Preparation of Polyimide/Ionic Liquid Hybrid Membrane for CO2/CH4 Separation
Abstract
1. Introduction
2. Experimental Sections
2.1. Experimental Materials
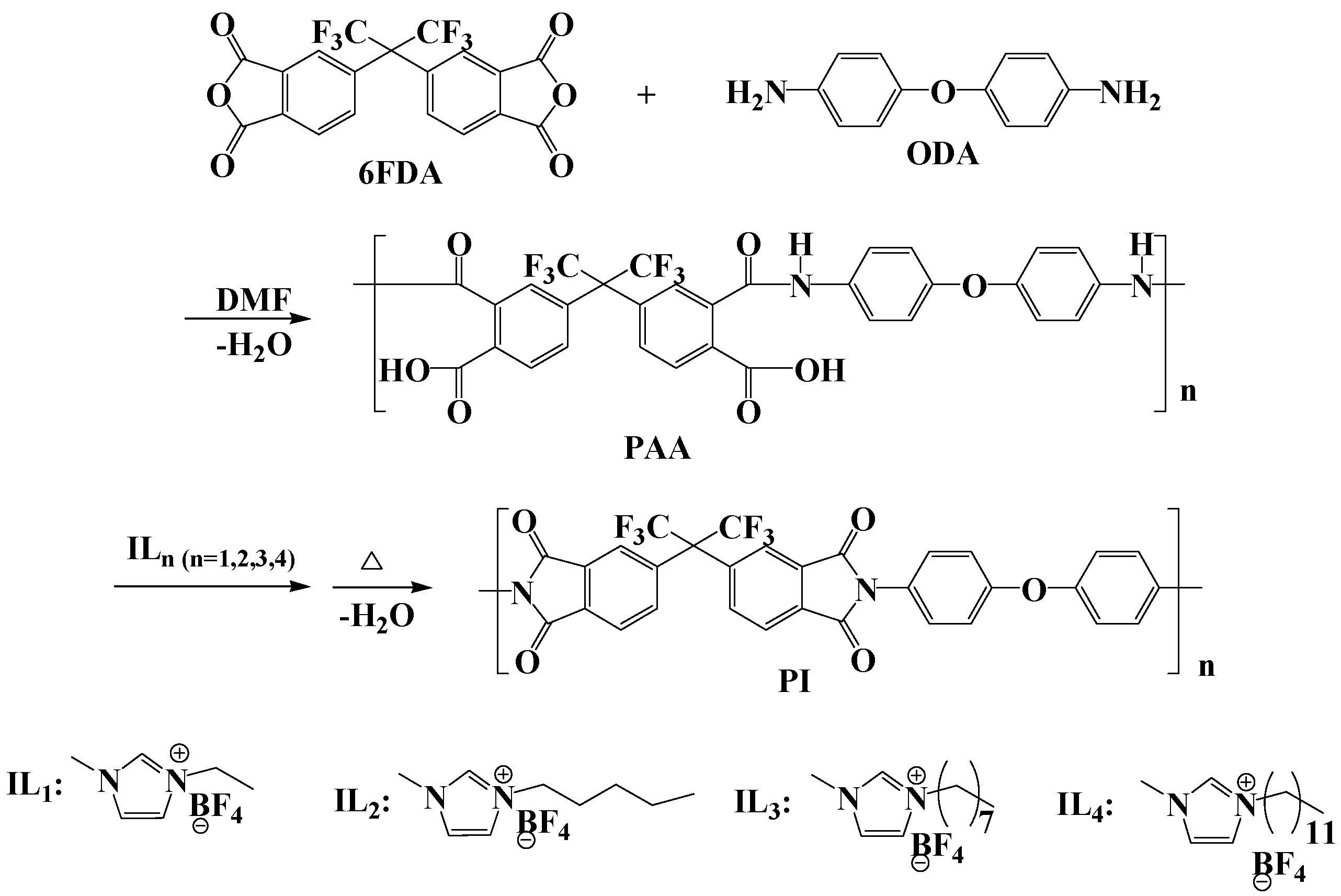
2.2. Experimental Steps
2.3. Gas Separation Measurement and Calculation Formula
Other Measurements
3. Results and Discussion
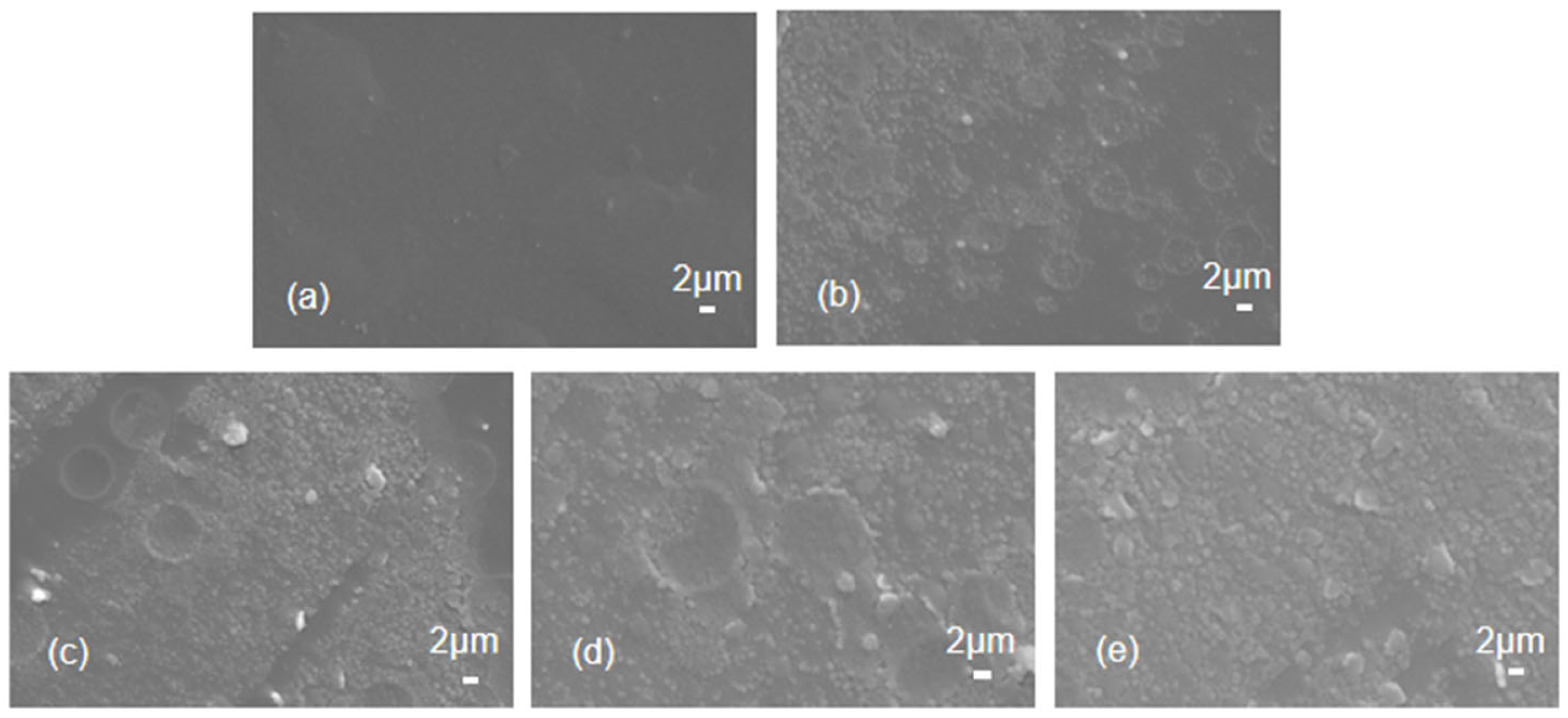
3.1. Surface Morphology of PI and PI/IL1 (x %) Membranes
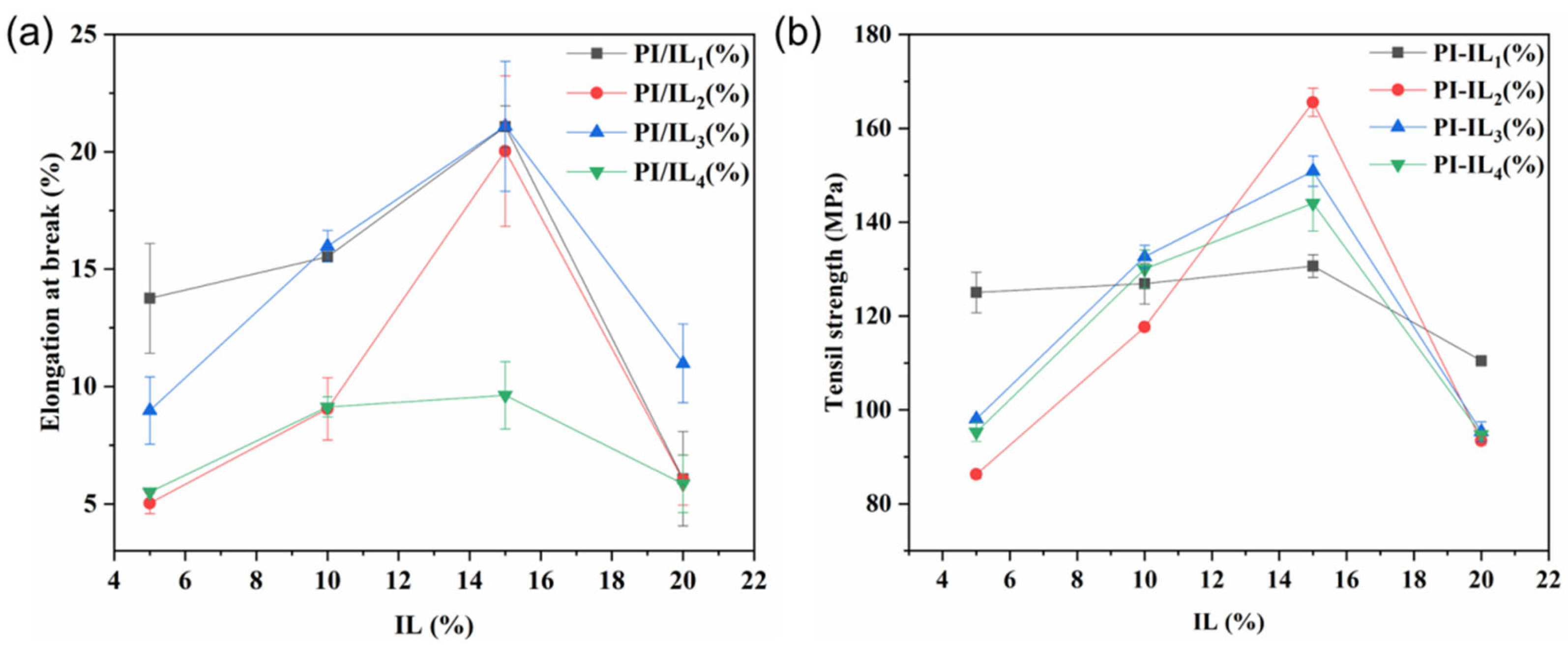
3.2. Mechanical Properties of PI and PI/ILn (x %) Membranes
- (1)
- The effect of IL content on the mechanical properties of PI membranes
- (2)
- The effect of IL types on the mechanical properties of PI membranes
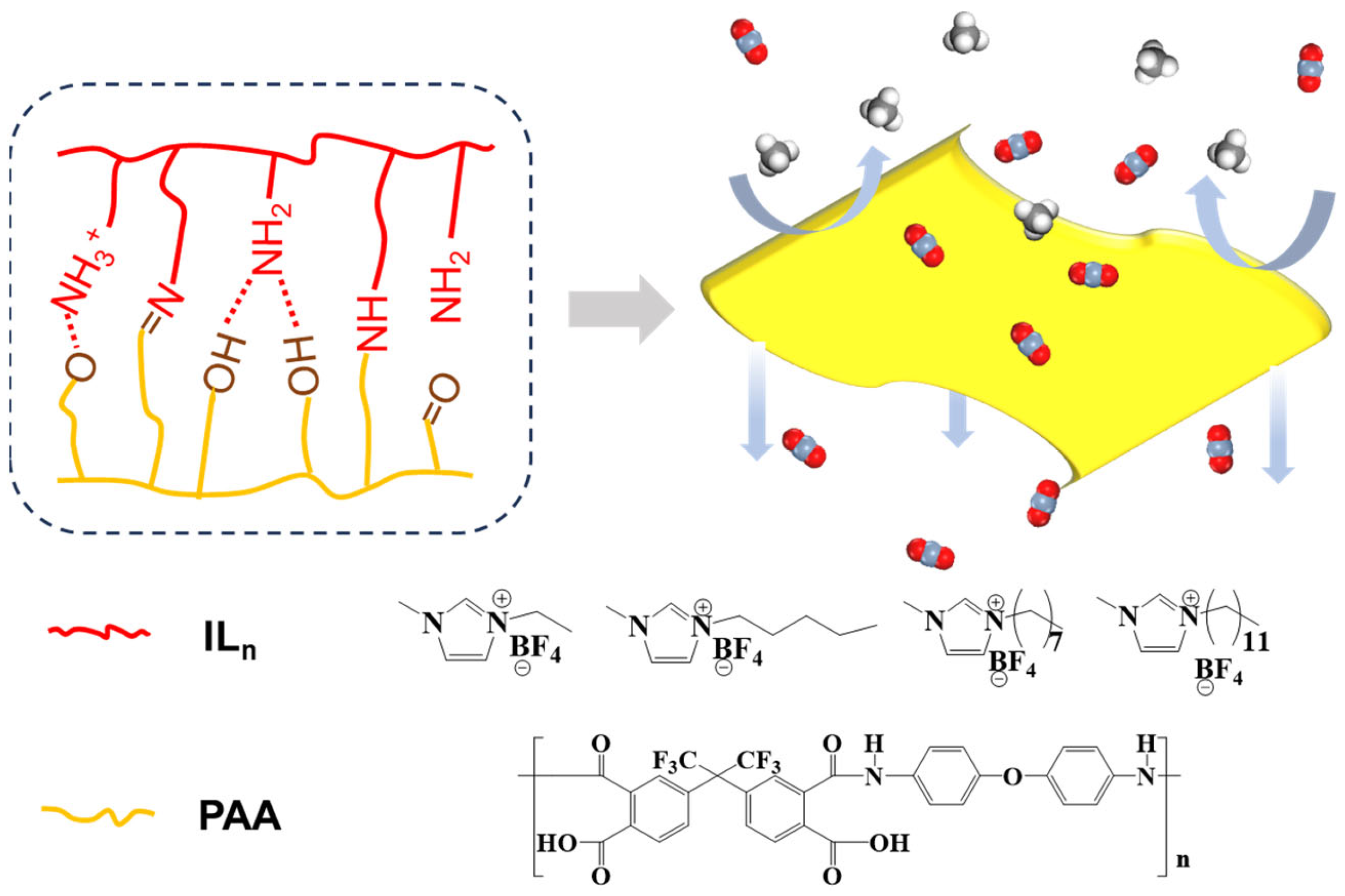
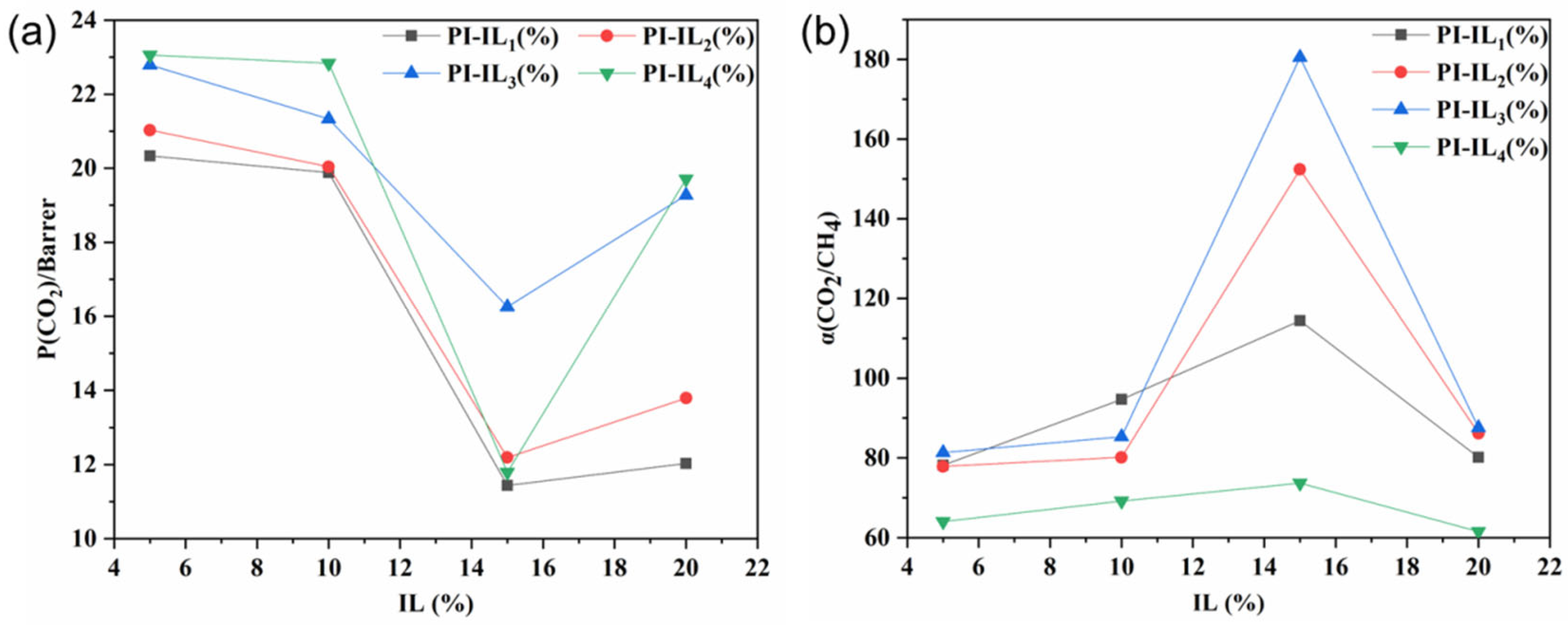
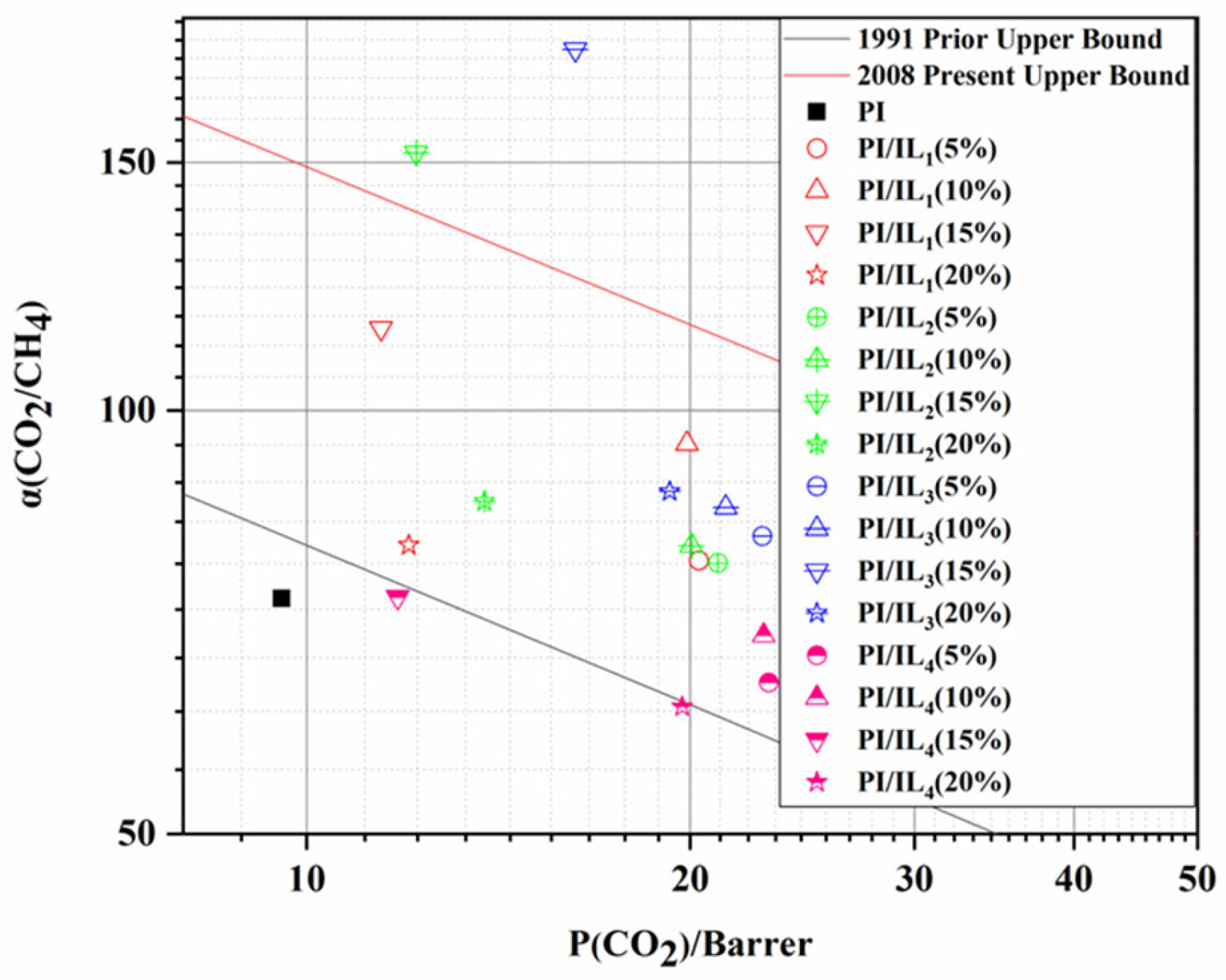
3.3. Gas Separation Performance of PI and PI/ILn (x %) Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benavides, M.; David, O.; Johnson, T. High-performance mixed matrix membranes (MMMs) composed of ZIF-94 filler and 6FDA-DAM polymer. J. Membr. Sci. 2018, 550, 198–207. [Google Scholar] [CrossRef]
- Muntha, S.; Kausar, A.; Siddiq, M. Progress on polymer-based membranes in gas separation technology. Polym. Plast. Technol. Eng. 2016, 55, 1282–1298. [Google Scholar] [CrossRef]
