Bio-Based Polymers for Environmentally Friendly Phase Change Materials
Abstract
1. Introduction
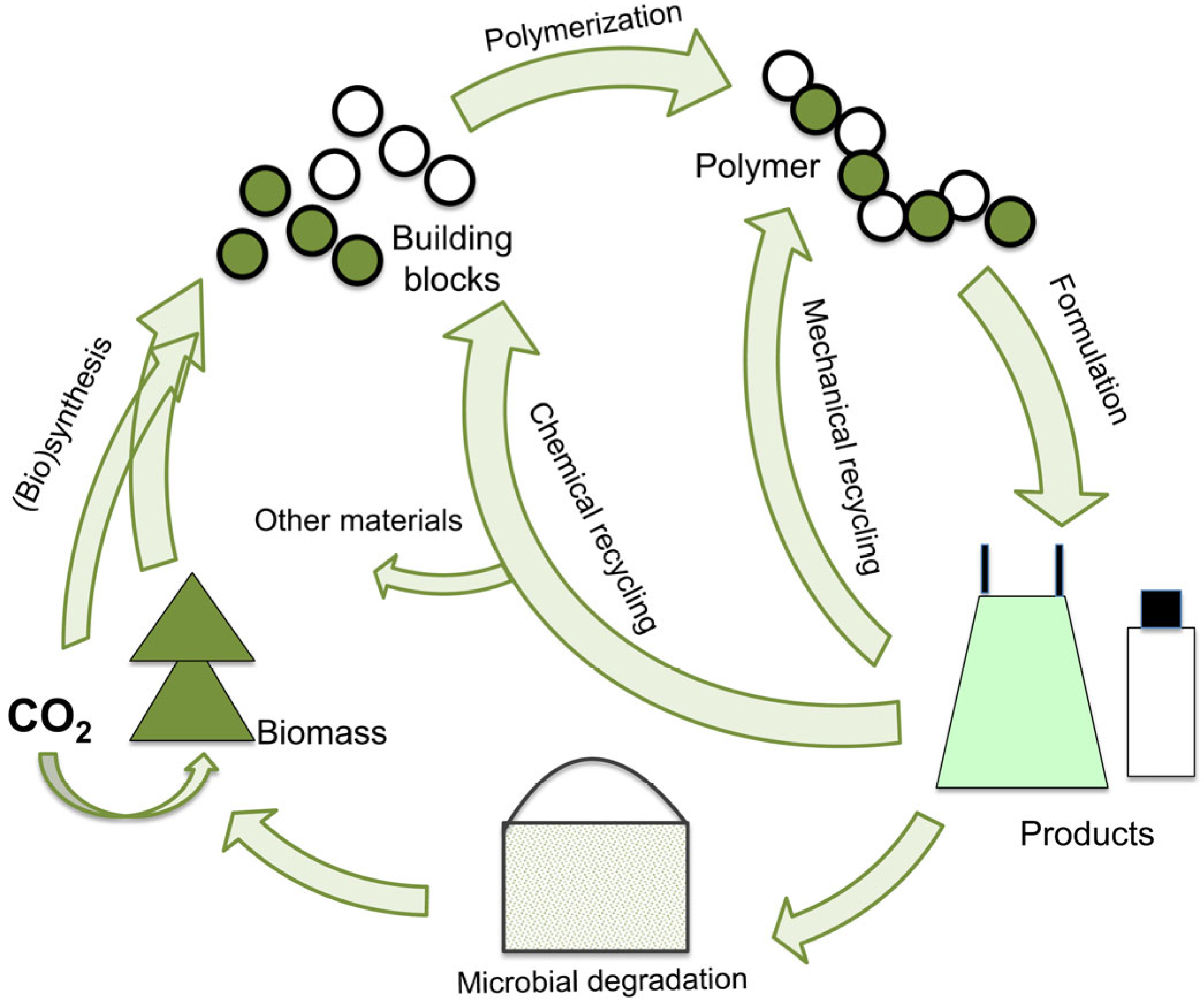
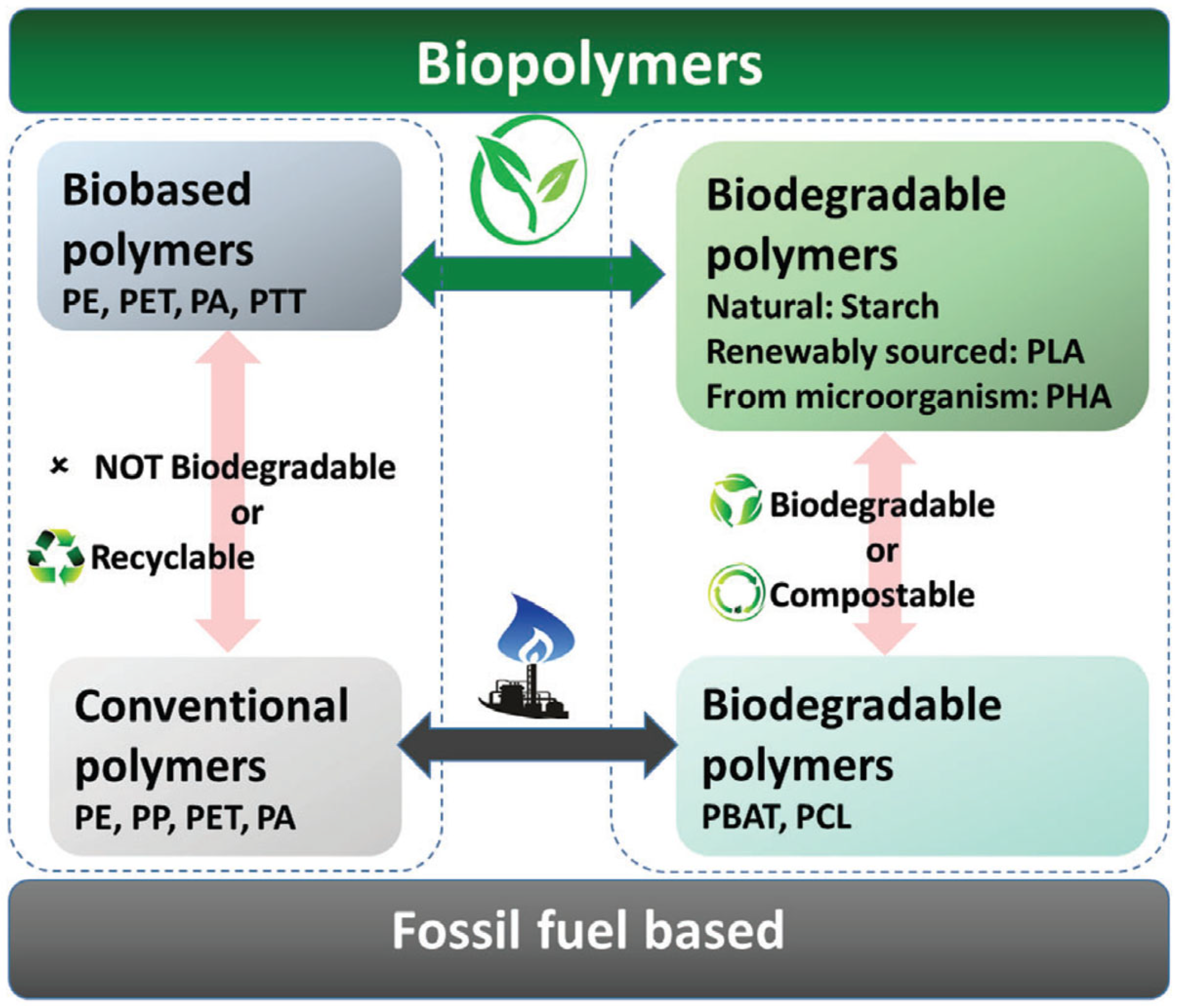
2. Bio-Based Polymers
- First class—polymers prepared from biomass, such as starch, cellulose, chitosan, chitin, sodium alginate, and natural rubber, including those polymers which are chemically modified;
- Second class—polymers obtained using microorganisms and plants, e.g., poly(hydroxyalkanoates) and poly(glutamic acid);
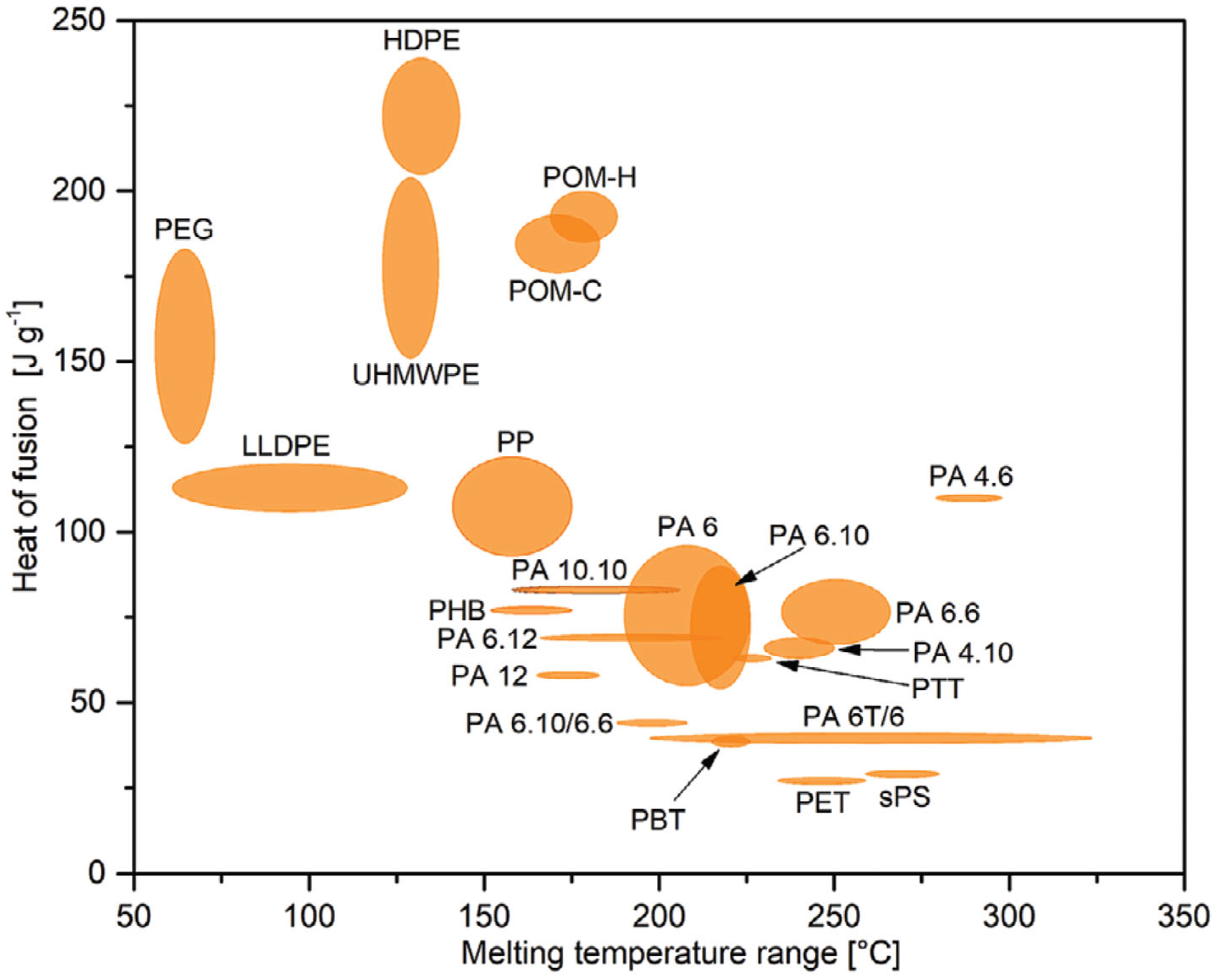
3. Bio-Based Polymers as Phase-Change Materials
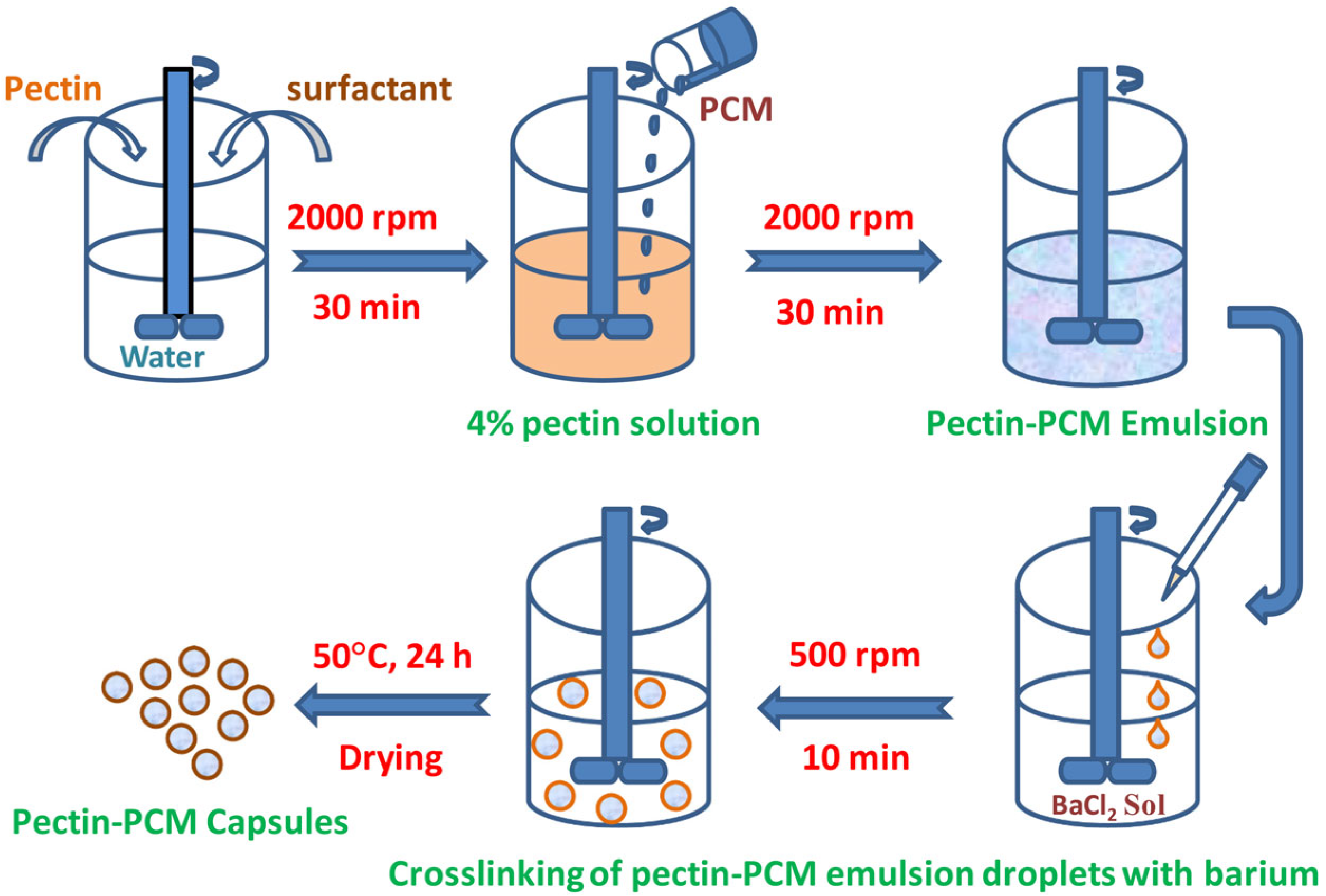
4. PCMs Encapsulation Using Bio-Based Polymers
5. PCMs Shape Stabilization Using Bio-Based Polymers
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | bacterial cellulose nanofibers |
| CA | capric acid |
| CAC | cellulose acetate |
| CCY | cotton yarn |
| CEL | cellulose |
| CET | methylhydroxycellulose |
| CMC | carboxymethylcellulose |
| CNFs | chitin nanofibers |
| CNT | carbon nanotubes |
| CS | chitosan |
| DCM | dichloromethane |
| DSC | differential scanning calorimetry |
| EC | ethyl cellulose |
| GO | graphene oxide |
| HPMC | hydroxylpropyl methyl cellulose |
| LA | lauric acid |
| MA | myristic acid |
| MC | methyl cellulose |
| PA | palmitic acid |
| PAN | polyacrylonitrile |
| PBAT | poly(butylene adipate-co-butylene terephthalate) |
| PBSA | poly(butylene succinate-co-butylene adipate) |
| PCL | poly(ε-caprolactone) |
| PCM | phase-change material |
| PDA | polydopamine |
| PEF | poly(ethylene furanoate) |
| PEG | poly(ethylene glycol) |
| PEO | poly(ethylene oxide) |
| PET | poly(ethylene terephthalate) |
| PGI | poly(glycerol-itaconic acid) |
| PLA | poly(lactic acid) |
| POM | polyoxymethylene |
| PPO | poly(propylene oxide) |
| PTHF | polytetrahydrofurane |
| PTT | poly(trimethylene terephthalate) |
| PVA | poly(vinyl alcohol) |
| SF | silk fibroin |
| TD | tetradecanol |
References
- Pielichowska, K.; Pielichowski, K. Phase Change Materials for Thermal Energy Storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on Thermal Energy Storage with Phase Change: Materials, Heat Transfer Analysis and Applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. (Eds.) Multifunctional Phase Change Materials; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780323857192. [Google Scholar]
- Confalonieri, C.; Gariboldi, E. 9—Shape-Stabilized and Form-Stable PCMs. In Multifunctional Phase Change Materials; Pielichowska, K., Pielichowski, K., Eds.; Woodhead Publishing: Southton, UK, 2023; pp. 369–410. ISBN 978-0-323-85719-2. [Google Scholar]
- Pielichowska, K.; Bieda, J.; Szatkowski, P. Polyurethane/Graphite Nano-Platelet Composites for Thermal Energy Storage. Renew. Energy 2016, 91, 456–465. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Mortazavi, S.M.; Khayamian, T. Preparation of Calcium Alginate Microcapsules Containing N-Nonadecane by a Melt Coaxial Electrospray Method. J. Electrost. 2015, 73, 56–64. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Novel Biodegradable Form Stable Phase Change Materials: Blends of Poly(Ethylene Oxide) and Gelatinized Potato Starch. J. Appl. Polym. Sci. 2010, 116, 1725–1731. [Google Scholar] [CrossRef]
- Okogeri, O.; Stathopoulos, V.N. What about Greener Phase Change Materials? A Review on Biobased Phase Change Materials for Thermal Energy Storage Applications. Int. J. Thermofluids 2021, 10, 100081. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Biobased Polymers BT—Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–7. ISBN 978-3-642-36199-9. [Google Scholar]
