1. Introduction
Over the past decades, the range of applied material selections has been wider because of the addition of reinforcements to polymer composite systems. The inorganic nanoparticles dispersed in polymeric matrices could improve the characteristics of polymer matrices, such as optical, thermal, mechanical, and magnetic properties. And the improvement is mainly decided by the properties of nanoparticles in the system [
1]. Because of polymers’ low viscosity and melting temperature, the reinforcements have a broad range of uses with various shapes, properties, and sizes. Both fiber and particle reinforcements enhance the properties of the polymer matrix or significantly introduce new functionalities into the polymer matrix [
2]. The primary processing of composites includes injection molding, filament winding, hand lay-up, bulk molding, resin transfer molding, etc. [
3]. These processes could be divided into two classes, considering the nature of the composites. In the first class, the additives are addressed independently, and the polymer matrix works like a simple medium in which the additives are dispersed. Composites synthesized through these techniques usually control the alignment of additives by external forces based on the properties of fillers. The second kind of polymer composites synthesized based on the interaction between additives and polymeric matrix, and the interactions between molecular guides the formation of designed arrangement. Extra field assisted process could be used to synthesize polymer composites combined with polymerization process. In the field of polymerization, atom transfer radical polymerization (ATRP) is a widely used and versatile strategy that was developed by Matyjaszewski and colleagues in 1990s [
4]. The ATRP technique allows for the precision and controlled synthesis of polymers containing characteristics of narrow molecular weight distributions, specific architectures, and predetermined molecular weights. This unique polymerization methodology brings a revolution to this field, enabling the polymerization of functional polymers with designed properties for different applications [
5]. ATRP has a controlled radical polymerization mechanism, in which a transition metal complex catalyst works as a mediation of the halogen atoms exchanging process between initiators and growing polymer chains. The controlled propagation of polymer chains relies on the equilibrium between active species and dormant, which could be turned by controlling the concentration of the copper catalyst and the initiator [
6,
7]. This controlled property of ATRP gives it reasonable control of polymer architecture and makes the synthesis of well-defined structures like block copolymers, brush polymers, and grafted polymers easier [
7,
8,
9]. The wide applications of ATRP rely on its ability to gain precise control over polymerization, resulting in polymers with well-defined characteristics and molecular weights. This nature of ATRP enables the turning of chemical, physical, and biological properties of materials, making ATRP an essential technique in designing advanced materials. However, despite its advantages, ATRP still faces challenges, such as difficulties in reducing agents and removing residual catalysts from the final product and the narrow scope of monomer selection [
10].
One of the challenges in synthesizing polymer composites is forming a homogeneous mixture during the production process [
11]. For particle-reinforced composites, the rapid coagulation of additives caused by their Van der Waals force, high surface energy, and capillary particles after mixing with polymer resin influence the final properties of composites [
12,
13]. In addition to these challenges, the micro-structure of polymer composites also dramatically affects the macro-properties of polymer composites. To overcome the above-mentioned problems, field-assisted additive manufacturing should be considered to synthesize polymer composites, and the manufacturing process has been used in some additive manufacturing processes, which is usually used by polymer composite materials. The history of field-assisted additive manufacturing (AM) could be traced back to the 1980s, when the concept of additive manufacturing was proposed. Additive manufacturing has been considered as a great breaking technology, which achieves the synthesis of complicated and tailored products with great design freedom. However, except for its potential, additive manufacturing often obtains challenges among limited speeds, material selected limitations, and accuracy. To solve these problems, external fields integrated into additive manufacturing systems have begun to attract researchers’ attention [
13,
14]. The crucial purpose of field-assisted AM technologies is to control and separate individual additives’ orientation together with position in a polymer resin to obtain minute-adjustment properties of the final products [
15,
16,
17]. By providing an additive field, the control of materials flow is more precise, the speed of additive manufacturing techniques is faster [
18,
19], and the compatibility of materials is wider [
20]. Magnetic field-assisted AM is one of the most promising techniques among field-assisted AM technologies. With the support of an additive magnetic field, the materials’ orientation and alignment could be controlled well.
Polymer nanocomposites consist of nanoscale additives, and polymeric matrices are a crucial part of materials, which has previously been explained. In order to fully utilize the characteristics of nanoparticles, it is critical to manage the additives’ distribution in the polymer composites system. The reason is that the structural arrangement has an influence on the interaction between the two components. Precisely, the generation of new techniques and manufacturing processes to control and create ordered arrays is a significant under-researched field. The reason is that nanoscale ordering could provide unique functionality that cannot currently be realized in random composites [
21]. Magnetic composites with a well-arranged structure have a wide range of applications. In the past few years, researchers have synthesized different polymer composites with various structures. For example, Lei Wang had synthesized Ti
3C
2Tx@Fe
3O
4/CNF aerogels (BTFCA)/epoxy with long-range aligned lamellar structures, which work as electromagnetic interference (EMI). The designed structure increases the EMI shielding effectiveness and reduces the secondary contamination [
22]. The EMI shielding property enables the material to be used in fifth-generation (5G) mobile communication technology [
23]. Magnetic particles in a composite system could be used to turn the functions of the materials by influencing and controlling the microstructure of the materials. For example, researchers synthesized Fe
2O
3@Au core-shell nanoparticles to turn the pKa of 11-mercaptoundecanoic acid by containing the thickness of the shell [
24]. Due to the advantages of magnetic polymer composites, such as bio-friendly, reusability, superparamagnetism, and the potential to turn the well-arranged microstructure, it has attracted increasing attention. In this project, Fe
3O
4 nanoparticles in the system were successfully used as a tool to guide and influence the molecular flow and further influence the arrangement of the microstructure of the synthesized polymer composites. The results of this experiment provide a potential for turning the macroscale characteristics by controlling the microstructure of the materials with the assistance of an extra applied field.
PMMA is one of the most widely used industrial polymer materials because of its mouldability, biocompatibility [
25], extraordinary optical transparency [
26], mechanical properties, and weatherability [
27]. Several nanoparticles/PMMA composites were synthesized through the ATRP technique in the past few decades to enhance or add various properties to PMMA [
28,
29,
30]. In this paper, compared with the typical synthesized process of magnetic polymer composites, a magnetic field-assisted ATRP technique was a novelty used to synthesize homogeneous magnetic polymer composites Fe
3O
4/PMMA containing well-arranged micro-structure. Compared with other methods of particle manipulation, magnetic field-assist technology has shown more flexibility and is more straightforward to use [
31,
32].
