Antibiofilm Effects of Modifying Polyvinylidene Fluoride Membranes with Polyethylenimine, Poly(acrylic acid) and Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
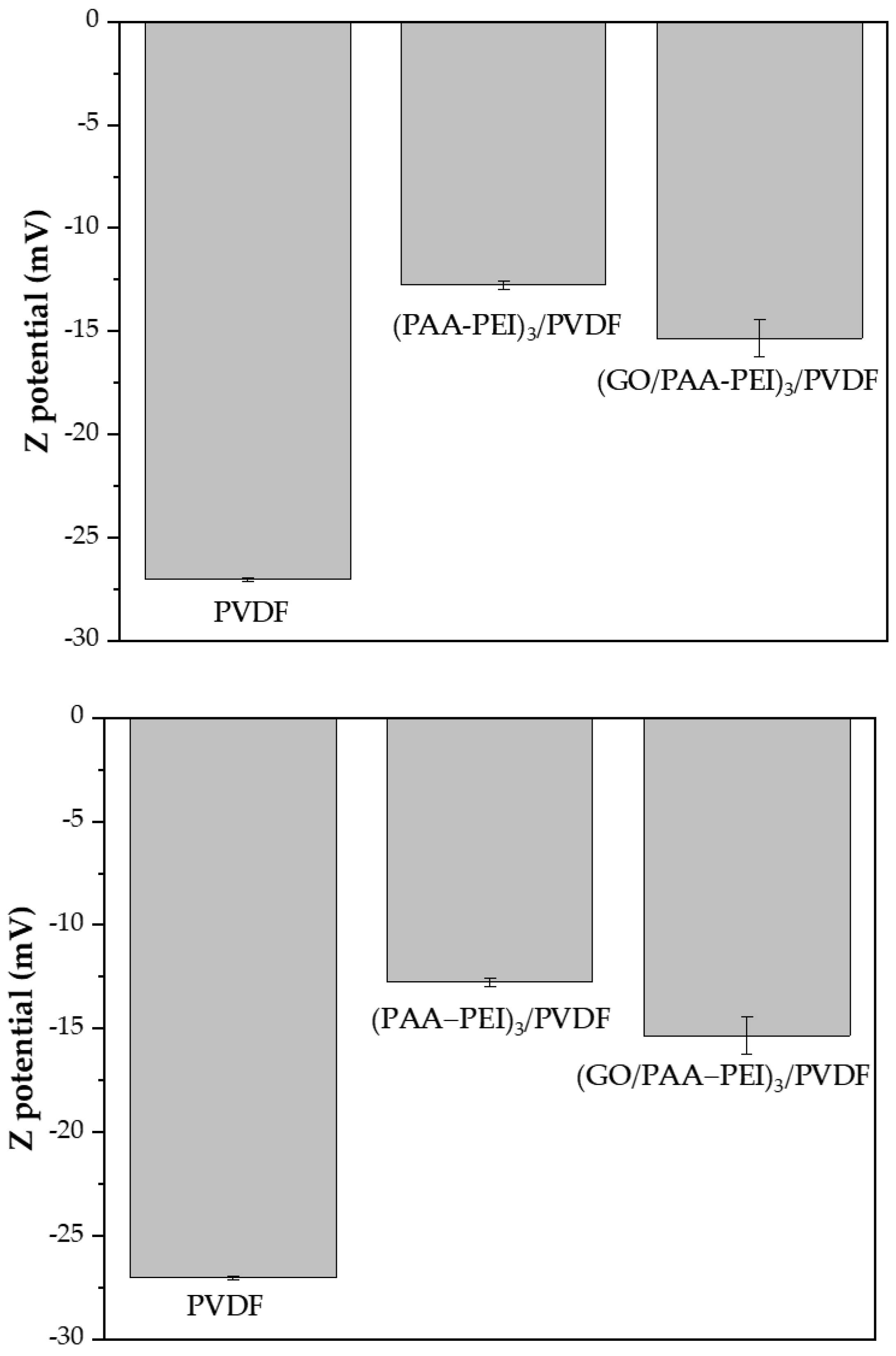
3.1. Membrane Characterization
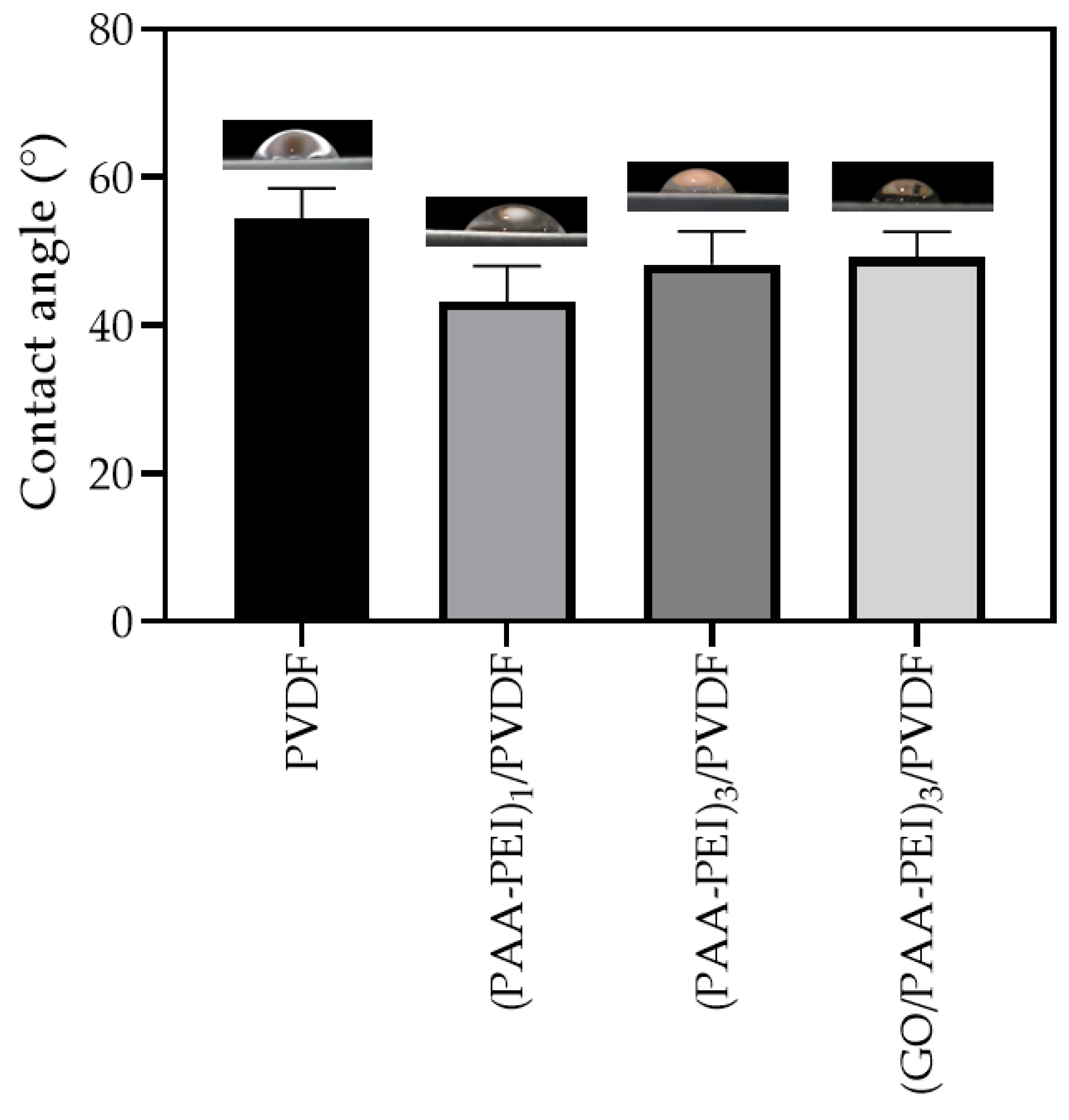
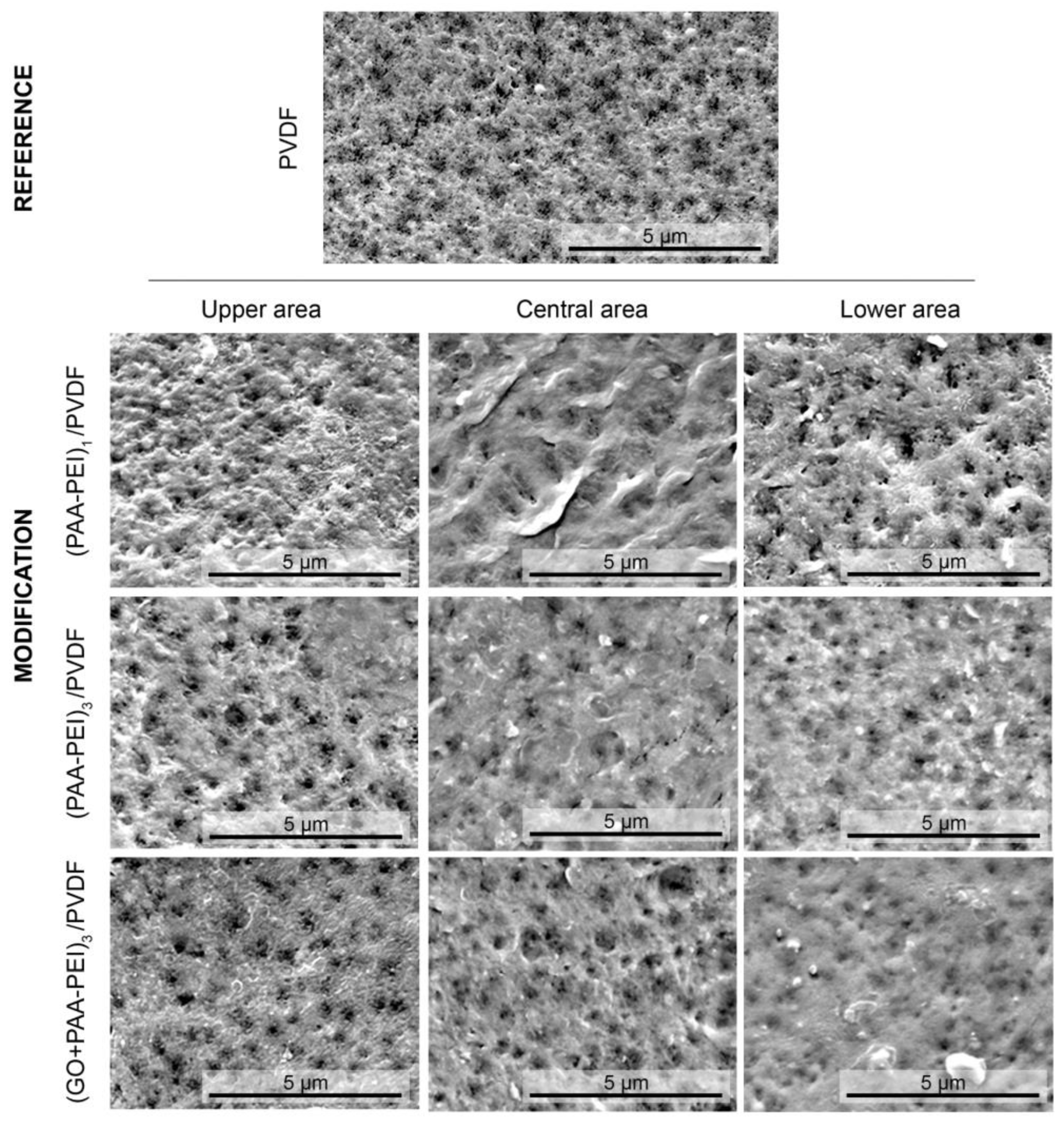
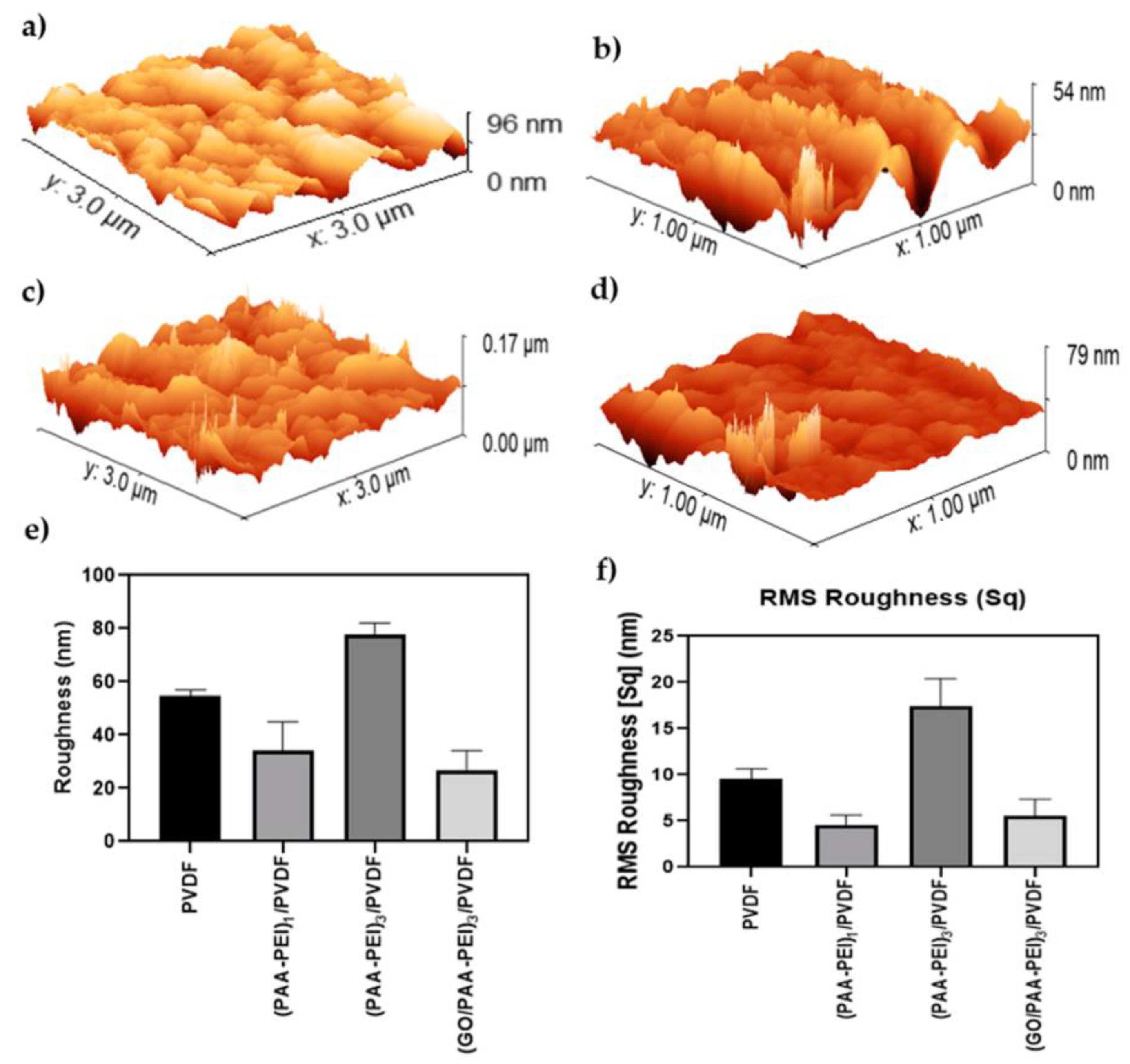
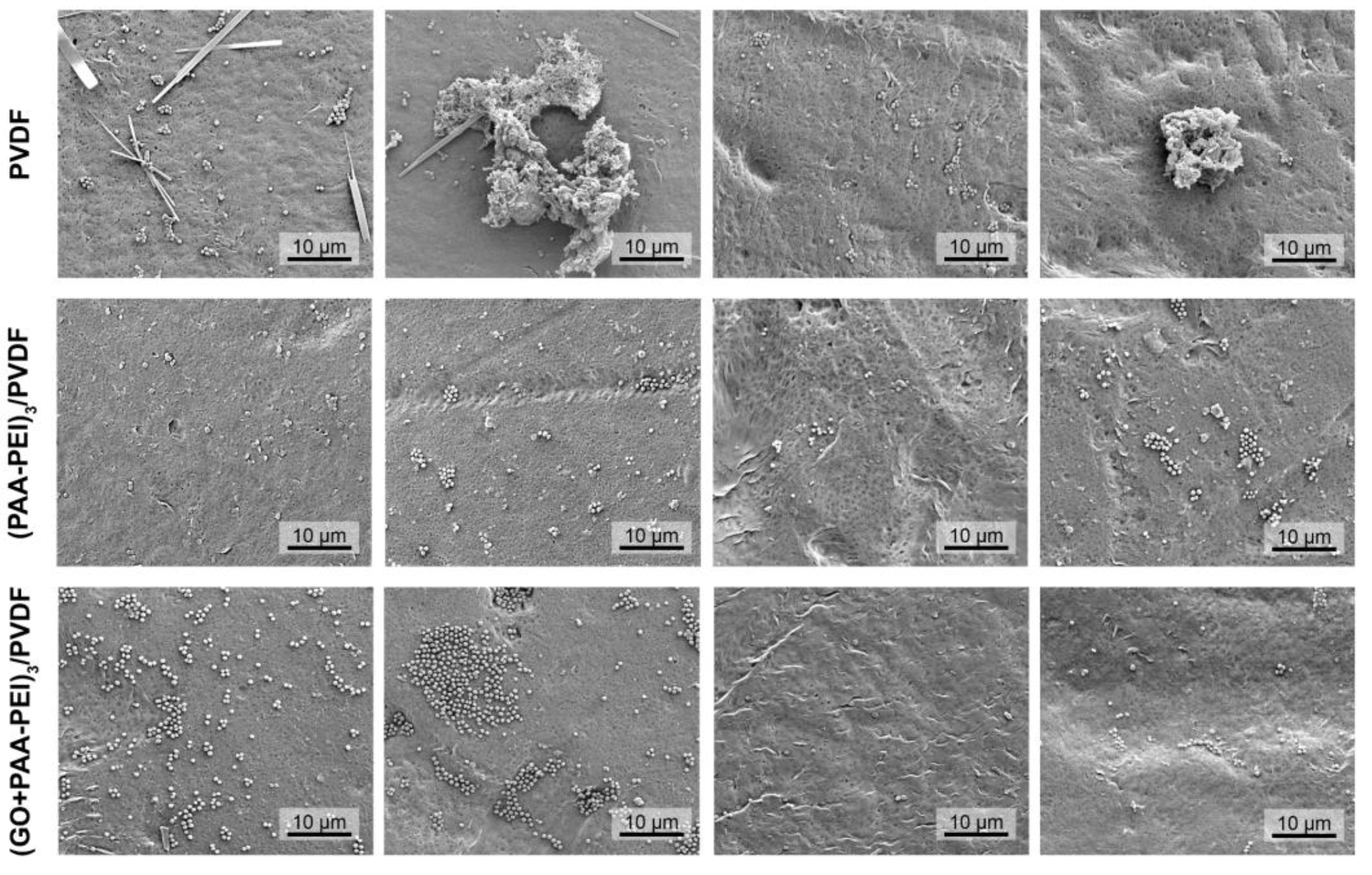
3.1.1. Hydrophilicity, Morphology and Roughness of Membrane Surface
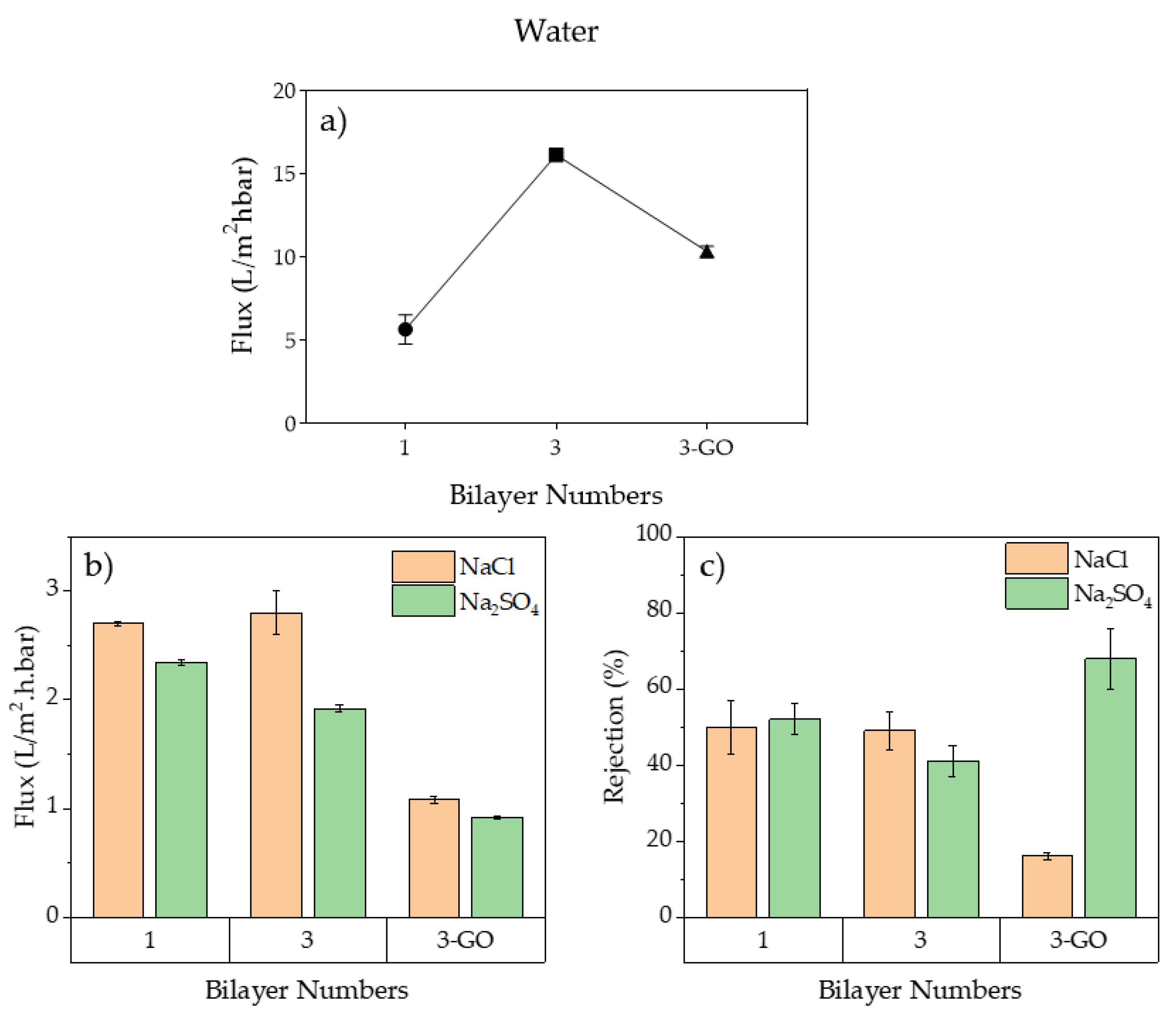
3.1.2. Membranes’ Performances
3.2. Antibiofilm and Antibacterial Activity
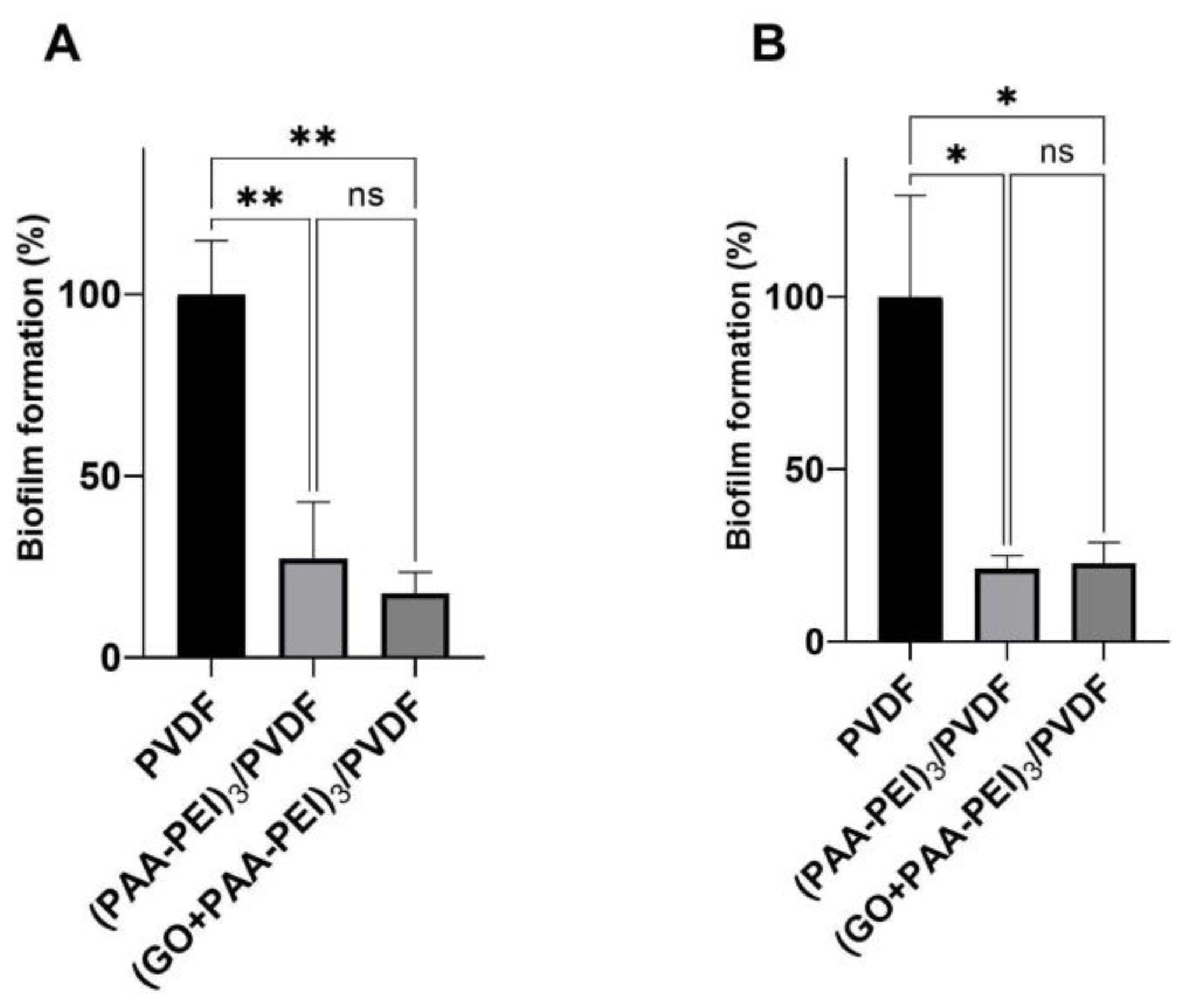
3.2.1. Antibiofilm Activity
3.2.2. Antibacterial Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, R.K. Fresh Water Availability and Its Global Challenge. Br. J. Multidiscip. Adv. Stud. 2023, 4, 1–78. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. mu The State of Desalination and Brine Production: A Global Outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, H. Recent Developments in Reverse Osmosis Desalination Membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel Technologies for Reverse Osmosis Concentrate Treatment: A Review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yang, S.; Li, P.; Sun, J.; Xing, D.; Peng, W.; Sun, F. Roles of Initial Bacterial Attachment and Growth in the Biofouling Development on the Microfiltration Membrane: From Viewpoints of Individual Cell and Interfacial Interaction Energy. J. Memb. Sci. 2021, 638, 119723. [Google Scholar] [CrossRef]
- Mario, C.-R.; D, V.E.; Camilo, M.; Jorge, F.; Manuel, D.J.; Carlos, H.J.; Mauricio, B. Isolation of a Novel Aggregatibacter Actinomycetemcomitans Serotype b Bacteriophage Capable of Lysing Bacteria within a Biofilm. Appl. Environ. Microbiol. 2011, 77, 3157–3159. [Google Scholar] [CrossRef]
- Bhowmik, P.; Rajagopal, S.; Hmar, R.V.; Singh, P.; Saxena, P.; Amar, P.; Thomas, T.; Ravishankar, R.; Nagaraj, S.; Katagihallimath, N.; et al. Validated In Silico Model for Biofilm Formation in Escherichia coli. ACS Synth. Biol. 2022, 11, 713–731. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2023, 12, 12. [Google Scholar] [CrossRef]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-Biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm Formation, and Related Impacts on Healthcare, Food Processing and Packaging, Industrial Manufacturing, Marine Industries, and Sanitation—A Review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Rho, H.; Cho, J.; Westerhoff, P.; Chon, K. Intrinsic PKa of Nanofiltration Membrane Surfaces to Assess Fouling and Cleaning Behaviors Induced by Foulant–Membrane Electrostatic Interactions. Environ. Sci. Technol. 