Sustained Release of Curcumin from Cur-LPs Loaded Adaptive Injectable Self-Healing Hydrogels
Abstract
1. Introduction
2. Experimental
2.1. Materials
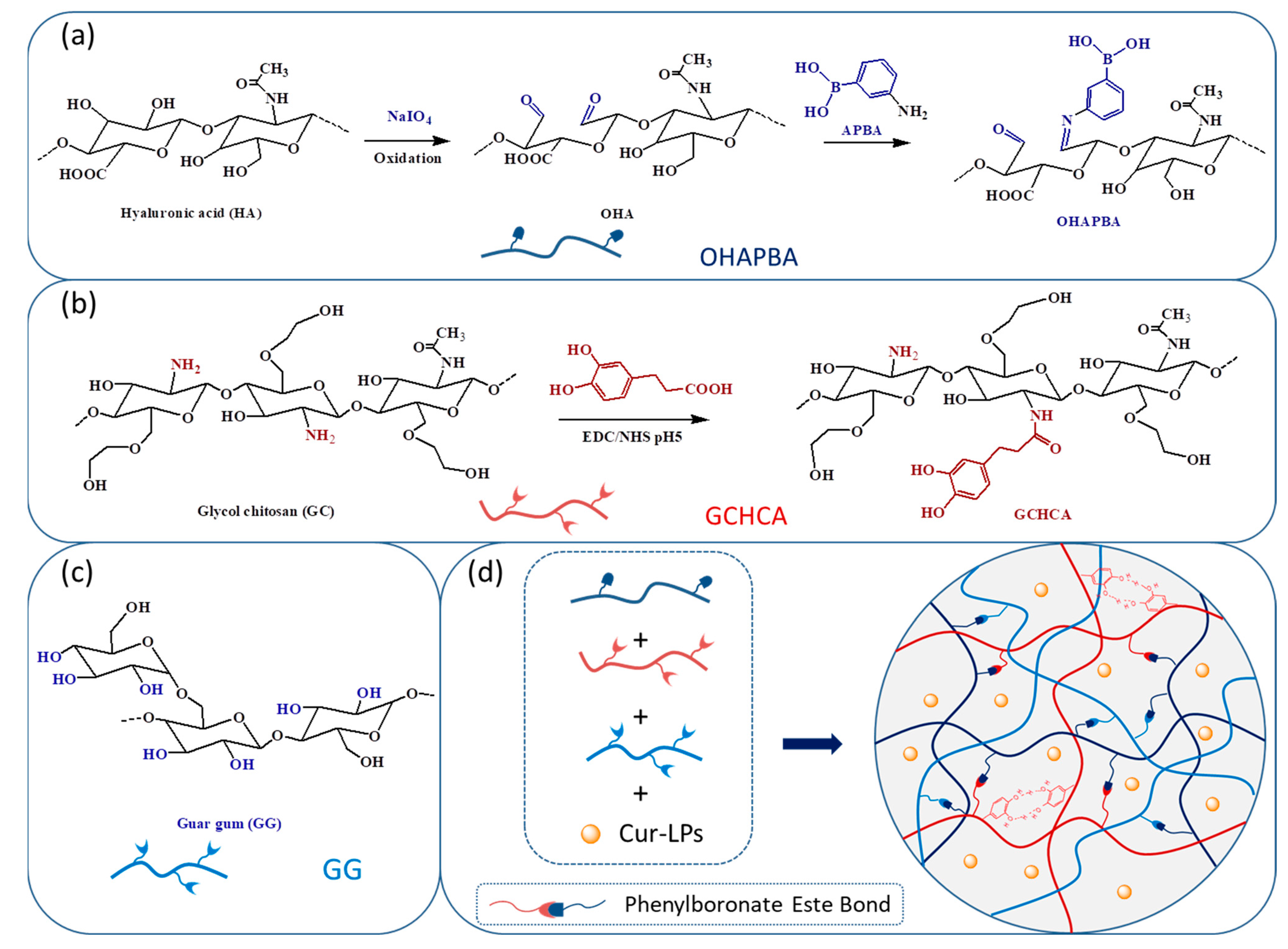
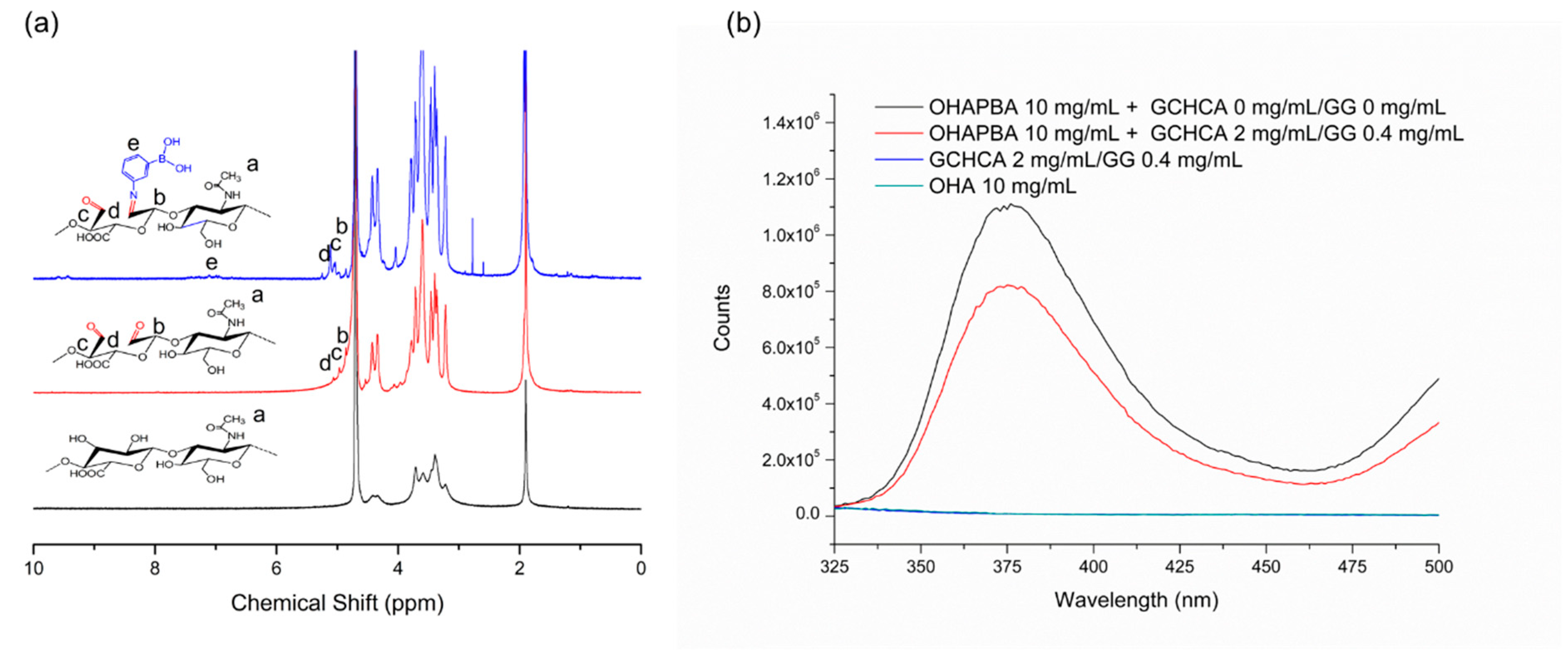
2.2. Preparation and Characterization of Catechol-Modified Glycol Chitosan (GCHCA)
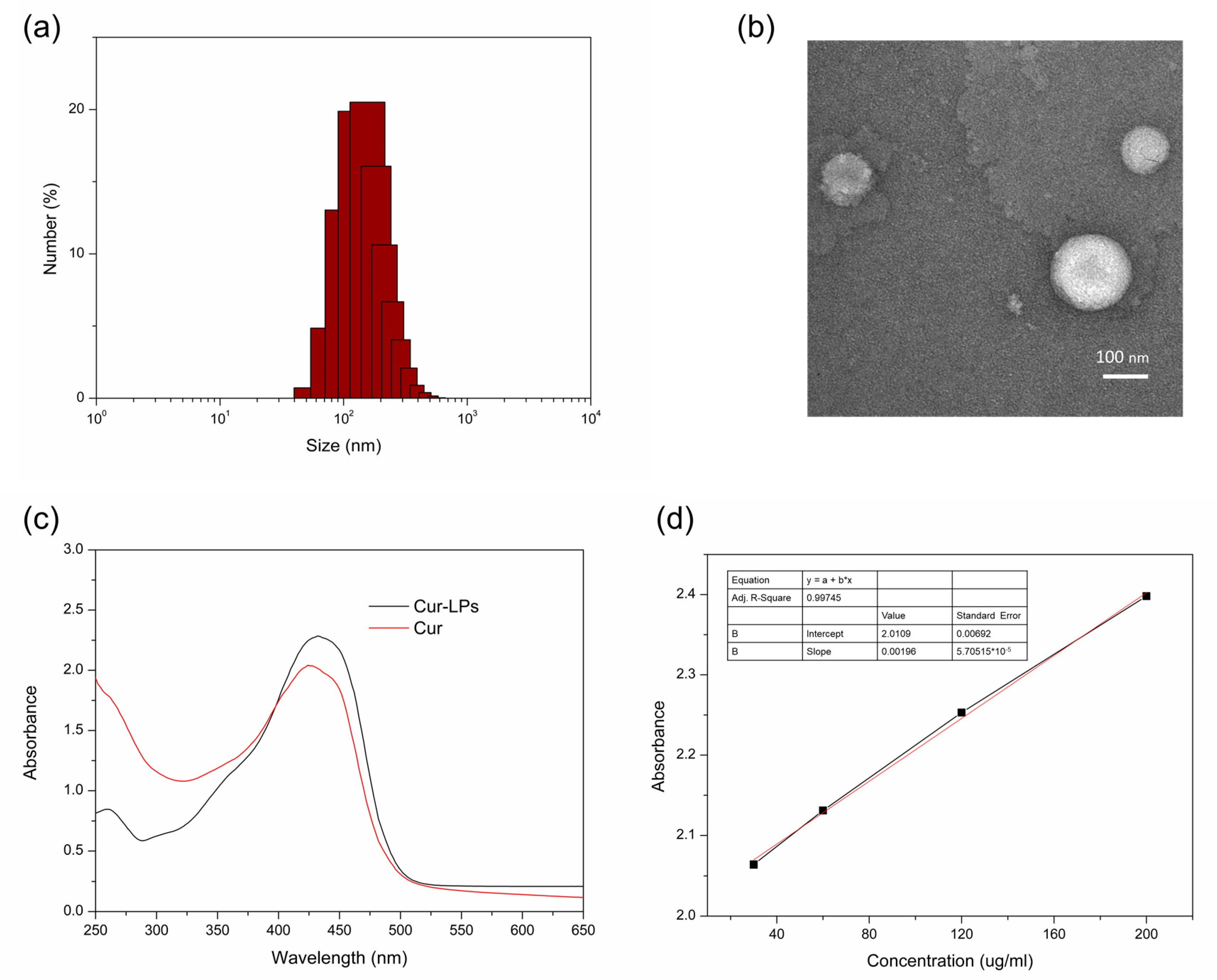
2.3. Preparation and Characterization of Curcumin Liposomes (Cur-LPs)
2.4. Preparation of Phenylboronic Acid Modified Oxidized Hyaluronic Acid Sodium Salt (OHAPBA) and Its Structural Characterization
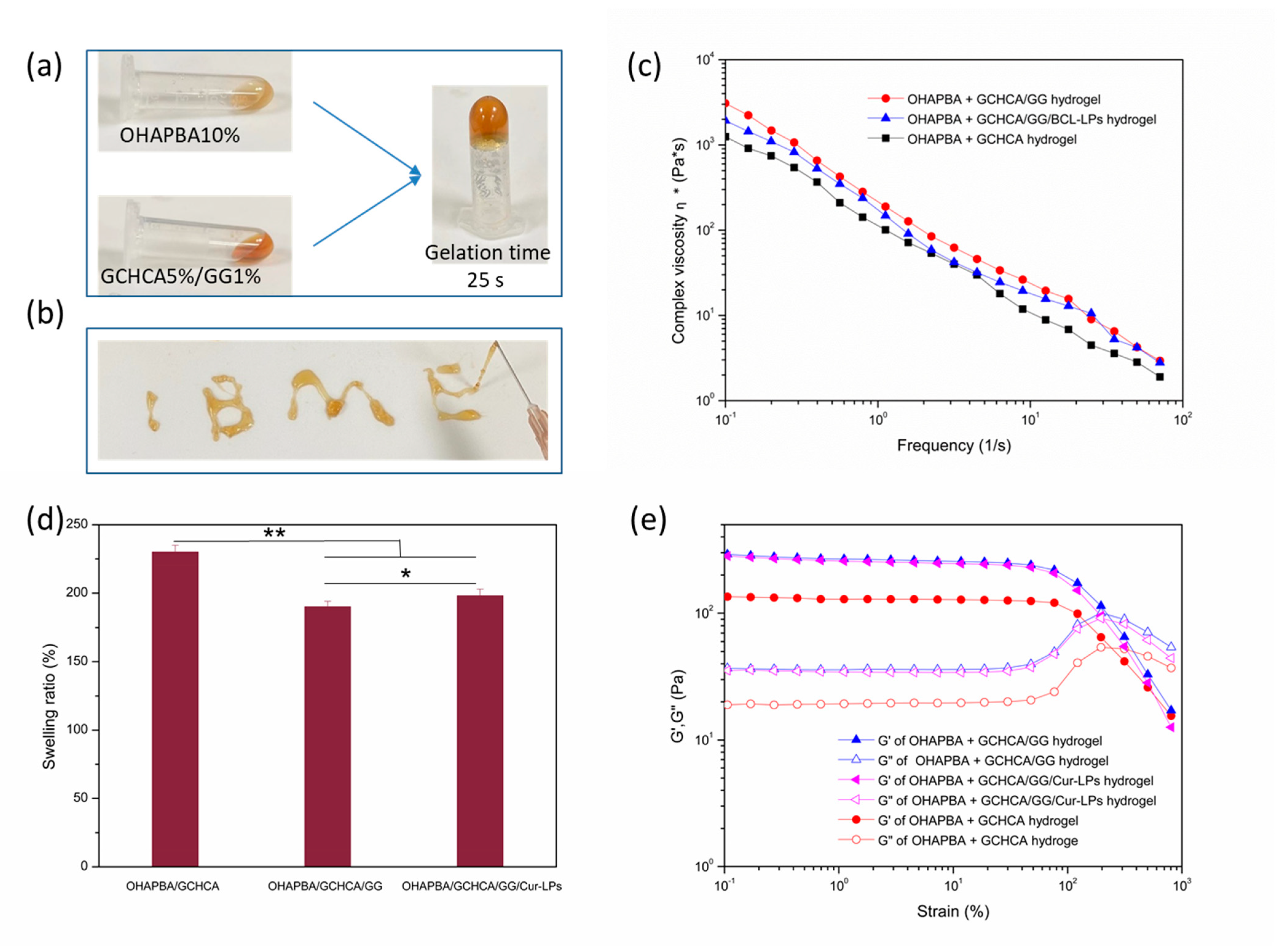
2.5. Preparation of the Hydrogels
2.6. Characterizations of the Hydrogels
2.6.1. Swelling Ratios of the Hydrogels
2.6.2. Rheological Tests
2.6.3. Cytotoxicity
2.6.4. Hemolysis Evaluation of Hydrogels
2.6.5. DPPH Scavenging Efficiency of Hydrogels
2.6.6. In Vitro Release of Curcumin from Cur-LPs Loaded Hydrogel
2.7. Statistical Analysis
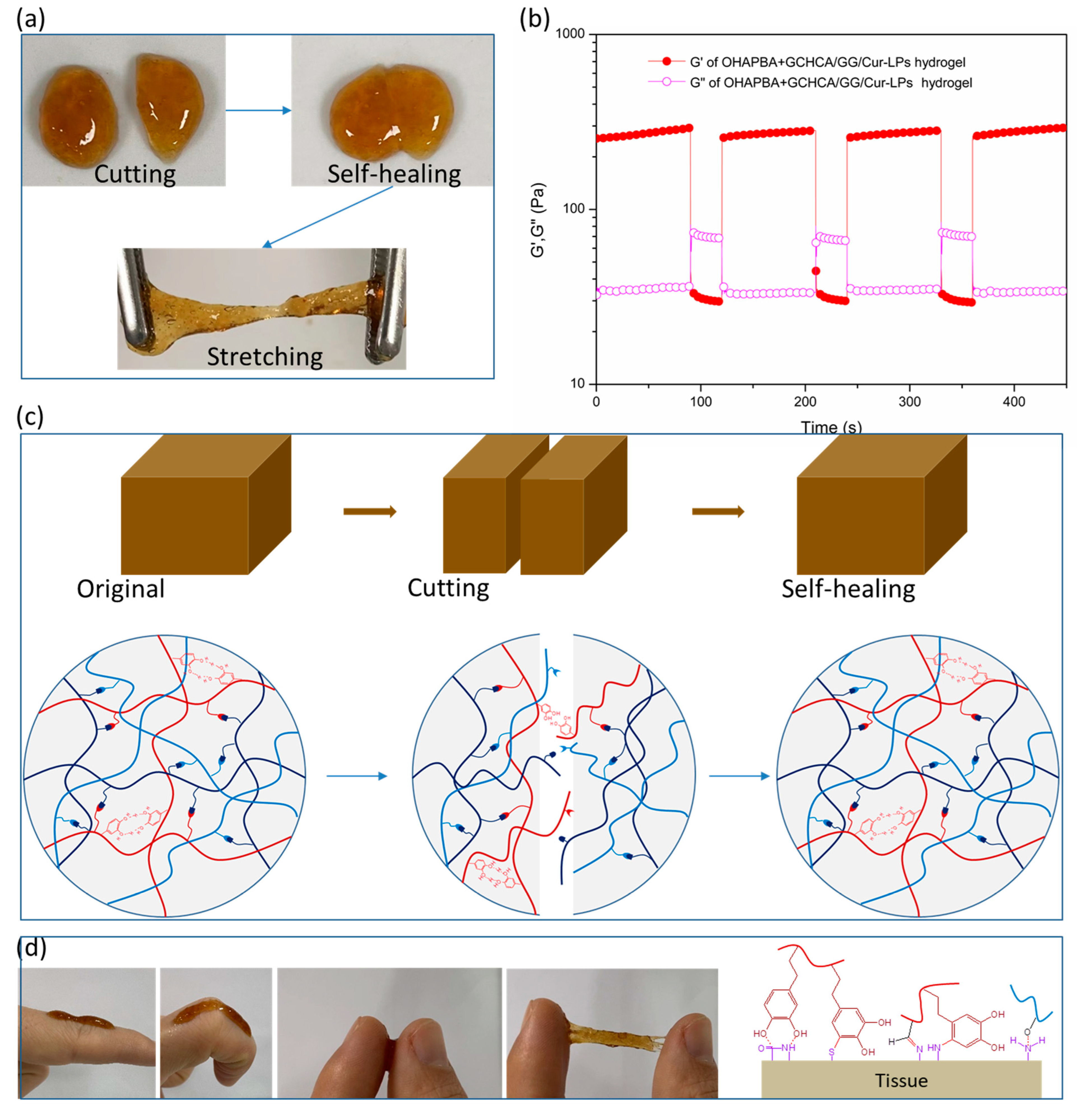
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, H.; Wang, Z.; Wang, R.; Chen, Q.; Yu, A.; Lu, A. Self-Healing, Injectable Hydrogel Dressing for Monitoring and Therapy of Diabetic Wound. Adv. Funct. Mater. 2024, 34, 2401209. [Google Scholar] [CrossRef]
