The Integration of Microwave-Synthesized Silver Colloidal Nanoparticles into Poly (Lactic Acid)-Based Textiles as Antimicrobial Agents via Pre- and Post-Electrospinning Processes
Abstract
1. Introduction
2. Experimental Methods
2.1. Material
2.2. Preparation of Silver Colloids
2.3. Preparation of PLA-Based Hydrophobic and Hydrophilic Dope Solutions
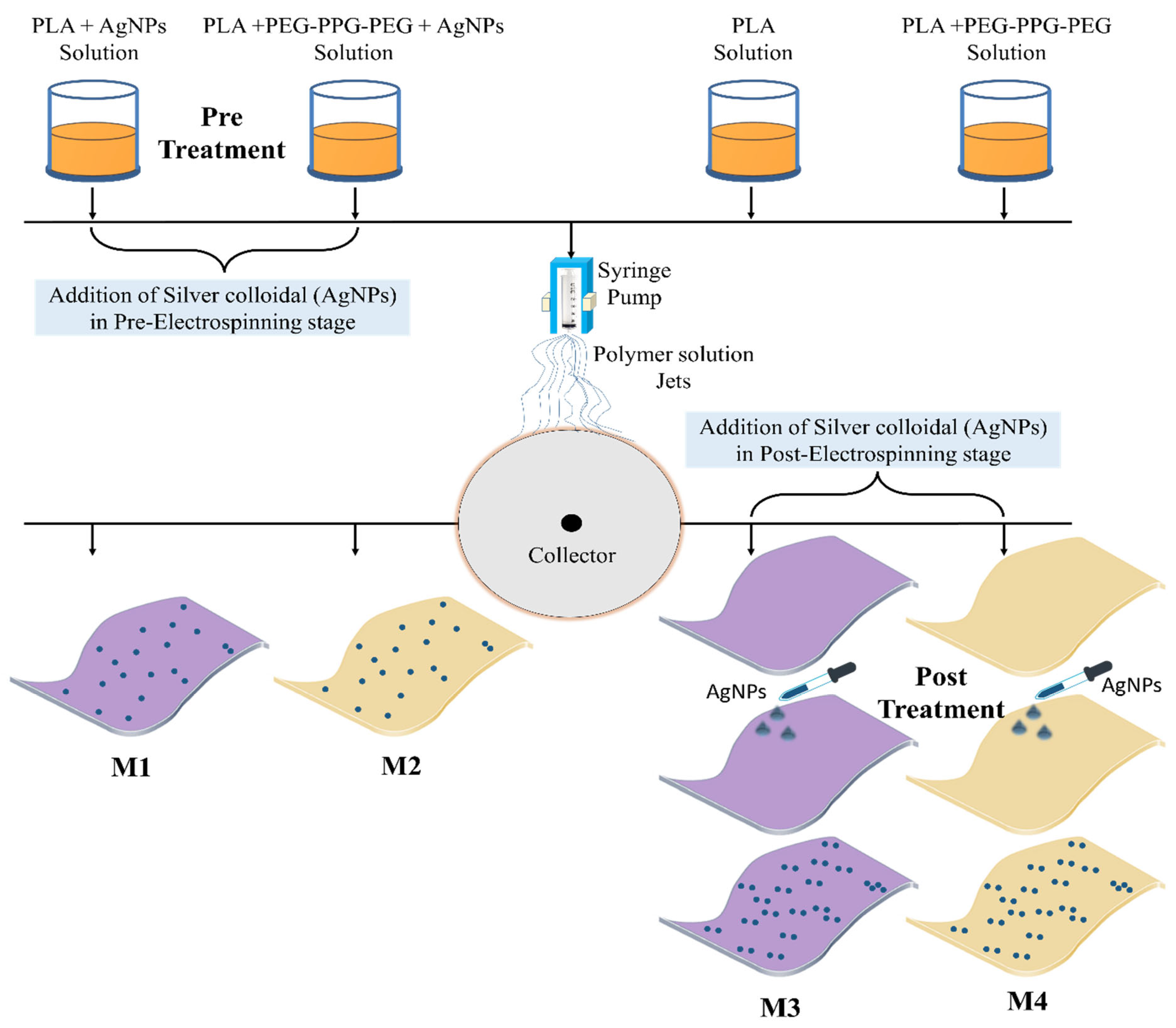
2.4. Integration of Silver Colloid into PLA-Based Textiles
2.4.1. Pre-Electrospinning Stage
