Nanocellulose Composite Films in Food Packaging Materials: A Review
Abstract
1. Introduction
2. Classification and Preparation of Nanocellulose
2.1. Cellulose and Nanocellulose
2.2. Preparation of Nanocellulose
2.2.1. Chemical Methods
2.2.2. Physical Methods
2.2.3. Biological Methods
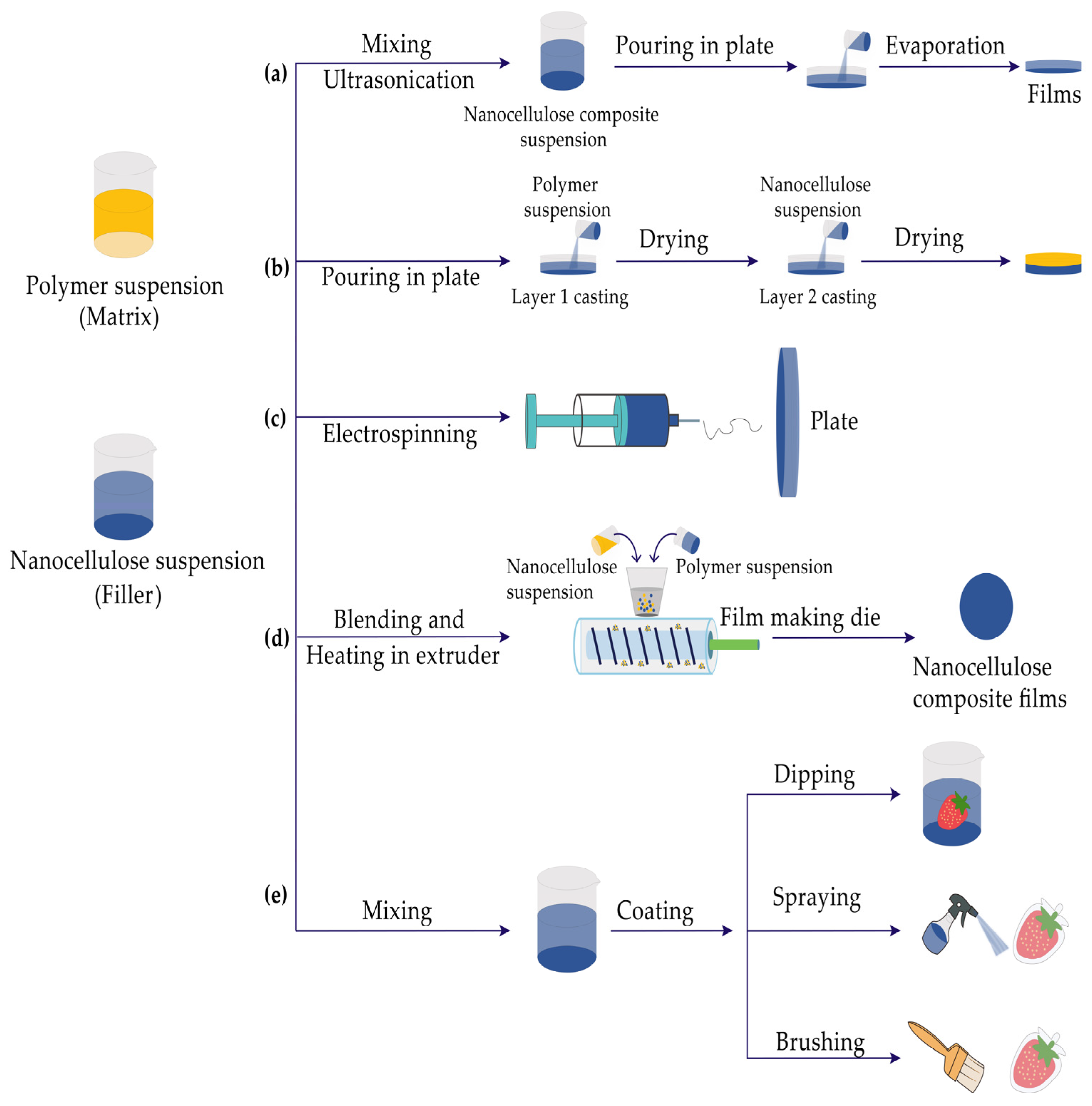
3. Preparation of Nanocellulose Composite Films
3.1. Wet Process
3.1.1. Solution Casting Method
3.1.2. Layer-by-Layer Assembly (LBL)
3.1.3. Electrospinning
3.2. Melt Process
3.3. Coating
4. Superior Performances of Nanocellulose Composite Films in Food Packaging
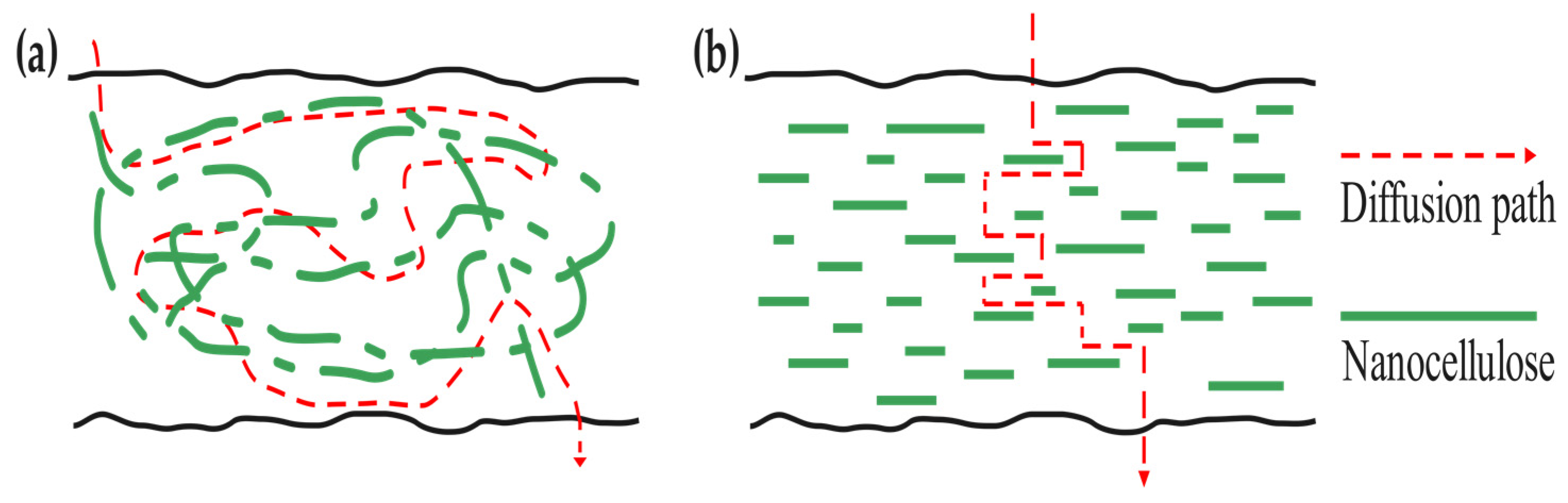
4.1. Oxygen Barrier Property
4.2. Water Vapor Barrier Property
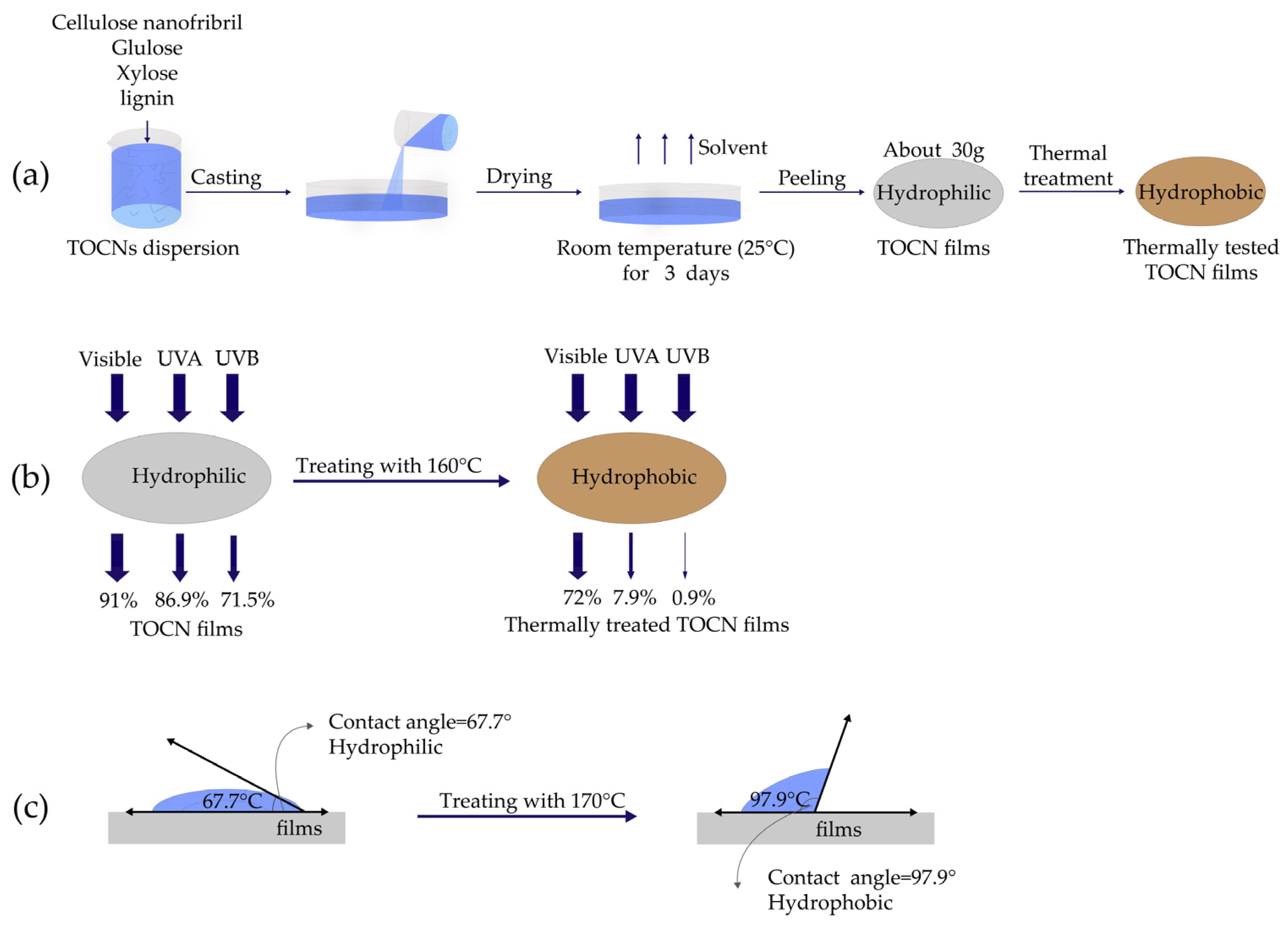
4.3. Ultraviolet Barrier Properties
4.4. Antibacterial Activity
4.5. Mechanical Properties
4.6. Thermal Stabilities
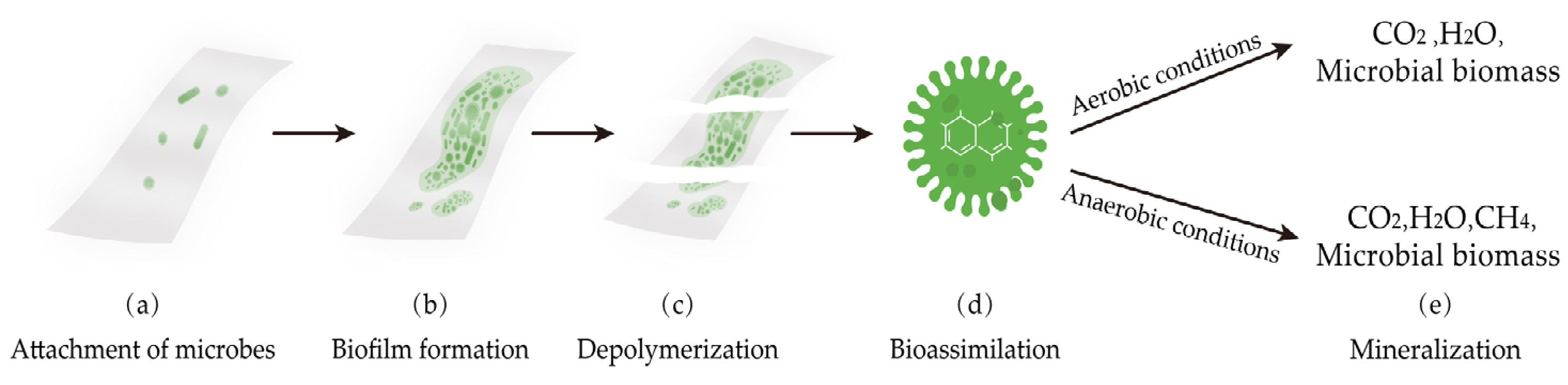
4.7. Biodegradation Properties
4.8. Edible Properties
5. Applications of Nanocellulose Composite Films in Food Packaging
5.1. Meat and Meat Products
5.2. Fruits and Vegetables
5.3. Aquatic Products
5.4. Edible Films in Food Products
6. Security Assessment of Nanocellulose Composite Films
7. Conclusion and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Nemat, B.; Razzaghi, M.; Bolton, K.; Rousta, K. The Potential of Food Packaging Attributes to Influence Consumers’ Decisions to Sort Waste. Sustainability 2020, 12, 2234. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging: A review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef] [PubMed]
- Sadalage, P.S.; Pawar, K.D. Production of microcrystalline cellulose and bacterial nanocellulose through biological valorization of lignocellulosic biomass wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Bartkowiak, M.; Peplińska, B.; Waliszewska, H.; Zborowska, M.; Borysiak, S.; Ratajczak, I. Miscanthus and Sorghum as sustainable biomass sources for nanocellulose production. Ind. Crops Prod. 2022, 186, 115177. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.V.; Gayathri, V.; Samanta, D.; Sampath, S. Bacterial cellulose: A promising biopolymer with interesting properties and applications. Int. J. Biol. Macromol. 2022, 220, 435–461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wu, S.; Zhou, J. Preparation and modification of nanocellulose and its application to heavy metal adsorption: A review. Int. J. Biol. Macromol. 2023, 236, 123916. [Google Scholar] [CrossRef]
- Lv, X.; Han, J.; Liu, M.; Yu, H.; Liu, K.; Yang, Y.; Sun, Y.; Pan, P.; Liang, Z.; Chang, L.; et al. Overview of preparation, modification, and application of tunicate-derived nanocellulose. Chem. Eng. J. 2023, 452, 139439. [Google Scholar] [CrossRef]
- Li, A.; Xu, D.; Luo, L.; Zhou, Y.; Yan, W.; Leng, X.; Dai, D.; Zhou, Y.; Ahmad, H.; Rao, J.; et al. Overview of nanocellulose as additives in paper processing and paper products. Nanotechnol. Rev. 2021, 10, 264–281. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Zhu, S.; Wu, Q.; Li, J.; Gao, Y.; Wang, B.; Li, J.; Gao, W.; Zeng, J.; et al. Preparation of nanocellulose in high yield via chemi-mechanical synergy. Carbohydr. Polym. 2021, 251, 117094. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, R.; Li, N.; Yang, X.; Lin, D. Nanocellulose: An amazing nanomaterial with diverse applications in food science. Carbohydr. Polym. 2023, 304, 120497. [Google Scholar] [CrossRef] [PubMed]
- Othman, H.; Rafat, R.; Shehdeh, J.; Abdalhadi, D.; Hisham, Q.; Omar, D.; Khalil, A.; Rana, A.K.; Rola, A.K.; Ghaleb, A. Design, synthesis and antimicrobial properties of cellulose-based amine film. Polym. Bull. 2022, 79, 627–641. [Google Scholar]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Silva, F.S.D.; Dourado, F.; Gama, M.; Poas, F. Nanocellulose Bio-Based Composites for Food Packaging. J. Nanomater. 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zeng, J.; Cheng, Z.; Wang, X.; Wang, B.; Zeng, Z.; Chen, K. Cellulose nanofibrils (CNFs) produced by different mechanical methods to improve mechanical properties of recycled paper. Carbohydr. Polym. 2021, 254, 117474. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Browne, C.; Batchelor, W.; Garnier, G. Self-assembly of cellulose nanocrystals of different lengths. J. Colloid Interface Sci. 2023, 630, 249–259. [Google Scholar] [CrossRef]
- Peng, S.; Luo, Q.; Zhou, G.; Xu, X. Recent Advances on Cellulose Nanocrystals and Their Derivatives. Polymers 2021, 13, 3247. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z. Research progress of nanocellulose-based food packaging. Trends Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: Types, properties, and emerging applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Teo, H.L.; Wahab, R.A. Towards an eco-friendly deconstruction of agro-industrial biomass and preparation of renewable cellulose nanomaterials: A review. Int. J. Biol. Macromol. 2020, 161, 1414–1430. [Google Scholar] [CrossRef] [PubMed]