- Han, G.; Yu, N.; Liu, D.; Yu, G.; Chen, X.; Zhong, C. Stepped enhancement of CO2 adsorption and separation in IL-ZIF-IL composites with shell-interlayer-core structure. AIChE J. 2020, 67, 17112–17117. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Z.; Ji, X. CO2 absorption using a hybrid 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide/titanium dioxide/polyethylene glycol absorbent. Fluid. Phase Equilibr. 2021, 188, 113011–113025. [Google Scholar] [CrossRef]
- Zeeshan, M.; Yalcin, K.; Oztuna, F.E.S.; Unal, U.; Keskin, S.; Uzun, A. A new class of porous materials for efficient CO2 separation: Ionic liquid/graphene aerogel composites. Carbon 2021, 171, 79–87. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of Ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- da Luz, M.; Dias, G.; Zimmer, H.; Bernard, F.L.; Nascimento, J.F.D.; Einloft, S. Poly(ionic liquid)s-based polyurethane blends: Efect of polyols structure and ILs counter cations in CO2 sorption performance of PILs physical blends. Polym. Bull. 2021, 79, 6123–6139. [Google Scholar] [CrossRef]
- Lei, L.; Lindbråthen, A.; Zhang, X.; Favvas, E.P.; Sandru, M.; Hillestad, M.; He, X. Preparation of carbon molecular sieve membranes with remarkable CO2/CH4 selectivity for high-pressure natural gas sweetening. J. Membr. Sci. 2020, 614, 118529. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Xu, L.; Zhang, G.; Zheng, J.; Tong, Z.; Shen, C.; Meng, Q. Preparation of amino-functional UiO-66/PIMs mixed matrix membranes with [bmim][Tf2N] as regulator for enhanced gas separation. Membranes 2021, 11, 35. [Google Scholar] [CrossRef]