2020, 54, 7706–7714. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, H.; You, C.; Zhou, W.; Chen, J.; Xiao, W.; Garba, Z.N.; Wang, L.; Yuan, Z. Construction of Functionalized Graphene Separation Membranes and Their Latest Progress in Water Purification. Sep. Purif. Technol. 2022, 285, 120301. [Google Scholar] [CrossRef]
- Kim, A.; Hak Kim, J.; Patel, R. Modification Strategies of Membranes with Enhanced Anti-Biofouling Properties for Wastewater Treatment: A Review. Bioresour. Technol. 2022, 345, 126501. [Google Scholar] [CrossRef] [PubMed]
- Allaoui, B.E.; Chakhtouna, H.; Zari, N.; Bouhfid, R.; Qaiss, A.E.K. Recent Developments in Functionalized Polymer NF Membranes for Biofouling Control. Emergent Mater. 2022, 5, 1345–1371. [Google Scholar] [CrossRef]
- Samree, K.; Srithai, P.; Kotchaplai, P.; Thuptimdang, P.; Painmanakul, P.; Hunsom, M.; Sairiam, S. Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles. Membranes 2020, 10, 289. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, W.N. Biofouling Formation and Structure on Original and Modified PVDF Membranes: Role of Microbial Species and Membrane Properties. RSC Adv. 2017, 7, 37990–38000. [Google Scholar] [CrossRef]
- Priya, S.; Murali, A.; Preeth, D.R.; Dharanibalaji, K.C.; Jeyajothi, G. Green Synthesis of Silver Nanoparticle-Embedded Poly(Methyl Methacrylate-Co-Methacrylic Acid) Copolymer for Fungal-Free Leathers. Polym. Bull. 2022, 79, 4607–4626. [Google Scholar] [CrossRef]
- Kim, T.; Kwon, S.; Lee, J.; Lee, J.S.; Kang, S. A Metallic Anti-Biofouling Surface with a Hierarchical Topography Containing Nanostructures on Curved Micro-Riblets. Microsyst. Nanoeng. 2022, 8, 6. [Google Scholar] [CrossRef]
- Vellwock, A.E.; Su, P.; Zhang, Z.; Feng, D.; Yao, H. Reconciling the Conflict between Optical Transparency and Fouling Resistance with a Nanowrinkled Surface Inspired by Zebrafish’s Cornea. ACS Appl. Mater. Interfaces 2022, 14, 7617–7625. [Google Scholar] [CrossRef] [PubMed]
- Pinem, J.A.; Wardani, A.K.; Aryanti, P.T.P.; Khoiruddin, K.; Wenten, I.G. Hydrophilic Modification of Polymeric Membrane Using Graft Polymerization Method: A Mini Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 12054. [Google Scholar] [CrossRef]
