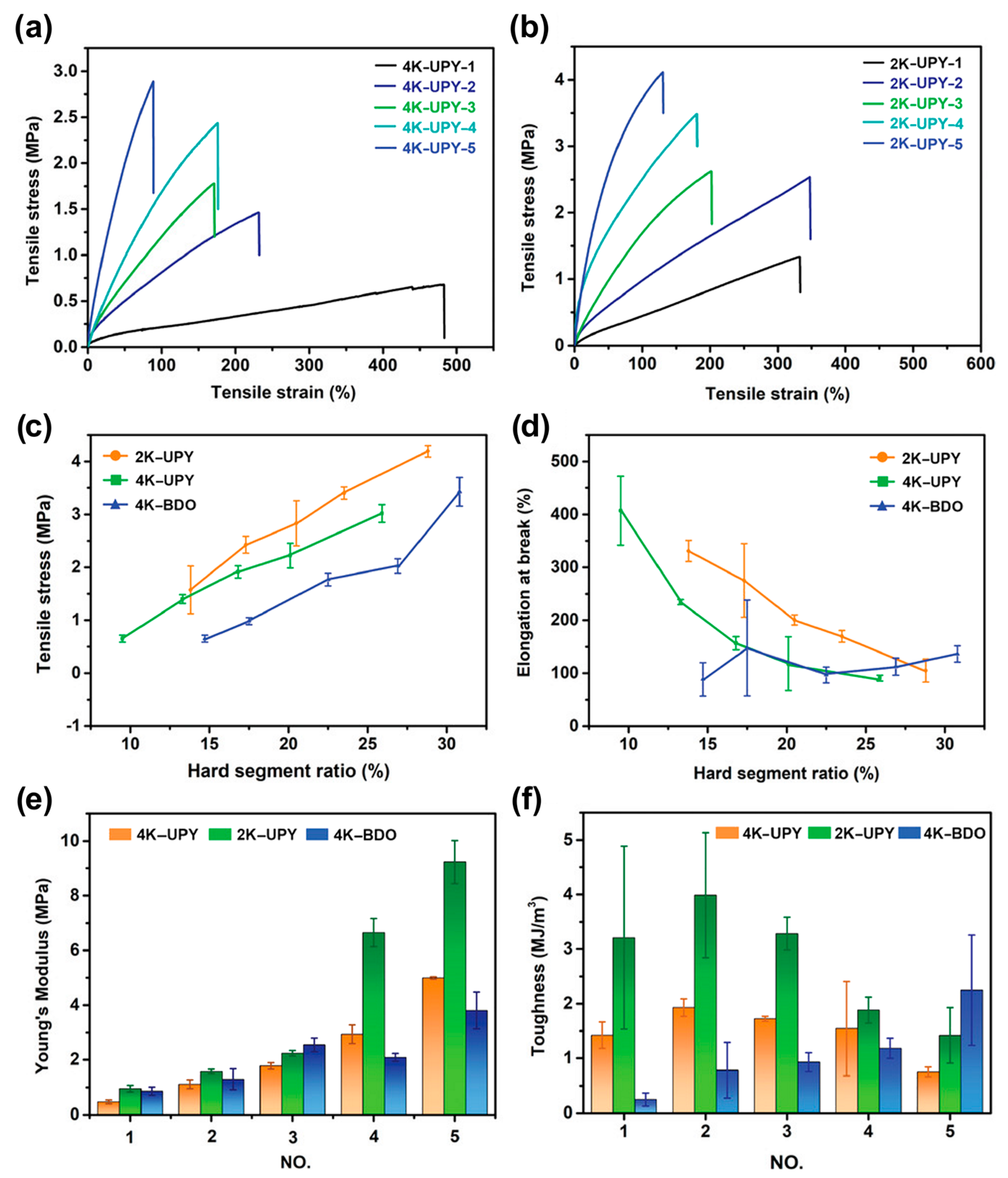
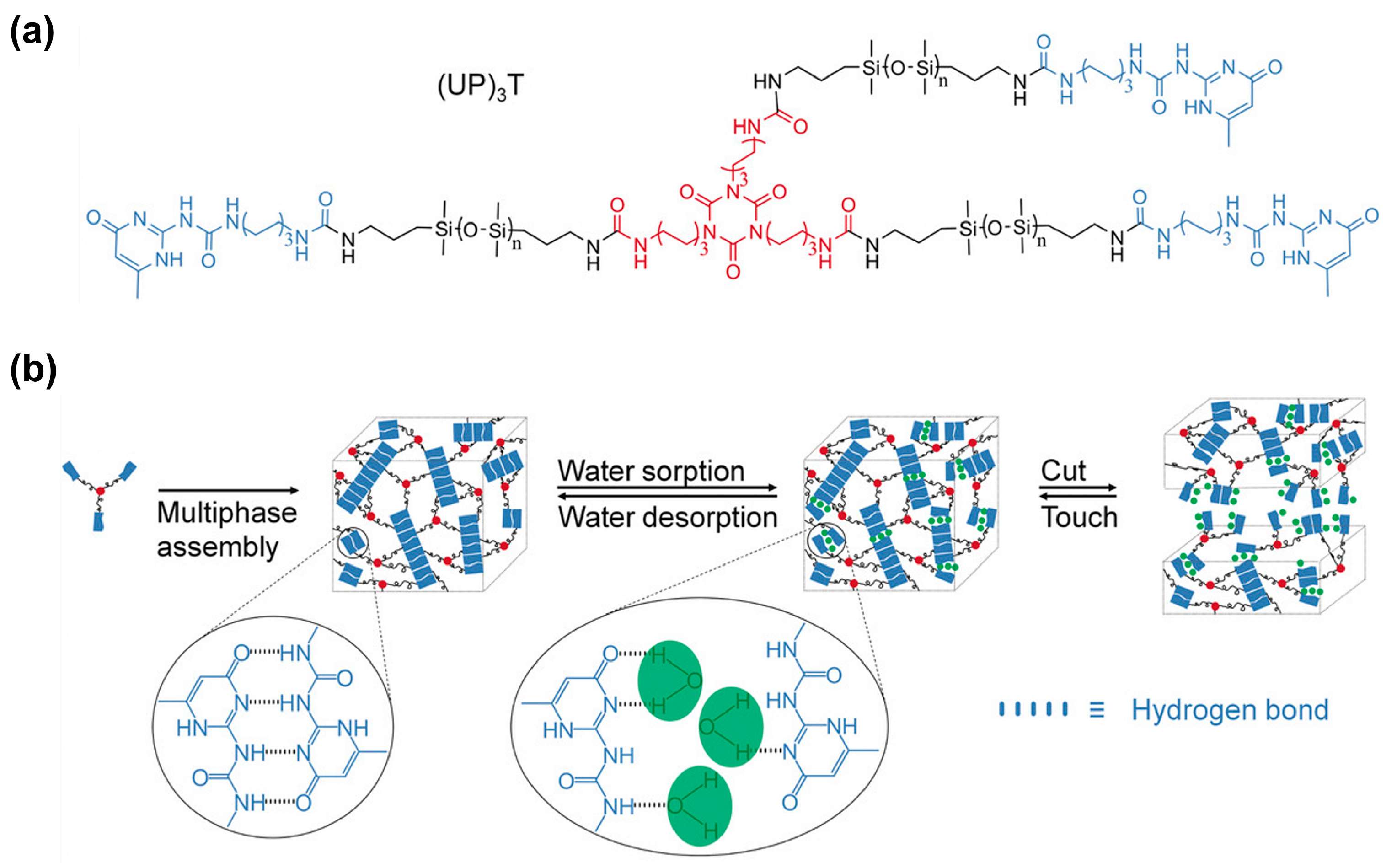

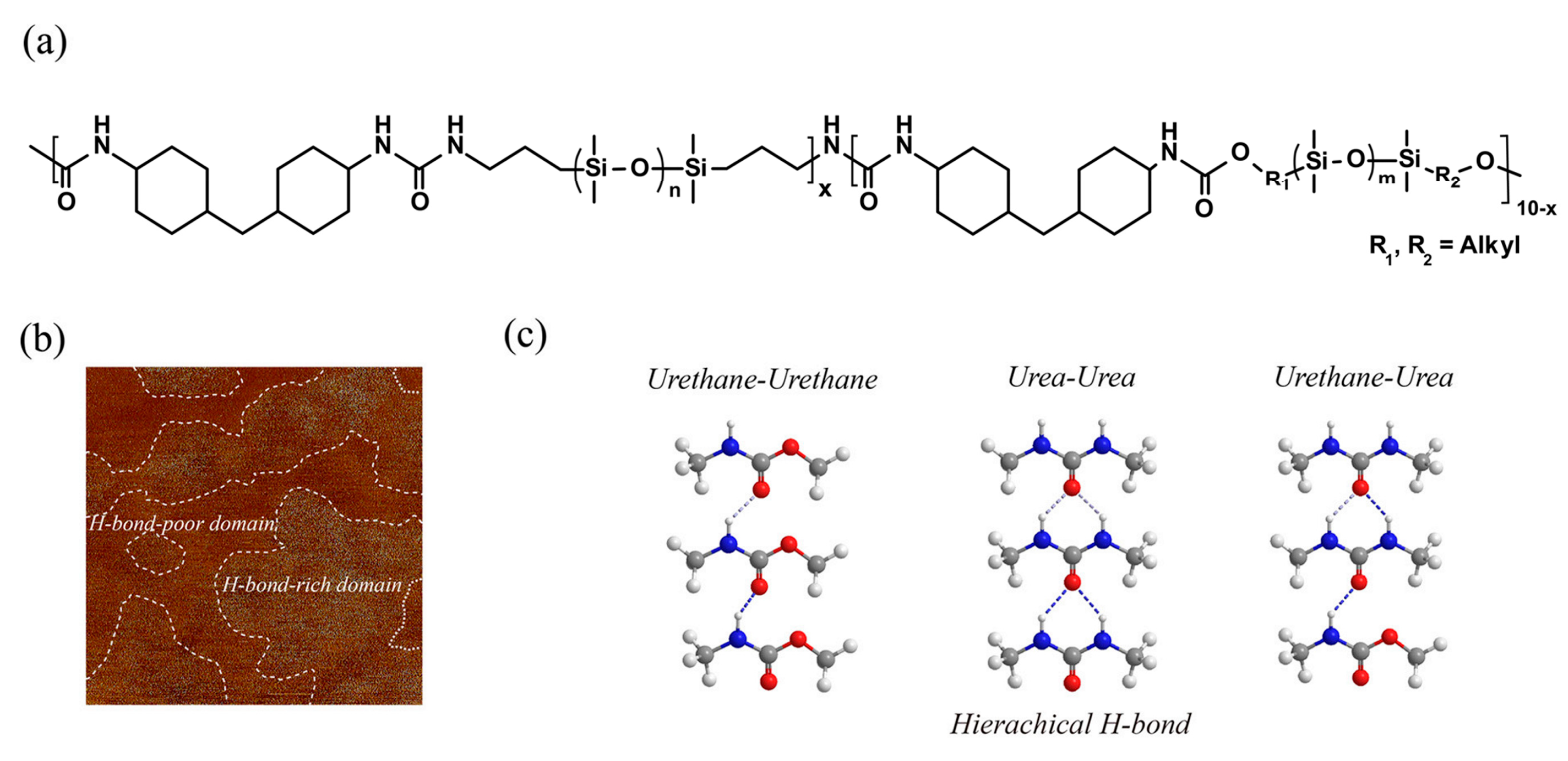
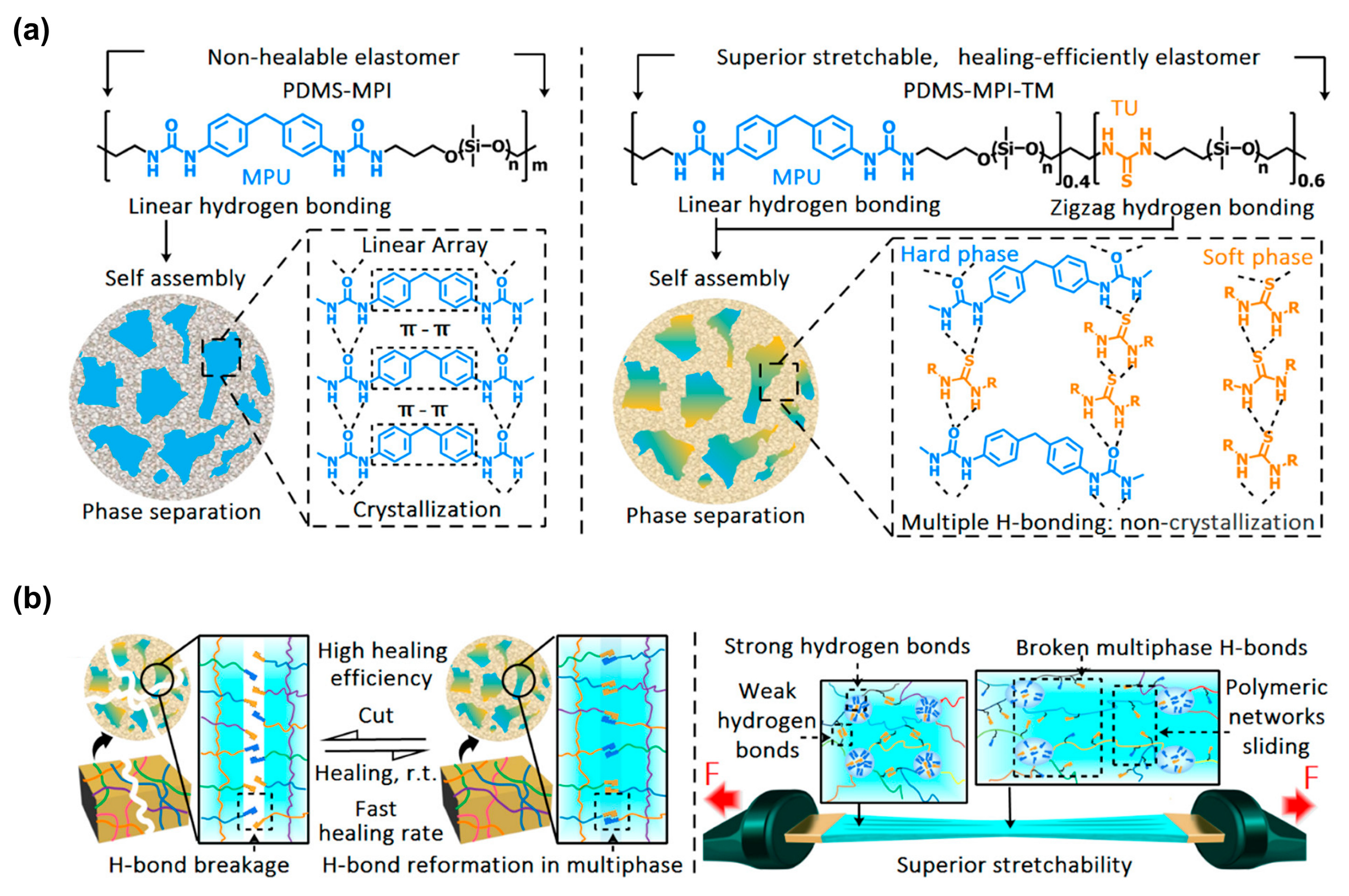
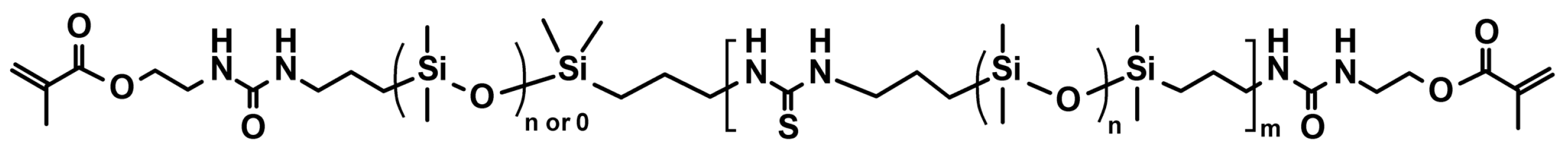
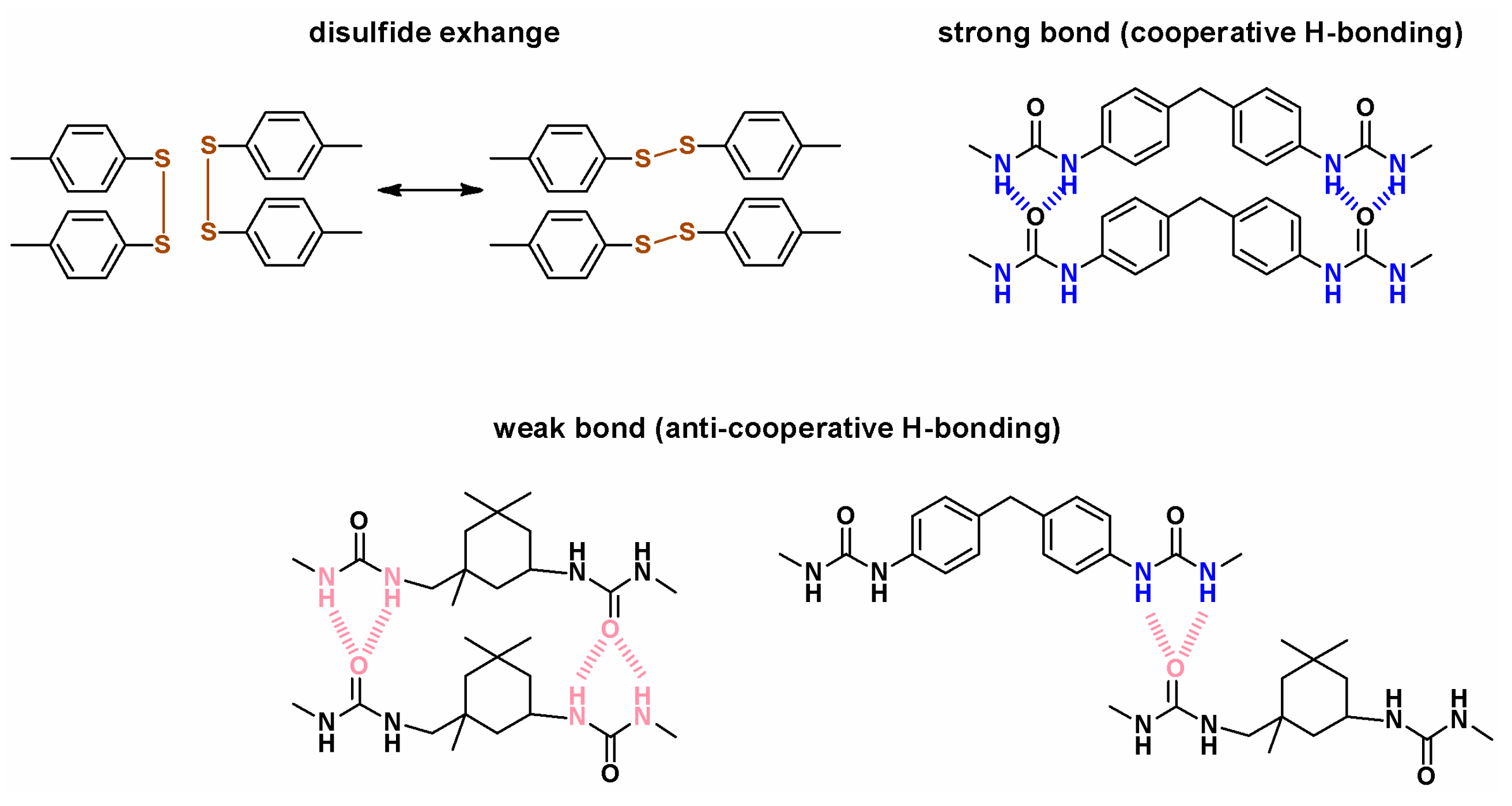