- Xue, W.-L.; Wang, L.; Li, Y.K.; Chen, H.; Fu, K.X.; Zhang, F.; He, T.; Deng, Y.H.; Li, J.R.; Wan, C.-Q. Reticular chemistry for ionic liquid-functionalized metal-organic frameworks with high selectivity for CO2. ACS Sustain. Chem. Eng. 2020, 8, 18558–18567. [Google Scholar] [CrossRef]
- Hu, T.; Dong, G.; Li, H.; Chen, V. Effect of PEG and PEO-PDMS copolymer additives on the structure and performance of Matrimid@ hollow fibers for CO2 separation. J. Membr. Sci. 2014, 468, 107–117. [Google Scholar] [CrossRef]
- Lin, W.; Chung, T. Gas permeability, diffusivity, solubility, and aging characteristics of 6FDA-durene polyimide membranes. J. Membr. Sci. 2001, 186, 183–193. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 1, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 468, 77–97. [Google Scholar] [CrossRef]
- Qu, Y.; Du, X.; Cheng, K.; Zang, Y. Synthesis and permselectivity of a soluble two-dimensional macromolecular sheet by solid-solid interfacial polycondensation followed by chemical exfoliation. ACS Mater. Lett. 2020, 2, 1121–1128. [Google Scholar] [CrossRef]
- Ravi, C.; Dutta, K.; Bhatia, K. Interfacial engineering of MOF-based mixed matrix membrane through atomistic simulations. J. Phys. Chem. C 2019, 124, 594–604. [Google Scholar] [CrossRef]