2.4.2. Electrospinning Process
2.4.3. Post-Electrospinning Stage
2.5. Characterization
2.5.1. Morphological Study
2.5.2. Crystalline Structure and Thermal Stability
2.5.3. Wettability
2.5.4. Mechanical Properties
2.5.5. Antibacterial Properties
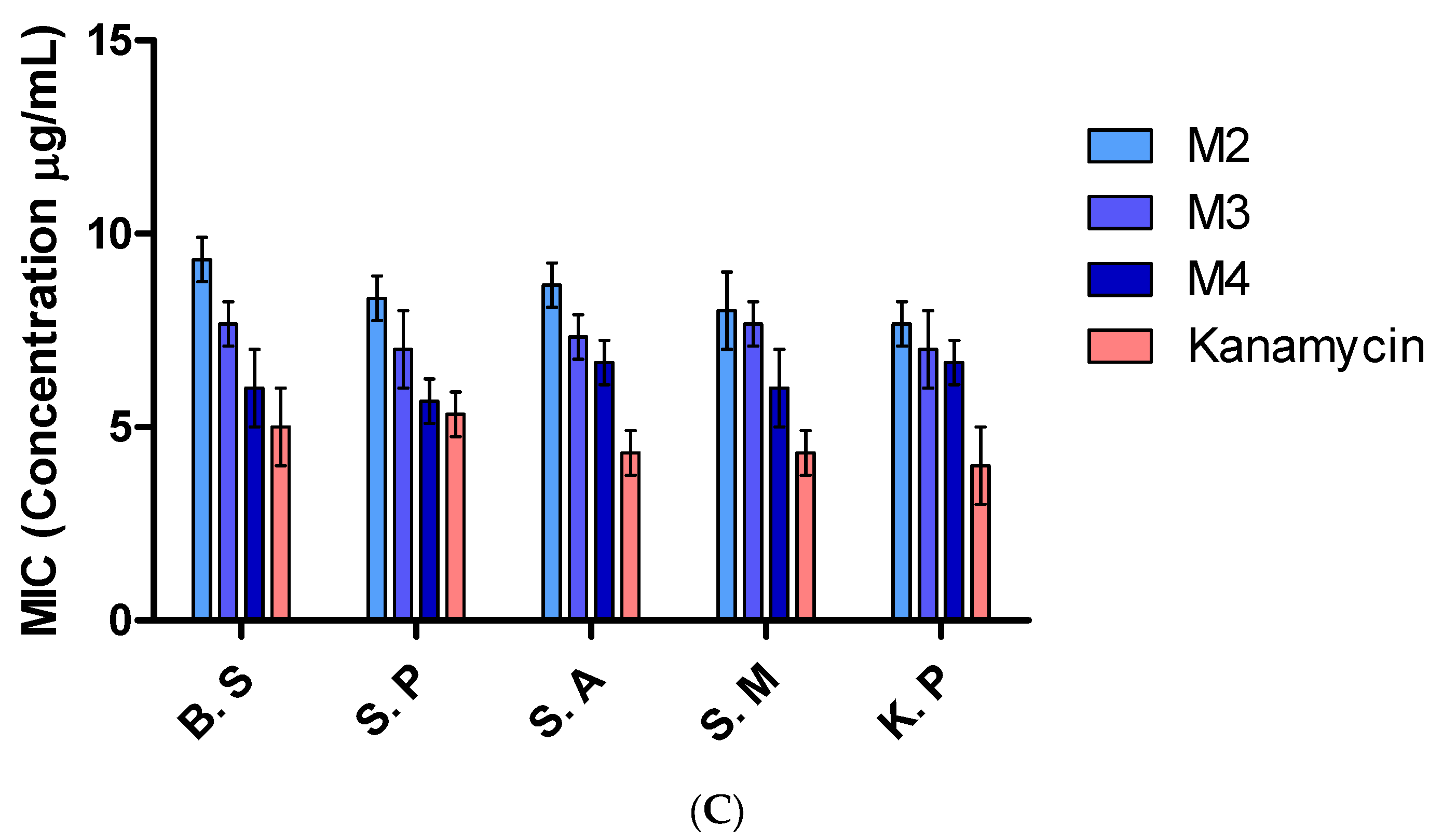
2.5.6. Determination of Minimum Inhibitory Concentration (MIC)
3. Results and Discussion
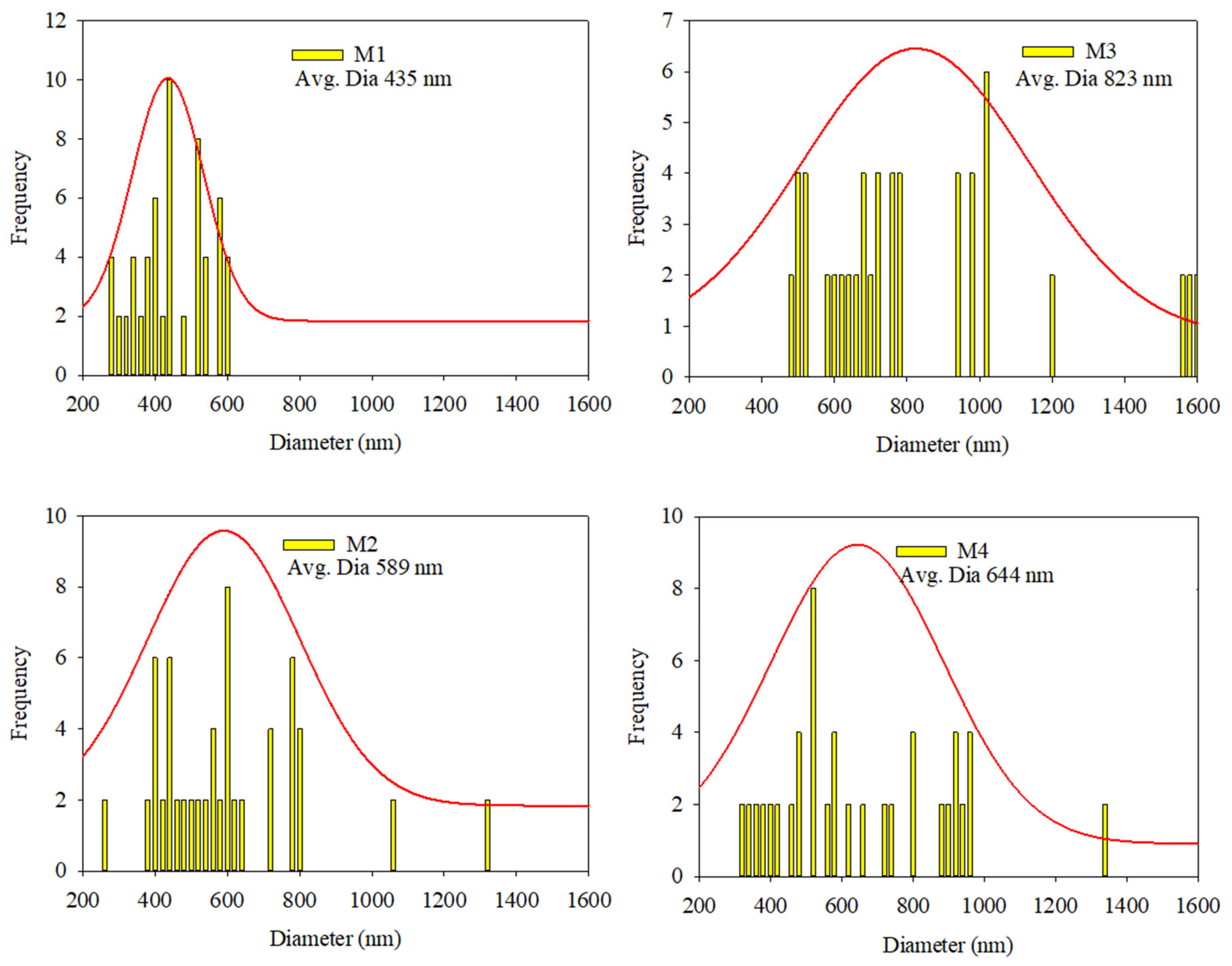
3.1. Morphological Study
3.2. Elemental Analysis