- Martin-Martinez, F.J. Designing nanocellulose materials from the molecular scale. Proc. Natl. Acad. Sci. USA 2018, 115, 7174–7175. [Google Scholar] [CrossRef]
- Zhong, C.; Zajki-Zechmeister, K.; Nidetzky, B. Reducing end thiol-modified nanocellulose: Bottom-up enzymatic synthesis and use for templated assembly of silver nanoparticles into biocidal composite material. Carbohydr. Polym. 2021, 260, 117772. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Hemraz, U.D. Preparation and Surface Functionalization of Carboxylated Cellulose Nanocrystals. Nanomaterials 2021, 11, 1641. [Google Scholar] [CrossRef]
- Xu, H.; Sanchez-Salvador, J.L.; Blanco, A.; Balea, A.; Negro, C. Recycling of TEMPO-mediated oxidation medium and its effect on nanocellulose properties. Carbohydr. Polym. 2023, 319, 121168. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.B.; Moniruzzaman, M. Ionic liquid assisted nanocellulose production from microcrystalline cellulose: Correlation between cellulose solubility and nanocellulose yield via COSMO-RS prediction. J. Mol. Liq. 2022, 368, 120591. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, C.; Huang, C.; Wang, S. Dissolution behavior of ionic liquids for different ratios of lignin and cellulose in the preparation of nanocellulose/lignin blends. J. Colloid Interface Sci. 2024, 657, 767–777. [Google Scholar] [CrossRef]
- Almeida, R.O.; Maloney, T.C.; Gamelas, J.A.F. Production of functionalized nanocelluloses from different sources using deep eutectic solvents and their applications. Ind. Crops Prod. 2023, 199, 116583. [Google Scholar] [CrossRef]
- Dias, I.K.R.; Lacerda, B.K.; Arantes, V. High-yield production of rod-like and spherical nanocellulose by controlled enzymatic hydrolysis of mechanically pretreated cellulose. Int. J. Biol. Macromol. 2023, 242, 125053. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, N.G.; Oyekanmi, A.A.; Hanafiah, M.M.; Olugbade, T.O.; Adeyeri, M.K.; Olaiya, F.G. Enzyme-assisted extraction of nanocellulose from textile waste: A review on production technique and applications. Bioresour. Technol. Rep. 2022, 19, 101183. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Samsalee, N.; Meerasri, J.; Sothornvit, R. Rice husk nanocellulose: Extraction by high-pressure homogenization, chemical treatments and characterization. Carbohydr. Polym. Technol. Appl. 2023, 6, 100353. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Chen, J.; Kong, L. Study on nanocellulose by high pressure homogenization in homogeneous isolation. Fibers Polym. 2015, 16, 572–578. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. Characterization of cellulose nanofibrillation by micro grinding. J. Nanoparticle Res. 2014, 16, 2349. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Syafri, E.; Kasim, A.; Abral, H.; Asben, A. Cellulose nanofibers isolation and characterization from ramie using a chemical-ultrasonic treatment. J. Nat. Fibers 2019, 16, 1145–1155. [Google Scholar] [CrossRef]
- Otoni, C.G.; Carvalho, A.S.; Cardoso, M.V.; Bernardinelli, O.D.; Lorevice, M.V.; Colnago, L.A.; Loh, W.; Mattoso, L.H. High-Pressure Microfluidization as a Green Tool for Optimizing the Mechanical Performance of All-Cellulose Composites. ACS Sustain. Chem. Eng. 2018, 6, 12727–12735. [Google Scholar] [CrossRef]
- Hu, C.; Xiong, Z.; Xiong, H.; Chen, L.; Zhang, Z. Effects of dynamic high-pressure microfluidization treatment on the functional and structural properties of potato protein isolate and its complex with chitosan. Food Res. Int. 2021, 140, 109868. [Google Scholar] [CrossRef] [PubMed]
- Fontes, A.M.; Pirich, C.L.; Tanobe, G.R.O.A.; Tarrés, Q.; Delgado-Aguilar, M.; Ramos, L.P. Micro/nanostructured lignonanocellulose obtained from steam-exploded sugarcane bagasse. Cellulose 2021, 28, 10163–10182. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, L.; Zhan, P.; Liu, N.; Wu, Z. Preparation of nanocellulose from steam exploded poplar wood by enzymolysis assisted sonication. Mater. Res. Express 2020, 7, 035010. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Q.; Han, Z.; Li, J.; Liao, B.; Hu, L.; Huang, J.; Zou, C.; Jia, C.; Huang, J.; et al. Comparison of bacterial nanocellulose produced by different strains under static and agitated culture conditions. Carbohydr. Polym. 2020, 227, 115323. [Google Scholar] [CrossRef] [PubMed]
- Barja, F. Bacterial nanocellulose production and biomedical applications. J. Biomed. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films—A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Nanocellulose Based Green Nanocomposites: Characteristics and Application in Primary Food Packaging. Food Rev. Int. 2023, 39, 7148–7179. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Alkenylation of cellulose nanocrystals (CNC) and their applications. Polymer 2016, 101, 338–346. [Google Scholar] [CrossRef]
