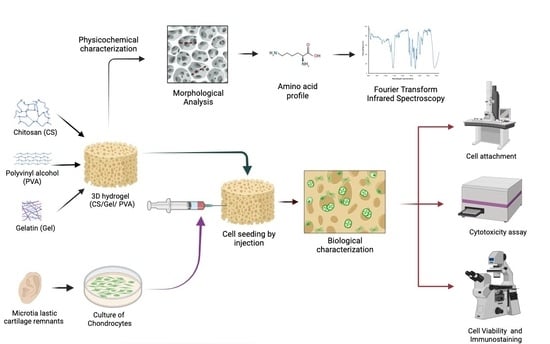
Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture
Abstract
1. Introduction
2. Materials and Methods
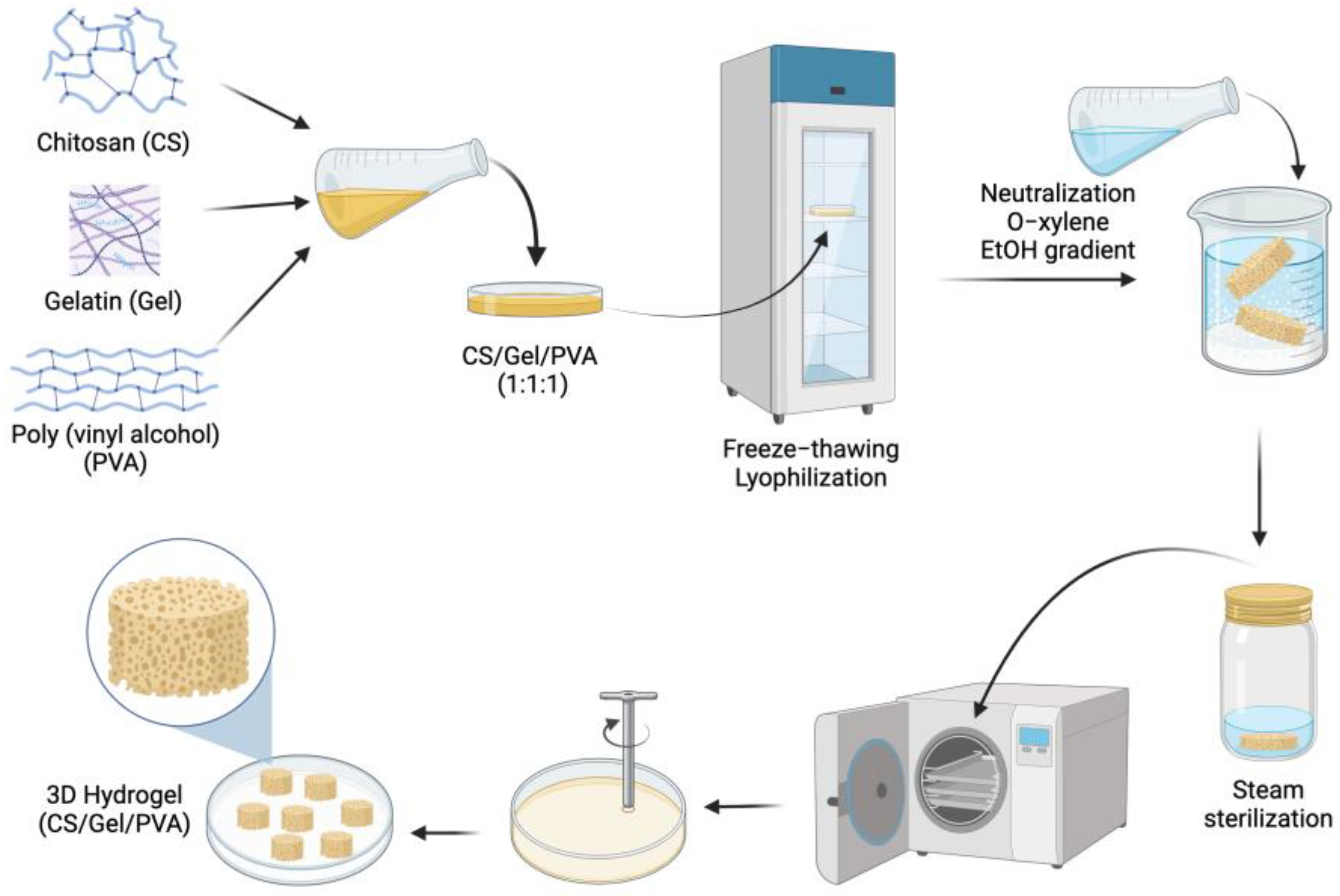
2.1. Chitosan/Gelatin/Poly(Vinyl Alcohol) Hydrogel Preparation and Sterilization
2.1.1. Ternary Polymer Solutions
2.1.2. Sterilized Hydrogel Preparation
2.2. Characterization of Chitosan/Gelatin/Poly(Vinyl Alcohol) Hydrogels
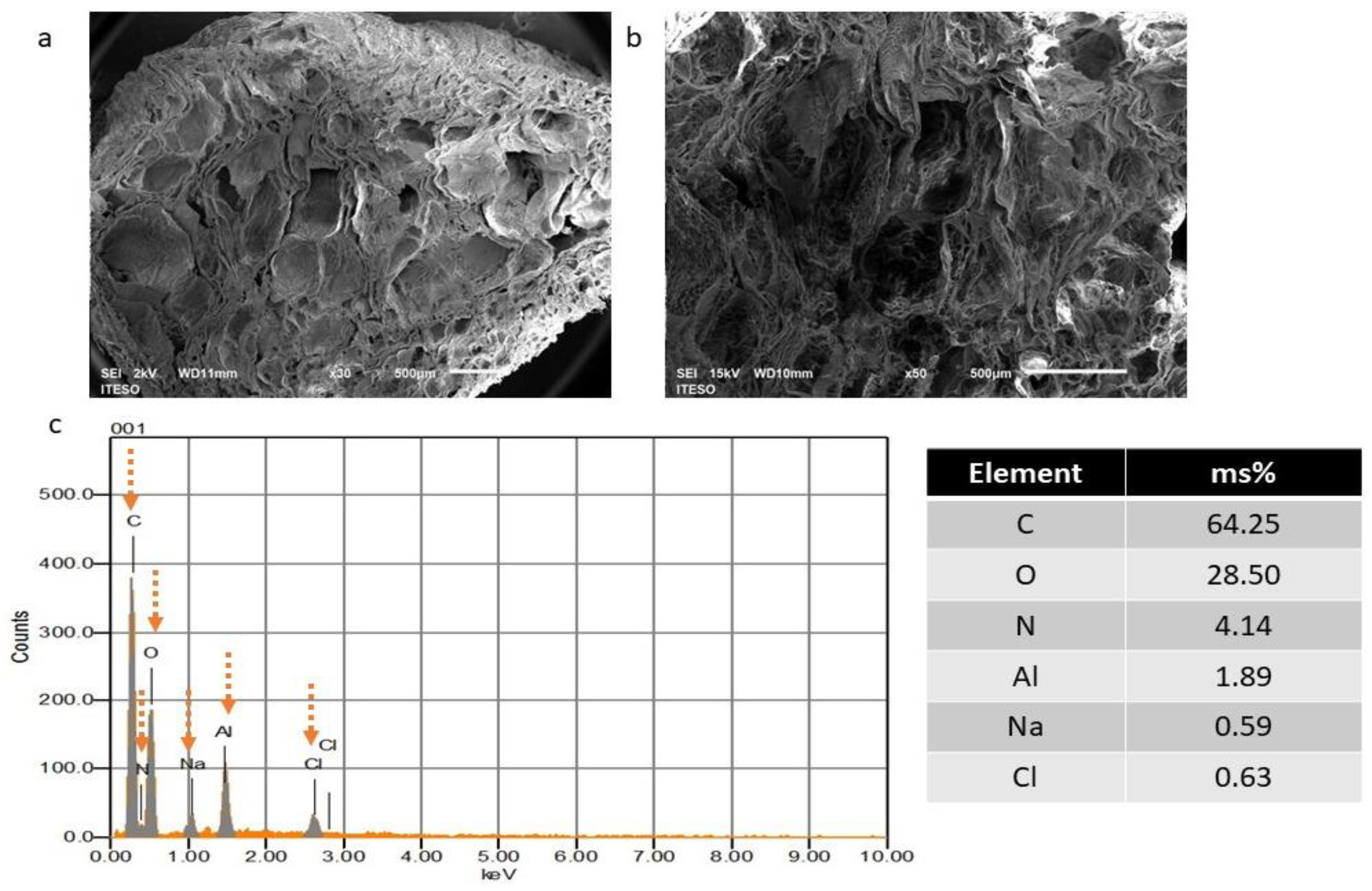
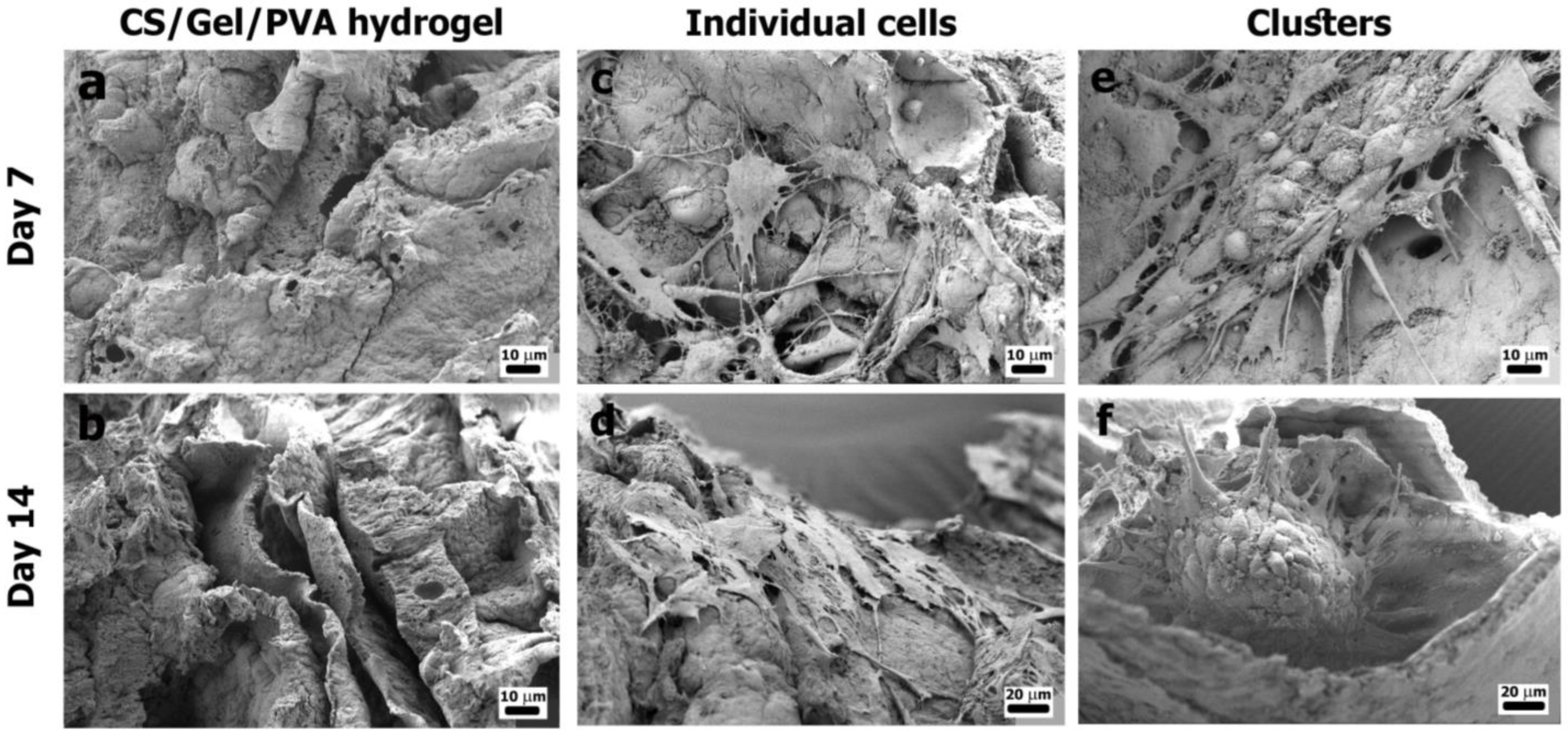
2.2.1. Morphological Analysis
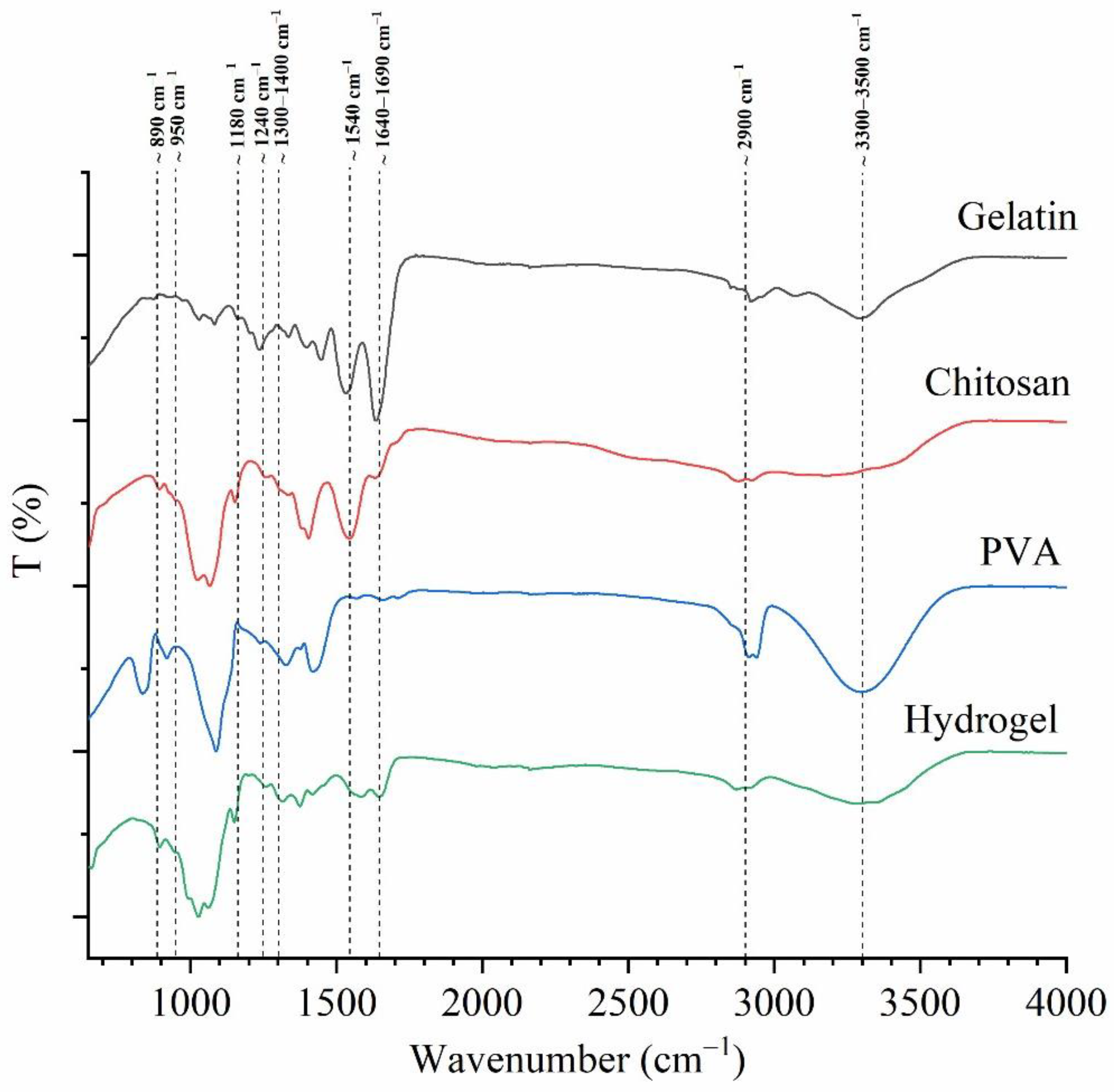
2.2.2. Fourier-Transform Infrared Spectroscopy
2.2.3. Amino Acid Profile
2.3. Culture of Chondrocytes in Chitosan/Gelatin/Poly(Vinyl Alcohol) Hydrogels
2.3.1. Isolation and Culture of Human Elastic Chondrocytes
2.3.2. Cytotoxicity Assay
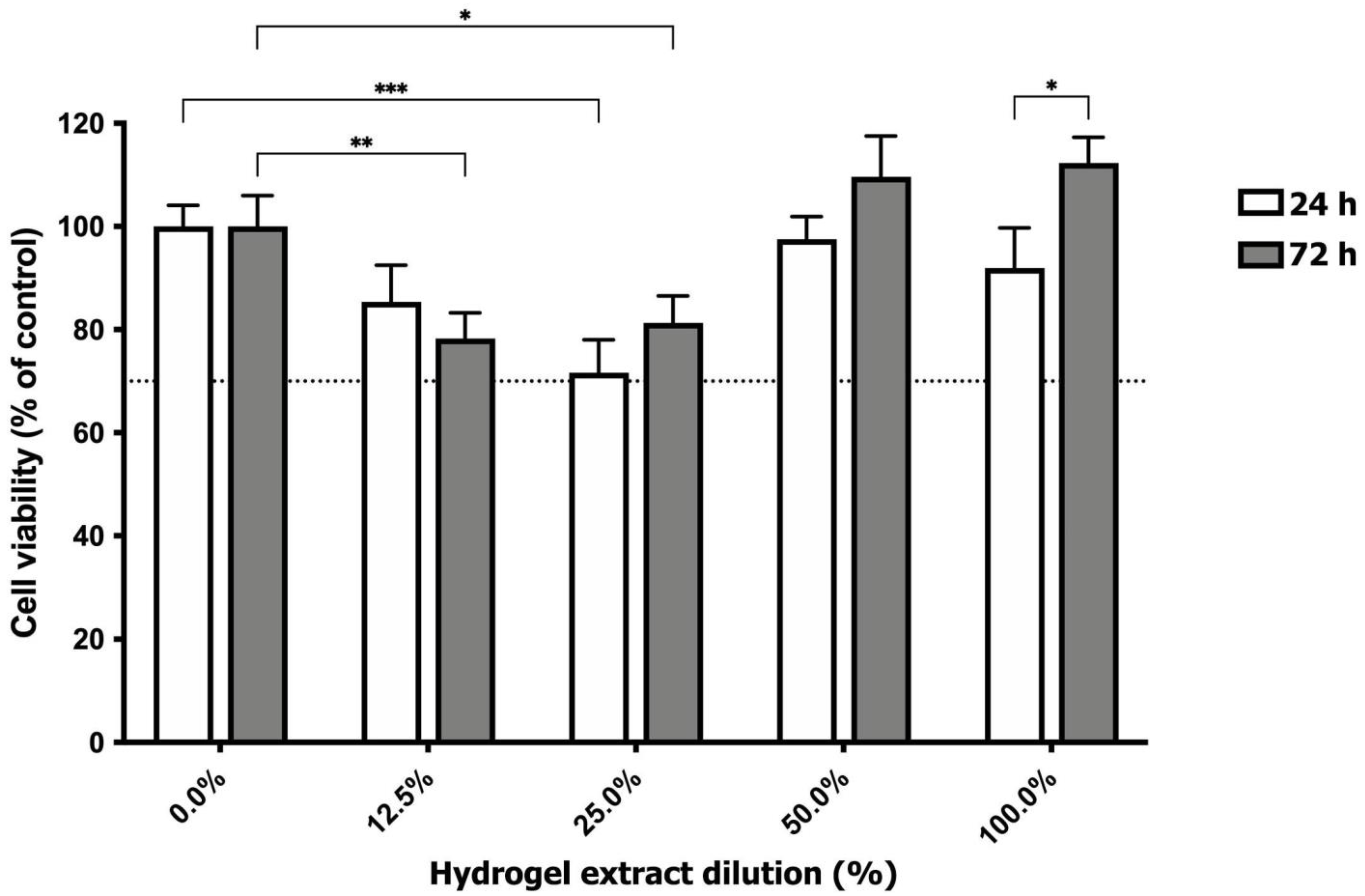
2.3.3. Cell Viability Assay
2.3.4. Cell Attachment
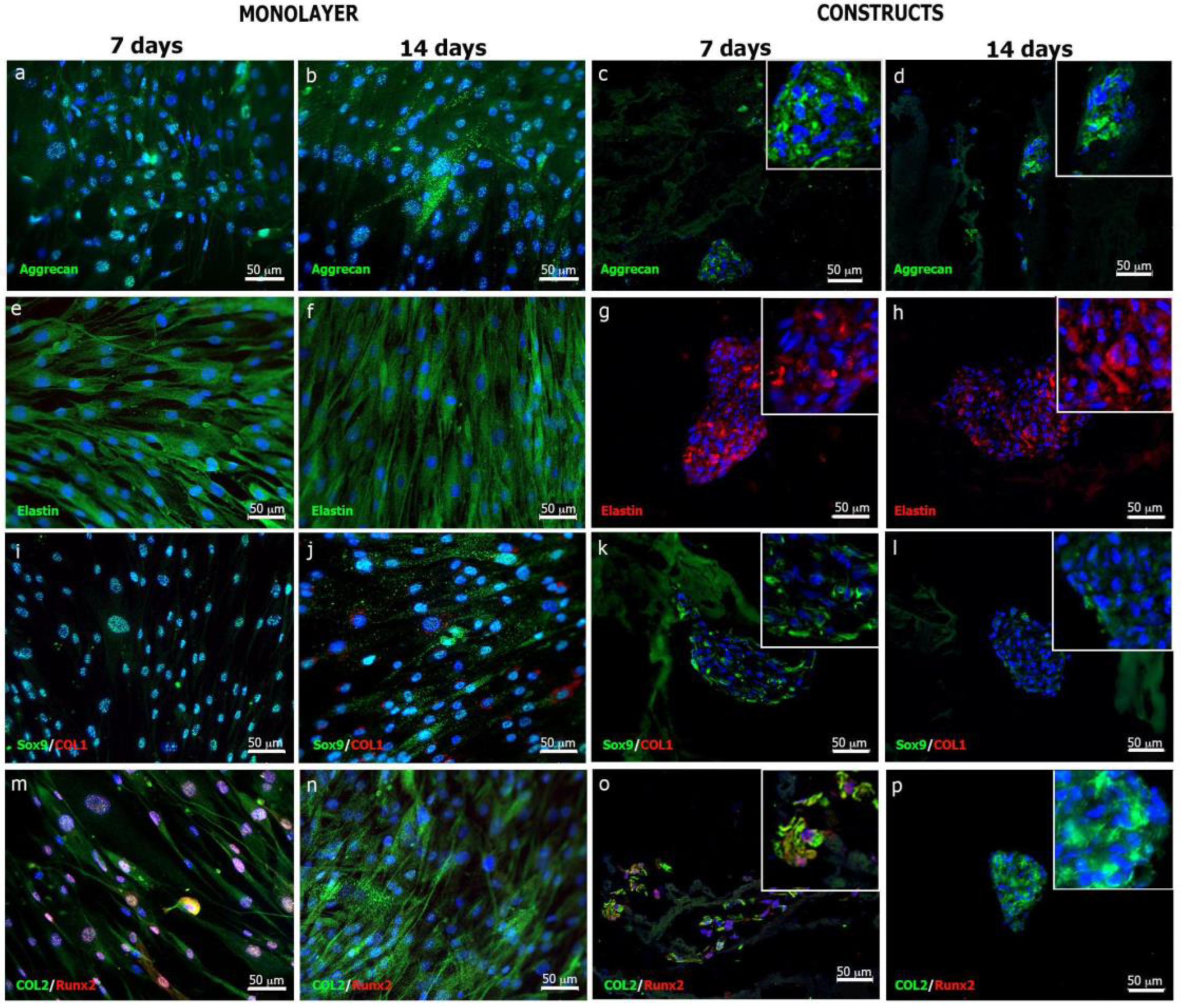
2.3.5. Immunofluorescence
3. Results and Discussion
3.1. Chitosan/Gelatin/Poly(Vinyl Alcohol) Hydrogel Preparation
3.2. Characterization of Chitosan/Gelatin/Poly(Vinyl Alcohol) Hydrogels
3.2.1. Morphological Analysis
3.2.2. Fourier-Transform Infrared Spectroscopy
| Polymer | Peak Region (Wavenumber cm−1) | Assignment | Reference |
|---|---|---|---|
| Chitosan | ~1655–1649 | Amide I: C=O stretch | [14] |
| ~1650–1580 | NH2 deformation | [14] | |
| ~1570–1510 (1554) | Amide II: N–H deformation and C–N stretching | [14] | |
| ~1625–1500 1560, 1548 | NH3+ deformation Symmetric deformation | [14] | |
| ~1420 | O–H and C–H deformation (ring) | [14] | |
| ~1383–1377 | CH3 deformation (bend) | [14] | |