- Piorkowska, E. Overview of Biobased Polymers. In Thermal Properties of Bio-Based Polymers; Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–35. ISBN 978-3-030-39962-7. [Google Scholar]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers That Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Narayan, R. Preparation of Bio-Based Polymers for Materials Applications. Appl. Biochem. Biotechnol. 1988, 17, 7–22. [Google Scholar] [CrossRef]
- Carafa, R.N.; Foucher, D.A.; Sacripante, G.G. Biobased Polymers from Lignocellulosic Sources. Green Chem. Lett. Rev. 2023, 16, 2153087. [Google Scholar] [CrossRef]
- Kimura, Y. Molecular, Structural, and Material Design of Bio-Based Polymers. Polym. J. 2009, 41, 797–807. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic Biopolymers and Their Composites: Advantages and Limitations—An Overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 20 November 2023).
- Available online: https://www.cas.org/resources/cas-insights/materials/biopolymers-manufacturings-latest-green-hero (accessed on 20 November 2023).
- Available online: https://www.european-bioplastics.org/market/ (accessed on 20 November 2023).
- Available online: https://bioplasticsnews.com/top-bioplastics-producers/ (accessed on 20 November 2023).
- Available online: https://biokunststofftool.de/materials/bio-pp/?lang=en (accessed on 20 November 2023).
- Available online: https://natureplast.eu/en/matiere/biobased-pa-2/ (accessed on 20 November 2023).
- Wijesena, R.N.; Tissera, N.D.; Rathnayaka, V.W.S.G.; Rajapakse, H.D.; de Silva, R.M.; de Silva, K.M.N. Shape-Stabilization of Polyethylene Glycol Phase Change Materials with Chitin Nanofibers for Applications in “Smart” Windows. Carbohydr. Polym. 2020, 237, 116132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zhang, Y.; Lu, A.; Li, X.; Liu, L.; Qin, G.; Fang, Z.; Zhang, J.; Liu, Y. A Novel Design and Synthesis of Multifunctional Magnetic Chitosan Microsphere Based on Phase Change Materials. Mater. Lett. 2019, 237, 185–187. [Google Scholar] [CrossRef]
- Li, J.; Meng, L.; Chen, J.; Chen, X.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Encapsulation of Polyethylene Glycol in Cellulose-Based Porous Capsules for Latent Heat Storage and Light-to-Thermal Conversion. Front. Chem. Sci. Eng. 2023, 17, 1038–1050. [Google Scholar] [CrossRef]
- Niu, L.; Li, X.; Zhang, Y.; Yang, H.; Feng, J.; Liu, Z. Electrospun Lignin-Based Phase-Change Nanofiber Films for Solar Energy Storage. ACS Sustain. Chem. Eng. 2022, 10, 13081–13090. [Google Scholar] [CrossRef]
- Baniasadi, H.; Madani, M.; Seppälä, J.; Zimmerman, J.B.; Yazdani, M.R. Form-Stable Phase Change Electrospun Nanofibers Mat with Thermal Regulation and Biomedical Multi-Functionalities. J. Energy Storage 2023, 68, 107660. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Huang, X.; Li, Y.; Xi, Z.; Hai, G.; Tao, Z.; Wang, G. Shape-Stabilized Phase-Change Materials Supported by Eggplant-Derived Porous Carbon for Efficient Solar-to-Thermal Energy Conversion and Storage. Sustain. Energy Fuels 2020, 4, 1764–1772. [Google Scholar] [CrossRef]
- Feng, L.; Ding, J.; Hu, H.; Lv, Z.; Zhang, Y.; Xu, B.; Quan, J.; Hao, S.; Fan, H.; Hang, Z. Preparation and Characterization of Bio-Based PLA/PEG/g-C3N4 Low-Temperature Composite Phase Change Energy Storage Materials. Polymers 2023, 15, 2872. [Google Scholar] [CrossRef]
- Sheng, X.; Dong, D.; Lu, X.; Zhang, L.; Chen, Y. MXene-Wrapped Bio-Based Pomelo Peel Foam/Polyethylene Glycol Composite Phase Change Material with Enhanced Light-to-Thermal Conversion Efficiency, Thermal Energy Storage Capability and Thermal Conductivity. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106067. [Google Scholar] [CrossRef]