- Bastante, C.C.; Silva, N.H.C.S.; Cardoso, L.C.; Serrano, C.M.; de la Ossa, E.J.M.; Freire, C.S.R.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Follain, N.; Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Marais, S.; Dufresne, A. Tunable gas barrier properties of filled-PCL film by forming percolating cellulose network. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 26–30. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Wågberg, L. Ultrastrong and flame-resistant freestanding films from nanocelluloses, self-assembled using a layer-by-layer approach. Appl. Mater. Today 2017, 9, 229–239. [Google Scholar] [CrossRef]
- Pasaoglu, M.E.; Koyuncu, I. Substitution of petroleum-based polymeric materials used in the electrospinning process with nanocellulose: A review and future outlook. Chemosphere 2021, 269, 128710. [Google Scholar] [CrossRef] [PubMed]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials 2020, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Xiao, Y.; Wang, K.; Cui, M.; Chen, D.; Guan, D.; Luan, G.; Xu, H. Development and evaluation of gum arabic-based antioxidant nanocomposite films incorporated with cellulose nanocrystals and fruit peel extracts. Food Packag. Shelf Life 2021, 30, 100768. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Mekonnen, T.H. Multilayer assemblies of cellulose nanocrystal—Polyvinyl alcohol films featuring excellent physical integrity and multi-functional properties. J. Colloid Interface Sci. 2020, 580, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostructure Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Park, Y.; You, M.; Shin, J.; Ha, S.; Kim, D.; Heo, M.H.; Nah, J.; Kim, Y.A.; Seol, J.H. Thermal conductivity enhancement in electrospun poly(vinyl alcohol) and poly(vinyl alcohol)/cellulose nanocrystal composite nanofibers. Sci. Rep. 2019, 9, 3026. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Gray, N.; Hamzeh, Y.; Kaboorani, A.; Abdulkhani, A. Influence of cellulose nanocrystal on strength and properties of low density polyethylene and thermoplastic starch composites. Ind. Crops Prod. 2018, 115, 298–305. [Google Scholar] [CrossRef]
- Hubbe, M.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. BioResources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Production of edible coatings with twin-nozzle electrospraying equipment and the effects on shelf-life stability of fresh-cut apple slices. J. Food Process Eng. 2018, 41, e12627. [Google Scholar] [CrossRef]
- Pacaphol, K.; Seraypheap, K.; Aht-Ong, D. Development and application of nanofibrillated cellulose coating for shelf life extension of fresh-cut vegetable during postharvest storage. Carbohydr. Polym. 2019, 224, 115167. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: Mechanical, thermal and optical properties. Compos. Sci. Technol. 2015, 106, 149–155. [Google Scholar] [CrossRef]
- Boone, L.; Préat, N.; Nhu, T.T.; Fiordelisi, F.; Guillard, V.; Blanckaert, M.; Dewulf, J. Environmental performance of plastic food packaging: Life cycle assessment extended with costs on marine ecosystem services. Sci. Total Environ. 2023, 894, 164781. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Mendes, A.C.; Pedersen, G.A. Perspectives on sustainable food packaging:– is bio-based plastics a solution? Trends Food Sci. Technol. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Su, G. Plastic packaging-associated chemicals and their hazards—An overview of reviews. Chemosphere 2023, 331, 138795. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Wang, J.; Gardner, D.J.; Tajvidi, M. Cellulose nanofibrils versus cellulose nanocrystals: Comparison of performance in flexible multilayer films for packaging applications. Food Packag. Shelf Life 2020, 23, 100464. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Azmi, A.; Nurazzi, N.M.; Atiqah, A.; Atikah, M.S.N.; Ibrahim, R.; Norrrahim, M.N.F.; Asyraf, M.R.M.; Sharma, S.; Punia, S.; et al. Oxygen permeability properties of nanocellulose reinforced biopolymer nanocomposites. Mater. Today Proc. 2022, 52, 2414–2419. [Google Scholar] [CrossRef]
- Mondragon, G.; Peña-Rodriguez, C.; González, A.; Eceiza, A.; Arbelaiz, A. Bionanocomposites based on gelatin matrix and nanocellulose. Eur. Polym. J. 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Kim, J.-K.; Choi, B.; Jin, J. Transparent, water-stable, cellulose nanofiber-based packaging film with a low oxygen permeability. Carbohydr. Polym. 2020, 249, 116823. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Xiao, Y.; Guo, X.; Huang, A.; Xu, H. Development of gum arabic-based nanocomposite films reinforced with cellulose nanocrystals for strawberry preservation. Food Chem. 2021, 350, 129199. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Aulin, C.; Salazar-Alvarez, G.; Lindström, T. High strength, flexible and transparent nanofibrillated cellulose-nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef]