| ~1325–1322 | Amide III: O–H and C–H deformation | [14] | |
| ~1265–1260 | C–H wag (ring) | [14] | |
| ~ 1200–950 | C–O–C and C–O stretching | [13] | |
| ~1160–1150 | Primary or secondary alcohol | [14] | |
| ~1080 | C–O stretching | [14] | |
| ~900 | Aliphatic aldehydes/ | [14] | |
| 860–650 | CH deformation of the β-glycosidic bond | [13] | |
| Type B gelatin from bovine skin | ~ 1650–1630 | Amide I: C=O and N–H stretching /hydrogen bonding coupled with C00- | [51,52] |
| ~1632 | Intermolecular associations with imide residues | [59] | |
| ~1560–1540 | Amide II: N–H bend coupled with C–N stretch | [51] | |
| ~1460–1430 | Amide II: CH2 bend | [51] | |
| ~1250–1180 | Amide III: N–H bend | [51] | |
| ~1130–1022 | Amide III: C–O stretch | [51] | |
| ~1200–950 | Amide III: skeletal stretch | [51] | |
| ~890–650 | Amide III: skeletal stretch | [51] | |
| PVA | ~1750–1735 | C=O vibration | [53] |
| ~1640 | Water absorption | [60] | |
| ~1460–1400 | CH2 bend | [53] | |
| ~1350–1300 | Scissoring O–H, rocking with C–H, wagging | [53] | |
| ~1140–1080 | C–O (crystallinity) | [53] | |
| C–O stretching of acetyl group present in the PVA backbone and O–H bending/ the amorphous sequence of PVA | [53] | ||
| ~920 | CH2 rocking | [53] | |
| ~820 | C–C stretching/skeletal vibration | [53] |
3.2.3. Amino Acid Profile
3.3. Culture of Chondrocytes in Chitosan/Gelatin/Poly(Vinyl Alcohol) Hydrogel
3.3.1. Cytotoxicity Assay
3.3.2. Cell Viability Assay
3.3.3. Cell Attachment
3.3.4. Elastic Cartilage Extracellular Matrix Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X.; Yang, Y. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef]