- Tanaka, K.; Islam, N.; Kido, M.; Kita, H.; Okamoto, K.-I. Gas permeation and separation properties of sulfonated polyimide membranes. Polymer 2006, 47, 4370–4377. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Dong, J.; Li, H.-B.; Zhang, Q.; Zhao, X. Ionic polyimide membranes containing Troger’s base: Synthesis, microstructure and potential application in CO2 separation. J. Membr. Sci. 2020, 602, 117967. [Google Scholar] [CrossRef]
- Xin, Q.; Shao, W.; Ma, Q.; Ye, X.; Huang, Z.; Li, B.; Wang, S.; Li, H.; Zhang, Y. Efficient CO2 separation of multi-permselective mixed matrix membranes with a unique interfacial structure regulated by mesoporous nanosheets. ACS Appl. Mater. Interfaces 2020, 2020, 12–42. [Google Scholar] [CrossRef]
- Klepić, M.; Jansen, J.C.; Fuoco, A.; Esposito, E.; Izák, P.; Petrusová, Z.; Vankelecom, I.F.; Randová, A.; Fíla, V.; Lanč, M.; et al. Gas separation performance of carbon dioxide-selective poly(vinyl alcohol)-ionic liquid blend membranes: The effect of temperature, feed pressure and humidity. Sep. Purif. 2021, 270, 13–33. [Google Scholar] [CrossRef]
- Bei, P.; Liu, H.; Zhang, Y.; Gao, Y.; Cai, Z.; Chen, Y. Preparation and characterization of polyimide membranes modified by a task-specific ionic liquid based on Schiff base for CO2/N2 separation. Environ. Sci. Pollut. Res. 2020, 28, 738–753. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Kwon, S. Synthesis and characterization of highly soluble and oxygen permeable new polyimides based on twisted biphenyl dianhydride and spirobifluorene diamine. Macromolecules 2005, 38, 7950–7956. [Google Scholar] [CrossRef]