- Lu, P.; Xiao, H.; Zhang, W.; Gong, G. Reactive coating of soybean oil-based polymer on nanofibrillated cellulose film for water vapor barrier packaging. Carbohydr. Polym. 2014, 111, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, Y.; Jiang, Y.; Miao, M.; Cao, S.; Fang, J. Use of carbon dots to enhance UV-blocking of transparent nanocellulose films. Carbohydr. Polym. 2017, 161, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.-C.; Chen, W.; Huang, R.; Hong, S.; Wu, Q.; Mei, C. Production of lignin-containing cellulose nanofibers using deep eutectic solvents for UV-absorbing polymer reinforcement. Carbohydr. Polym. 2020, 246, 116548. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Vázquez, M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020, 99, 105323. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Y.; Zuo, C.; Deng, Y.; Dai, H. Thermally-induced cellulose nanofibril films with near-complete ultraviolet-blocking and improved water resistance. Carbohydr. Polym. 2019, 223, 115050. [Google Scholar] [CrossRef] [PubMed]
- Madivoli, E.S.; Kareru, P.G.; Gichuki, J.; Elbagoury, M.M. Cellulose nanofibrils and silver nanoparticles enhances the mechanical and antimicrobial properties of polyvinyl alcohol nanocomposite film. Sci. Rep. 2022, 12, 19005. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and Conductive Nanocellulose-Based Films for Active and Intelligent Food Packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef]
- Sundaram, J.; Pant, J.; Goudie, M.J.; Mani, S.; Handa, H. Antimicrobial and Physicochemical Characterization of Biodegradable, Nitric Oxide-Releasing Nanocellulose-Chitosan Packaging Membranes. J. Agric. Food Chem. 2016, 64, 5260–5266. [Google Scholar] [CrossRef]
- Zahan, K.A.; Azizul, N.M.; Mustapha, M.; Tong, W.Y.; Rahman, M.S.A.; Sahuri, I.S. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Mater. Today Proc. 2020, 31, 83–88. [Google Scholar] [CrossRef]
- Hassan, E.; Fadel, S.; Abou-Elseoud, W.; Mahmoud, M.; Hassan, M. Cellulose Nanofibers/Pectin/Pomegranate Extract Nanocomposite as Antibacterial and Antioxidant Films and Coating for Paper. Polymers 2022, 14, 4605. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, I.; Hokkanen, A.; Österberg, M.; Vähä-Nissi, M.; Harlin, A.; Orelma, H. Hybrid films from cellulose nanomaterials—Properties and defined optical patterns. Cellulose 2022, 29, 8551–8567. [Google Scholar] [CrossRef]
- Raj, L.F.A.A.; Shanmugapriya, R.; Jeslin, J. Biosynthesis of cellulose microfibre from peanut shell for the preparation of bio-nanocomposite films for food-packaging application. Bull. Mater. Sci. 2019, 42, 63. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Chen, G. A comparative study on the starch-based biocomposite films reinforced by nanocellulose prepared from different non-wood fibers. Cellulose 2019, 26, 2425–2435. [Google Scholar] [CrossRef]
- Nair, S.S.; Dartiailh, C.; Levin, D.B.; Yan, N. Highly Toughened and Transparent Biobased Epoxy Composites Reinforced with Cellulose Nanofibrils. Polymers 2019, 11, 612. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; Abdullah, M.F.B.; Omar, M.F. Thermal properties of nanocellulose-reinforced composites: A review. J. Appl. Polym. Sci. 2020, 137, 48544. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly(vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Cheikh, S.B.; Cheikh, R.B.; Cunha, E.; Lopes, P.E.; Paiva, M.C. Production of cellulose nanofibers from Alfa grass and application as reinforcement for polyvinyl alcohol. Plast. Rubber Compos. 2018, 47, 297–305. [Google Scholar] [CrossRef]
- Hai, L.; Choi, E.S.; Zhai, L.; Panicker, P.S.; Kim, J. Green nanocomposite made with chitin and bamboo nanofibers and its mechanical, thermal and biodegradable properties for food packaging. Int. J. Biol. Macromol. 2020, 144, 491–499. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Wu, J.; Du, X.; Yin, Z.; Xu, S.; Xu, S.; Zhang, Y. Preparation and characterization of cellulose nanofibrils from coconut coir fibers and their reinforcements in biodegradable composite films. Carbohydr. Polym. 2019, 211, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, H.; He, Y.; Fei, X.; Peng, L. Preparation and characterization of carboxymethyl cellulose-based composite films reinforced by cellulose nanocrystals derived from pea hull waste for food packaging applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw. Appl. Surf. Sci. 2018, 449, 591–602. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-L.; Tran, T.H.; Hao, L.T.; Jeon, H.; Koo, J.M.; Shin, G.; Hwang, D.S.; Hwang, S.Y.; Park, J.; Oh, D.X. Biorenewable, transparent, and oxygen/moisture barrier nanocellulose/nanochitin-based coating on polypropylene for food packaging applications. Carbohydr. Polym. 2021, 271, 118421. [Google Scholar] [CrossRef] [PubMed]
- Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar]
- Arun, R.; Shruthy, R.; Preetha, R.; Sreejit, V. Biodegradable nano composite reinforced with cellulose nano fiber from coconut industry waste for replacing synthetic plastic food packaging. Chemosphere 2022, 291, 132786. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Influence of Nanocellulose Additive on the Film Properties of Native Rice Starch-based Edible Films for Food Packaging. Recent. Pat. Nanotechnol. 2019, 13, 222–233. [Google Scholar] [CrossRef]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef]
- Fritz, C.; Jeuck, B.; Salas, C.; Gonzalez, R.; Jameel, H.; Rojas, O.J. Nanocellulose and Proteins: Exploiting Their Interactions for Production, Immobilization, and Synthesis of Biocompatible Materials. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 271, pp. 207–224. [Google Scholar]
- Shi, S.-C.; Mandal, P.K.; Chen, T.-H. Mechanical Properties and Tribological Behavior of MoS2-Enhanced Cellulose-Based Biocomposites for Food Packaging. Polymers 2021, 13, 1838. [Google Scholar] [CrossRef]
- Chen, Q.-J.; Zhou, L.-L.; Zou, J.-Q.; Gao, X. The preparation and characterization of nanocomposite film reinforced by modified cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 132, 1155–1162. [Google Scholar] [CrossRef]
- Garavand, F.; Nooshkam, M.; Khodaei, D.; Yousefi, S.; Cacciotti, I.; Ghasemlou, M. Recent advances in qualitative and quantitative characterization of nanocellulose-reinforced nanocomposites: A review. Adv. Colloid Interface Sci. 2023, 318, 102961. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Bharimalla, A.K.; Nadanathangam, V.; Dhakane-Lad, J.; Mahapatra, A.; Jagajanantha, P.; Saxena, S. Nanocellulose reinforced corn starch-based biocomposite films: Composite optimization, characterization and storage studies. Food Packag. Shelf Life 2022, 33, 100860. [Google Scholar] [CrossRef]
- Vilarinho, F.; Stanzione, M.; Buonocore, G.G.; Barbosa-Pereira, L.; Sendón, R.; Vaz, M.F.; Silva, A.S. Green tea extract and nanocellulose embedded into polylactic acid film: Properties and efficiency on retarding the lipid oxidation of a model fatty food. Food Packag. Shelf Life 2021, 27, 100609. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mater. Res. Technol. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Firmanda, A.; Fahma, F.; Warsiki, E.; Syamsu, K.; Arnata, I.W.; Sartika, D.; Suryanegara, L.; Qanytah; Suyanto, A. Antimicrobial mechanism of nanocellulose composite packaging incorporated with essential oils. Food Control 2023, 147, 109617. [Google Scholar] [CrossRef]
- Salimiraad, S.; Safaeian, S.; Basti, A.A.; Khanjari, A.; Nadoushan, R.M. Characterization of novel probiotic nanocomposite films based on nano chitosan/ nano cellulose/ gelatin for the preservation of fresh chicken fillets. LWT 2022, 162, 113429. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T. Intelligent and active packaging of chicken thigh meat by conducting nano structure cellulose-polypyrrole-ZnO film. Mater. Sci. Eng. C 2019, 102, 798–809. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, S.; Xu, S.; Li, J.; Wang, L. Characterization of antioxidant properties of soy bean protein-based films with Cortex Phellodendri extract in extending the shelf life of lipid. Food Packag. Shelf Life 2019, 22, 100413. [Google Scholar] [CrossRef]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Bian, L.; Wang, K.; Chia, W.Y.; Khoo, K.S.; Zhang, C.; Chew, K.W. Preparation and characterization of curdlan/nanocellulose blended film and its application to chilled meat preservation. Chemosphere 2021, 266, 128948. [Google Scholar] [CrossRef] [PubMed]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial nanocomposite films made of poly(lactic acid)-cellulose nanocrystals (PLA-CNC) in food applications: Part A—Effect of nisin release on the inactivation of Listeria monocytogenes in ham. Cellulose 2014, 21, 1837–1850. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- He, X.; Pu, Y.; Chen, L.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. A comprehensive review of intelligent packaging for fruits and vegetables: Target responders, classification, applications, and future challenges. Compr. Rev. Food Sci. Food Saf. 2023, 22, 842–881. [Google Scholar] [CrossRef]