- Onat, B.; Ulusan, S.; Banerjee, S.; Erel-Goktepe, I. Multifunctional layer-by-layer modified chitosan/poly(ethylene glycol) hydrogels. Eur. Polym. J. 2019, 112, 73–86. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional In Vitro Models. Int. J. Mol. Sci. 2022, 23, 2662. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Gong, X.; Mills, K.L. Large-scale patterning of single cells and cell clusters in hydrogels. Sci. Rep. 2018, 8, 3849. [Google Scholar] [CrossRef]
- Hao, D.; Lopez, J.M.; Chen, J.; Iavorovschi, A.M.; Lelivelt, N.M.; Wang, A. Engineering Extracellular Microenvironment for Tissue Regeneration. Bioengineering 2022, 9, 202. [Google Scholar] [CrossRef]
- Gao, J.; Yu, X.; Wang, X.; He, Y.; Ding, J. Biomaterial–Related Cell Microenvironment in Tissue Engineering and Regenerative Medicine. Engineering 2022, 13, 31–45. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Wang, T.; Turhan, M.; Gunasekaran, S. Selected properties of pH-sensitive, biodegradable chitosan-poly(vinyl alcohol) hydrogel. Polym. Int. 2004, 53, 911–918. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and peptides as important modifiers of the polymer scaffolds for tissue engineering applications—A review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Martínez-Colin, E.J.; González-Torres, M.; Ortega-Sánchez, C.; Sánchez-Sánchez, R.; Delgado-Meza, J.; Machado-Bistraín, F.; Martínez-López, V.; Giraldo, D.; Márquez-Gutiérrez, É.A.; et al. Chondrogenic Potential of Human Adipose-Derived Mesenchymal Stromal Cells in Steam Sterilized Gelatin/Chitosan/Polyvinyl Alcohol Hydrogels. Polymers 2023, 15, 3938. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Velasquillo-Martínez, C.; Knauth, P.; López, Z.; Moreno-Valtierra, M.; Bravo-Madrigal, J.; Jiménez-Palomar, I.; Luna-Bárcenas, G.; Espinosa-Andrews, H.; García-Carvajal, Z.Y. Sterilized chitosan-based composite hydrogels: Physicochemical characterization and in vitro cytotoxicity. J. Biomed. Mater. Res.-Part A 2020, 108, 81–93. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; García-Carvajal, Z.Y.; Jiménez-Palomar, I.; Jiménez-Avalos, J.A.; Espinosa-Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136, 47149. [Google Scholar] [CrossRef]
- Sánchez-Cardona, Y.; Echeverri-Cuartas, C.E.; López, M.E.L.; Moreno-Castellanos, N. Chitosan/gelatin/pva scaffolds for beta pancreatic cell culture. Polymers 2021, 13, 2372. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Javed, M.; Otto, I.A.; Combellack, E.J.; Morgan, S.; Breugem, C.C.; Archer, C.W.; Khan, I.M.; Lineaweaver, W.C.; Kon, M.; et al. Combining regenerative medicine strategies to provide durable reconstructive options: Auricular cartilage tissue engineering. Stem Cell Res. Ther. 2016, 7, 19. [Google Scholar] [CrossRef]
- Visscher, D.O.; Lee, H.; Van Zuijlen, P.P.; Helder, M.N.; Atala, A.; Yoo, J.J.; Lee, S.J. A photo-crosslinkable cartilage-derived extracellular matrix (ECM) bioink for auricular cartilage tissue engineering. Acta Biomater. 2020, 121, 193–203. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Kazemi, A.; Rasouli, H.R.; Kazemi, M.; Motamedi, M.H.K. Reconstructive surgery of auricular defects: An overview. Trauma Mon. 2015, 20, e28202. [Google Scholar] [CrossRef]