- Xiao, W.; Wan, X.; Shi, L.; Ye, M.; Zhang, Y.; Wang, S. A Viscous-Biofluid Self-Pumping Organohydrogel Dressing to Accelerate Diabetic Wound Healing. Adv. Mater. 2024, 36, e2401539. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, L.; Dong, J.; Yuan, X.; Ye, J.; Fan, Y.; Liu, B.; Xie, J.; Ji, X. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat. Commun. 2024, 15, 1042. [Google Scholar] [CrossRef]
- Pranantyo, D.; Yeo, C.K.; Wu, Y.; Fan, C.; Xu, X.; Yip, Y.S.; Vos, M.I.G.; Mahadevegowda, S.H.; Lim, P.L.K.; Yang, L.; et al. Hydrogel dressings with intrinsic antibiofilm and antioxidative dual functionalities accelerate infected diabetic wound healing. Nat. Commun. 2024, 15, 954. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Grinstaff, M.W. On-Demand Dissolution of Chemically Cross-Linked Hydrogels. Acc. Chem. Res. 2017, 50, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yang, Z.; Zhai, Q.; Li, D.; Zhu, X.; He, Q.; Li, L.; Cannon, R.D.; Wang, H.; Tang, H.; et al. An All-in-One “4A Hydrogel”: Through First-Aid Hemostatic, Antibacterial, Antioxidant, and Angiogenic to Promoting Infected Wound Healing. Small 2023, 19, 2207437. [Google Scholar] [CrossRef]