- Vo, T.S.; Lwin, K.M.; Kim, K. Recent Developments of Nano-Enhanced Composite Membranes Designed for Water/Wastewater Purification—A Review. Adv. Compos. Hybrid. Mater. 2024, 7, 127. [Google Scholar] [CrossRef]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-Layer Preparation of Polyelectrolyte Multilayer Membranes for Separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-Driven Layer-by-Layer Assembly of Nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.Q.; Yang, X.; Deng, H. Preparation of Layer-by-Layer Nanofiltration Membranes by Dynamic Deposition and Crosslinking. Membranes 2019, 9, 20. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and Modification of Poly(Vinylidene Fluoride) (PVDF) Membranes—A Review. J. Memb. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.; Tong, T.; Epsztein, R.; Sun, M.; Verduzco, R.; Ma, J.; Elimelech, M. Selective Removal of Divalent Cations by Polyelectrolyte Multilayer Nanofiltration Membrane: Role of Polyelectrolyte Charge, Ion Size, and Ionic Strength. J. Memb. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef]
- Shan, W.; Bacchin, P.; Aimar, P.; Bruening, M.L.; Tarabara, V.V. Polyelectrolyte Multilayer Films as Backflushable Nanofiltration Membranes with Tunable Hydrophilicity and Surface Charge. J. Memb. Sci. 2010, 349, 268–278. [Google Scholar] [CrossRef]
- Junker, M.A.; Regenspurg, J.A.; Valdes Rivera, C.I.; te Brinke, E.; de Vos, W.M. Effects of Feed Solution PH on Polyelectrolyte Multilayer Nanofiltration Membranes. ACS Appl. Polym. Mater. 2023, 5, 355–369. [Google Scholar] [CrossRef]
- Park, S.J.; Cheedrala, R.K.; Diallo, M.S.; Kim, C.; Kim, I.S.; Goddard, W.A. Nanofiltration Membranes Based on Polyvinylidene Fluoride Nanofibrous Scaffolds and Crosslinked Polyethyleneimine Networks. In Nanotechnology for Sustainable Development; Mamadou, S.D., Fromer, N.A., Jhon, M.S., Eds.; Springer International Publishing: Springer Cham, Switzerland, 2014; pp. 33–46. [Google Scholar]
- Wang, J.; Han, L.; Yang, H.; Xu, Z. liang Anti-Bacterial PVA@Ag/PVDF NF Membrane Made by Filtration-Coating plus in-Situ Reduction of NPs for Dye Removal. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134289. [Google Scholar] [CrossRef]
- Nan, Q.; Li, P.; Cao, B. Fabrication of Positively Charged Nanofiltration Membrane via the Layer-by-Layer Assembly of Graphene Oxide and Polyethylenimine for Desalination. Appl. Surf. Sci. 2016, 387, 521–528. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Gu, P.; Ng, A.J.; Wang, M.; Wei, Y.; Urban, J.J.; Mi, B. Graphene-Polyelectrolyte Multilayer Membranes with Tunable Structure and Internal Charge. Carbon. N. Y. 2020, 160, 219–227. [Google Scholar] [CrossRef]