- Wei, D.; Wu, C.; Jiang, G.; Sheng, X.; Xie, Y. Lignin-Assisted Construction of Well-Defined 3D Graphene Aerogel/PEG Form-Stable Phase Change Composites towards Efficient Solar Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 224, 111013. [Google Scholar] [CrossRef]
- Le, W.T.; Kankkunen, A.; Rojas, O.J.; Yazdani, M.R. Leakage-Free Porous Cellulose-Based Phase Change Cryogels for Sound and Thermal Insulation. Sol. Energy Mater. Sol. Cells 2023, 256, 112337. [Google Scholar] [CrossRef]
- Baniasadi, H.; Seppälä, J.; Kankkunen, A.; Seppälä, A.; Yazdani, M.R. Water-Resistant Gum-Based Phase Change Composite for Thermo-Regulating Insulation Packaging. J. Energy Storage 2023, 61, 106725. [Google Scholar] [CrossRef]
- Liu, L.; Fan, X.; Zhang, Y.; Zhang, S.; Wang, W.; Jin, X.; Tang, B. Novel Bio-Based Phase Change Materials with High Enthalpy for Thermal Energy Storage. Appl. Energy 2020, 268, 114979. [Google Scholar] [CrossRef]
- Yin, G.Z.; Díaz Palencia, J.L.; Wang, D.Y. Fully Bio-Based Poly (Glycerol-Itaconic Acid) as Supporter for PEG Based Form Stable Phase Change Materials. Compos. Commun. 2021, 27, 100893. [Google Scholar] [CrossRef]
- Ke, G.; Jin, X.; Cai, G.; Li, W.; Xu, A. A Novel Composite Cotton Yarn with Phase Change and Electrical Conductivity Functions. J. Ind. Text. 2022, 51, 554S–568S. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Huang, H.; Dong, D.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Bio-Based Radish@PDA/PEG Sandwich Composite with High Efficiency Solar Thermal Energy Storage. ACS Sustain. Chem. Eng. 2020, 8, 8448–8457. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Y.; Liu, W. Electrospun Polyethylene Glycol/Cellulose Acetate Phase Change Fibers with Core-Sheath Structure for Thermal Energy Storage. Renew. Energy 2013, 60, 222–225. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Kang, B.-h.; Sheng, X.; Lu, X. Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage. Polymers 2019, 11, 2043. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Biodegradable PEO/Cellulose-Based Solid-Solid Phase Change Materials. Polym. Adv. Technol. 2011, 22, 1633–1641. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhao, S.; Wang, X.; Zheng, J.; Zeng, W.; Yuan, M.; Zhao, N.; Li, Q.; Wang, Z.; et al. Biobased Phase Change Material with Reduced Thermal Conductivity: From Preparation to Analysis of Thermal Insulation Performance. ACS Appl. Polym. Mater. 2023, 5, 3728–3736. [Google Scholar] [CrossRef]
- Singh, J.; Vennapusa, J.R.; Dixit, P.; Maiti, T.K.; Chattopadhyay, S. A Novel Strategy for Temperature Controlling of Chocolates through 1-Dodecanol Embedded Polyurea Coated Barium Alginate Beads. J. Taiwan Inst. Chem. Eng. 2022, 138, 104497. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yang, S.; Yun, B.Y.; Kang, Y.; Kim, S. Use of Biochar Co-Mediated Chitosan Mesopores to Encapsulate Alkane and Improve Thermal Properties. Environ. Res. 2022, 212, 113539. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Chen, H.; Lidfeldt, M.; Gunnarsson, J.; Olsén, P.; Berglund, L.A. Sustainable Thermal Energy Batteries from Fully Bio-Based Transparent Wood. Small 2023, 19, 2301262. [Google Scholar] [CrossRef]
- Qu, M.; Guo, C.; Li, L.; Zhang, X. Preparation and Investigation on Tetradecanol and Myristic Acid/Cellulose Form-Stable Phase Change Material. J. Therm. Anal. Calorim. 2017, 130, 781–790. [Google Scholar] [CrossRef]
- Reddy, V.J.; Dixit, P.; Singh, J.; Chattopadhyay, S. Understanding the Core-Shell Interactions in Macrocapsules of Organic Phase Change Materials and Polysaccharide Shell. Carbohydr. Polym. 2022, 294, 119786. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yeol Yun, B.; Uk Kim, Y.; Wi, S.; Kim, S. Introduction of Eicosane into Biochar Derived from Softwood and Wheat Straw: Influence of Porous Structure and Surface Chemistry. Chem. Eng. J. 2021, 415, 128887. [Google Scholar] [CrossRef]