- Shamsipur, H.; Dawood, B.A.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Thermally rearrangeable PIM-polyimides for gas separation membranes. Macromolecules 2014, 47, 5595–5606. [Google Scholar] [CrossRef]
- Shrimant, B.; Dangat, Y.; Kharul, U.K.; Wadgaonkar, P.P. Intrinsically microporous polyimides containing spirobisindane and phenazine units: Synthesis, characterization and gas permeation properties. J. Polym. Sci. A Polym. Chem. 2018, 56, 766–775. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, J.; Song, J.; Bae, T.-H. CO2/N2 Separation properties of polyimide-based mixed-matrix membranes comprising UiO-66 with various functionalities. Membranes 2020, 10, 154. [Google Scholar] [CrossRef]
- An, H.; Lee, A.S.; Kammakakam, I.; Sang Hwang, S.; Kim, J.H.; Lee, J.H.; Suk Lee, J. Bromination/debromination-induced thermal crosslinking of 6FDA-durene for aggressive gas separations. J. Membr. Sci. 2018, 545, 358–366. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Vera, A.T.; de Moura, B.A.; Esteves, L.M.; Tariq, M.; Esperança, J.M.S.S.; Esteves, I.A.A.C. Paramagnetic ionic liquid/metal organic framework composites for CO2/CH4 and CO2/N2 separations. Front. Chem. 2020, 8, 590191–590210. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, H.; Wang, Q.; Ma, W.; Yang, G.; Xu, S.; Li, S.; Su, G.; Qu, Y.; Zhang, M.; et al. Optimization of a MOF blended with modified polyimide membrane for high-performance gas separation. Membranes 2021, 12, 34. [Google Scholar] [CrossRef]