- Melgarejo-Ramírez, Y.; Sánchez-Sánchez, R.; García-Carvajal, Z.; García-López, J.; Gutiérrez-Gómez, C.; Luna-Barcenas, G.; Ibarra, C.; Velasquillo, C. Biocompatibility of Human Auricular Chondrocytes Cultured onto a Chitosan/Polyvynil Alcohol/Epichlorohydrin-Based Hydrogel for Tissue Engineering Application. Int. J. Morphol. 2014, 32, 1347–1356. [Google Scholar] [CrossRef][Green Version]
- Zeng, J.; Jia, L.; Wang, D.; Chen, Z.; Liu, W.; Yang, Q.; Liu, X.; Jiang, H. Bacterial nanocellulose-reinforced gelatin methacryloyl hydrogel enhances biomechanical property and glycosaminoglycan content of 3D-bioprinted cartilage. Int. J. Bioprinting 2023, 9, 131–143. [Google Scholar] [CrossRef]
- Tang, P.; Song, P.; Peng, Z.; Zhang, B.; Gui, X.; Wang, Y.; Liao, X.; Chen, Z.; Zhang, Z.; Fan, Y.; et al. Chondrocyte-laden GelMA hydrogel combined with 3D printed PLA scaffolds for auricle regeneration. Mater. Sci. Eng. C 2021, 130, 112423. [Google Scholar] [CrossRef]
- Otto, I.; Capendale, P.; Garcia, J.; De Ruijter, M.; Van Doremalen, R.; Castilho, M.; Lawson, T.; Grinstaff, M.; Breugem, C.; Kon, M.; et al. Biofabrication of a shape-stable auricular structure for the reconstruction of ear deformities. Mater. Today Bio 2021, 9, 100094. [Google Scholar] [CrossRef]
- Visscher, D.O.; Gleadall, A.; Buskermolen, J.K.; Burla, F.; Segal, J.; Koenderink, G.H.; Helder, M.N. Design and fabrication of a hybrid alginate hydrogel/poly(ε-caprolactone) mold for auricular cartilage reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1711–1721. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Liu, H.; Wang, Z.; Li, G.; Liu, Q.; Wang, C. Chondrocyte-laden gelatin/sodium alginate hydrogel integrating 3D printed PU scaffold for auricular cartilage reconstruction. Int. J. Biol. Macromol. 