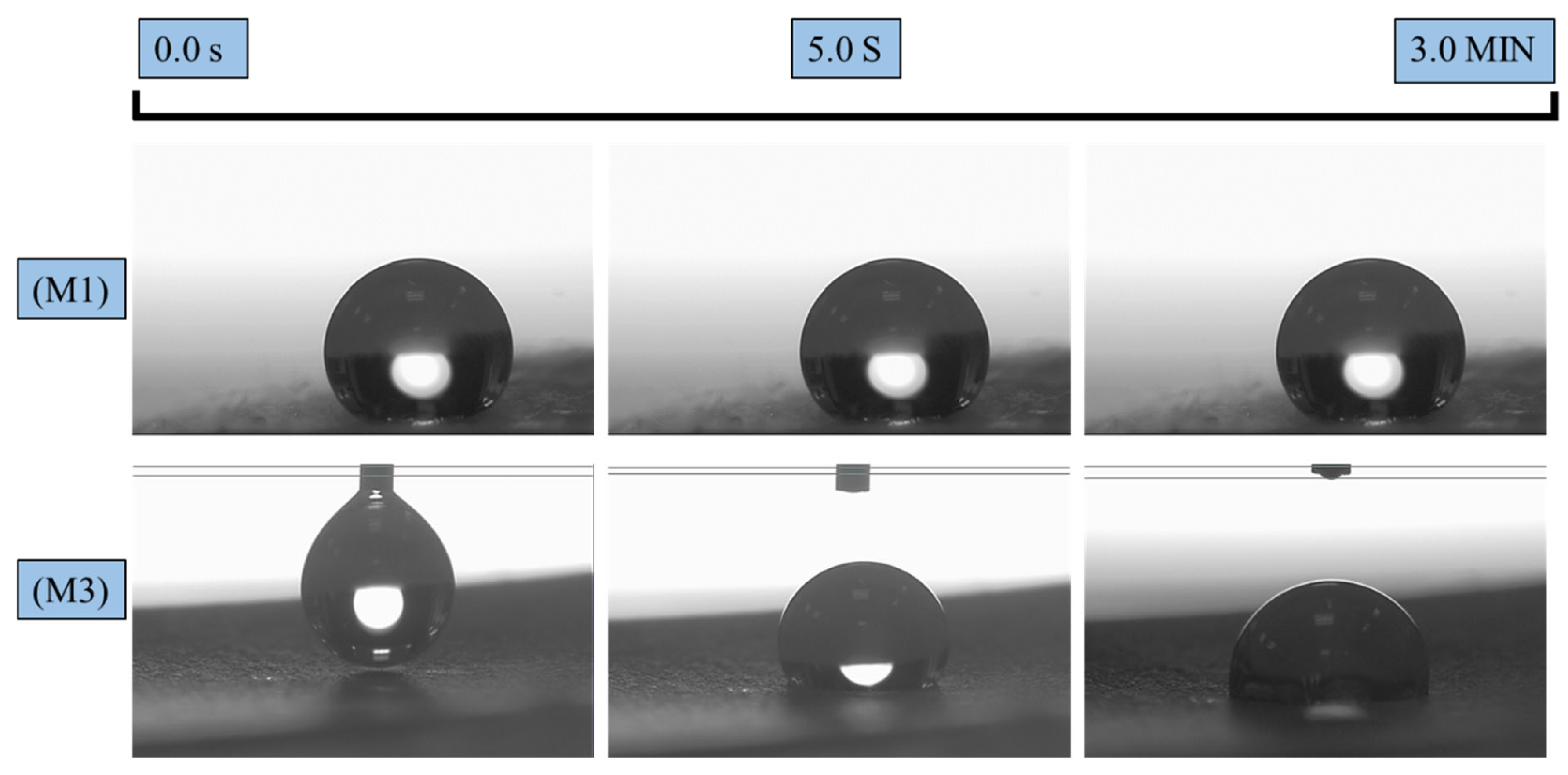
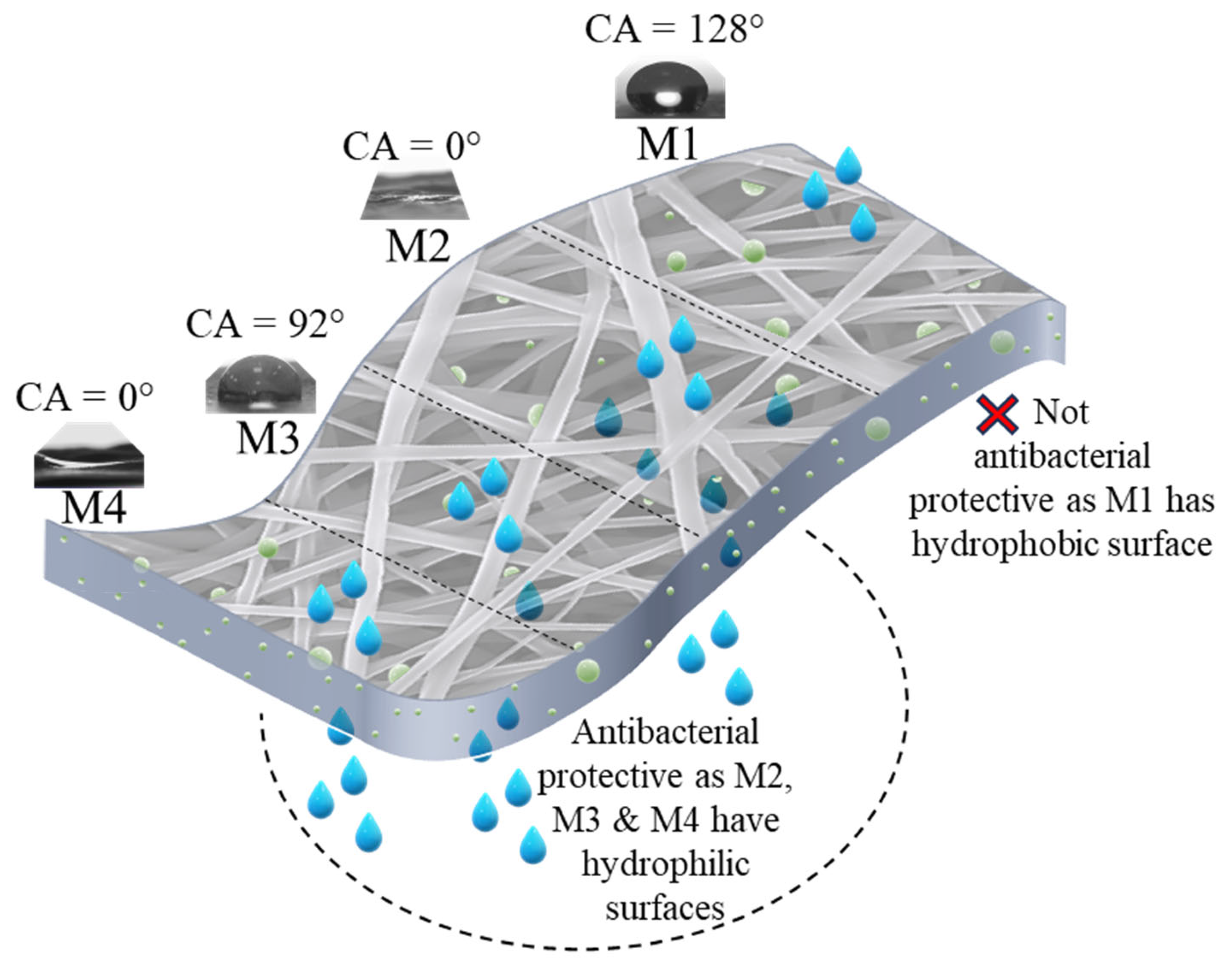
3.3. Wettability
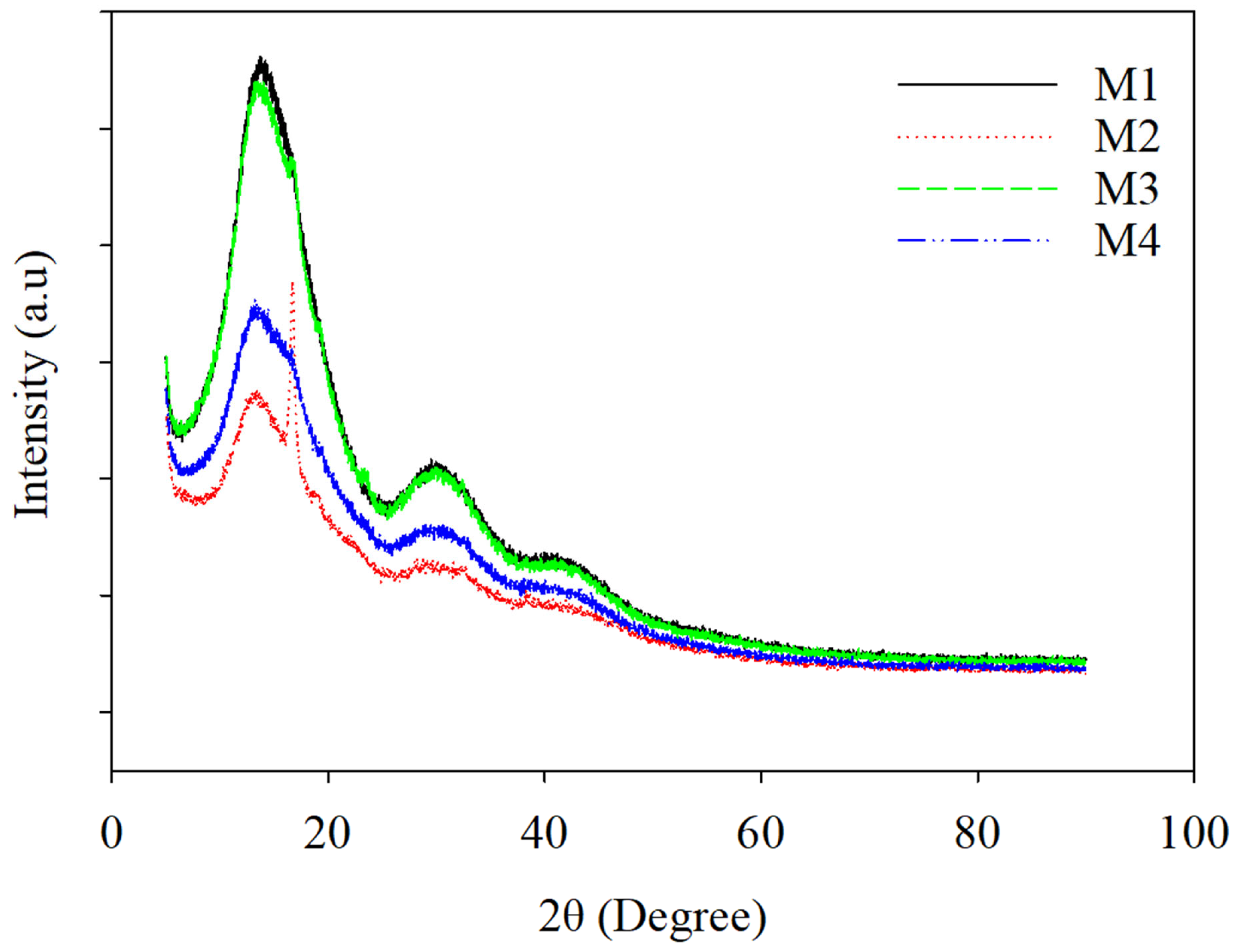
3.4. X-ray Diffraction
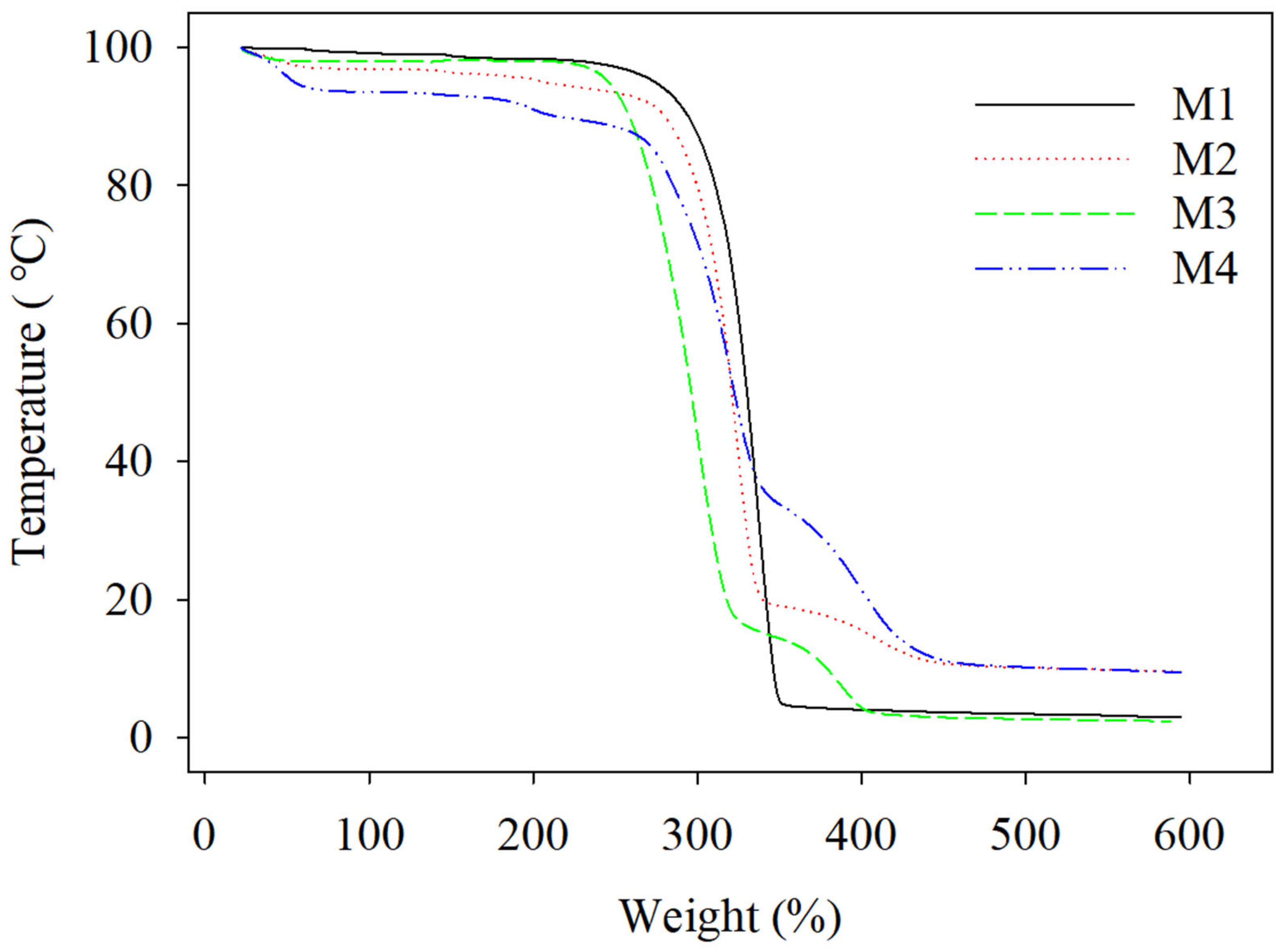
3.5. Thermal Analysis
3.6. Mechanical Strength