- Vu, M.-T.; Lin, R.; Diao, H.; Zhu, Z.; Bhatia, S.K.; Smart, S. Effect of ionic liquids (ILs) on MOFs/polymer interfacial enhancement in mixed matrix membranes. J. Membr. Sci. 2019, 587, 117157. [Google Scholar] [CrossRef]
- Clara, C. Mixed Matrix Membranes. Membranes 2019, 9, 149. [Google Scholar] [CrossRef]
- Ahmad, N.; Leo, C.; Mohammad, A. Enhancement on the CO2 separation performance of mixed matrix membrane using ionic liquid. Mater. Lett. 2021, 304, 148–157. [Google Scholar] [CrossRef]
- Paola, B.; Daniela, Z.; Gabriele, C. Triggering the gas transport in PVdF-HFP membranes via Imidazolium Ionic Liquids. Sep. Purif. 2020, 250, 117201–117206. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W.; Shaari, N.; Ang, W.L. Recent progress in the development of ionic liquid-based mixed matrix membrane for CO2 separation: A review. Int. J. Energy Res. 2021, 45, 9800–9830. [Google Scholar] [CrossRef]
- Hayashi, E.; Hashimoto, K.; Thomas, M.L.; Tsuzuki, S.; Watanabe, M. Role of Cation Structure in CO2 Separation by Ionic Liquid/Sulfonated Polyimide Composite Membrane. Membranes 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zha, Y.; Du, X.; Xu, S.; Zhang, M.; Jia, H. Interfacial polymerization of self-standing covalent organic framework membranes at alkane/ionic liquid interfaces for dye separation. ACS Appl. Polym. Mater. 2022, 10, 7528–7536. [Google Scholar] [CrossRef]