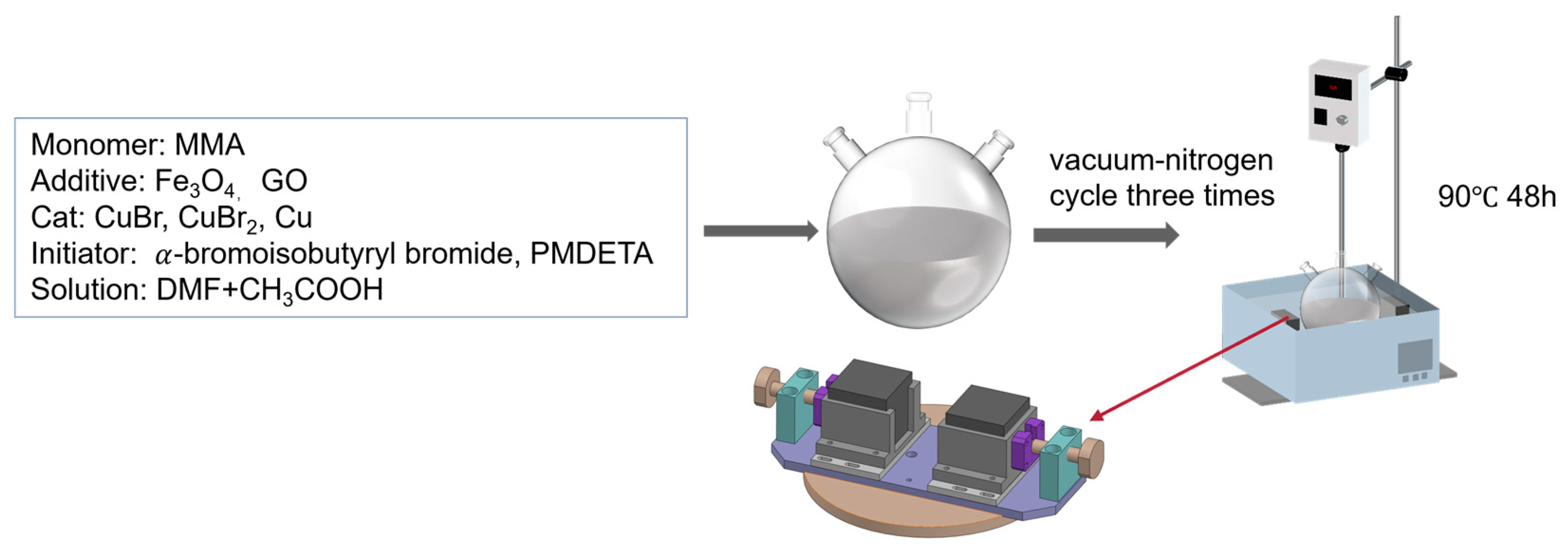
The scheme of this paper is shown in
Figure 1 below. Generally, the polymerization was assisted by an extra magnetic field. During the polymerization, the magnetic particles in the system draw and push the polymer chains to move according to the direction of the magnetic field. As a result, a well-arranged structure could be synthesized.
3. Experiments
Generally, Fe
3O
4 was dispersed into N,N-Dimethylformamide (DMF) by an ultrasonic process to form a uniform solution. The solution containing methyl methacrylate (MMA), catalyst, and initiator of the ATRP reaction was added into the system. Through the ATRP reaction, the magnetic polymer composite, Fe
3O
4/PMMA, was fabricated. The schematic diagram is shown in
Figure 3 below.
- I.
Purification of catalyst
Firstly, pretreatment of CuBr was needed. CuBr was dissolved into enough CH3COOH by magnetic stirring at room temperature overnight to remove impurities in CuBr. Next, methyl alcohol was used to wash the purified CuBr 3 times, and the gained CuBr was placed in a vacuum oven and dried for 12 h at 40 °C. After drying, the purified CuBr was kept under vacuum in a desiccator.
- II.
Polymerization of Fe3O4/PMMA
Different weight concentrations of Fe3O4 (0.35 wt%, 0.7 wt%, 2.5 wt%, 5 wt%, 10 wt%, 20 wt%, which are 0.065 g, 0.130 g, 0.511 g, 0.93 g, 1.80 g, 3.76 g, respectively) were dispersed into 15 mL of DMF by an ultrasonic process for 30 min. The solution was kept as solution A. Then, 0.152 g of CuBr, 0.088 g of CuBr2, and 0.036 g of L-ascorbic acid were dissolved in a mixed solution consisting of 10 mL of DMF and 4 mL of CH3COOH by mechanical stirring. The dissolving process proceeded in a 100 mL two-neck spherical reaction flask, and the solution was kept as solution B. Subsequently, 20 mL of MMA and solution A, with a particular concentration of Fe3O4, were added into solution B, and the mixed solution was well dispersed through mechanical stirring to form solution AB. Three vacuum–nitrogen cycles were purged into the system to remove the oxygen in solution AB before starting the ATRP process.
Following this, 80 μL of α-bromoisobutyryl bromide and 142.4 μL of 1,1,4,7,7-Pentamethyldiethylenetriamine (PMDETA) were added into the solution AB in a two-neck spherical reaction flask by dropping through an injection syringe. Then, the apparatus was heated to 90 °C and bathed in oil for 48 h to facilitate the reaction under a 200 mT magnetic field. The reactants were added according to a molar ratio of 287[MMA]:1[-Br]: 324[CH3COOH]:2[PMDETA].
- III.
Polymerization of pure PMMA
A similar process synthesized pristine PMMA. Firstly, 10 mL of DMF and 4 mL of CH3COOH were mixed together to form a solution A. Then, 0.152 g of CuBr, 0.088 g of CuBr2, and 0.036 g of L-Ascorbic acid were added and stirred well to obtain a homogeneous solution B, followed by adding 20 mL of MMA and solution to form a homogeneous solution AB by mechanical stirring. To remove oxygen in the system, three vacuum–nitrogen cycles were taken before the polymerization.
Next, 80 μL of α-bromoisobutyryl bromide and 142.4 μL of PMDETA were injected into the solution AB in a two-neck spherical reaction flask by a syringe. Then, polymerization was triggered by heating the apparatus to 90 °C and keeping it in an oil bath for 48 h. The reaction system schematic diagrams are drawn in
Figure 3.
- IV.
Collection of synthesized samples
After polymerization, the synthesized polymer composites were collected through a vacuum filter. The products were washed three times through the dissolving and filtering process. The purified products were then dried overnight at 80 °C. After drying, a hot press process was taken to gain the polymer sheet at 145 °C, followed by a pressing process at 150 °C with 30 KN to further deform the sheet into circular disks with 50 mm diameter and 1 mm thickness.
- V.
Testing techniques and conditions
The morphologies of obtained samples were studied by a scanning electron microscope (TESCAN VEGA3) and field emission transmission electron microscope (JEOL JEM-2100F)). The samples were learned through Fourier-transform infrared spectroscopy (FTIR) spectrometers (Perkin Elmer) with a wavenumber ranging from 500 to 4000 cm−1. X-ray diffraction (XRD, Rigaku Smartlab, Tokyo, Japan) was used to study the crystallization structures scanning from 15° to 85°. Differential scanning calorimetry (DSC, METTLER 1100LF) was used to learn the thermal properties, and the temperature range was from 30 °C to 140 °C in Ar flow with a temperature rising rate of 10 °C/min. The molecular weight and distribution of the polymer chain were detected by a Shimadzu Rid-20A (Kyoto, Japan), and the polymer was dissolved in tetrahydrofuran (THF) to make a solution of 20 mg/mL. This study used Zygo Nexview Optical Surface Profiler to test the surface roughness (Sa) of Fe3O4/PMMA samples with different amounts of Fe3O4. All the samples were machined at a feed rate of 400 mm/min and 3 μm of cut.