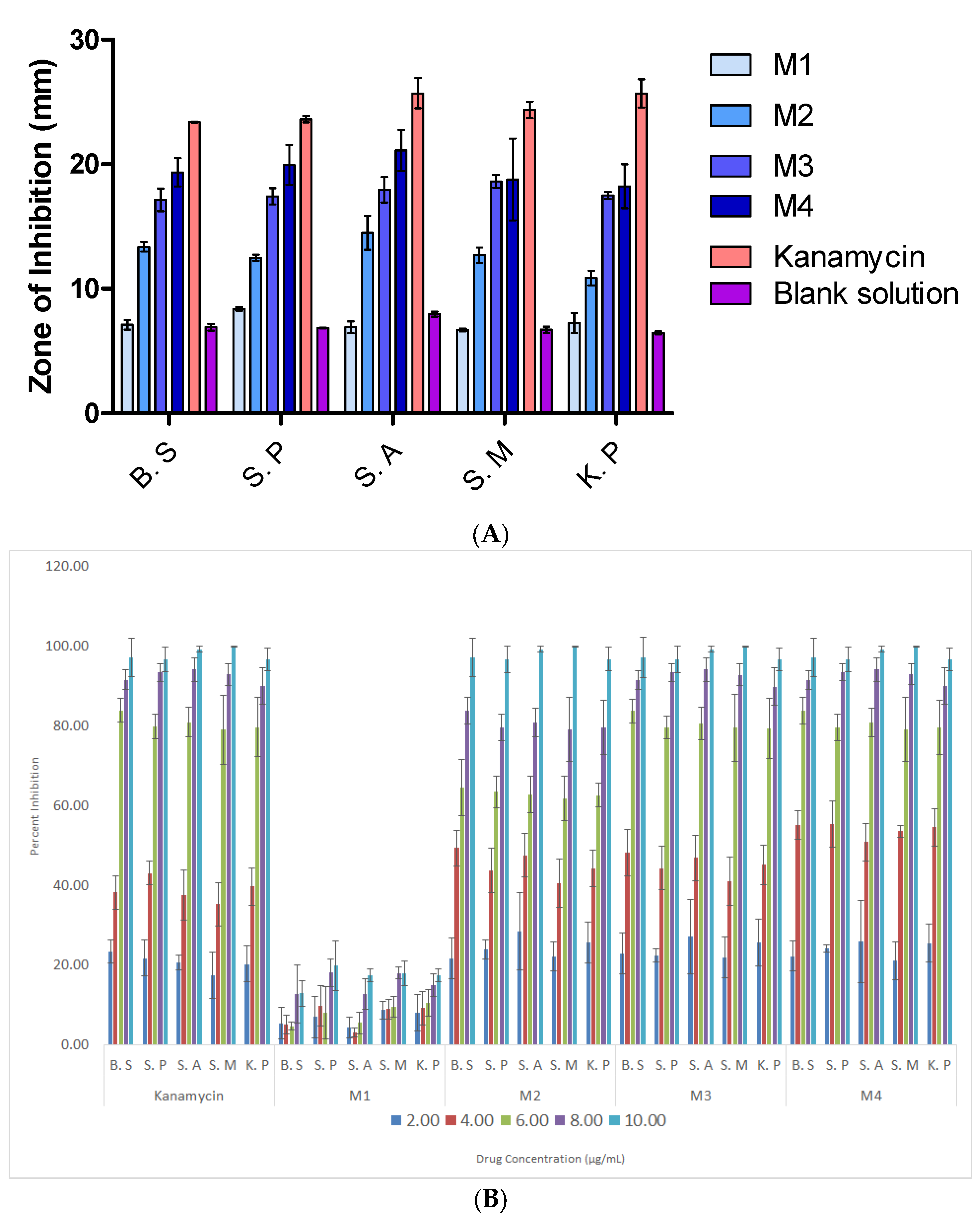
3.7. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BIOPLASTICS Facts and Figures. 2022. Available online: https://www.european-bioplastics.org/news/publications/ (accessed on 1 January 2023).