- Chang, L.; Wang, C.; Han, S.; Sun, X.; Xu, F. Chemically Triggered Hydrogel Transformations through Covalent Adaptable Networks and Applications in Cell Culture. ACS Macro Lett. 2021, 10, 901–906. [Google Scholar] [CrossRef]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Yang, X.; Wu, J.; Kirk, T.B.; Xu, J.; Xu, A.; Xue, W. Injectable and Self-Healing Hydrogels with Double-Dynamic Bond Tunable Mechanical, Gel-Sol Transition and Drug Delivery Properties for Promoting Periodontium Regeneration in Periodontitis. ACS Appl. Mater. Interfaces 2021, 13, 61638–61652. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, X.; Fan, L.; Filppula, A.M.; Li, Q.; Zhang, H.; Zhao, Y.; Shang, L. Self-Healing Dynamic Hydrogel Microparticles with Structural Color for Wound Management. Nano-Micro Lett. 2024, 16, 232. [Google Scholar] [CrossRef]
- Hua, S.; Zhang, Y.; Zhu, Y.; Fu, X.; Meng, L.; Zhao, L.; Kong, L.; Pan, S.; Che, Y. Tunicate cellulose nanocrystals strengthened injectable stretchable hydrogel as multi-responsive enhanced antibacterial wound dressing for promoting diabetic wound healing. Carbohydr. Polym. 2024, 343, 122426. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable Adhesive Self-Healing Multiple-Dynamic-Bond Crosslinked Hydrogel with Photothermal Antibacterial Activity for Infected Wound Healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Wang, J.; Du, P.; Hsu, Y.I.; Uyama, H. Rapid preparation of dynamic-crosslinked nanocomposite hydrogel sensors with efficiency self-healing and adhesion properties for elderly health and sleep management. Chem. Eng. J. 2024, 480, 148324. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, B.; An, Z.; Zheng, P.; Liu, Y.; Zhao, H.; Zhang, Z.; Gao, T.; Cao, Y.; Zhang, Y.; et al. MMP-Responsive Nanoparticle-Loaded, Injectable, Adhesive, Self-Healing Hydrogel Wound Dressing Based on Dynamic Covalent Bonds. Biomacromolecules 2023, 24, 5769–5779. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, M.; Zhang, D.; He, Y.; Chang, R.; Ren, Y.; Guan, F. One-Step Synthesis of Multifunctional Chitosan Hydrogel for Full-Thickness Wound Closure and Healing. Adv. Healthc. Mater. 2022, 11, 2101808. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, X.; Chen, Y.; Chang, H.; Lu, D.; Pei, D.; Geng, Z.; Zeng, Z.; Guo, C.; Huang, J.; et al. Dual Glucose/ROS-Sensitive Injectable Adhesive Self-Healing Hydrogel with Photothermal Antibacterial Activity and Modulation of Macrophage Polarization for Infected Diabetic Wound Healing. ACS Mater. Lett. 2023, 5, 3142–3155. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef]
- He, Y.; Liu, K.; Guo, S.; Chang, R.; Zhang, C.; Guan, F.; Yao, M. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomater. 2023, 155, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, L.; Wu, S.; Yang, S.; Ren, L.; Cheng, B.; Xia, J. A Shape-Programmable Hierarchical Fibrous Membrane Composite System to Promote Wound Healing in Diabetic Patients. Small 2022, 18, 2107544. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Li, D.; Chen, K.; Tang, H.; Hu, S.; Xin, L.; Jing, X.; He, Q.; Wang, S.; Song, J.; Mei, L.; et al. A Logic-Based Diagnostic and Therapeutic Hydrogel with Multistimuli Responsiveness to Orchestrate Diabetic Bone Regeneration. Adv. Mater. 2022, 34, 2108430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pei, D.; Yang, Y.; Xu, K.; Yu, J.; Zhang, Y.; Zhang, Q.; He, G.; Zhang, Y.; Li, A.; et al. Green Tea Derivative Driven Smart Hydrogels with Desired Functions for Chronic Diabetic Wound Treatment. Adv. Funct. Mater. 2021, 31, 2009442. [Google Scholar] [CrossRef]
- Feng, X.; Pi, C.; Fu, S.; Yang, H.; Zheng, X.; Hou, Y.; Wang, Y.; Zhang, X.; Zhao, L.; Wei, Y. Combination of Curcumin and Paclitaxel Liposomes Exhibits Enhanced Cytotoxicity Towards A549/A549-T Cells and Unaltered Pharmacokinetics. J. Biomed. Nanotechnol. 2020, 16, 1304–1313. [Google Scholar] [CrossRef]
- Li, Z.-L.; Peng, S.-F.; Chen, X.; Zhu, Y.-Q.; Zou, L.-Q.; Liu, W.; Liu, C.-M. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, J.-K.; Zhao, L.; Zheng, Z.-Y.; Li, J.; Pu, W.; Liu, S.; Liu, X.-S.; Liu, S.