- Jashni, E.; Hosseini, S.M.; Shabanian, M.; Sadrzadeh, M. Silane Functionalized Graphene Oxide-Bound Polyelectrolyte Layers for Producing Monovalent Cation Permselective Membranes. Sep. Purif. Technol. 2021, 278, 119583. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, Y.; Xu, J.; Guo, L.; Ding, H.; Chen, Y.; Shi, Y.; Liao, J.; Huixiang Ang, E.; Shen, Z.; et al. Enhancing Anti-Biofouling Activity in Electrodialysis by Spraying GO@Ag Nanosheets on Anion Exchange Membranes. Sep. Purif. Technol. 2025, 353, 128611. [Google Scholar] [CrossRef]
- Khajouei, M.; Najafi, M.; Jafari, S.A.; Latifi, M. Membrane Surface Modification via In Situ Grafting of GO/Pt Nanoparticles for Nitrate Removal with Anti-Biofouling Properties. Micromachines 2023, 14, 128. [Google Scholar] [CrossRef]
- Geng, C.; Zhao, F.; Wang, Q.; Zheng, S.; Liu, Y.; Niu, H.; Zhang, J.; Dong, H. Anti-Biofouling Property and Anti-Leaching Investigation of Modifier for PVDF Ultrafiltration Membrane by Incorporating Antibacterial Graphene Oxide Derivatives. J. Environ. Chem. Eng. 2022, 10, 108558. [Google Scholar] [CrossRef]
- Faria, A.F.; Perreault, F.; Elimelech, M. Elucidating the Role of Oxidative Debris in the Antimicrobial Properties of Graphene Oxide. ACS Appl. Nano Mater. 2018, 1, 1164–1174. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Kavousi Heidari, M.; Tavakoli, O. Performance Comparison of Thin-Film Nanocomposite Polyamide Nanofiltration Membranes for Heavy Metal/Salt Wastewater Treatment. J. Nanoparticle Res. 2023, 25, 77. [Google Scholar] [CrossRef]
- Murali, A.; Sampath, S.; Appukutti Achuthan, B.; Sakar, M.; Chandrasekaran, S.; Suthanthira Vanitha, N.; Joseph Bensingh, R.; Abdul Kader, M.; Jaisankar, S.N. Copper (0) Mediated Single Electron Transfer-Living Radical Polymerization of Methyl Methacrylate: Functionalized Graphene as a Convenient Tool for Radical Initiator. Polymers 2020, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, H.; Zhao, H.; Cheng, F.; Li, J. Enhanced Water Permeability and Antifouling Properties of Cross-Linked Graphene Oxide Composite Membranes with Tunable d-Spacings. J. Memb. Sci. 2024, 708, 123044. [Google Scholar] [CrossRef]