2023, 253, 126294. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Bi, B.; Hou, M.; Yao, L.; Du, Q.; He, A.; Liu, Y.; Miao, C.; Liang, X.; et al. A moldable thermosensitive hydroxypropyl chitin hydrogel for 3D cartilage regeneration in vitro and in vivo. Acta Biomater. 2020, 108, 87–96. [Google Scholar] [CrossRef]
- Griffin, M.F.; Premakumar, Y.; Seifalian, A.M.; Szarko, M.; Butler, P.E.M. Biomechanical Characterisation of the Human Auricular Cartilages; Implications for Tissue Engineering. Ann. Biomed. Eng. 2016, 44, 3460–3467. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, X.; Li, X.; Guo, C.; Li, C.; Liu, L.; Zhou, Z. Non-mulberry silk fiber-based scaffolds reinforced by PLLA porous microspheres for auricular cartilage: An in vitro study. Int. J. Biol. Macromol. 2021, 182, 1704–1712. [Google Scholar] [CrossRef]
- Wong, C.; Chen, H.; Chiu, H.; Tsuang, H.; Bai, Y.; Chung, J.; Lin, H.; Hsieh, J.; Chen, T.; Yang, L. Facilitating In Vivo Articular Cartilage Repair by Tissue-Engineered Cartilage Grafts Produced from Auricular Chondrocytes. Am. J. Sports Med. 2018, 46, 713–727. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Otto, I.A.; Levato, R.; Webb, W.R.; Khan, I.M.; Breugem, C.C.; Malda, J. Progenitor cells in auricular cartilage demonstrate cartilage-forming capacity in 3D hydrogel culture. Eur. Cells Mater. 2018, 35, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, D.; Li, X.; Meng, L. Chromatographic method for determination of the free amino acid content of chamomile flowers. Pharmacogn. Mag. 2015, 11, 176–179. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 23 November 2023).