4. Results and Discussion
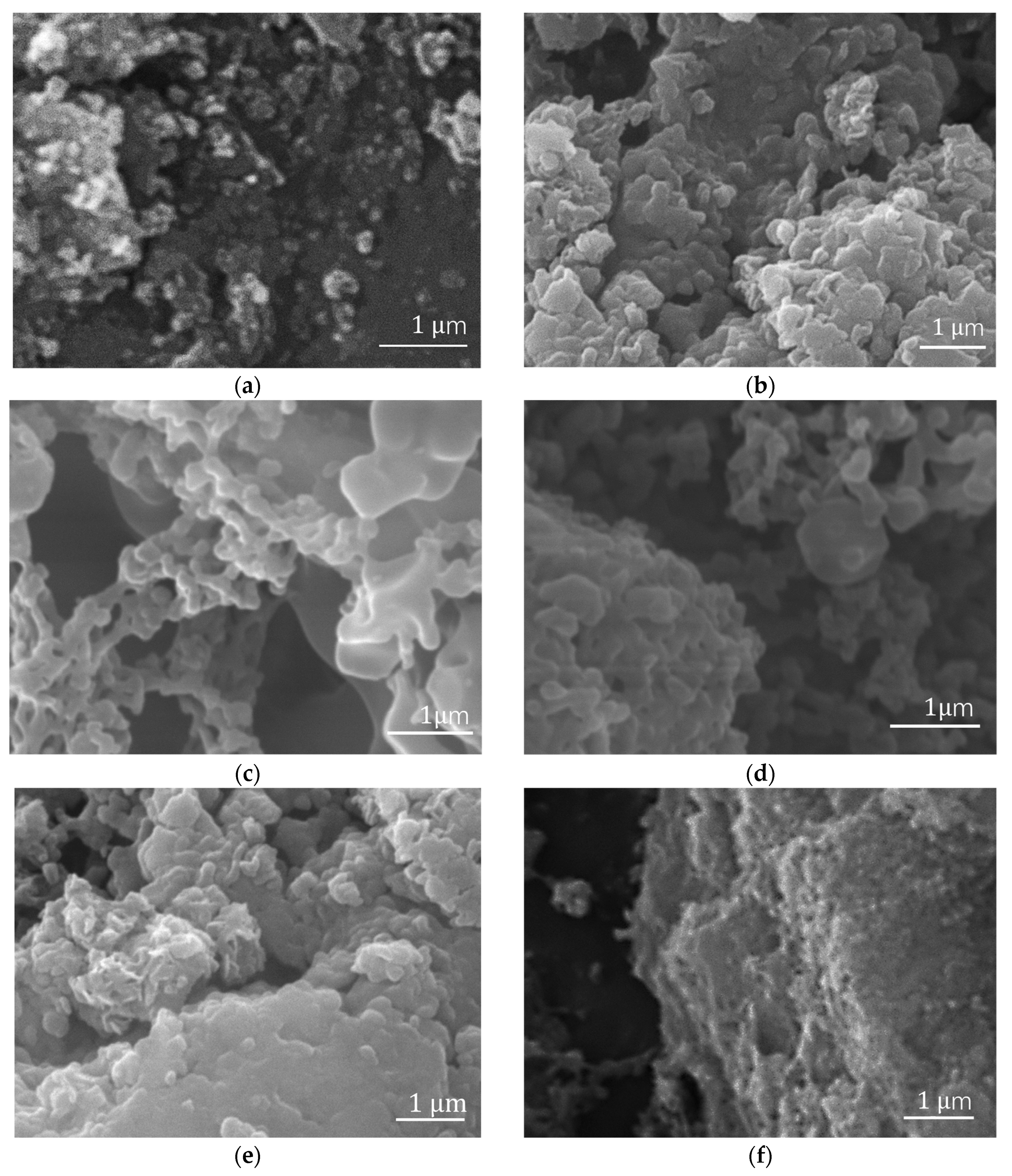
The morphology and structure of each reaction product were observed by a TESCAN VEGA3 scanning electron microscope and a transmission electron microscope.
Figure 4a shows the morphology of Fe
3O
4 nanoparticles. Because of their magnetic properties, these nanoparticles have a solid intermolecular force to reunite. After ultrasonic mixing, Fe
3O
4 particles were dispersed. After Fe
3O
4 is added to the ATRP system, polymer chains grow under a magnetic field, with the magnetic fillers affecting the chains’ arrangement.
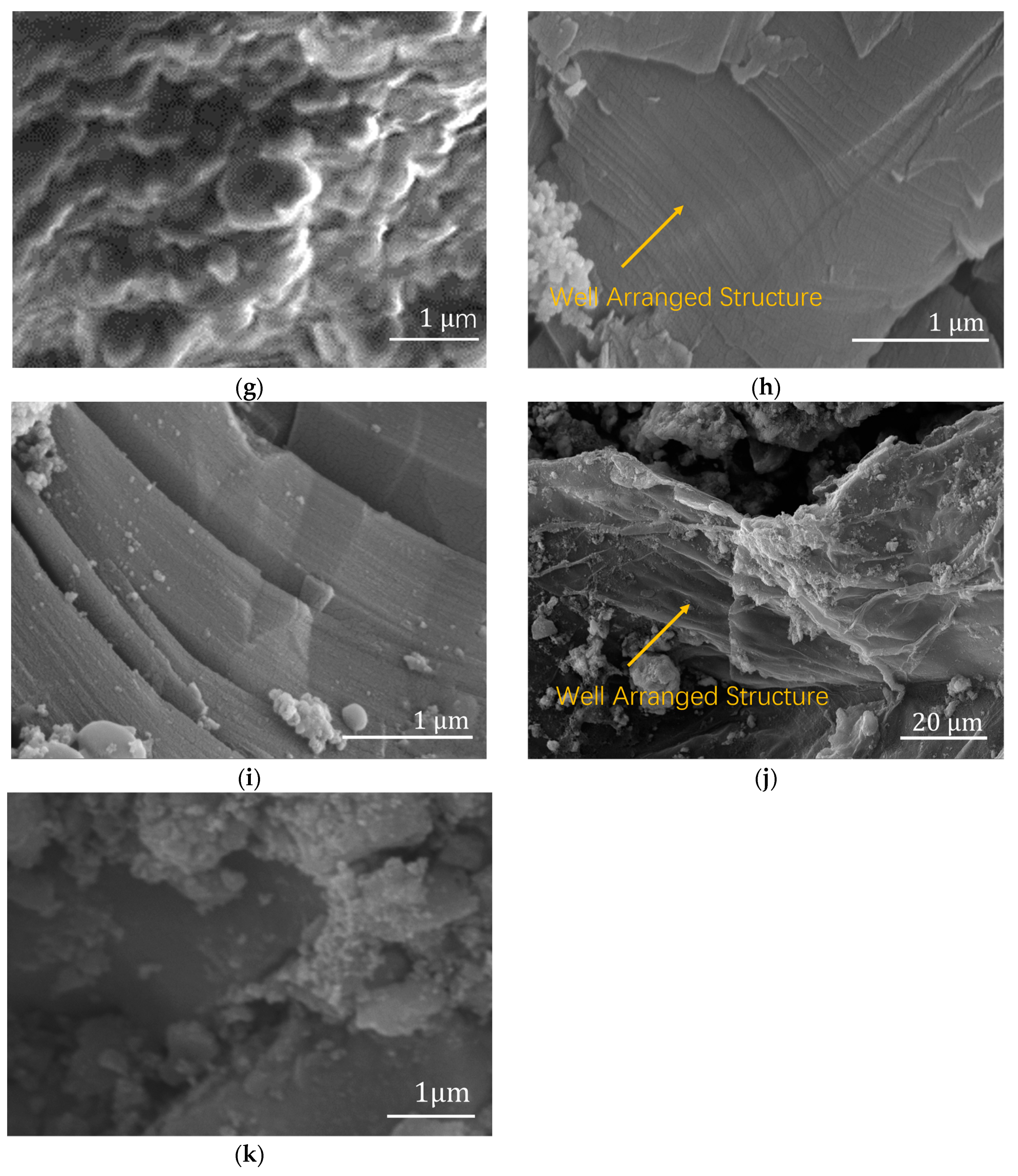
Compared with the morphology of pure PMMA in
Figure 4b and Fe
3O
4/PMMA (20 wt%) synthesized without magnetic field,
Figure 4h–j show the obvious oriented structure of the polymer composite with 20 wt% weight concentration of magnetic additives Fe
3O
4. When the weight concentration of magnetic fillers increases from 0.35 wt% to 20 wt%, the arrangement of polymer chains grows from a small plate structure to a well-arranged