- Huang, H.; Song, Z.; Wei, N.; Shi, L.; Mao, Y.; Ying, Y.; Sun, L.; Xu, Z.; Peng, X. Ultrafast Viscous Water Flow through Nanostrand-Channelled Graphene Oxide Membranes. Nat. Commun. 2013, 4, 2979. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective Ion Penetration of Graphene Oxide Membranes. ACS Nano 2013, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.-M.; Ren, W. Highly Stable Graphene-Oxide-Based Membranes with Superior Permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Ali, A.; Rehman, F.; Ali Khan, M.; Memon, F.H.; Soomro, F.; Iqbal, M.; Yang, J.; Thebo, K.H. Functionalized Graphene Oxide-Based Lamellar Membranes with Tunable Nanochannels for Ionic and Molecular Separation. ACS Omega 2022, 7, 32410–32417. [Google Scholar] [CrossRef]
- Zubair, M.; Farooq, S.; Hussain, A.; Riaz, S.; Ullah, A. A Review of Current Developments in Graphene Oxide–Polysulfone Derived Membranes for Water Remediation. Environ. Sci. Adv. 2024, 3, 983–1003. [Google Scholar] [CrossRef]
- Islam, S.S.; Jose, T.; Seikh, A.H.; Karim, M.R.; Alnaser, I.A.; Bose, S. Shear-Aligned Graphene Oxide Nanosheets Incorporated PVDF Composite Membranes for Selective Dye Rejection with High Water Flux. RSC Adv. 2024, 14, 27852–27861. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Venault, A.; Huang, Y.-T.; Wu, B.-W.; Chou, C.-J.; Ishihara, K.; Chang, Y. Toward Antibiofouling PVDF Membranes. Langmuir 2019, 35, 6782–6792. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP Nanofibrous Mats with Antibacterial and Anti-Biofouling Properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, D.; Sun, X.; Jiang, S.; Liu, Y.; Zhang, D.; Zhang, L. Surface Modified by Green Synthetic of Cu-MOF-74 to Improve the Anti-Biofouling Properties of PVDF Membranes. Chem. Eng. J. 2021, 411, 128524. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Y.; Feng, X.; Bi, S.; Ding, W.; Chen, L. Improved Antifouling Properties of PVDF Membranes Modified with Oppositely Charged Copolymer. Biofouling 2013, 29, 331–343. [Google Scholar] [CrossRef]
- Wang, C.; Park, M.J.; Seo, D.H.; Phuntsho, S.; Gonzales, R.R.; Matsuyama, H.; Drioli, E.; Shon, H.K. Inkjet Printed Polyelectrolyte Multilayer Membrane Using a Polyketone Support for Organic Solvent Nanofiltration. J. Memb. Sci. 2022, 642, 119943. [Google Scholar] [CrossRef]
- Rodríguez, B.; Oztürk, D.; Rosales, M.; Flores, M.; García, A. Antibiofouling Thin-Film Composite Membranes (TFC) by in Situ Formation of Cu-(m-Phenylenediamine) Oligomer Complex. J. Mater. Sci. 2018, 53, 6325–6338. [Google Scholar] [CrossRef]