- Montero, Y.; Souza, A.G.; Oliveira, É.R.; Rosa, D.D.S. Nanocellulose functionalized with cinnamon essential oil: A potential application in active biodegradable packaging for strawberry. Sustain. Mater. Technol. 2021, 29, e00289. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H. Preparation and characterization of a biodegradable starch-based antibacterial film containing nanocellulose and polyhexamethylene biguanide. Food Packag. Shelf Life 2021, 30, 100718. [Google Scholar] [CrossRef]
- Dey, D.; Dharini, V.; Selvam, S.P.; Sadiku, E.R.; Kumar, M.M.; Jayaramudu, J.; Gupta, U.N. Physical, antifungal, and biodegradable properties of cellulose nanocrystals and chitosan nanoparticles for food packaging application. Mater. Today Proc. 2021, 38, 860–869. [Google Scholar] [CrossRef]
- Xiang, F.; Xia, Y.; Wang, Y.; Wang, Y.; Wu, K.; Ni, X. Preparation of konjac glucomannan based films reinforced with nanoparticles and its effect on cherry tomatoes preservation. Food Packag. Shelf Life 2021, 29, 100701. [Google Scholar] [CrossRef]
- Chen, F.; Chang, X.; Xu, H.; Fu, X.; Ding, S.; Wang, R. Gellan gum-based functional films integrated with bacterial cellulose and nano-TiO2/CuO improve the shelf life of fresh-cut pepper. Food Packag. Shelf Life 2023, 38, 101103. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Becher, D.; Pereira, F.G.M.; Salmieri, S.; Lacroix, M. Modified cellulose nanocrystals (CNCs) loaded in gellan gum matrix enhance the preservation of Agaricus bisporus mushrooms. J. Food Process. Preserv. 2020, 44, e14846. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, R.; Zhou, C.; Gao, Z.; Gu, Y.; Chen, S.; Yang, Q.; Yan, B. Dynamically crosslinked chitosan/cellulose nanofiber-based films integrated with γ-cyclodextrin/curcumin inclusion complex as multifunctional packaging materials for perishable fruit. Food Hydrocoll. 2023, 144, 108996. [Google Scholar] [CrossRef]
- Li, L.; Wang, W.; Sun, J.; Chen, Z.; Ma, Q.; Ke, H.; Yang, J. Improved properties of polyvinyl alcohol films blended with aligned nanocellulose particles induced by a magnetic field. Food Packag. Shelf Life 2022, 34, 100985. [Google Scholar] [CrossRef]
- Ponni, P.; Subramanian, K.S.; Janavi, G.J.; Subramanian, J. Synthesis of Nano-film from Nanofibrillated Cellulose of Banana Pseudostem (Musa spp.) to Extend the Shelf life of Tomato. BioResources 2020, 15, 2882–2905. [Google Scholar] [CrossRef]
- Garg, R.; Rana, H.; Singh, N.; Goswami, S. Guargum/nanocellulose based novel crosslinked antimicrobial film with enhanced barrier and mechanical properties for food packaging. J. Environ. Chem. Eng. 2023, 11, 109254. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.K.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Liao, W.; Wang, Q.; Xia, W. Chitosan/bacterial cellulose films incorporated with tea polyphenol nanoliposomes for silver carp preservation. Carbohydr. Polym. 2022, 297, 120048. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, J.; Jiang, L.; Zhu, Z.; He, S.; He, S.; Shao, W. Development of intelligent/active food packaging film based on TEMPO-oxidized bacterial cellulose containing thymol and anthocyanin-rich purple potato extract for shelf life extension of shrimp. Food Packag. Shelf Life 2021, 29, 100709. [Google Scholar] [CrossRef]
- Li, L.; Wang, W.; Zheng, M.; Sun, J.; Chen, Z.; Wang, J.; Ma, Q. Nanocellulose-enhanced smart film for the accurate monitoring of shrimp freshness via anthocyanin-induced color changes. Carbohydr. Polym. 2023, 301, 120352. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin doped functionalized cellulose nanofibers based edible chitosan coating on kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano 2019, 6, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- DeLoid, G.; Casella, B.; Pirela, S.; Filoramo, R.; Pyrgiotakis, G.; Demokritou, P.; Kobzik, L. Effects of engineered nanomaterial exposure on macrophage innate immune function. NanoImpact 2016, 2, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, M.; Kangas, H.; Laitinen, O.; Sneck, A.; Lahtinen, P.; Peresin, M.S.; Niinimäki, J. Characteristics and safety of nano-sized cellulose fibrils. Cellulose 2014, 21, 3871–3886. [Google Scholar] [CrossRef]
- Shazali, N.A.H.; Zaidi, N.E.; Ariffin, H.; Abdullah, L.C.; Ghaemi, F.; Abdullah, J.M.; Takashima, I.; Rahman, N.M.A.N.A. Characterization and Cellular Internalization of Spherical Cellulose Nanocrystals (CNC) into Normal and Cancerous Fibroblasts. Materials 2019, 12, 3251. [Google Scholar] [CrossRef]
- Khare, S.; Deloid, G.M.; Molina, R.M.; Gokulan, K.; Demokritou, P. Effects of ingested nanocellulose on intestinal microbiota and homeostasis in Wistar Han rats. NanoImpact 2020, 18, 100216. [Google Scholar] [CrossRef]
- Arvidsson, R.; Peters, G.; Hansen, S.F.; Baun, A. Prospective environmental risk screening of seven advanced materials based on production volumes and aquatic ecotoxicity. NanoImpact 2022, 25, 100393. [Google Scholar] [CrossRef]