- Aijaz, M.O.; Karim, M.R.; Alnaser, I.A.; Siddiqui, I.H.; Assaifan, A.K. Silica NPs in PLA-Based Electrospun Nanofibrous Non-Woven Protective Fabrics with Dual Hydrophilicity/Hydrophobicity, Breathability, and Thermal Insulation Characteristics for Individuals with Disabilities. Polymers 2023, 15, 4139. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Aijaz, M.O.; Alharthi, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly(vinyl alcohol)/chitosan for selective lead(II) and cadmium(II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef]
- Karim, M.R. Fabrication of electrospun aligned nanofibers from conducting polyaniline copolymer/polyvinyl alcohol/chitosan oligossacaride in aqueous solutions. Synth. Met. 2013, 178, 34–37. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Karim, M.R.; Omar, N.M.A.; Othman, M.H.D.; Wahab, M.A.; Uzzaman, M.A.; Alharbi, H.M.; Wazeer, I. Recent Progress, Challenges, and Opportunities of Membrane Distillation for Heavy Metals Removal. Chem. Rec. 2022, 22, e202100323. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Oh, T.H. Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications. Crystals 2021, 11, 199. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; Dandurand, J.; Pepe, A.; Bochicchio, B. Thermal and dynamic mechanical behavior of poly(lactic acid) (PLA)-based electrospun scaffolds for tissue engineering. J. Appl. Polym. Sci. 2021, 138, 51313. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.; Vallés-Lluch, A. PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous polymer-based composites obtained by electrospinning for bone tissue engineering. Polymers 2021, 14, 96. [Google Scholar] [CrossRef]
- Hajikhani, M.; Emam-Djomeh, Z.; Askari, G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021, 172, 143–153. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Yang, S.B.; Karim, M.R.; Othman, M.H.D.; Alnaser, I.A. Preparation and Characterization of Poly(Lactic Acid)/Poly (ethylene glycol)-Poly(propyl glycol)-Poly(ethylene glycol) Blended Nanofiber Membranes for Fog Collection. Membranes 2023, 13, 32. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, G.; Wang, Y.; Guan, D. Hierarchical structured PVA-PLA nanofibrous membrane with “water-chestnut-like” surface morphology for water harvesting. Microporous Mesoporous Mater. 2021, 324, 111260. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Song, J.; Gong, H.; Meng, J.; Li, Z.; Li, J. Porous poly (L–lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef]
- Chang, W.-M.; Zhao, Y.-X.; Guo, R.-P.; Wang, Q.; Gu, X.-D. Design and Study of Clothing Structure for People with Limb Disabilities. J. Fiber Bioeng. Inform. 2009, 2, 62–67. [Google Scholar] [CrossRef]
- Kosinski, K.; Orzada, B.T.; Kim, H.-S. Commercialization of Adaptive Clothing: Toward a Movement of Inclusive Design. International Textile and Apparel Association Annual Conference Proceedings; Iowa State University Digital Press: Ames, IA, USA, 2018; Volume 75. [Google Scholar]
- Dana, A.N.; Bauman, W.A. Bacteriology of pressure ulcers in individuals with spinal cord injury: What we know and what we should know. J. Spinal Cord Med. 2015, 38, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface Modification of Polycaprolactone Membrane via Aminolysis and Biomacromolecule Immobilization for Promoting Cytocompatibility of Human Endothelial Cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef]
- You, X.; Piao, C.; Chen, L. Preparation of a magnetic molecularly imprinted polymer by atom-transfer radical polymerization for the extraction of parabens from fruit juices. J. Sep. Sci. 2016, 39, 2831–2838. [Google Scholar] [CrossRef]
- Alves, P.; Pinto, S.; de Sousa, H.C.; Gil, M.H. Surface modification of a thermoplastic polyurethane by low-pressure plasma treatment to improve hydrophilicity. J. Appl. Polym. Sci. 2011, 122, 2302–2308. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Li, D.; Qin, J.; Zhang, M.; Wang, K.; Zhao, J.; Zhang, L. LBL deposition of chitosan/heparin bilayers for improving biological ability and reducing infection of nanofibers. Int. J. Biol. Macromol. 2020, 154, 999–1006. [Google Scholar] [CrossRef]