- Rodríguez, B.E.; Armendariz-Ontiveros, M.M.; Quezada, R.; Huitrón-Segovia, E.A.; Estay, H.; García García, A.; García, A. Influence of Multidimensional Graphene Oxide (GO) Sheets on Anti-Biofouling and Desalination Performance of Thin-Film Composite Membranes: Effects of GO Lateral Sizes and Oxidation Degree. Polymers 2020, 12, 2860. [Google Scholar] [CrossRef]
- Reurink, D.M.; Willott, J.D.; Roesink, H.D.W.; de Vos, W.M. Role of Polycation and Cross-Linking in Polyelectrolyte Multilayer Membranes. ACS Appl. Polym. Mater. 2020, 2, 5278–5289. [Google Scholar] [CrossRef]
- Jiang, B.-B.; Sun, X.-F.; Wang, L.; Wang, S.-Y.; Liu, R.-D.; Wang, S.-G. Polyethersulfone Membranes Modified with D-Tyrosine for Biofouling Mitigation: Synergistic Effect of Surface Hydrophility and Anti-Microbial Properties. Chem. Eng. J. 2017, 311, 135–142. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; Yang, J.; Ma, A. Advancing Strategies of Biofouling Control in Water-Treated Polymeric Membranes. Polymers 2022, 14, 1167. [Google Scholar] [CrossRef]
- Fontananova, E.; Bahattab, M.A.; Aljlil, S.A.; Alowairdy, M.; Rinaldi, G.; Vuono, D.; Nagy, J.B.; Drioli, E.; Di Profio, G. From Hydrophobic to Hydrophilic Polyvinylidenefluoride (PVDF) Membranes by Gaining New Insight into Material’s Properties. RSC Adv. 2015, 5, 56219–56231. [Google Scholar] [CrossRef]
- Choi, W.; Gu, J.-E.; Park, S.-H.; Kim, S.; Bang, J.; Baek, K.-Y.; Park, B.; Lee, J.S.; Chan, E.P.; Lee, J.-H. Tailor-Made Polyamide Membranes for Water Desalination. ACS Nano 2015, 9, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ghasemi, H. Hydrophilic Polymer-Based Anti-Biofouling Coatings: Preparation, Mechanism, and Durability. Adv. Colloid. Interface Sci. 2020, 284, 102264. [Google Scholar] [CrossRef]
- Freitas de Oliveira, F.; Schneider, R.P. Slow Sand Filtration for Biofouling Reduction in Seawater Desalination by Reverse Osmosis. Water Res. 2019, 155, 474–486. [Google Scholar] [CrossRef]
- Miao, A.; Wei, M.; Xu, F.; Wang, Y. Influence of Membrane Hydrophilicity on Water Permeability: An Experimental Study Bridging Simulations. J. Memb. Sci. 2020, 604, 118087. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Giraldo, H.; Quintero, Y.; Quezada, R.; Hassan, N.; Estay, H. Copper-Modified Polymeric Membranes for Water Treatment: A Comprehensive Review. Membranes 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Rehl, B.; Ma, E.; Parshotam, S.; DeWalt-Kerian, E.L.; Liu, T.; Geiger, F.M.; Gibbs, J.M. Water Structure in the Electrical Double Layer and the Contributions to the Total Interfacial Potential at Different Surface Charge Densities. J. Am. Chem. Soc. 2022, 144, 16338–16349. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Oztürk, D.; Rosales, M.; Diaz, D.I.; Mautner, A. Incorporation of CuO Nanoparticles into Thin-Film Composite Reverse Osmosis Membranes (TFC-RO) for Antibiofouling Properties. Polym. Bull. 2018, 75, 2053–2069. [Google Scholar] [CrossRef]