- Phadungphatthanakoon, S.; Poompradub, S.; Wanichwecharungruang, S.P. Increasing the Thermal Storage Capacity of a Phase Change Material by Encapsulation: Preparation and Application in Natural Rubber. ACS Appl. Mater. Interfaces 2011, 3, 3691–3696. [Google Scholar] [CrossRef]
- Jin, L.; Tan, Y.; Yuan, S.; Wang, S.; Cheng, X.; Wang, H.; Du, Z.; Du, X. Phytic Acid–Decorated κ-Carrageenan/Melanin Hybrid Aerogels Supported Phase Change Composites with Excellent Photothermal Conversion Efficiency and Flame Retardancy. Renew. Energy 2023, 206, 148–156. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, J.; Li, Y.; Wang, H.; Song, G.; Tang, G. Emulsion-Electrospinning n-Octadecane/Silk Composite Fiber as Environmental-Friendly Form-Stable Phase Change Materials. J. Appl. Polym. Sci. 2017, 134, 45538. [Google Scholar] [CrossRef]
- Baştürk, E.; Kahraman, M.V. Photocrosslinked Biobased Phase Change Material for Thermal Energy Storage. J. Appl. Polym. Sci. 2016, 133, 43757. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Cheng, C.; Sun, K.; Jin, T.; Shi, Q. Flexible and Biocompatible Silk Fiber-Based Composite Phase Change Material for Personal Thermal Management. ACS Sustain. Chem. Eng. 2022, 10, 16368–16376. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Lee, D.-E.; Park, T. Shape-Stabilized Orange Peel/Myristic Acid Phase Change Materials for Efficient Thermal Energy Storage Application. Energy Rep. 2022, 8, 9618–9628. [Google Scholar] [CrossRef]
- Fashandi, M.; Leung, S.N. Preparation and Characterization of 100% Bio-Based Polylactic Acid/Palmitic Acid Microcapsules for Thermal Energy Storage. Mater. Renew. Sustain. Energy 2017, 6, 14. [Google Scholar] [CrossRef]
- M’ghari, O.; Hassani, F.S.A.; Mekhzoum, M.E.M.; Zari, N.; Bouhfid, R.; el Kacem Qaiss, A. Elaboration of a Composite Material Based on Plaster Reinforced with Phase Change Material/Oakum Fiber: Physical, Thermal and Mechanical Properties. J. Energy Storage 2021, 35, 102321. [Google Scholar] [CrossRef]
- Tangsiriratana, E.; Skolpap, W.; Patterson, R.J.; Sriprapha, K. Thermal Properties and Behavior of Microencapsulated Sugarcane Wax Phase Change Material. Heliyon 2019, 5, e02184. [Google Scholar] [CrossRef] [PubMed]
- Abdulmunem, A.R.; Samin, P.M.; Sopian, K.; Hoseinzadeh, S.; Al-Jaber, H.A.; Garcia, D.A. Waste Chicken Feathers Integrated with Phase Change Materials as New Inner Insulation Envelope for Buildings. J. Energy Storage 2022, 56, 106130. [Google Scholar] [CrossRef]
- Souissi, M.; Trigui, A.; Jedidi, I.; Loukil, M.S.; Abdelmouleh, M. Bio-Based Composite as Phase Change Material Including Spent Coffee Grounds and Beeswax Paraffin. Korean J. Chem. Eng. 2023, 40, 2342–2355. [Google Scholar] [CrossRef]
- Meng, L.; Ivanov, A.S.; Kim, S.; Zhao, X.; Kumar, N.; Young-Gonzales, A.; Saito, T.; Bras, W.; Gluesenkamp, K.; Bocharova, V. Alginate-Sodium Sulfate Decahydrate Phase Change Composite with Extended Stability. ACS Appl. Polym. Mater. 2022, 4, 6563–6571. [Google Scholar] [CrossRef]