- Melgarejo-Ramírez, Y.; Sánchez-Sánchez, R.; García-López, J.; Brena-Molina, A.M.; Gutiérrez-Gómez, C.; Ibarra, C.; Velasquillo, C. Characterization of pediatric microtia cartilage: A reservoir of chondrocytes for auricular reconstruction using tissue engineering strategies. Cell Tissue Bank. 2016, 17, 481–489. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, A.; Bhagwat, A.; Bangde, P.; Jain, R.; Dandekar, P. Chitosan/gelatin/PVA membranes for mammalian cell culture. Carbohydr. Polym. Technol. Appl. 2021, 2, 100163. [Google Scholar] [CrossRef]
- López-Velázquez, J.C.; Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Qui-Zapata, J.A.; García-Morales, S.; Navarro-López, D.E.; Luna-Bárcenas, G.; Vassallo-Brigneti, E.C.; García-Carvajal, Z.Y. Gelatin–chitosan–PVA hydrogels and their application in agriculture. J. Chem. Technol. Biotechnol. 2019, 94, 3495–3504. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Posniak, S.; Chung, J.H.Y.; Liu, X.; Mukherjee, P.; Wallace, G.G. The importance of elastin and its role in auricular cartilage tissue engineering. Bioprinting 2023, 32, e00276. [Google Scholar] [CrossRef]
- Lee, J.M.; Sultan, M.T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; Park, C.H. Artificial auricular cartilage using silk fibroin and polyvinyl alcohol hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. [Google Scholar] [CrossRef]
- Almajidi, Y.Q.; Abdullaev, S.S.; Alani, B.G.; Saleh, E.A.M.; Ahmad, I.; Ramadan, M.F.; Al-Hasnawi, S.S.; Romero-Parra, R.M. Chitosan-gelatin hydrogel incorporating polyvinyl alcohol and MnFe double-layered hydroxide nanocomposites with biological activity. Int. J. Biol. Macromol. 2023, 246, 125566. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mahmoud, A.A.; Osman, O.; El-Aal, M.A.; Eid, M. Molecular spectroscopic analyses of gelatin. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 81, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Corona-Escalera, A.F.; Tinajero-Díaz, E.; García-Reyes, R.A.; Luna-Bárcenas, G.; Seyfoddin, A.; Padilla-de la Rosa, J.D.; González-Ávila, M.; García-Carvajal, Z.Y. Enzymatic Crosslinked Hydrogels of Gelatin and Poly (Vinyl Alcohol) Loaded with Probiotic Bacteria as Oral Delivery System. Pharmaceutics 2022, 14, 2759. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-García, B.M.; Argüelles-Monal, W.M.; Hernández, J.; Félix-Valenzuela, L.; Acosta, N.; Goycoolea, F.M. Molecularly imprinted Chitosan—Genipin hydrogels with recognition capacity toward o-Xylene. Biomacromolecules 2007, 8, 3355–3364. [Google Scholar] [CrossRef]
- Ananthalakshmi, R.; Ravi, S.; Jeddy, N.; Thangavelu, R.; Janardhanan, S. Natural alternatives for chemicals used in histopathology lab—A literature review. J. Clin. Diagn. Res. 2016, 10, EE01–EE04. [Google Scholar] [CrossRef]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and physicochemical characterization of a diclofenac sodium-dual layer polyvinyl alcohol patch. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef]
- Chen, H.L.; Wu, L.G.; Tan, J.; Zhu, C.L. PVA membrane filled β-cyclodextrin for separation of isomeric xylenes by pervaporation. Chem. Eng. J. 2000, 78, 159–164. [Google Scholar] [CrossRef]
- Nkhwa, S.; Lauriaga, K.F.; Kemal, E.; Deb, S. Poly(vinyl alcohol): Physical Approaches to Designing Biomaterials for Biomedical Applications. Conf. Pap. Sci. 2014, 2014, 403472. [Google Scholar] [CrossRef]
- Jipa, I.; Stoica, A.; Stroescu, M.; Dobre, L.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)-bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Aykin-Dinçer, E.; Koç, A.; Erbas, M. Extraction and physicochemical characterization of broiler (Gallus gallus domesticus) skin gelatin compared to commercial bovine gelatin. Poult. Sci. 2017, 96, 4124–4131. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kee, L.T.; Awad, E.A.; Sazili, A.Q. Extraction, characterization and molecular structure of bovine skin gelatin extracted with plant enzymes bromelain and zingibain. J. Food Sci. Technol. 2020, 57, 3772–3781. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, L.; Chen, X.G.; Lai, Y.; Liu, K.; Xue, K. Comparative study of alginate and type I collagen as biomaterials for cartilage stem/progenitor cells to construct tissue-engineered cartilage in vivo. Front. Bioeng. Biotechnol. 2023, 10, 1057199. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Silva, D.; Pimenta, A.; Fernandes, A.; Mata, J.; Armês, H.; Salema-Oom, M.; Saramago, B.; Serro, A. Polyvinyl alcohol/chitosan wound dressings loaded with antiseptics. Int. J. Pharm. 2021, 593, 120110. [Google Scholar] [CrossRef]
- García-Martínez, L.; Campos, F.; Godoy-Guzmán, C.; Del Carmen Sánchez-Quevedo, M.; Garzón, I.; Alaminos, M.; Campos, A.; Carriel, V. Encapsulation of human elastic cartilage-derived chondrocytes in nanostructured fibrin-agarose hydrogels. Histochem. Cell Biol. 2017, 147, 83–95. [Google Scholar] [CrossRef]