- Tachibana, S.; Hashimoto, K.; Mizuno, H.; Ueno, K.; Watanabe, M. Effects of polyimide sequence and monomer structures on CO2 permeation and mechanical properties of sulfonated polyimide/ionic liquid composite membranes. Polymer 2022, 15, 124533–124555. [Google Scholar] [CrossRef]
- You, L.; Guo, Y.; He, Y.; Huo, F.; Zeng, S.; Li, C.; Zhang, X.; Zhang, X. Molecular level understanding of CO2 capture in ionic liquid/polyimide composite membrane. Front. Chem. Sci. Eng. 2021, 16, 141–151. [Google Scholar] [CrossRef]
- Kanehashi, S.; Kishida, M.; Kidesaki, T.; Shindo, R.; Sato, S.; Miyakoshi, T.; Nagai, K. CO2 separation properties of a glassy aromatic polyimide composite membranes containing high-content 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquid. J. Membr. Sci. 2013, 430, 211–222. [Google Scholar] [CrossRef]
- Iqra, S.; Arshad, H.; Sarah, F. Effect analysis of nickel ferrite (NiFe2O4) and titanium dioxide (TiO2) nanoparticles on CH4/CO2 gas permeation properties of cellulose acetate based mixed matrix membranes. J. Polym. Environ. 2019, 27, 1449–1464. [Google Scholar] [CrossRef]
- Ana, M.; Pascual, D.; Angel, L.; Vicente, D. Poly(3-hydroxybutyrate)/ZnO bionano composites with improved mechanical, barrier and antibacterial properties. Int. J. Mol. Sci. 2014, 15, 10950–10960. [Google Scholar] [CrossRef]
- Sona, R.; Cor, J. Carbon Dioxide Solubility in the Homologous 1-Alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide Family. J. Chem. Eng. Data 2009, 54, 382–386. [Google Scholar] [CrossRef]