-J.; Zheng, Y.; et al. Novel Curcumin Liposome Modified with Hyaluronan Targeting CD44 Plays an Anti-Leukemic Role in Acute Myeloid Leukemia In Vitro and In Vivo. ACS Appl. Mater. Inter. 2017, 9, 16858–16869. [Google Scholar] [CrossRef]
- Mimica, B.; Popovic, V.B.; Banjari, I.; Kadic, A.J.; Puljak, L. Methods Used for Enhancing the Bioavailability of Oral Curcumin in Randomized Controlled Trials: A Meta-Research Study. Pharmaceuticals 2022, 15, 939. [Google Scholar] [CrossRef]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Increasing the bioavailability of curcumin using a green supercritical fluid technology-assisted approach based on simultaneous starch aerogel formation-curcumin impregnation. Food Chem. 2024, 455, 139468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zheng, S.; Wang, Z. Enhancement of Oral Bioavailability of Curcumin Loaded PLGA Nanoparticles. Chin. J. Mod. Appl. Pharm. 2014, 31, 717–721. [Google Scholar]
- Guo, H.; Dai, W.; Miao, Y.; Wang, Y.; Ma, D.; Xue, W. Sustained Heparin Release Actuator Achieved from Thermal and Water Activated Shape Memory Hydrogels Containing Main-chain LC Units. Chem. Eng. J. 2018, 339, 459–467. [Google Scholar] [CrossRef]