- Asadian, M.; Dhaenens, M.; Onyshchenko, I.; De Waele, S.; Declercq, H.; Cools, P.; Devreese, B.; Deforce, D.; Morent, R.; De Geyter, N. Plasma Functionalization of Polycaprolactone Nanofibers Changes Protein Interactions with Cells, Resulting in Increased Cell Viability. ACS Appl. Mater. Interfaces 2018, 10, 41962–41977. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-Coated Poly(lactic acid) Nanofibres Loaded with Essential Oils for Wound Healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Anaraki, N.A.; Rad, L.R.; Irani, M.; Haririan, I. Fabrication of PLA/PEG/MWCNT electrospun nanofibrous scaffolds for anticancer drug delivery. J. Appl. Polym. Sci. 2015, 132, 41286. [Google Scholar] [CrossRef]
- González, E.; Shepherd, L.M.; Saunders, L.; Frey, M.W. Surface Functional Poly(lactic Acid) Electrospun Nanofibers for Biosensor Applications. Materials 2016, 9, 47. [Google Scholar] [CrossRef]
- Mojdeh Azarnia, V.H.-A. Water Exposed Electrospinning of Super Hydrophilic Polylactic Acid/Pluronic Blend Nano-Fibers. Int. J. Adv. Sci. Eng. Technol. 2018, 6, 6. [Google Scholar]
- Karim, M.R.; Aijaz, M.O.; Al-Harthi, N. Antiseptic and Fragrance-Free Soap. U.S. Patent 9896651B1, 20 February 2018. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Xi, H.; Yu, Y.; Song, Y.; Wu, C.; Zhou, Y. Evaluation methods of inhibition to microorganisms in biotreatment processes: A review. Water Cycle 2023, 4, 70–78. [Google Scholar] [CrossRef]
- Şener, S.Ö.; Yilmaz, S.S.; Doganci, M.D.; Doganci, E. Fabrication of tetra-n-butylammonium hydrogen sulfate-based poly(lactic acid)-poly(ethylene glycol) composite electrospun wound dressing. J. Appl. Polym. Sci. 2024, 141, e56139. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, M.O.; Yang, S.B.; Karim, M.R.; Alnaser, I.A.; Alahmari, A.D.; Almubaddel, F.S.; Assaifan, A.K. Preparation and Characterization of Electrospun Poly (lactic acid)/Poly (ethylene glycol)–b–poly (propylene glycol)–b–poly (ethylene glycol)/Silicon Dioxide Nanofibrous Adsorbents for Selective Copper (II) Ions Removal from Wastewater. Membranes 2023, 13, 54. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Ansel, H.C.; Norred, W.P.; Roth, I.L. Antimicrobial activity of dimethyl sulfoxide against Escherichia coli, Pseudomonas aeruginosa, and Bacillus megaterium. J. Pharm. Sci. 1969, 58, 836–839. [Google Scholar] [CrossRef] [PubMed]




| Sample Names | PLA (wt.%) | PEG-PPG-PEG (wt.%) | Silver Colloidal | Method of AgNP Integration | Electrospinning Dope Solution |
|---|---|---|---|---|---|
| M1 | 100 | 0 | 20 (v/v. %) | Electrospinning (E) | PLA/AgNPs |
| M2 | 80 | 20 | 20 (v/v. %) | Electrospinning (E) | PLA/PEG-PPG-PEG/AgNPs |
| M3 | 100 | 0 | 0.083 mL/cm2 | Solution casting (S) | PLA |
| M4 | 80 | 20 | 0.083 mL/cm2 | Solution casting (S) | PLA/PEG-PPG-PEG |
| Samples | Inhibition Zone (mm) ± SD, n = 3 | ||||
|---|---|---|---|---|---|
| B.S | S.P | S.A | S.M | K.P | |
| M1 | 6.63 ± 0.80 | 6.57 ± 1.02 | 6.83 ± 1.16 | 6.69 ± 1.43 | 6.75 ± 0.84 |
| M2 | 13.25 ± 0.85 | 12.26 ± 0.44 | 13.51 ± 1.74 | 12.49 ± 0.91 | 10.36 ± 1.55 |
| M3 | 16.91 ± 2.02 | 16.83 ± 0.70 | 18.54 ± 1.77 | 17.99 ± 1.10 | 19.22 ± 1.51 |
| M4 | 19.30 ± 2.63 | 20.11 ± 2.02 | 20.09 ± 2.10 | 19.09 ± 2.95 | 16.86 ± 2.04 |
| Kanamycin | 22.80 ± 0.93 | 24.04 ± 1.47 | 25.42 ± 1.33 | 24.40 ± 0.93 | 25.81 ± 1.23 |
| Blank | 7.06 ± 0.46 | 6.96 ± 0.27 | 7.61 ± 0.35 | 6.88 ± 0.42 | 6.60 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aijaz, M.O.; Alnaser, I.A.; Haque Siddiqui, M.I.; Karim, M.R. The Integration of Microwave-Synthesized Silver Colloidal Nanoparticles into Poly (Lactic Acid)-Based Textiles as Antimicrobial Agents via Pre- and Post-Electrospinning Processes. Polymers 2024, 16, 3613. https://doi.org/10.3390/polym16243613
Aijaz MO, Alnaser IA, Haque Siddiqui MI, Karim MR. The Integration of Microwave-Synthesized Silver Colloidal Nanoparticles into Poly (Lactic Acid)-Based Textiles as Antimicrobial Agents via Pre- and Post-Electrospinning Processes. Polymers. 2024; 16(24):3613. https://doi.org/10.3390/polym16243613
Chicago/Turabian StyleAijaz, Muhammad Omer, Ibrahim A. Alnaser, Md Irfanul Haque Siddiqui, and Mohammad Rezaul Karim. 2024. "The Integration of Microwave-Synthesized Silver Colloidal Nanoparticles into Poly (Lactic Acid)-Based Textiles as Antimicrobial Agents via Pre- and Post-Electrospinning Processes" Polymers 16, no. 24: 3613. https://doi.org/10.3390/polym16243613
APA StyleAijaz, M. O., Alnaser, I. A., Haque Siddiqui, M. I., & Karim, M. R. (2024). The Integration of Microwave-Synthesized Silver Colloidal Nanoparticles into Poly (Lactic Acid)-Based Textiles as Antimicrobial Agents via Pre- and Post-Electrospinning Processes. Polymers, 16(24), 3613. https://doi.org/10.3390/polym16243613