structure. Compared with polymer composites with 10 wt% weight concentrations of magnetic additives, the well-arranged structure is more apparent when the weight concentration grows to 20 wt%. When monomers begin to grow, the small chains are separated and guided by the magnetic nanoparticles in the system under a specific magnetic field of 200 mT. The direction of polymer chains’ growth is assisted by the combination of magnetic fillers and magnetic fields. As a result, when the concentration of magnetic fillers increases to 20 wt%, a large, well-arranged PMMA layered structure is formed, as shown in
Figure 4f–h.
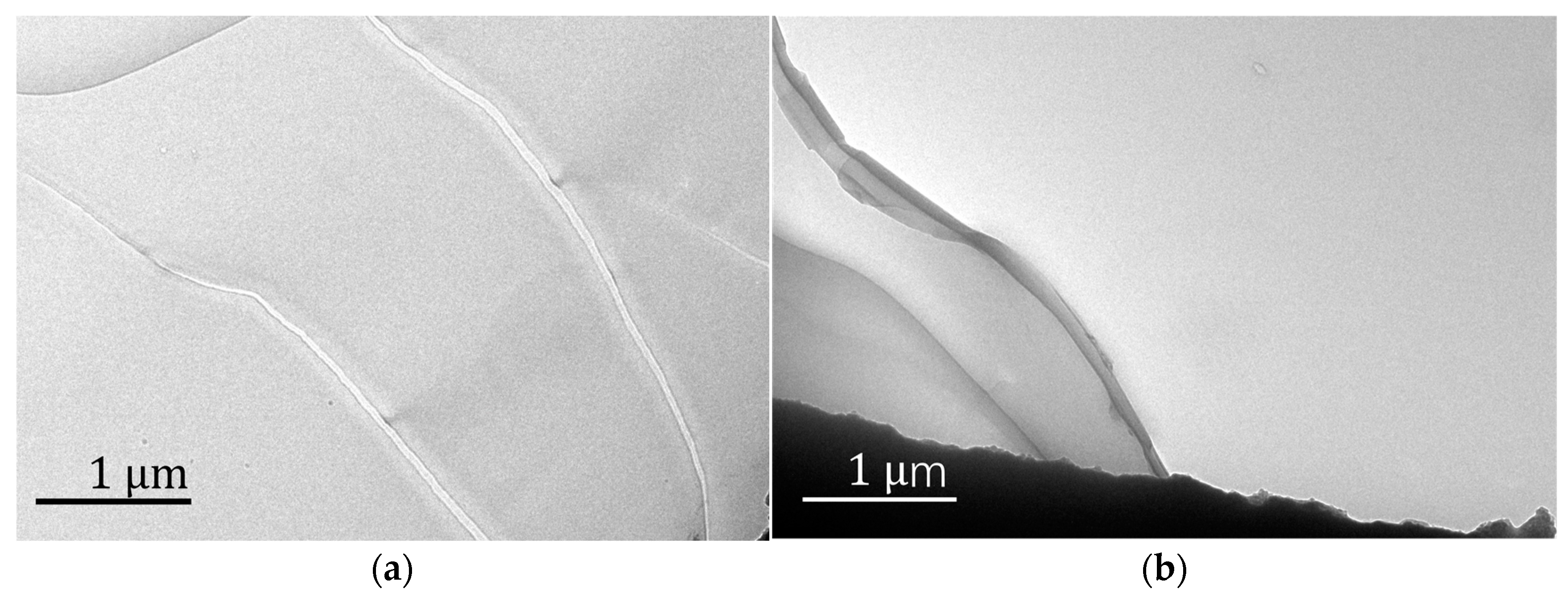
Figure 5 shows the TEM pictures of the well-arranged structure of Fe
3O
4/PMMA (20 wt%). The TEM results clearly show the well-arranged layered structure of polymer composites, which confirms the functions of added magnetic nanoparticles and magnetic field. Under the magnetic field, magnetics, Fe
3O
4 particles have an influence on the structure of polymer composites during the polymerization process.