- Lee, J.J.C.; Sugiarto, S.; Ong, P.J.; Soo, X.Y.D.; Ni, X.; Luo, P.; Hnin, Y.Y.K.; See, J.S.Y.; Wei, F.; Zheng, R.; et al. Lignin-g-Polycaprolactone as a Form-Stable Phase Change Material for Thermal Energy Storage Application. J. Energy Storage 2022, 56, 106118. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Yang, W.; Kim, J. Fabrication of Biobased Advanced Phase Change Material and Multifunctional Composites for Efficient Thermal Management. ACS Sustain. Chem. Eng. 2023, 11, 1178–1189. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; DeWitt, S.J.A.; McGuire, S.; Lively, R.P. Incorporation of Phase Change Materials into Fibers for Sustainable Thermal Energy Storage. Ind. Eng. Chem. Res. 2021, 60, 3374–3384. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Fernandes, M.; Carneiro, N.; Pedro Souto, A. Functionalization of Wool Fabric with Phase-Change Materials Microcapsules after Plasma Surface Modification. J. Appl. Polym. Sci. 2013, 128, 2638–2647. [Google Scholar] [CrossRef]
- Pinto, S.C.; Silva, N.H.C.S.; Pinto, R.J.B.; Freire, C.S.R.; Duarte, I.; Vicente, R.; Vesenjak, M.; Marques, P.A.A.P. Multifunctional Hybrid Structures Made of Open-Cell Aluminum Foam Impregnated with Cellulose/Graphene Nanocomposites. Carbohydr. Polym. 2020, 238, 116197. [Google Scholar] [CrossRef] [PubMed]
- Kizildag, N. Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality. Coatings 2023, 13, 414. [Google Scholar] [CrossRef]
- Kamimoto, M.; Abe, Y.; Sawata, S.; Tani, T.; Ozawa, T. Latent Thermal Storage Unit Using Form-Stable High Density Polyethylene; Part I: Performance of the Storage Unit. J. Sol. Energy Eng. 1986, 108, 282–289. [Google Scholar] [CrossRef]
- Weingrill, H.M.; Resch-Fauster, K.; Lucyshyn, T.; Zauner, C. High-Density Polyethylene as Phase-Change Material: Long-Term Stability and Aging. Polym. Test. 2019, 76, 433–442. [Google Scholar] [CrossRef]
- Weingrill, H.M.; Resch-Fauster, K.; Zauner, C. Applicability of Polymeric Materials as Phase Change Materials. Macromol. Mater. Eng. 2018, 303, 1800355. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Ikai, S. New Polymeric Material for Latent Heat Thermal Energy Storage. In Proceedings of the 5th Workshop of the IEA ECES IA Annex 10, Tsu, Japan, 10 November 2000. [Google Scholar]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Bailey, F.E.; Koleske, J.V. Poly(Ethylene Oxide); Academic Press: Cambridge, MA, USA, 1976; ISBN 9780120732500. [Google Scholar]
- Pielichowski, K.; Flejtuch, K. Differential Scanning Calorimetry Study of Blends of Poly(Ethylene Glycol) with Selected Fatty Acids. Macromol. Mater. Eng. 2003, 288, 259–264. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Differential Scanning Calorimetry Studies on Poly(Ethylene Glycol) with Different Molecular Weights for Thermal Energy Storage Materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Flejtuch, K. Badanie Przemian Fazowych Wybranych Ukladów Polieterów Pod Kątem Akumulacji Energii Cieplnej. PhD Thesis, Cracow University of Technology, Krakow, Poland, 2004. [Google Scholar]
- Pielichowski, K.; Flejtuch, K. Binary Blends of Polyethers with Fatty Acids: A Thermal Characterization of the Phase Transitions. J. Appl. Polym. Sci. 2003, 90, 861–870. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation Methods for Phase Change Materials—A Critical Review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Ghasemi, K.; Tasnim, S.; Mahmud, S. PCM, Nano/Microencapsulation and Slurries: A Review of Fundamentals, Categories, Fabrication, Numerical Models and Applications. Sustain. Energy Technol. Assess. 2022, 52, 102084. [Google Scholar] [CrossRef]
- Deveci, S.S.; Basal, G. Preparation of PCM Microcapsules by Complex Coacervation of Silk Fibroin and Chitosan. Colloid Polym. Sci. 2009, 287, 1455–1467. [Google Scholar] [CrossRef]
- Jeon, I.K.; Azzam, A.; Al Jebaei, H.; Kim, Y.-R.; Aryal, A.; Baltazar, J.-C. Effects of Shape-Stabilized Phase Change Materials in Cementitious Composites on Thermal-Mechanical Properties and Economic Benefits. Appl. Therm. Eng. 2023, 219, 119444. [Google Scholar] [CrossRef]