- Dai, W.; Guo, H.; Gao, B.; Ruan, M.; Xu, L.; Wu, J.; Kirk, T.B.; Xu, J.; Ma, D.; Xue, W. Double Network Shape Memory Hydrogels Activated by Near-Infrared with High Mechanical Toughness, Nontoxicity, and 3D Printability. Chem. Eng. J. 2019, 356, 934–949. [Google Scholar] [CrossRef]
- Jin, H.-H.; Lu, Q.; Jiang, J.-G. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J. Dairy. Sci. 2016, 99, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, M.; Fang, H.; Wang, B. A new boronic acid based fluorescent reporter for catechol. Bioorg. Med. Chem. Lett. 2012, 22, 7179–7182. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, F.; Kong, D.; Zhang, J.; Zhou, X.; Xu, J.; Chen, L. Constructing a fluorescent probe for specific detection of catechol based on 4-carboxyphenylboronic acid-functionalized carbon dots. Sens. Actuators B Chem. 2017, 250, 712–720. [Google Scholar] [CrossRef]
- Wu, M.; Chen, J.; Huang, W.; Yan, B.; Peng, Q.; Liu, J.; Chen, L.; Zeng, H. Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive pH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery. Biomacromolecules 2020, 21, 2409–2420. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, L.; Gong, L.; Hu, W.; Huang, W.; Chen, Y.; Asha, A.B.; Srinivas, S.; Chen, L.; Narain, R.; et al. Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties. Chem. Mater. 2019, 31, 2366–2376. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel Mussel-Inspired Injectable Self-Healing Hydrogel with Anti-Biofouling Property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef]