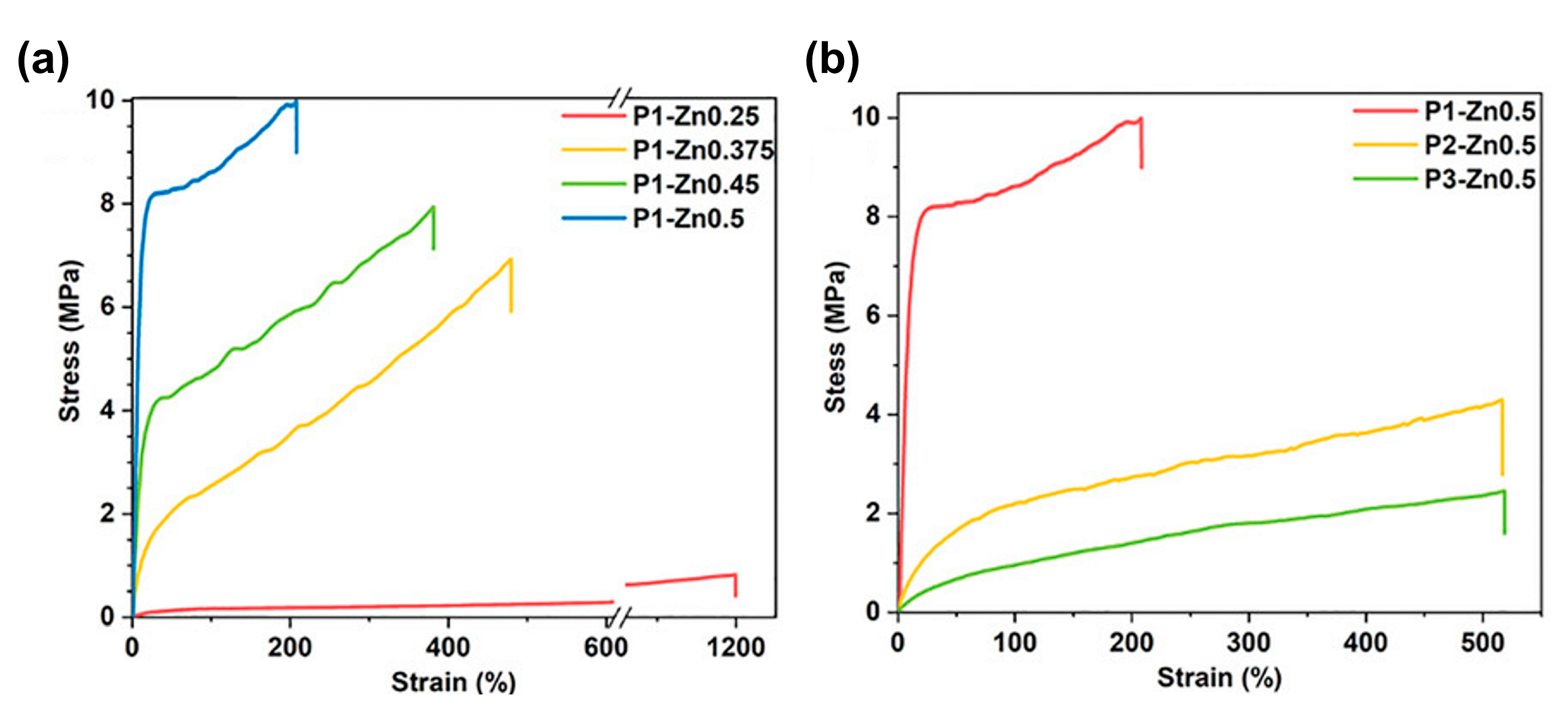

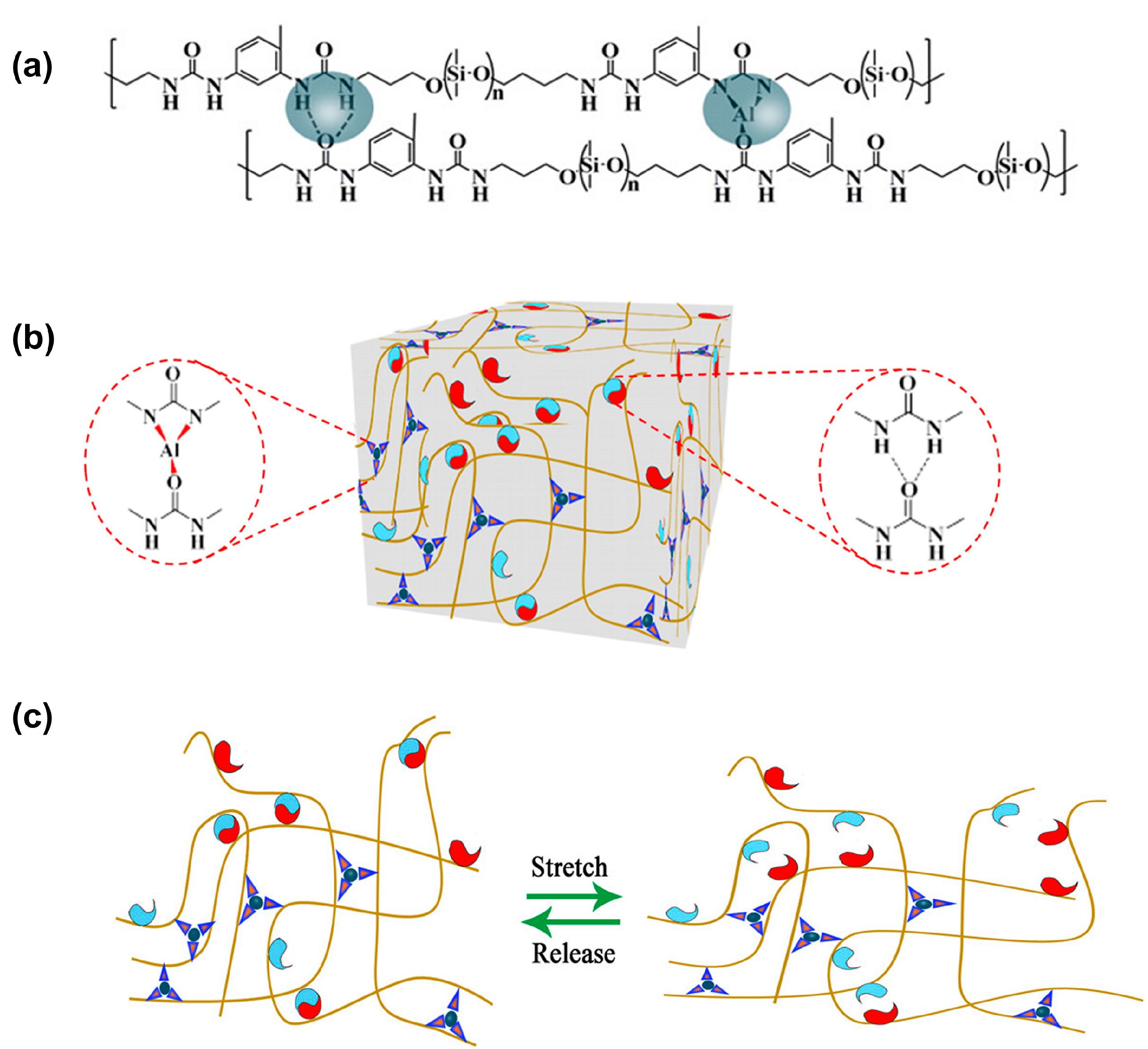
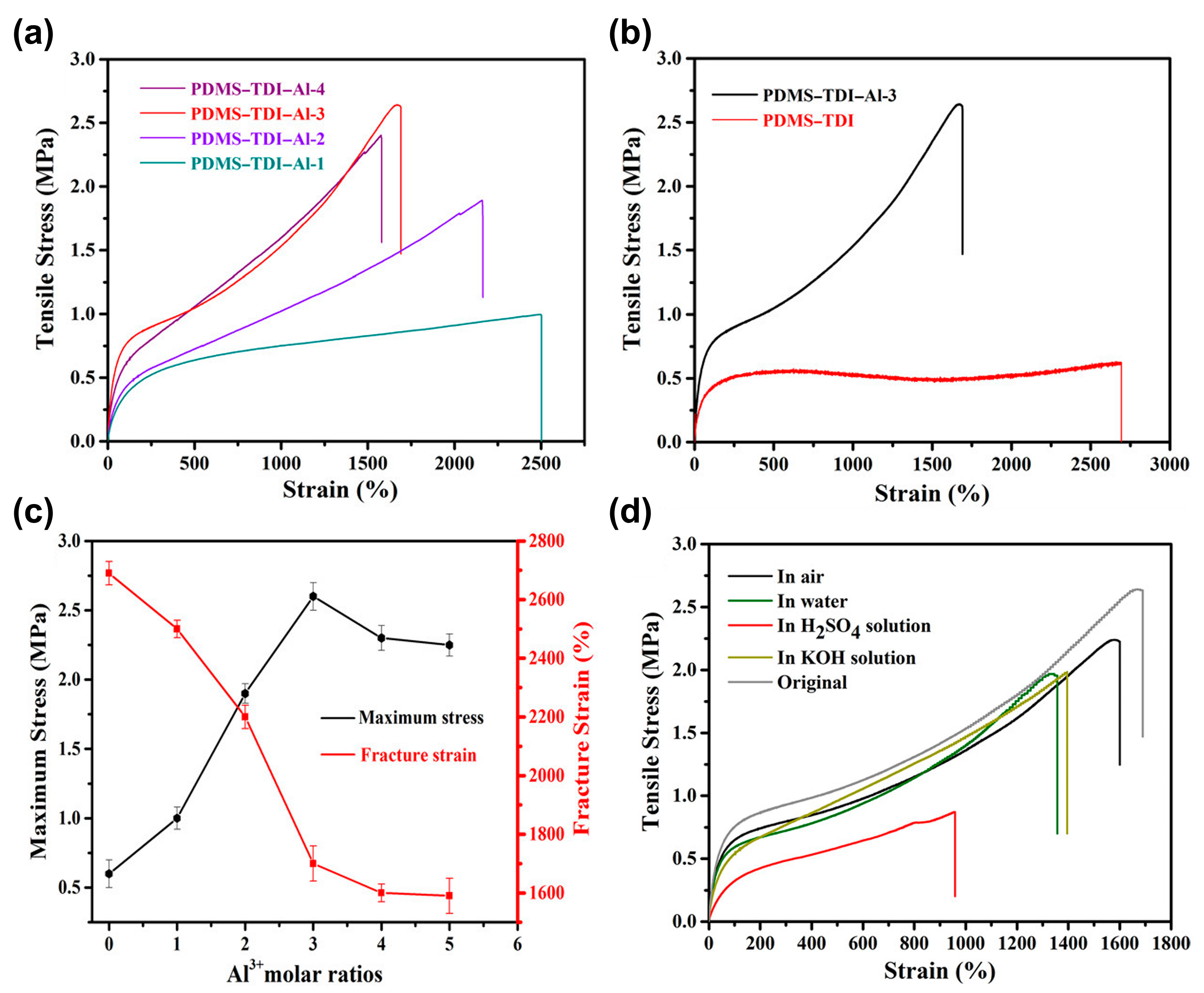
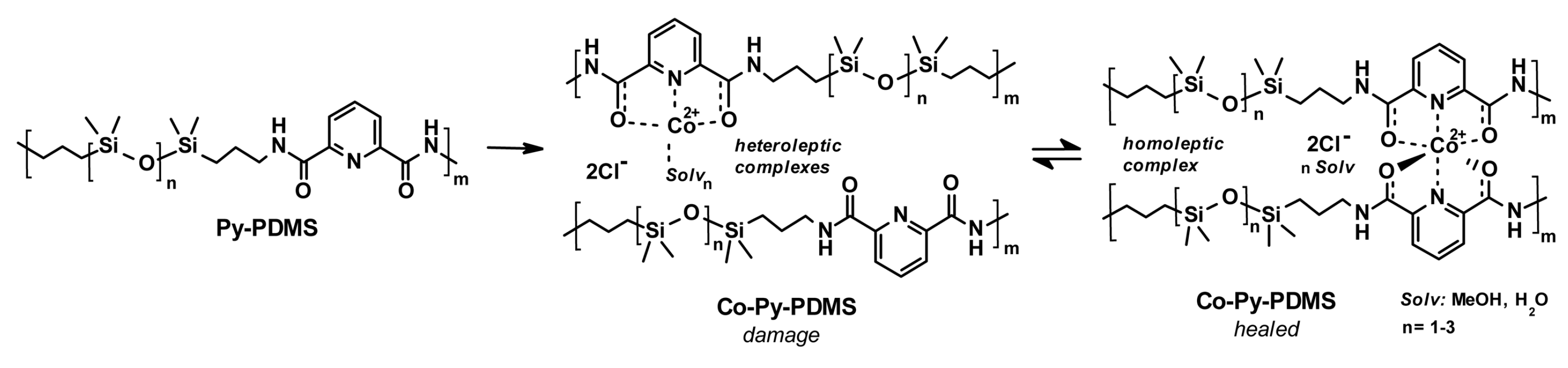

| Sample | DOPA/IPDI/PDMS (mmol) | Mn | U Units | Cat Units | Modulus (MPa) | Hardness (MPa) |
|---|---|---|---|---|---|---|
| PDMS-Cat1-Zn | 1/5.5/5 | 4450 | 6 | 2 | 172.0 | 10.7 |
| PDMS-Cat2-Zn | 0.5/5.25/5 | 8387 | 9 | 2 | 136.7 | 7.0 |
| PDMS-Cat3-Zn | 0.25/5.125/5 | 20,640 | 20 | 2 | 90.2 | 4.5 |
| PDMS-Cat1-Ca | 1/5.5/5 | 4450 | 6 | 2 | 162.5 | 12.4 |
| PDMS-Cat1-Co | 1/5.5/5 | 4450 | 6 | 2 | 166.9 | 13.1 |
| PDMS-Cat1-Fe | 1/5.5/5 | 4450 | 6 | 2 | 190.3 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalewska, A.; Majewska-Smolarek, K. Synergistic Self-Healing Enhancement in Multifunctional Silicone Elastomers and Their Application in Smart Materials. Polymers 2024, 16, 487. https://doi.org/10.3390/polym16040487
Kowalewska A, Majewska-Smolarek K. Synergistic Self-Healing Enhancement in Multifunctional Silicone Elastomers and Their Application in Smart Materials. Polymers. 2024; 16(4):487. https://doi.org/10.3390/polym16040487
Chicago/Turabian StyleKowalewska, Anna, and Kamila Majewska-Smolarek. 2024. "Synergistic Self-Healing Enhancement in Multifunctional Silicone Elastomers and Their Application in Smart Materials" Polymers 16, no. 4: 487. https://doi.org/10.3390/polym16040487
APA StyleKowalewska, A., & Majewska-Smolarek, K. (2024). Synergistic Self-Healing Enhancement in Multifunctional Silicone Elastomers and Their Application in Smart Materials. Polymers, 16(4), 487. https://doi.org/10.3390/polym16040487