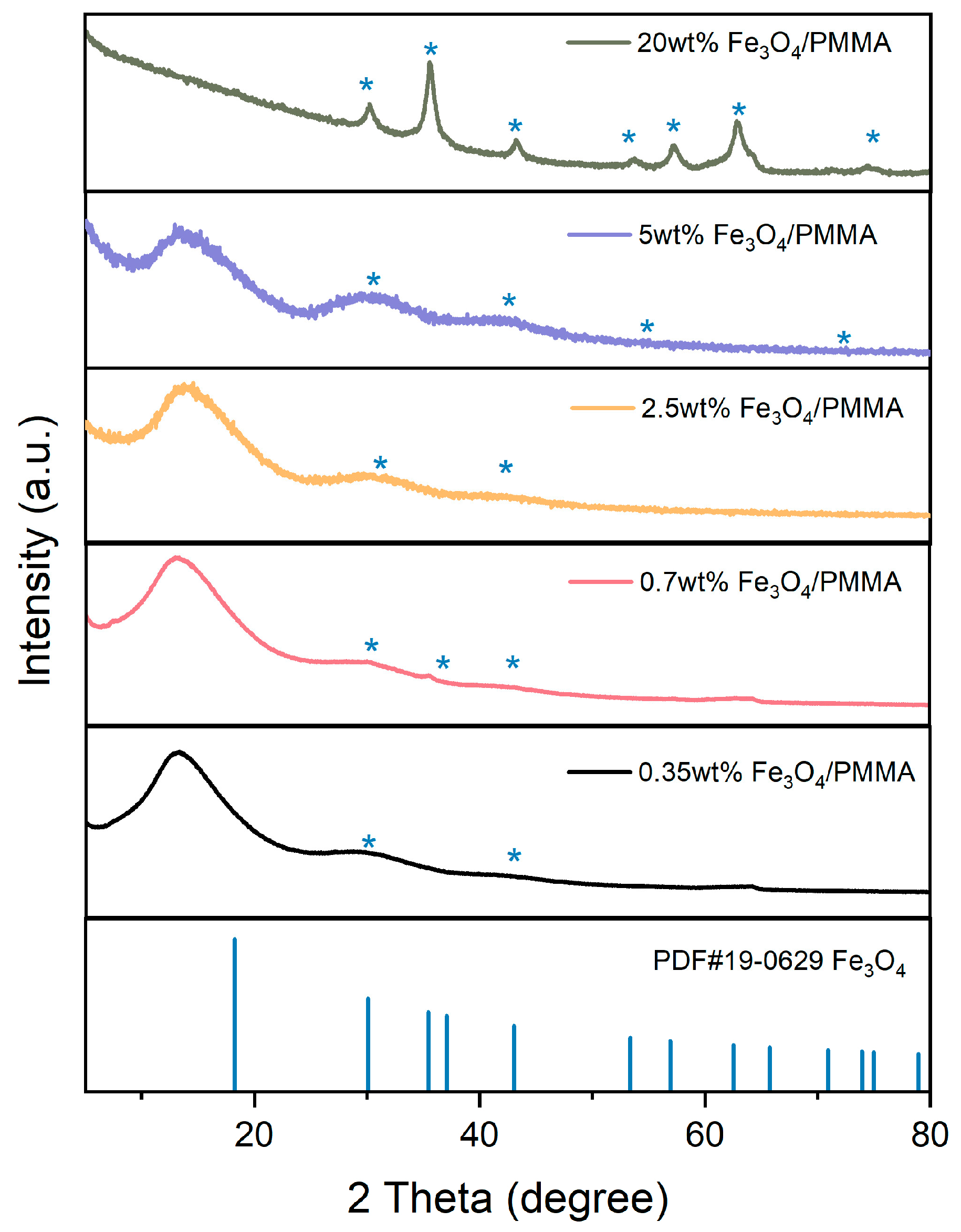
The crystallization structures of products were analyzed by X-ray diffraction. The spectra of synthesized composites, Fe
3O
4/PMMA, with different weight percentages of magnetic additives Fe
3O
4, are detected from 15° to 85° and shown in
Figure 6.
The PDF card of Fe3O4PDF#19-0629 is represented by a blue line. Peaks of 30°, 35° 53°, 56°, 71°, and 73° represent Fe3O4, and it could be found that these typical peaks are observed in XRD patterns of Fe3O4/PMMA composites; with the addition of magnetic fillers, peaks of Fe3O4 are more significant. When the concentration of Fe3O4 is added to 20 wt%, the XRD spectra of polymer composites show clear and significant typical peaks of Fe3O4.
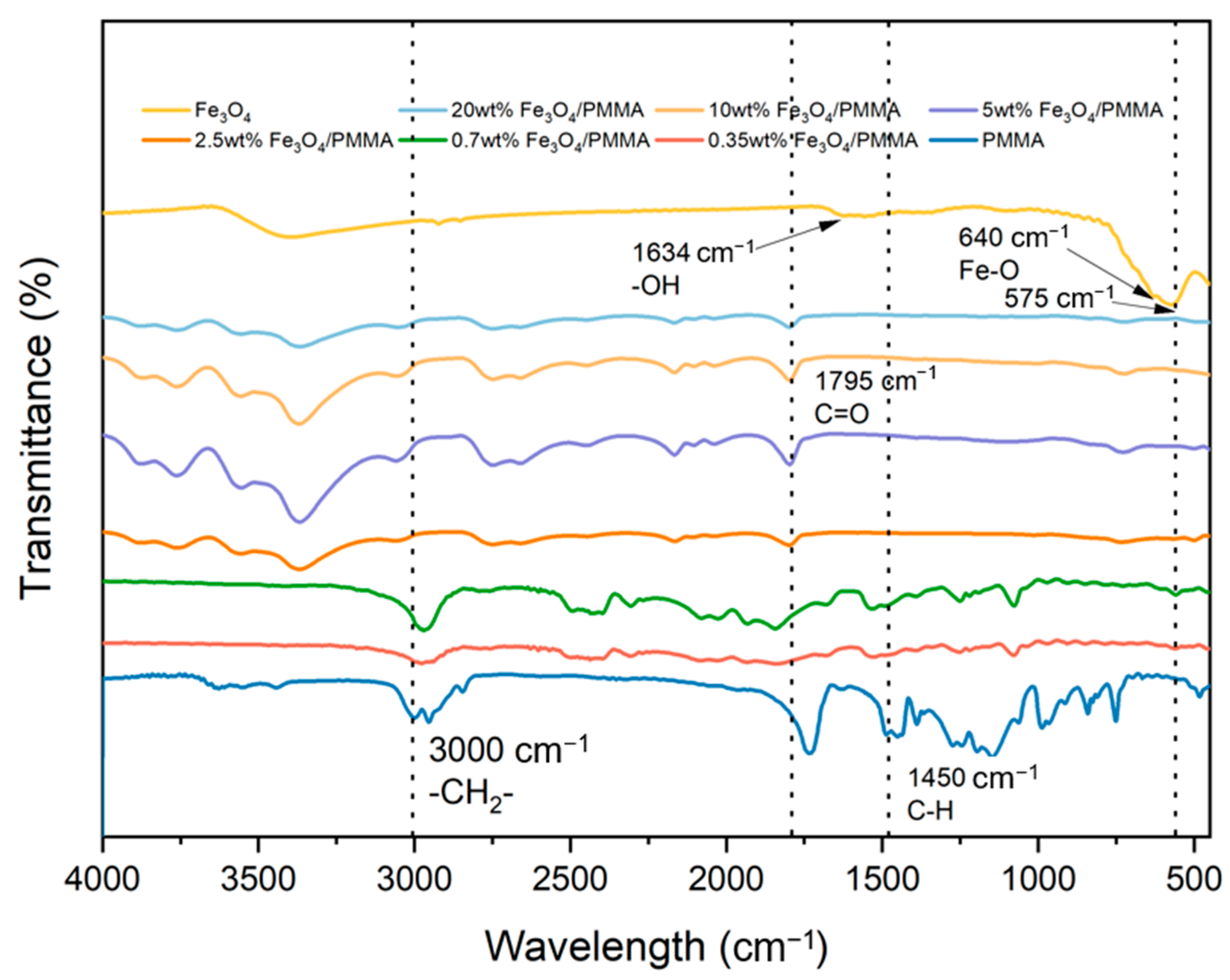
A Perkin Elmer Fourier-transform infrared spectrometer was adopted to study the polymer structure with a wavenumber ranging from 500 to 4000 cm
−1.
Figure 7 shows the measured results for Fe
3O
4 and Fe
3O
4/PMMA with different weight percentages of Fe
3O
4 and pure PMMA.