- Qiu, Y.; Ren, J.; Zhao, D.; Li, H.; Deng, M. Poly(amide-6-b-ethylene oxide)/[Bmim][Tf2N] blend membranes for carbon dioxide separation. J. Energy Chem. 2016, 25, 122–130. [Google Scholar] [CrossRef]
- Liliana, C.; Tome, M.; Isabel, M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Chen, H.; Li, P.; Chung, T. PVDF/ionic liquid polymer blends with superior separation performance for removing CO2 from hydrogen and flue gas. Int. J. Hydrogen Energy 2012, 37, 11796–11804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zhao, S.; Qu, Y.; Jia, H.; Xu, S.; Zhang, M.; Geng, G. Preparation of Polyimide/Ionic Liquid Hybrid Membrane for CO2/CH4 Separation. Polymers 2024, 16, 393. https://doi.org/10.3390/polym16030393
Du X, Zhao S, Qu Y, Jia H, Xu S, Zhang M, Geng G. Preparation of Polyimide/Ionic Liquid Hybrid Membrane for CO2/CH4 Separation. Polymers. 2024; 16(3):393. https://doi.org/10.3390/polym16030393
Chicago/Turabian StyleDu, Xiaoyu, Shijun Zhao, Yanqing Qu, Hongge Jia, Shuangping Xu, Mingyu Zhang, and Guoliang Geng. 2024. "Preparation of Polyimide/Ionic Liquid Hybrid Membrane for CO2/CH4 Separation" Polymers 16, no. 3: 393. https://doi.org/10.3390/polym16030393
APA StyleDu, X., Zhao, S., Qu, Y., Jia, H., Xu, S., Zhang, M., & Geng, G. (2024). Preparation of Polyimide/Ionic Liquid Hybrid Membrane for CO2/CH4 Separation. Polymers, 16(3), 393. https://doi.org/10.3390/polym16030393