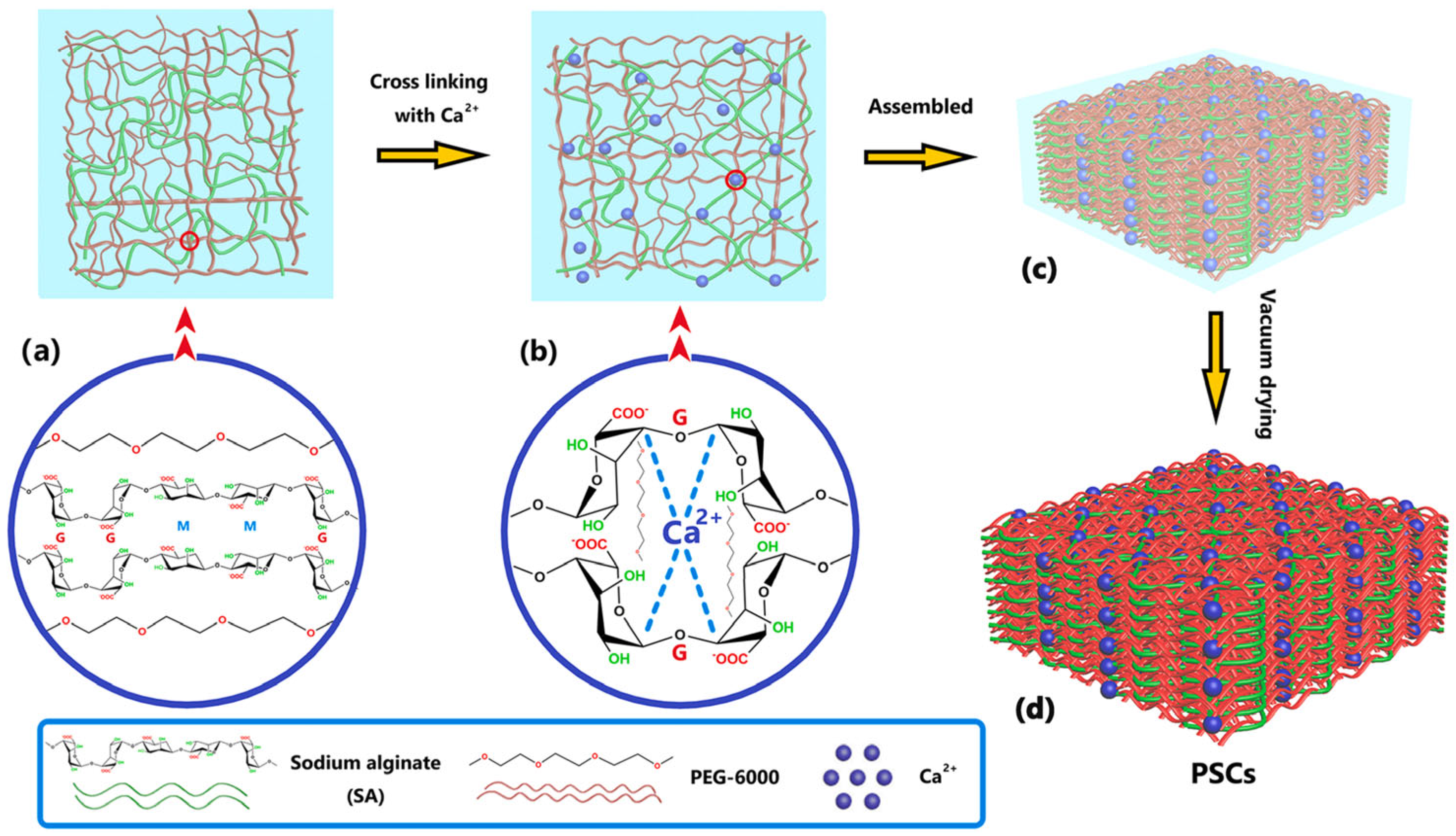
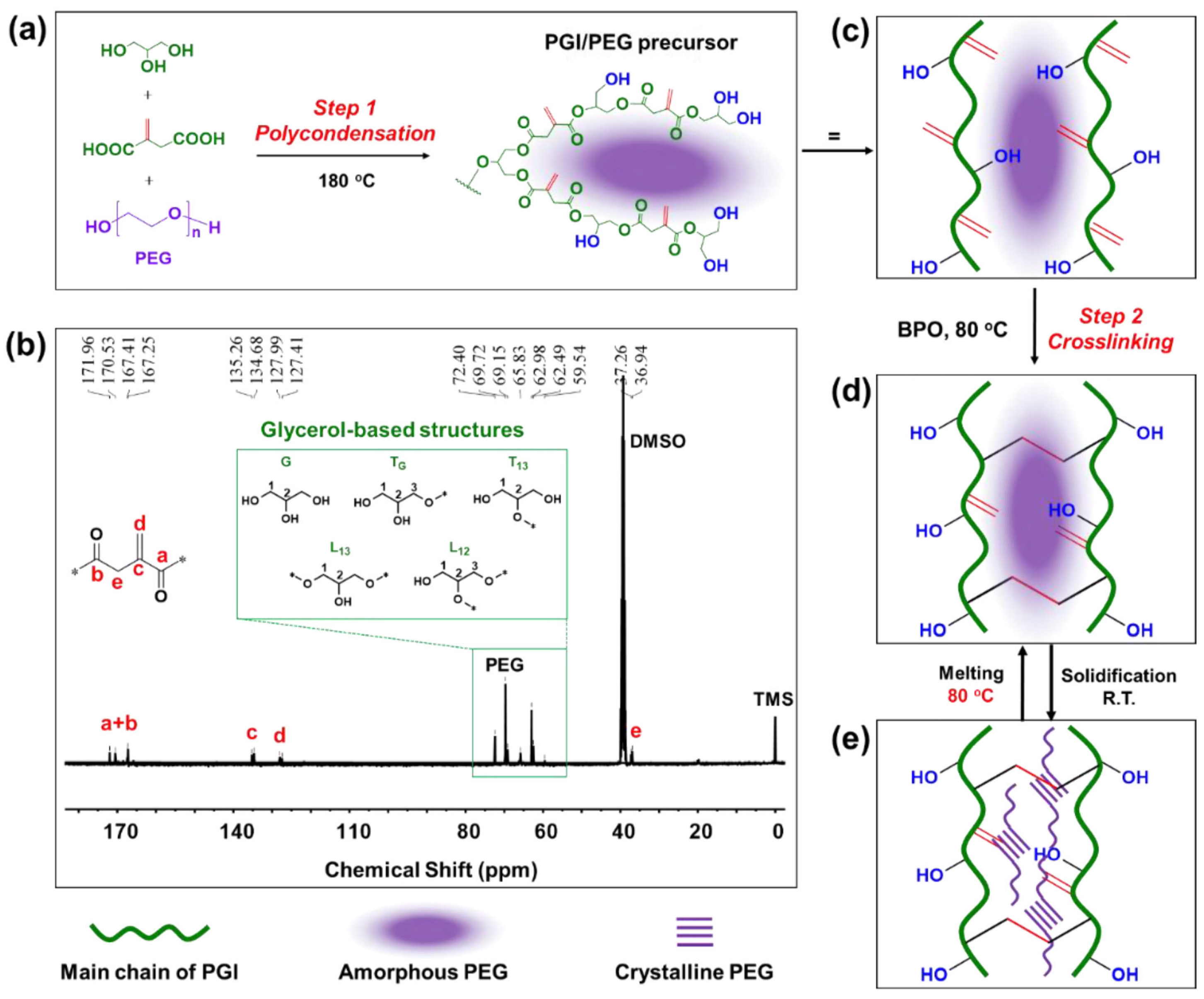
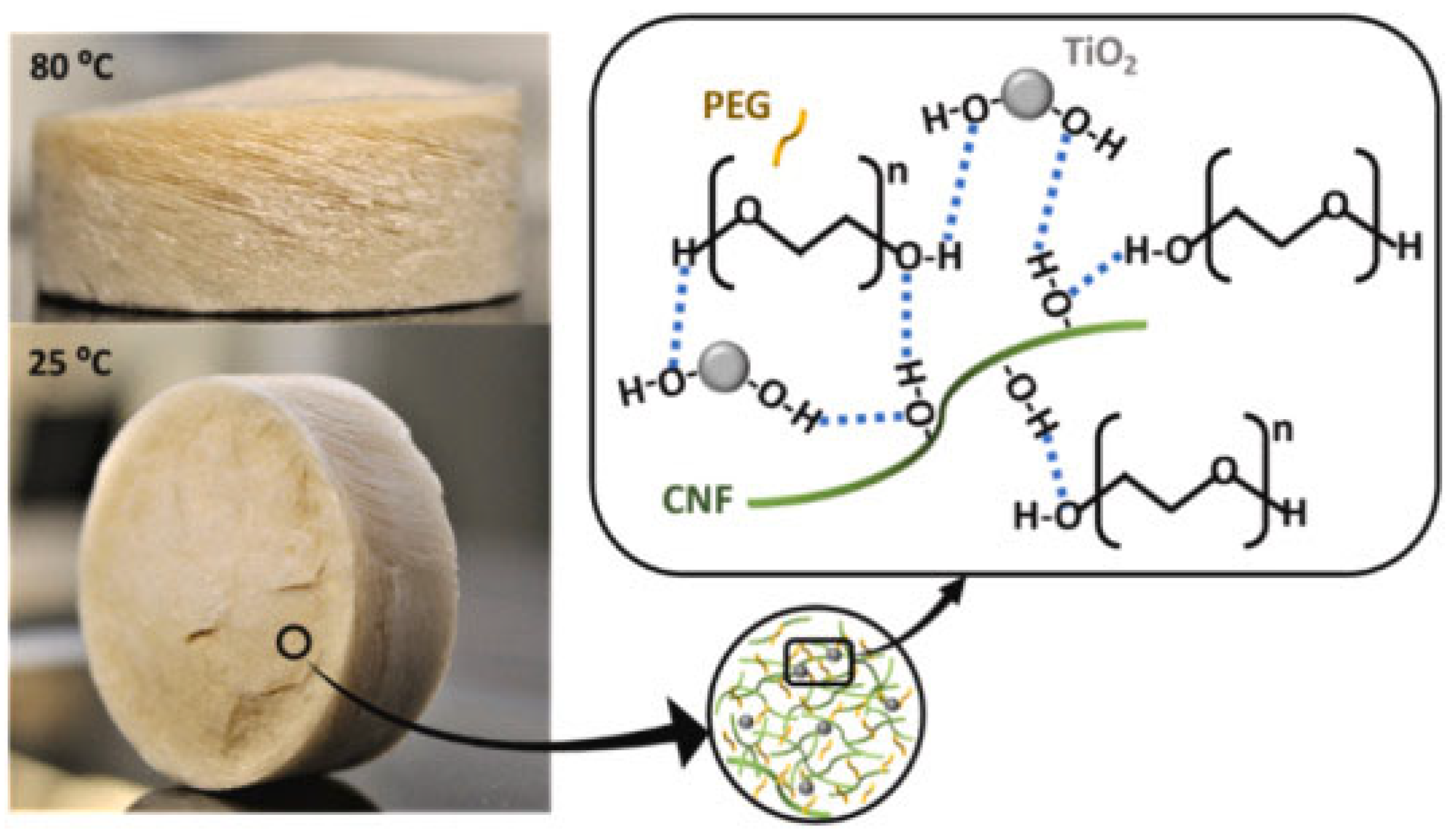
| Biopolymer | Biobased Content (%) | Major Producers | Applications | Biodegradability | Ref. |
|---|---|---|---|---|---|
| Starch | 100 | Novamont; Futerro; Biome | Flexible packaging, consumer goods, agriculture | Yes | [18,19] |
| PLA | Up to 100 | NatureWorks; Total Corbion; Shimadzu Cor.; Toyobo; Evonik, | Flexible packaging, rigid packaging, consumer goods | Yes | [18,19] |
| PHA | 100 | Bio- On; Kaneka; Tepha; Danimer Scientific; Newlight Technologies; Yield10 Bioscience, Tianjin GreenBio Materials, | Flexible packaging, rigid packaging | Yes | [18,19] |
| PHB | n/a | Mitsubishi Chemical; Showa Denko K.K.; SK Chemicals; PTT MCC Biochem | Flexible packaging, rigid packaging | Yes | [19] |
| PBS | Till 100 | Showa Denko K.K.; SK Chemicals; Mitsubishi Chemical | Flexible packaging, agriculture | Yes | [18,19] |
| PBS | Til 50 | Natureplast; | Flexible packaging, rigid packaging, regular consumption goods, horticulture/agriculture | Yes | [20] |
| PEG | Til 100 | Sirius; Croda; Solvay; Clariant IGL Specialty Chemicals; BASF; Helvetic; Bioscience | Consumer goods | Yes | [18] |
| PBSA | Till 100 | Showa Denko K.K.; SK Chemicals; | Flexible packaging, agriculture | Yes | [18,19] |
| PBAT | Till 50 | BASF; Zhuhai Wango Chemical Co.; JinHui ZhaoLong; Eastman; Novamont | Flexible packaging, rigid packaging, agriculture | Yes | [18,19] |
| PCL | n/a | Perstorp until 2018, acquired by Ingevity | Consumer goods | Yes | [18,19] |
| PTT | 27–37 | Dupont | Fibers (including woven and non-woven), consumer goods | No | [18,19] |
| PEF | Till 100 | Avantium; Corbion; BASF; DuPont, Synvina | Packaging: replacement for PET | No | [18,19] |
| PE | n/a | Braskem; Neste; LyondellBasell | Flexible packaging, rigid packaging, building and construction | No | [19,21] |
| PP | 30 | Borealis; Neste; LyondellBasell | Flexible packaging, rigid packaging, | No | [21] |
| PA | Till 100 | Natureplast; Technoform; Nexis Fibers; Acro; Avient; Fulgar; Akeme; Hengshui | Technical parts, regular consumption goods, sports and leisure, transport | No | [20,22] |
| PET | Till 75 | Toray Industries, The Coca-Cola Company, M&G Chemicals; Virent; PepsiCo; Toyota Tsusho | Rigid packaging | No | [18,19] |
| PCM | Bio-Based Polymer | Tm [°C] | Tc [°C] | Heat of Melting (J/g) | Heat of Freezing (J/g) | Ref. |
|---|---|---|---|---|---|---|
| PEG 1000 | Chitin nanofibers (CNFs) from crab shells of Portunus pelagicus | N/A | 24.47 | N/A | 109.65 | [23] |
| PEG 1000 | Chitosan with a deacetylation degree of 80–95% | 40 | 73 | 24/31 | 75 | [24] |
| PEG 1000 | Microcrystalline cellulose (MCC) | 65.47 | 142.2 | 42.32 | 137.4 | [25] |
| PEG 1000 | Lignin | 45.20 | 42.16 | 43.06 | 37.88 | [26] |
| PEG 1000 | Gelatin from bovine skin (Type B) | 33.2 | 26.4 | 61.7 | 52.6 | [27] |
| PEG 2000 | Spongy-like porous carbon derived from eggplants | 61.55 | 149.00 | 19.90 | 138.14 | [28] |
| PEG 2000 | PLA | 64.54 174.15 | 37.51 68.73 | 189.80 16.3 | 96.43 11.32 | [29] |
| PEG 4000 | Dried pomelo peel foam | 62.7 | 158.1 | 41.5 | 156.1 | [30] |
| PEG 4000 | Enzymatic lignin | 61 | 41 | 168.7 | 165.12 | [31] |
| PEG 4000 | Cellulose nanofibrils (CNF) never dried and partially delignified birch pulp fibers ACNF: The CNF was modified by heterogeneous acetylation LCNF: lignin-containing nanofibril suspension | 58.4 58.2 | N/A | 115.1 142.6 | 109.7 137.2 | [32] |
| PEG 6000 | Gum tragacanth | 55.3 | 106.8 | 39.2 | 111.3 | [33] |
| PEG 6000 | Ca2+- Crosslinked SA | N/A | 156.8 | N/A | 150.3 | [34] |