| Types | Other Nomenclature | Micromorphology | Typical Sources | Average Size | Preparation Method | Ref. |
|---|---|---|---|---|---|---|
| Cellulose nanocrystals (CNCs) | Cellulose whiskers (CNWs), cellulose nanocrystalline (CNC), nanocrystalline cellulose (NCC) | Whiskers, rod-shaped | Plants (wood, cotton, hemp, flax, etc.) | Diameter: 5–70 nm Length: 100–250 nm | Acid hydrolysis | [18,19] |
| Cellulose nanofibrils (CNFs) | Micro-fibrillated cellulose (MFC), nano-fibrillated cellulose (NFC) | Twisted filamentous | Plants (wood, beet, cotton, hemp, flax, etc.) | Diameter: 5–60 nm Length: several micrometers | Mechanical treatment | [20,21] |
| Bacterial nanocellulose (BNC) | Bacterial cellulose (BC), regenerated bacterial celllose(RBC) | Gel-like, ribbon-like | Acetobacter, xylinum, pasteurii, etc. | Diameter: 20–100 nm Length: unfixed | Bacterial synthesis | [7,22] |
| Preparation Methods | Main Sources | Treatment | Advantages | Shortcomings | Product | Ref. |
|---|---|---|---|---|---|---|
| Top-down approach | Plant sources, by-products of agricultural products | Physical methods | Easy operation, mature technology | Environmentally unfriendly | Cellulose nanocrystals (CNCs) | [23,24] |
| Chemical methods | Easy operation, easy control | Uneven product size, damage the structure | Cellulose nanofibrils (CNFs) | [23,24] | ||
| Bottom-up approach | Culture medium containing carbon sources | Biological methods | Without other ingredients, high purity | Low yield, time consuming | Bacterial nanocellulose (BNC) | [25,26] |
| Method | Mechanism | Advantages | Shortcomings | Ref. |
|---|---|---|---|---|
| TEMPO method | The hydroxyl groups of the cellulose are oxidized to carboxylic groups by strong oxidizing agents, weakening the binding force between fiber molecules and making them more prone to micro-fibrillation | Mild conditions, low environmental contamination, good operability | Costly (reagents are expensive), incomplete reaction | [27,28] |
| Ionic liquid method | Anions in ionic liquids and hydroxyl groups of cellulose will interact, dissolving cellulose effectively and separating the cellulose chain | Good thermal stability and solubility, recyclable | High cost for recycling | [29,30] |
| Deep eutectic solvent method | The eutectic mixture can destroy the hydrogen bonds inside cellulose so as to degrade it | Biodegradability, recyclable, low cost, low melting point | Weak swelling ability and solubility of product | [30,31] |
| Enzymatic hydrolysis | Enzymatic reaction takes place between cellulose hydrolases and the amorphous region of cellulose, increasing the proportion of the crystallization zone | Pollution-free, low energy consumption | Low efficiency | [32,33] |
| Method | Conditions | Advantages | Shortcomings | Ref. |
|---|---|---|---|---|
| High-pressure homogenization | The energy produced by high-pressure and high-speed motion is used to break up the tight structure of the fiber, thereby gradually reducing the fiber size to the nanoscale | Good dispersibility | High energy consumption, the machine is easy to clog up | [35,36] |
| Microgrinding | Shear forces produced by grinding machine and grindstone are employed to split up hydrogen bonds to decrease the size of cellulosic material | Mass production, Low cost | Inefficiency, changes the crystallinity | [37,38] |
| High-intensity ultrasonication | The shock wave generated by ultrasound can be exploited to destroy fibrous tissue | Good thermal stability | Low production yield | [39,40] |
| High-pressure micro-fluidization | High pressure can be deployed to inject fiber suspension through micro channels, and then the fibers will be discharged through the channel, with the shear forces among them promoting nano-fibrillation | High efficiency | Decrease the crystallinity | [41,42] |
| Steam explosion | The tremendous explosive force produced by the release of high-pressure steam in a short time helps destroy the fiber structure and produce nanofibers of cellulose | Less use of chemical reagents, high efficiency | Need specific equipment | [43,44] |
| Cryocrushing | Extreme temperature reduction is conducted to embrittle the interior of the fiber, and then fiber fibrillation is realized | Improve heat stability | High cost for equipment | [24,39] |
| Method | Significant | Product Morphology | Properties | Advantages | Shortcomings | Ref. |
|---|---|---|---|---|---|---|
| Static culture | Incubator and culture medium are both in a static state | Gel-like | Higher in crystallinity, polymerization, fracture strength, Young’s modulus | Thickness and shape could be controlled | Time-consuming, space-occupying | [45,46] |