- Ferreira, K.D.; Cardoso, L.D.; Oliveira, L.P.; Franzo, V.S.; Pancotti, A.; Miguel, M.P.; Silva, L.A.F.; Vulcani, V.A.S. Histological analysis of elastic cartilages treated with alkaline solution. Arq. Bras. Med. Vet. Zootec. 2020, 72, 647–654. [Google Scholar] [CrossRef]
- Kang, N.; Liu, X.; Guan, Y.; Wang, J.; Gong, F.; Yang, X.; Yan, L.; Wang, Q.; Fu, X.; Cao, Y.; et al. Effects of co-culturing BMSCs and auricular chondrocytes on the elastic modulus and hypertrophy of tissue engineered cartilage. Biomaterials 2012, 33, 4535–4544. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, H.; Wang, Y.; Bi, S.; Zhou, K.; Li, H.; Zhou, C.; Wang, Y.; Wu, W.; Peng, B.; et al. The application and progress of tissue engineering and biomaterial scaffolds for total auricular reconstruction in microtia. Front. Bioeng. Biotechnol. 2023, 11, 1089031. [Google Scholar] [CrossRef]
- Zopf, D.A.; Flanagan, C.L.; Nasser, H.B.; Mitsak, A.G.; Huq, F.S.; Rajendran, V.; Green, G.E.; Hollister, S.J. Biomechanical evaluation of human and porcine Auricular cartilage. Laryngoscope 2015, 125, E262–E268. [Google Scholar] [CrossRef]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Hall, B.K.; Miyake, T. Divide, accumulate, differentiate: Cell condensation skeletal development revisited. Int. J. Dev. Biol. 1995, 39, 881–893. [Google Scholar] [PubMed]
- Fowler, D.A.; Larsson, H.C.E. The tissues and regulatory pattern of limb chondrogenesis. Dev. Biol. 2020, 463, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Melrose, J. Aggrecan, the primary weight-bearing cartilage proteoglycan, has context-dependent, cell-directive properties in embryonic development and neurogenesis: Aggrecan glycan side chain modifications convey interactive biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Korntner, S.H.; Mullen, A.M.; Zeugolis, D.I. Collagen type II: From biosynthesis to advanced biomaterials for cartilage engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef] [PubMed]
- Ba, R.; Kong, L.; Wu, G.; Liu, S.; Dong, Y.; Li, B.; Zhao, Y. Increased Expression of Sox9 during Balance of BMSCs/Chondrocyte Bricks in Platelet-Rich Plasma Promotes Construction of a STable 3-D Chondrogenesis Microenvironment for BMSCs. Stem Cells Int. 2020, 2020, 5492059. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Yang, C.Y.; Hsiao, H.Y.; Chen, L.; Chu, I.M.; Cheng, M.H.; Tsao, C.H. Cultivation of auricular chondrocytes in poly(ethylene glycol)/poly(ε-caprolactone) hydrogel for tracheal cartilage tissue engineering in a rabbit model. Eur. Cells Mater. 2018, 35, 350–364. [Google Scholar] [CrossRef]

| Amino Acid | Pristine Type B Gelatin from Bovine Skin% (g/100 g) | CS/Gel/PVA Hydrogel % (g/100 g) |
|---|---|---|
| Histidine (His) | 10.38 | 0.45 |
| Serine (Ser) | 1.13 | 0.08 |
| Arginine (Arg) | 0.29 | 0.01 |
| Glycine (Gly) | 14.40 | 0.73 |
| Aspartic acid (Asp) | ND | ND |
| Glutamic acid (Glx) | 0.98 | 0.03 |
| Threonine (Thr) | 4.59 | 0.33 |
| Alanine (Ala) | 9.07 | 0.39 |
| Proline (Pro) | 16.24 | 0.82 |
| Lysine (Lys) | 0.01 | 0.01 |
| Tyrosine (Tyr) | ND | ND |
| Valine (Val) | 0.63 | 0.08 |
| Isoleucine (Ile) | 3.09 | 0.20 |
| Leucine (Leu) | 1.54 | 0.01 |
| Phenylalanine (Phe) | 0.01 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Sánchez, C.; Melgarejo-Ramírez, Y.; Rodríguez-Rodríguez, R.; Jiménez-Ávalos, J.A.; Giraldo-Gomez, D.M.; Gutiérrez-Gómez, C.; Rodriguez-Campos, J.; Luna-Bárcenas, G.; Velasquillo, C.; Martínez-López, V.; et al. Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture. Polymers 2024, 16, 479. https://doi.org/10.3390/polym16040479
Ortega-Sánchez C, Melgarejo-Ramírez Y, Rodríguez-Rodríguez R, Jiménez-Ávalos JA, Giraldo-Gomez DM, Gutiérrez-Gómez C, Rodriguez-Campos J, Luna-Bárcenas G, Velasquillo C, Martínez-López V, et al. Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture. Polymers. 2024; 16(4):479. https://doi.org/10.3390/polym16040479
Chicago/Turabian StyleOrtega-Sánchez, Carmina, Yaaziel Melgarejo-Ramírez, Rogelio Rodríguez-Rodríguez, Jorge Armando Jiménez-Ávalos, David M. Giraldo-Gomez, Claudia Gutiérrez-Gómez, Jacobo Rodriguez-Campos, Gabriel Luna-Bárcenas, Cristina Velasquillo, Valentín Martínez-López, and et al. 2024. "Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture" Polymers 16, no. 4: 479. https://doi.org/10.3390/polym16040479
APA StyleOrtega-Sánchez, C., Melgarejo-Ramírez, Y., Rodríguez-Rodríguez, R., Jiménez-Ávalos, J. A., Giraldo-Gomez, D. M., Gutiérrez-Gómez, C., Rodriguez-Campos, J., Luna-Bárcenas, G., Velasquillo, C., Martínez-López, V., & García-Carvajal, Z. Y. (2024). Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture. Polymers, 16(4), 479. https://doi.org/10.3390/polym16040479