| PEG 6000 | PGI | 58.35 | 86.93 | 14.15 | 83.65 | [35] |
| PEG 2000 to PEG 10,000 | Cotton composite yarn (PPCCY) was fabricated by impregnating PEG2000–10,000 into CNT/cotton yarn (CCY) and coating electrospun PAN on its surface Composite PPCCY with various PEG types: PEG 2000 PEG 4000 PEG 6000 PEG 10,000 | PEG 2000–56.5 PEG 4000–65.3 PEG 6000–62.9 PEG 10,000–66.3 | PEG 2000–135.3 PEG 4000–136.0 PEG 6000–150.8 PEG 10,000–126.0 | PEG 2000–29.8 PEG 4000–35.8 PEG 6000–35.6 PEG 10,000–39.5 | PEG 2000–128.8 PEG 4000–128.4 PEG 6000–150.9 PEG 10,000–125.3 | [36] |
| PEG 4000 to PEG 10,000 | To produce a PDA layer on the surface of the freeze-dried radish, the self-polymerization of dopamine was initiated | 64.77 | 161.52 | 44.32 | 158.41 | [37] |
| PEG 10,000 | CAC | 62.3 | 60.6 | 37.8 | 58.7 | [38] |
| Isocyanate terminated PEG 6000 | Pomelo peel flour (PPF) with an average particle size of about 55 μm | 62.5 | 182.3 | 36.1 | 173.5 | [39] |
| PEO | Cellulose (microcrystalline powder 20 mm, degree of polymerization: 229) CAC (40% substitution acetyl groups) CMC (60% substitution, powder < 400 mm) CET (Cellulose ether; 30% substitution, powder 350 mm) PEO/CEL PEO/CMC PEO/CAC PEO/CET | PEO/CEL–63.4 PEO/CMC–58.4 PEO/CAC–62.7 PEO/CET–64.7 | PEO/CEL–134.7 PEO/CMC–140.2 PEO/CAC–156.3 PEO/CET–156.8 | PEO/CEL–32.5 PEO/CMC–36.6 PEO/CAC–27.6 PEO/CET–37.2 | PEO/CEL–127.3 PEO/CMC–138.0 PEO/CAC–152.8 PEO/CET–153.3 | [40] |
| PEO | Potato starch | 67.3 | 96.9 | 33.5 | 94.6 | [7] |
| PEO with a viscosity average molecular weight (Mv) of 1 × 105 g/mol | PLLA | 60.95 | 58.79 | 46.31 | 57.59 | [41] |
| 1-Dodecanol | SA | 25.69 | 149.15 ± 3 | 14.65 | 147.79 ± 3 | [42] |
| Dodecane | CS (low MW with a degree of deacetylation of 75–85%), to obtain the biochar papermill sludge, was used as biomass | −9.9 | N/A | 91 | N/A | [43] |
| 1-Dodecanol from coconut extract (70 wt.%) | Bio-based transparent wood: delignified and succinylated birch wood | 25.2 ± 1 | 141 ± 5 | 19.3 ± 0.6 | 128 ± 7 | [44] |
| TD and MA | Hydroxylpropyl methyl cellulose (HPMC). | 34.61 | 206.45 | 31.09 | 204.59 | [45] |
| Hexadecane, butyl stearate, caprylic acid | Poly(D-galacturonic acid methyl ester) | H5—23.99 B5—21.60 C5—18.50 | H5—184.89 B5—116.03 C5—118.04 | H5—1.49 B5—10.34 C5—−19.85 | H5—185.89 B5—118.26 C5—114.42 | [46] |
| n-Nonadecane | Alginic acid sodium salt | 32.10 | 28.71 | 81.67 | 18.67 | [6] |
| Eicosane | Biochars obtained by the pyrolysis of softwood and wheat straw at 550 °C and 700 °C | Softwood biochar/eicosane–37.0 Wheat straw biochar/eicosane—37.0 | N/A | Softwood biochar/eicosane–53.4 Wheat straw biochar/eicosane–75.0 | N/A | [47] |
| n-Eicosane | Natural rubber-latex | 35–45 | 20–35 | encap-C20 prepared at polymer weight contents of 20 wt.%: 204.5 ± 8.5 and encap-C20 prepared at polymer weight contents of 9 wt.% 239.8 ± 3.8 | encap-C20 prepared at polymer weight contents of 20 wt.%:208.2 ± 9.4 and encap-C20 prepared at polymer weight contents of 9 wt.% 251.4 ± 6.2 J g_1 | [48] |
| n-Octacosane | κ-Carrageenan | 63.6 | 157.7 | 51.1 | 249.3 | [49] |
| n-Octadecane | Aqueous silk solution | 22.95 ± 60.73 | 4.63 ± 60.38 | 34.98 ± 61.40 | 37.58 ± 62.72 | [50] |
| 1-Octadecanol, 1-Eicosanol, 1-Docosanol | Acrylated soybean oil (ASO) F2—(ASO/1- Octadecanol) F3—(ASO/1- Eicosanol) F2—(ASO/1- Docosanol) | F2—60.46 F3—66.67 F2—70.07 | F2—30.06 F3—45.58 F2—67.51 | F2—49.39 F3—56.18 F2—60.82 | F2—−17.56 F3—−41.01 F2—−70.07 | [51] |
| Capric acid | Silkworm cocoons | 30.6 | 123.4 | 26.7 | 122.6 | [52] |
| Myristic acid | Orange peel biochar | 55.37 | 67.20 | 49 | 65.14 | [53] |
| Vegetable-derived PA | Bio-based PLA | 62.3 | N/A | 59.9 | N/A | [54] |