- Huang, S.-H.; Lin, G.-L.; Gallardo, M.R.; Chu, Y.-T.; Wang, C.-H.; Millare, J.C.; Lee, K.-R. Incorporation of Graphene Oxide in the Layer-by-Layer Self-Assembly of Polyacrylic Acid and Poly(Diallyldimethylammonium Chloride) to Fabricate Nanocomposite Membrane for Forward Osmosis. Mater. Chem. Phys. 2024, 315, 128989. [Google Scholar] [CrossRef]
- Avila-Novoa, M.-G.; Iñíguez-Moreno, M.; Solís-Velázquez, O.-A.; González-Gómez, J.-P.; Guerrero-Medina, P.-J.; Gutiérrez-Lomelí, M. Biofilm Formation by Staphylococcus aureus Isolated from Food Contact Surfaces in the Dairy Industry of Jalisco, Mexico. J. Food Qual. 2018, 2018, 1746139. [Google Scholar] [CrossRef]
- Feng, Y.; Ming, T.; Zhou, J.; Lu, C.; Wang, R.; Su, X. The Response and Survival Mechanisms of Staphylococcus aureus under High Salinity Stress in Salted Foods. Foods 2022, 11, 1503. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia Coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on SEM and VP-SEM Pros and Cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef]
- Ma, G.; Xu, X.; Tesfai, M.; Wang, H.; Xu, P. Developing Anti-Biofouling and Energy-Efficient Cation-Exchange Membranes Using Conductive Polymers and Nanomaterials. J. Memb. Sci. 2020, 603, 118034. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; An, J.; Fu, H.; Wu, X.; Pang, C.; Gao, H. Construction of an Antibacterial Membrane Based on Dopamine and Polyethylenimine Cross-Linked Graphene Oxide. ACS Biomater. Sci. Eng. 2019, 5, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Lu, X.; Cheng, W.; Zhou, X.; Bernstein, R.; Zhang, W.; Kimura, K.; Elimelech, M. Photografting Graphene Oxide to Inert Membrane Materials to Impart Antibacterial Activity. Environ. Sci. Technol. Lett. 2019, 6, 141–147. [Google Scholar] [CrossRef]
- Kafil, V.; Omidi, Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. Bioimpacts 2011, 1, 23–30. [Google Scholar] [CrossRef]
- Florea, B.I.; Meaney, C.; Junginger, H.E.; Borchard, G. Transfection Efficiency and Toxicity of Polyethylenimine in Differentiated Calu-3 and Nondifferentiated COS-1 Cell Cultures. AAPS PharmSci 2015, 4, 12. [Google Scholar] [CrossRef]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(Ethylene Imine)s as Antimicrobial Agents with Selective Activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef]