The two intense peaks with small wavenumbers at 575 cm
−1 and 640 cm
−1 represent the stretching vibration mode of Fe-O metal–oxygen bonds in the Fe
3O
4 crystalline lattice. These two peaks are also found in polymer composites, which proves the successful connection between polymer resin and Fe
3O
4 additives. The typical peaks of PMMA can also be observed in
Figure 5. Peaks at 1450 cm
−1, 1634 cm
−1, 1765 cm
−1, and 3000 cm
−1 represent C-H, -OH, C=O, and -CH2- of PMMA, respectively. The FTIR spectra of Fe
3O
4/PMMA show clear peaks of PMMA and Fe
3O
4. Combined with the results from XRD, the successful synthesis of Fe
3O
4/PMMA could be proven.
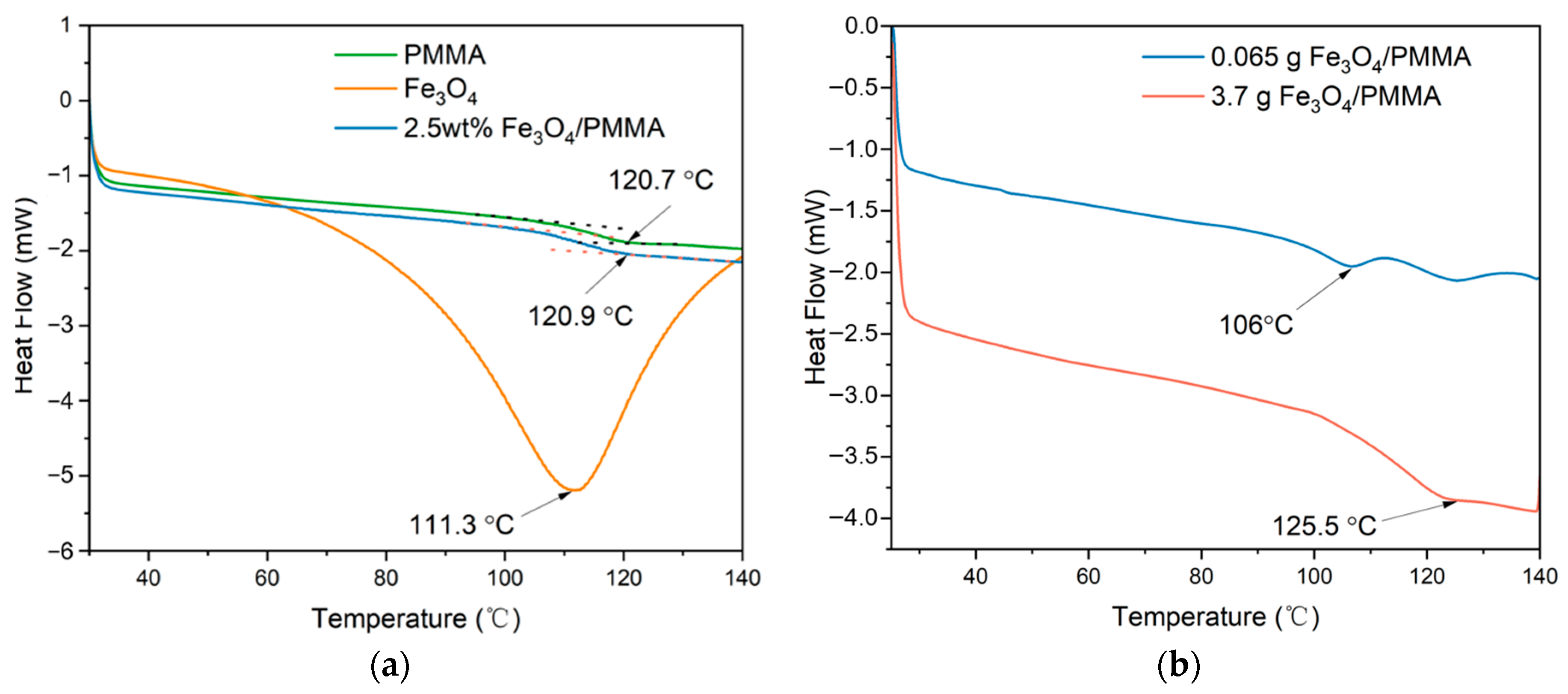
Differential scanning calorimetry (DSC) was tested to observe the thermal characteristics of different composites, and the results are shown in
Figure 8 below. The tested temperature ranges from 30 °C to 140 °C at an increment of 10 °C /min under N
2 atmosphere. The DSC results of PMMA, Fe
3O
4, 0.35 wt% of Fe
3O
4/PMMA, 2.5 wt% of Fe
3O
4/PMMA, and 20 wt% of Fe
3O
4/PMMA are shown in
Figure 8. From the DSC results, it can be found that when the concentration of magnetic fillers is low, the glass-transition temperature (T
g) decreases considerably from 120.7 °C to 106 °C compared with pure PMMA. When the concentration of magnetic fillers increases to 2.5 wt%, the T
g of Fe
3O
4/PMMA rises to 120.9 °C, which is close to the T
g of pure PMMA. The decrease in T
g with low weight concentration of magnetic additives could be explained by the fact that nanoparticles between the polymer chains decrease the intermolecular force between macromolecules.
With the further increase in the concentration of Fe3O4 to 20 wt%, the Tg of Fe3O4/PMMA rises to 125.5 °C. The growth of Tg, when the concentration of magnetic fillers becomes higher, may be caused by the restrictions of polymer chains’ movement by rigid Fe3O4 nanoparticles inside the polymer composites. The larger attractive forces between polymer chains from magnetic particles also lead to the higher Tg of polymer composites. The application of an extra magnetic field is to control nanoscale organization. In this experiment, magnetic fields could help to arrange the alignment of magnetically responsive Fe3O4 nanoparticles. Combined with SEM and TEM results, it could be found that the polymer composites have a well-arranged microstructure when the weight percentage of additives reached 20 wt%. With the well-ordered microstructure, the interaction between polymer chains makes the movement of polymer chains harder, which increases the Tg of the polymer matrix. Although the differences between pure PMMA and polymer composites are insignificant, this observation illuminates a potential strategy to modify composite characteristics by controlling micro-organization within the materials.
The molecular weight of PMMA and Fe
3O
4/PMMA with varying Fe
3O
4 weight percentages was measured by GPC. The testing solution was prepared by dissolving the products into tetrahydrofuran (THF), forming a 20 mg/mL concentration solution, and then the results were tested by a Shimadzu Rid-20A (Kyoto, Japan) machine.