| CA and LA | SA | 16.54 | 35.18 | 11.84 | N/A | [55] |
| Sugarcane wax/Al2O3 composite | Gelatin, gum Arabic | 68.00 | 61.00 | 59.66 | 44.45 | [56] |
| Paraffin wax type Heptacosane | Waste chicken feathers | 77.58 | N/A | 156.56 | N/A | [57] |
| Beeswax | Coffee grounds collected after brewing in an automatic coffee machine | 50.1 | 121.08 | 34.23 | 129.36 | [58] |
| Sodium sulfate decahydrate-Na2SO4·10H2O | SA | 32.6 | N/A | 37.1 | N/A | [59] |
| Lignin-g-PCL copolymers | Alkaline lignin | 51.33 | N/A | 61.16 | N/A | [60] |
| Erythritol (ET)-grafted-PLA | Meso-erythritol, PLA | 163.8 | 170.7 | N/A | N/A | [61] |
| Microencapsulated PCM (mixtures of branched-chain hydrocarbons encapsulated in melamine formaldehyde shell) | Microcrystalline cellulose 20 μm | N/A | 130.34 | N/A | N/A | [62] |
| Microcapsule PCMs (paraffins encapsulated in melamine shells) | PCM microcapsules on wool fabric | N/A | enthalpy after 10 washing cycles—2.92 | N/A | N/A | [63] |
| Microencapsulated PCMs (paraffin in PMMA shells) | Bacterial cellulose (BC) | 23.75 | 6.73 | 22.58 | 6.58 | [64] |
| Microencapsulated PCM (mixtures of branched-chain hy-drocarbons encapsu-lated in melamine formaldehyde shell) | Pullulan | 29.36 | 104.85 | 16.02/18.82 | 103.58 | [65] |
| Sample | Heating Cycle | Melting Point [°C] | ΔH [J/g] |
|---|---|---|---|
| PEG 1000 | 1 2 | 44.2 40.0 | 165.3 168.6 |
| PEG 3400 | 1 2 (2 peaks) | 63.0 56.4/61.9 | 191.7 171.6 |
| PEG 10,000 | 1 2 | 66.6 66.2 | 188.7 180.6 |
| PEG 20,000 | 1 2 | 68.5 67.7 | 177.4 165.0 |
| PEG 35,000 | 1 2 | 71.1 68.3 | 199.7 183.4 |
| PEG 3400/PEG 10,000 | 1 2 (2 peaks) | 66.4 55.6 64.3 | 169.2 173.5 |
| PEG 1000/PEG 10,000 | 1 2 (2 peaks) | 36.5 60.8 35.3 | 161.3 156.8 |
| PEG 1000/PEG 3400 | 1 (2 peaks) 2 (3 peaks) | 33.5 60.0 34.3 51.3 59.1 | 199.7 183.4 |
| Polyether | Melting | Freezing | ||||
|---|---|---|---|---|---|---|
| Melting Range [°C] | Tmax [°C] | Heat of Fusion [J/g] | Freezing Range [°C] | Tmax [°C] | Heat of Freezeing [J/g] | |
| PEG 3400 | 51.9 ÷ 65.2 | 63.4 | 166.8 | 30.4 ÷ 39.0 | 36.7 | 158.3 |
| PPO 4000 | −11.5 ÷ −6.0 | −9.2 | 0.6 | - | - | - |
| PTHF 2900 | 23.9 ÷ 33.7 | 29.7 | 91.0 | −3.8 ÷ 5.7 | 0.2 | 85.4 |
| Advantages | Disadvantages |
|---|---|
| Protection from light, heat, moisture, and high oxygen concentration that might lead to the decomposition of PCMs | Micro and nanoencapsulation are complicated to conduct, and macro encapsulation has a lower structure stability and fracture resistance |
| Prevent evaporation of volatile compounds that harm the environment and users’ health | Needs a standard selection procedure of PCMs and shell materials |
| Cover unpleasant odours | |
| Control the speeds of internal materials’ release | Low thermal conductivity due to the polymer used as the shell |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pielichowska, K.; Nowicka-Dunal, K.; Pielichowski, K. Bio-Based Polymers for Environmentally Friendly Phase Change Materials. Polymers 2024, 16, 328. https://doi.org/10.3390/polym16030328
Pielichowska K, Nowicka-Dunal K, Pielichowski K. Bio-Based Polymers for Environmentally Friendly Phase Change Materials. Polymers. 2024; 16(3):328. https://doi.org/10.3390/polym16030328
Chicago/Turabian StylePielichowska, Kinga, Katarzyna Nowicka-Dunal, and Krzysztof Pielichowski. 2024. "Bio-Based Polymers for Environmentally Friendly Phase Change Materials" Polymers 16, no. 3: 328. https://doi.org/10.3390/polym16030328
APA StylePielichowska, K., Nowicka-Dunal, K., & Pielichowski, K. (2024). Bio-Based Polymers for Environmentally Friendly Phase Change Materials. Polymers, 16(3), 328. https://doi.org/10.3390/polym16030328