- Venault, A.; Yang, H.-S.; Chiang, Y.-C.; Lee, B.-S.; Ruaan, R.-C.; Chang, Y. Bacterial Resistance Control on Mineral Surfaces of Hydroxyapatite and Human Teeth via Surface Charge-Driven Antifouling Coatings. ACS Appl. Mater. Interfaces 2014, 6, 3201–3210. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G.A. A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes 2022, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial Susceptibility Testing in Biofilm-Growing Bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed]

| Staphylococcus aureus | Escherichia coli | |
|---|---|---|
| Inoculum | Log cfu/mL | |
| 5.75 | 5.29 | |
| PVDF membrane | ||
| Contact time | Mean log cfu/cm2 | |
| 0 h | 5.17 | 4.8 |
| 24 h | 5.02 | 4.9 |
| (PAA-PEI)3/PVDF membrane | ||
| Mean log cfu/cm2 | ||
| Contact time 24 h | 1.29 | 3.75 |
| Microbial kill (% reduction) | 99.98% | 93.05 |
| (GO + PAA-PEI)3/PVDF membrane | ||
| Mean log cfu/cm2 | ||
| Contact time 24 h | 1.32 | 4.42 |
| Microbial kill (% reduction) | 99.98% | 67.42% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Ruiz, M.; Negrete, C.; Espinoza, J.P.; Martínez, I.; Daille, L.K.; González, C.; Rodríguez, B. Antibiofilm Effects of Modifying Polyvinylidene Fluoride Membranes with Polyethylenimine, Poly(acrylic acid) and Graphene Oxide. Polymers 2024, 16, 3418. https://doi.org/10.3390/polym16233418
Castillo-Ruiz M, Negrete C, Espinoza JP, Martínez I, Daille LK, González C, Rodríguez B. Antibiofilm Effects of Modifying Polyvinylidene Fluoride Membranes with Polyethylenimine, Poly(acrylic acid) and Graphene Oxide. Polymers. 2024; 16(23):3418. https://doi.org/10.3390/polym16233418
Chicago/Turabian StyleCastillo-Ruiz, Mario, Constanza Negrete, Juan Pablo Espinoza, Iván Martínez, Leslie K. Daille, Christopher González, and Bárbara Rodríguez. 2024. "Antibiofilm Effects of Modifying Polyvinylidene Fluoride Membranes with Polyethylenimine, Poly(acrylic acid) and Graphene Oxide" Polymers 16, no. 23: 3418. https://doi.org/10.3390/polym16233418
APA StyleCastillo-Ruiz, M., Negrete, C., Espinoza, J. P., Martínez, I., Daille, L. K., González, C., & Rodríguez, B. (2024). Antibiofilm Effects of Modifying Polyvinylidene Fluoride Membranes with Polyethylenimine, Poly(acrylic acid) and Graphene Oxide. Polymers, 16(23), 3418. https://doi.org/10.3390/polym16233418