| Stirred culture | Continuous oxygen is input into the culture medium | Granular, star-like | Lower in crystallization, polymerization, fracture strength, Young’s modulus | Good water absorption, rehydration properties | Low yield | [45,46] |
| Process | Mechanism | Advantages | Shortcomings | Ref. |
|---|---|---|---|---|
| Solvent casting method | Nanocellulose and polymer are mixed in a corresponding solvent, and then the mixed suspension is cast on a flat plate. After the solvent evaporates, a uniform film takes shape | Nano-film is well distributed and the thickness is easy to control | Time-consuming, solvent needs to be recycled | [49,50] |
| Layer-by-layer assembly (LBL) | Polymers with opposite charges continuously deposit on the substrate through electrostatic interaction to form a multilayer film with uniform thickness | The composition, thickness, and structure of the film can be controllable at the molecular level | Complicated procedures, large-scale equipment is required | [51,52] |
| Electrospinning | By using a high-voltage electrostatic field, the polymer solution is stretched and overcomes the surface tension to form a jet stream, and then the solvent will evaporate. Finally, a nanoscale thin film is collected on the receiving device | Process is controllable, bioactive substances can be embedded easily | Complicated process, low yield | [53,54] |
| Type of NC | NC source | Type of Matrix | Preparation Method | Finding | Ref. |
|---|---|---|---|---|---|
| Cellulose nanofiber (CNF) | Bamboo | Chitin nanofiber | Blending | It has better thermal stability than pure CNF | [100] |
| Cellulose nanocrystals (CNCs) | Water hyacinth stem fiber | Polyvinyl alcohol, gelatin | Casting method | Degradation temperature rises from 380 °C to 385 °C | [101] |
| Cellulose nanofibrils (CNFs) | Waste coconut husk | Poly(vinyl alcohol) (PVA) | Solution casting | CNF-reinforced films have higher stability than pure PVA film | [102] |
| Cellulose nanocrystals (CNCs) | Pea hull | Carboxymethyl cellulose(CMC) | Solution casting | The addition of CNCs into CMC increases the melting temperature | [103] |
| Cellulose nanocrystals (CNCs) | Rice straw | Poly(vinyl alcohol) (PVA), Chitosan(CS) | Solution casting and evaporation technique | CNCs have a positive effect on the improvement in thermal stability | [104] |
| Bacterial cellulose (BNCs) | Komagataeibacter hansenii | Konjac glucomannan | Solution casting | The addition of BNC increases thermal stability | [105] |
| Cellulose nanofibers (CNF) and nanocrystals (CNCs) | Commercial product | Gelatine | Casting method | The degradation temperature increases by 7–9 °C | [75] |
| Cellulose nanofibers (CNFs) | Laboratory | Polyproppylene | Layer-by-layer assembly | The composite films need more energy to melt | [106] |
| Compositions | Application | Findings | Ref. |
|---|---|---|---|
| Bacterial cellulose/chitosan/gelatin/probiotic bacteria | Chicken fillets |
| [121] |
| Bacterial cellulose/polypyrrole/zinc oxide (ZnO) | Chicken thigh meat |
| [122] |
| Cortex phellodendri/soybean protein/nano-cellulose crystals | Beef tallow |
| [123] |
| Polylactic acid (PLA)/ziziphora clinopodioide essential oil/cellulose nanoparticle | Minced beef |
| [124] |
| Cellulose nanofiber/whey protein/TiO2 /rosemary essential oil | Lamb meat |
| [125] |
| Nanocomposite Formulation | Application | Findings | Ref. |
|---|---|---|---|
| Cellulose nanofibers/cinnamon essential oil | Strawberry |
| [131] |
| Starch-nanocellulose/polyhexamethylene biguanide | Grape |
| [132] |
| Cellulose nanocrystals/chitosan nanoparticle/poly vinyl alcohol | Mango |
| [133] |
| konjac glucomannan/zein nanoparticles/ nanocellulose/nano-TiO2/nano-SiO2 | Cherry tomato |
| [134] |
| Bacterial cellulose/nano-TiO2/copper oxide (CuO) | Fresh-cut pepper |
| [135] |
| Cellulose nanocrystals/gellan gum/gallic acid | Mushroom |
| [136] |
| Chitosan/cellulose nanofiber/γ-cyclodextrin/curcumin | Banana, tomato and cut apple slices |
| [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wu, Z.; Li, A.; Chen, N.; Rao, J.; Zeng, Q. Nanocellulose Composite Films in Food Packaging Materials: A Review. Polymers 2024, 16, 423. https://doi.org/10.3390/polym16030423
Xu Y, Wu Z, Li A, Chen N, Rao J, Zeng Q. Nanocellulose Composite Films in Food Packaging Materials: A Review. Polymers. 2024; 16(3):423. https://doi.org/10.3390/polym16030423
Chicago/Turabian StyleXu, Yanting, Zhenzeng Wu, Ao Li, Nairong Chen, Jiuping Rao, and Qinzhi Zeng. 2024. "Nanocellulose Composite Films in Food Packaging Materials: A Review" Polymers 16, no. 3: 423. https://doi.org/10.3390/polym16030423
APA StyleXu, Y., Wu, Z., Li, A., Chen, N., Rao, J., & Zeng, Q. (2024). Nanocellulose Composite Films in Food Packaging Materials: A Review. Polymers, 16(3), 423. https://doi.org/10.3390/polym16030423