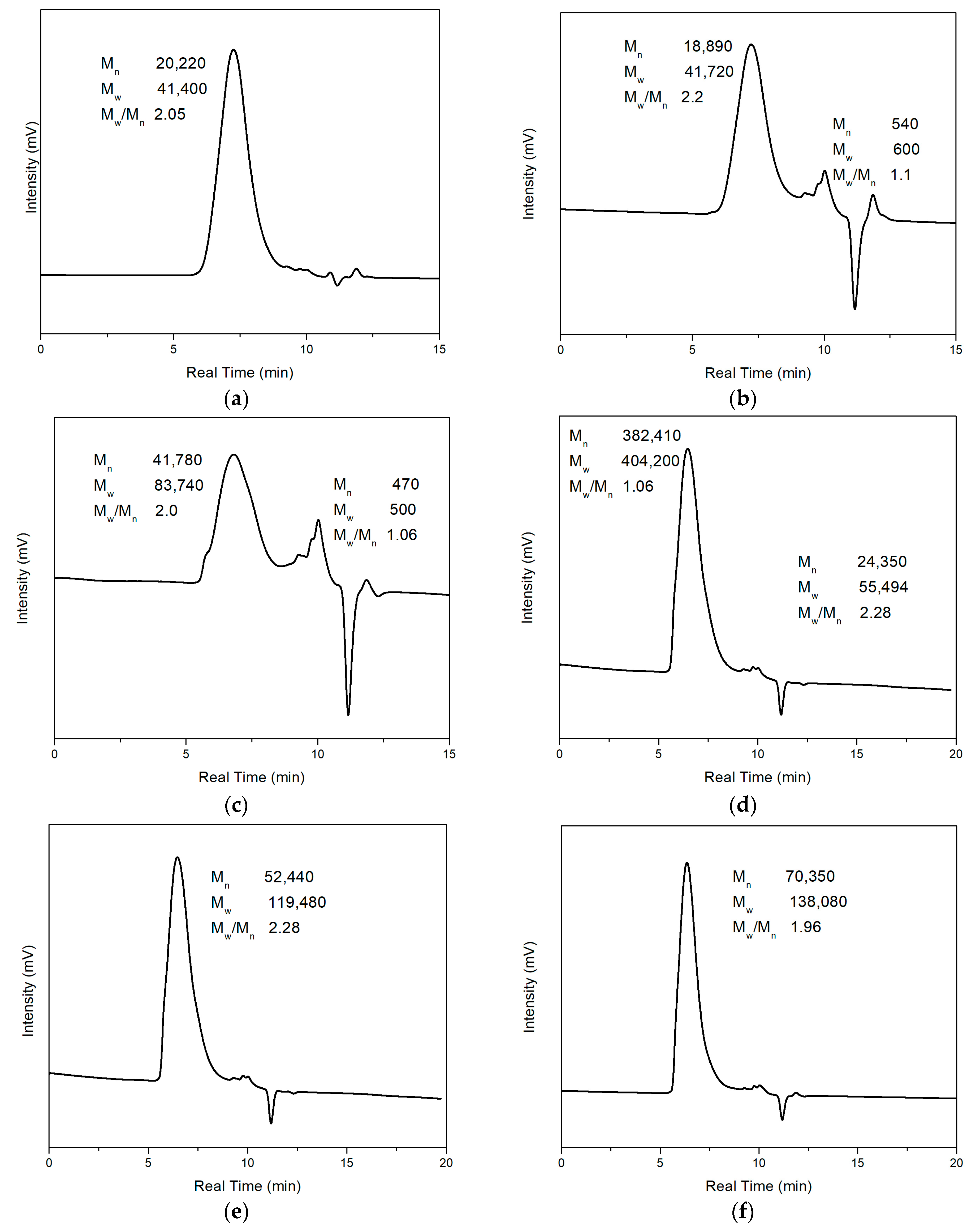
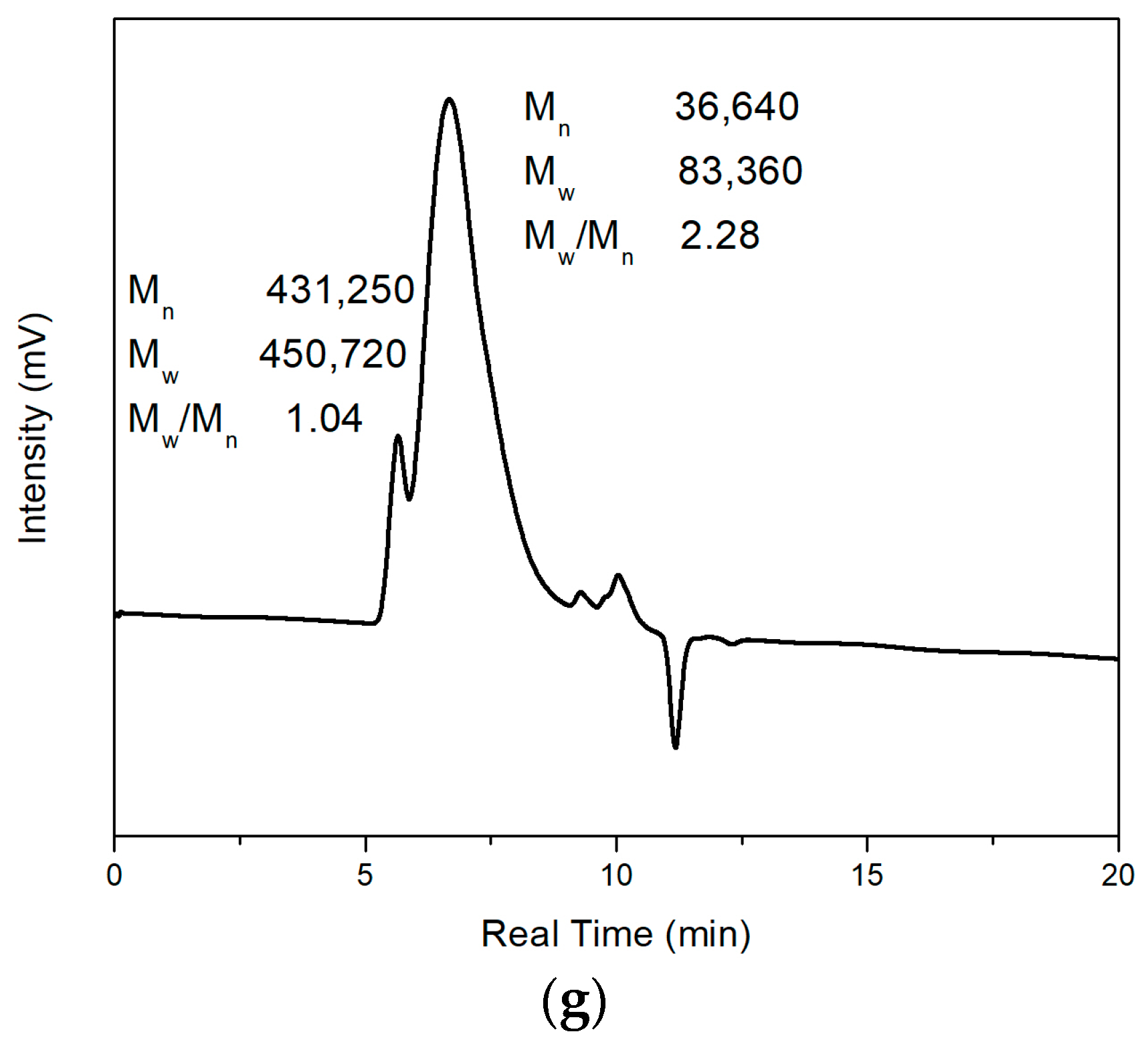
Figure 9a shows the GPC result of pure PMMA, and
Figure 9b–g shows the GPC results of synthesized composites with different weight percentages of Fe
3O
4 particles.