- Xu, Q.; A, S.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Bapat, A.P.; Roy, D.; Ray, J.G.; Savin, D.A.; Sumerlin, B.S. Dynamic-Covalent Macromolecular Stars with Boronic Ester Linkages. J. Am. Chem. Soc. 2011, 133, 19832–19838. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Ning, X.; Liu, Q.; Zhou, X.; Guo, H. Sustained Release of Curcumin from Cur-LPs Loaded Adaptive Injectable Self-Healing Hydrogels. Polymers 2024, 16, 3451. https://doi.org/10.3390/polym16243451
Wu C, Ning X, Liu Q, Zhou X, Guo H. Sustained Release of Curcumin from Cur-LPs Loaded Adaptive Injectable Self-Healing Hydrogels. Polymers. 2024; 16(24):3451. https://doi.org/10.3390/polym16243451
Chicago/Turabian StyleWu, Caixia, Xiaoqun Ning, Qunfeng Liu, Xiaoyan Zhou, and Huilong Guo. 2024. "Sustained Release of Curcumin from Cur-LPs Loaded Adaptive Injectable Self-Healing Hydrogels" Polymers 16, no. 24: 3451. https://doi.org/10.3390/polym16243451
APA StyleWu, C., Ning, X., Liu, Q., Zhou, X., & Guo, H. (2024). Sustained Release of Curcumin from Cur-LPs Loaded Adaptive Injectable Self-Healing Hydrogels. Polymers, 16(24), 3451. https://doi.org/10.3390/polym16243451