Figure 9b,c shows two significant peaks among these figures. The left peak represents larger-molecular-weight polymer chains, and the right peak represents molecules with small molecular weights of around 500.
Figure 9a shows the GPC results of pure PMMA. The number average molecular weight (M
n) and weight average molecular weight (M
w) of PMMA are 20,220 and 41,400, respectively, and M
w/M
n = 2.2 could calculate the dispersity of pure PMMA.
Figure 9b–g shows the polymer composites with different concentrations of magnetic particles (0.35 wt%, 0.7 wt%, 2.5 wt%, 5 wt%, 10 wt%, 20 wt%, respectively). The M
n of magnetic polymer composites is 18,890, 41,780, 382,410, 52,440, 70,350, and 36,640, respectively, and the M
w is 41,720, 83,740, 404,200, 119,480, 138,080, and 83,360, respectively. It could be found that except for Fe
3O
4/PMMA with 0.35 wt% Fe
3O
4 additives, other composites have larger M
n compared with pure PMMA. The larger molecular weight may come from adding an external magnetic field. Under the magnetic field direction, the flow of molecules was affected, and the unified direction of molecular flow led to easier elongation of polymer chains. However, when the concentration of magnetic additives was insufficient, the influence from the magnetic field was not significant, which led to the similar molecular weight of pure PMMA and Fe
3O
4/PMMA (0.35 wt%). The GPC results have been concluded and shown in
Table 1 below. The large dispersity may be because of the long polymerization time. With longer polymerization time, the polymerization period before termination of polymer chains may be significantly different, leading to a large dispersity of molecular weight.
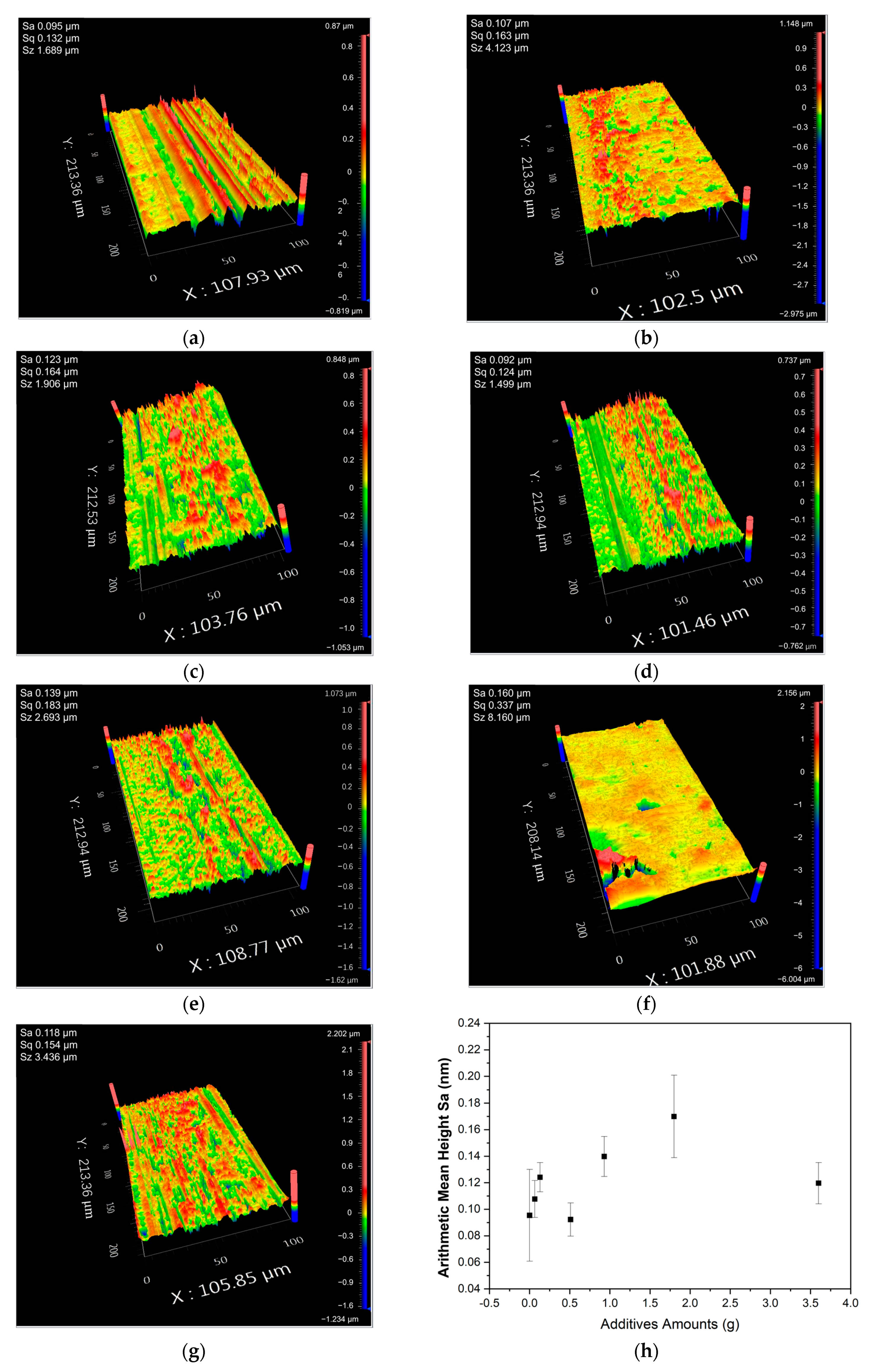
This study used Zygo Nexview to test the surface roughness (S
a) of Fe
3O
4/PMMA samples with different amounts of Fe
3O
4. All the samples were machined at a feed rate of 400 mm/min and 3 μm of cut.
Figure 10 shows the surfaces of workplaces after machining. From
Figure 10a–g, the concentrations of additives are 0 wt%, 0.35 wt%, 0.7 wt%, 2.5 wt%, 5 wt%, 10 wt%, and 20 wt%, respectively.
The surface roughness in terms of arithmetical mean height (Sa) results with the error bar are summarized in
Figure 10h. The results show that the resultant surface roughness is positively proportional to the additive amount, despite an exceptional case found in 2.5 wt% Fe
3O
4. However, when the additive concentration reached 20 wt%, a significant decrease in surface roughness was found in the workplace. The additives in composites break the Van der Waals force between polymer chains, which causes an increase in brittleness. When the concentration of additives is small, the particles inside the composites work as impurities, decreasing the ductility and machinability. However, when the concentration of magnetic fillers increases to 20 wt%, the magnetic fillers surrounded by the polymer chains move according to the magnetic field’s direction. As a result, the polymer composites form a well-arranged structure with a more significant Van der Waals force, affecting the macro-properties of polymer composites. The well-arranged structure enhances the attraction between polymer chains, increasing the ductility and decreasing the brittleness of polymer composites, leading to better machinability.