Study on the Mechanical and Thermal Properties and Shape Memory Behaviors of Blends of Bio-Based Polybenzoxazine and Polycaprolactone with Different Molecular Weights
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Preparation of Bio-Based Benzoxazine Monomers and PCL/V-fa Blends
2.3. Characterizations
3. Results and Discussion
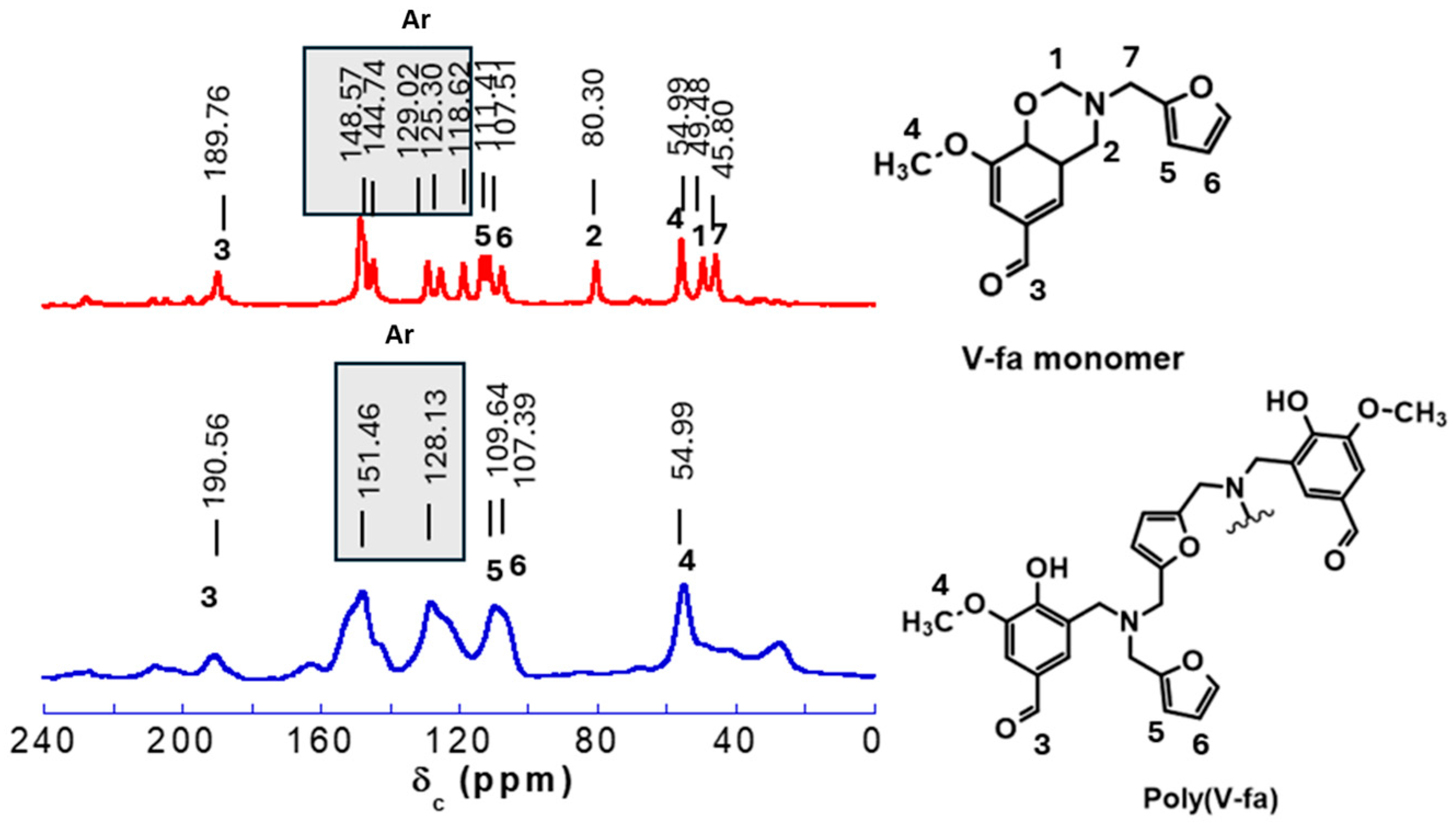
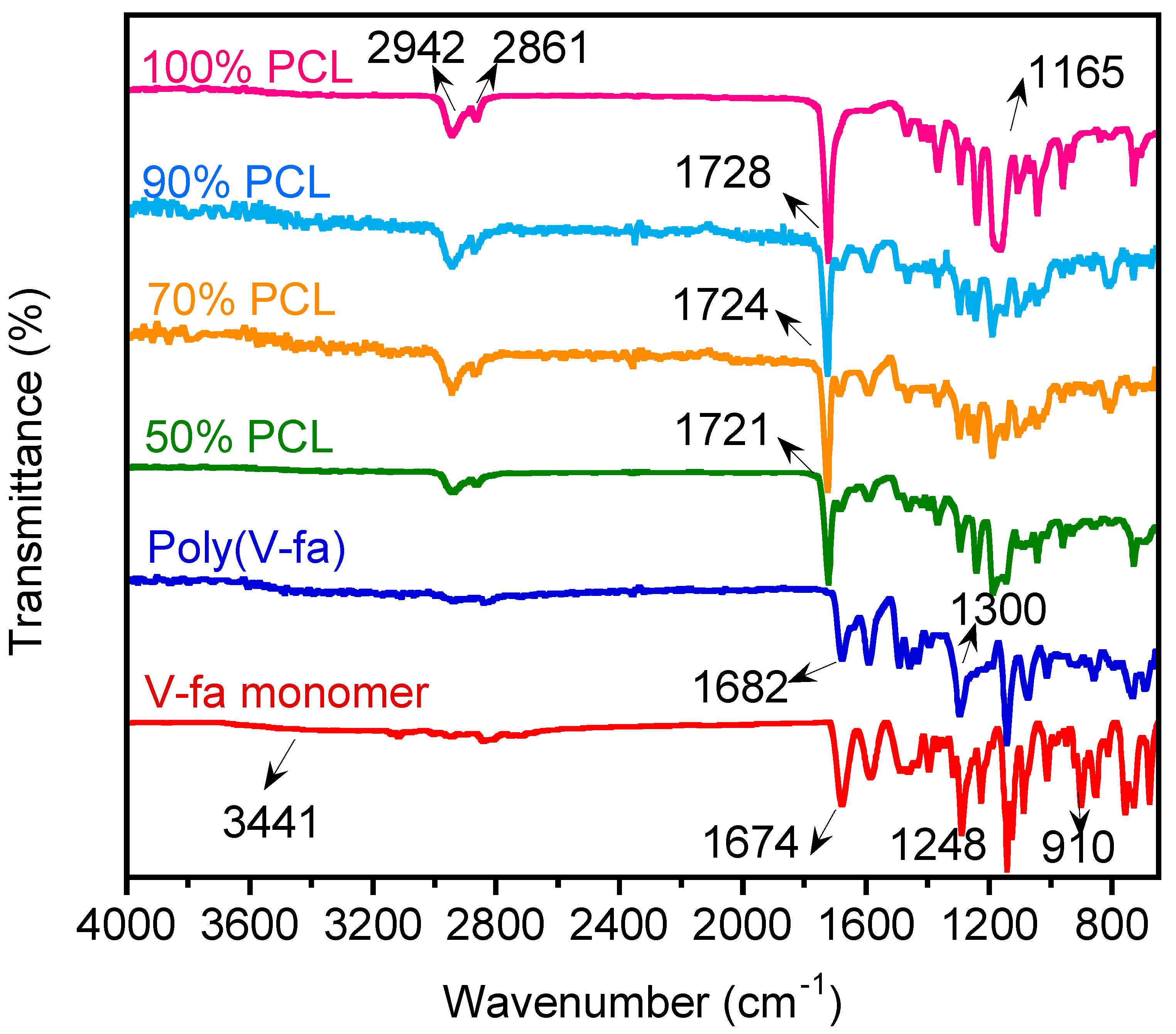
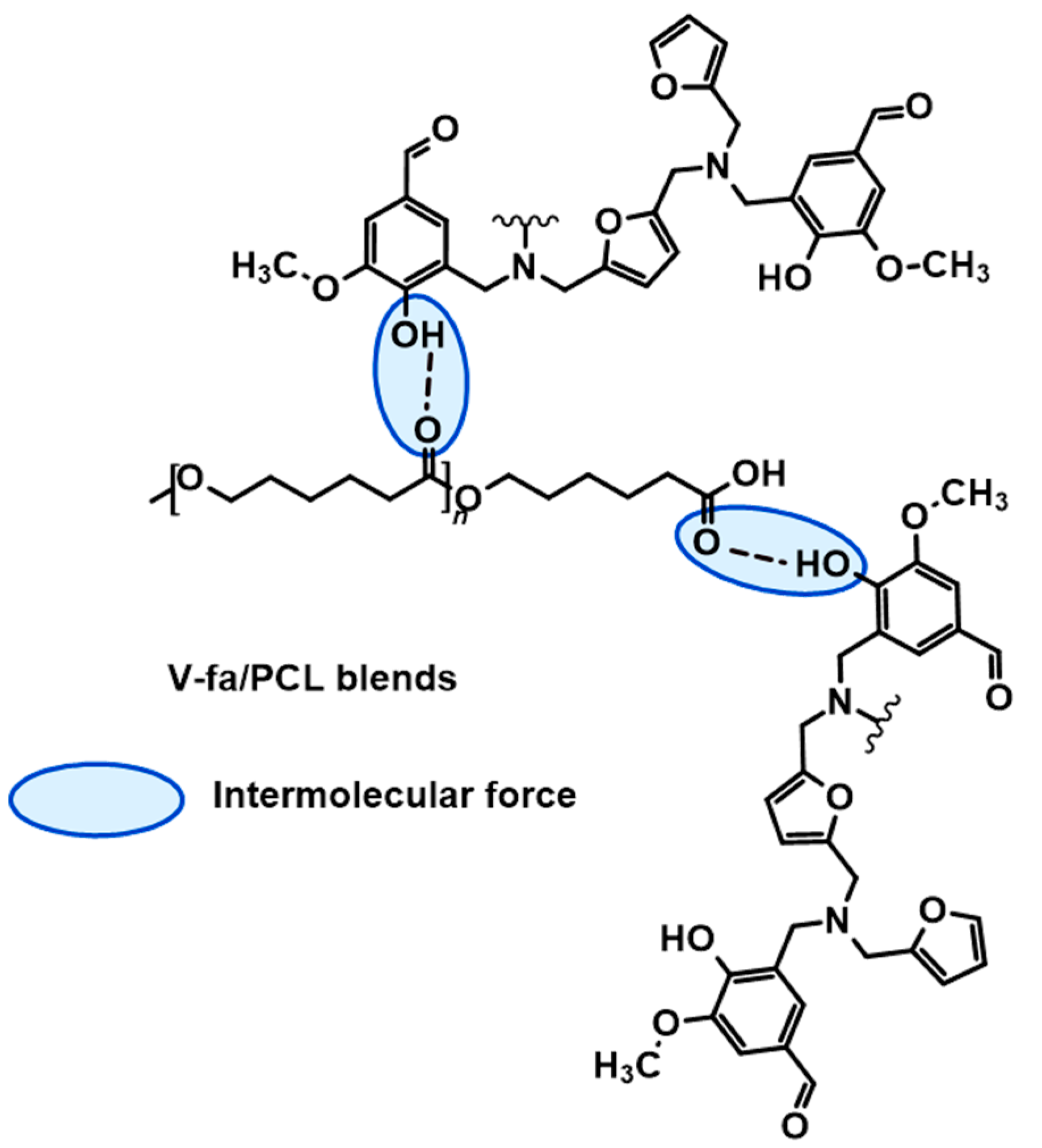
3.1. Investigation of Chemical Structures
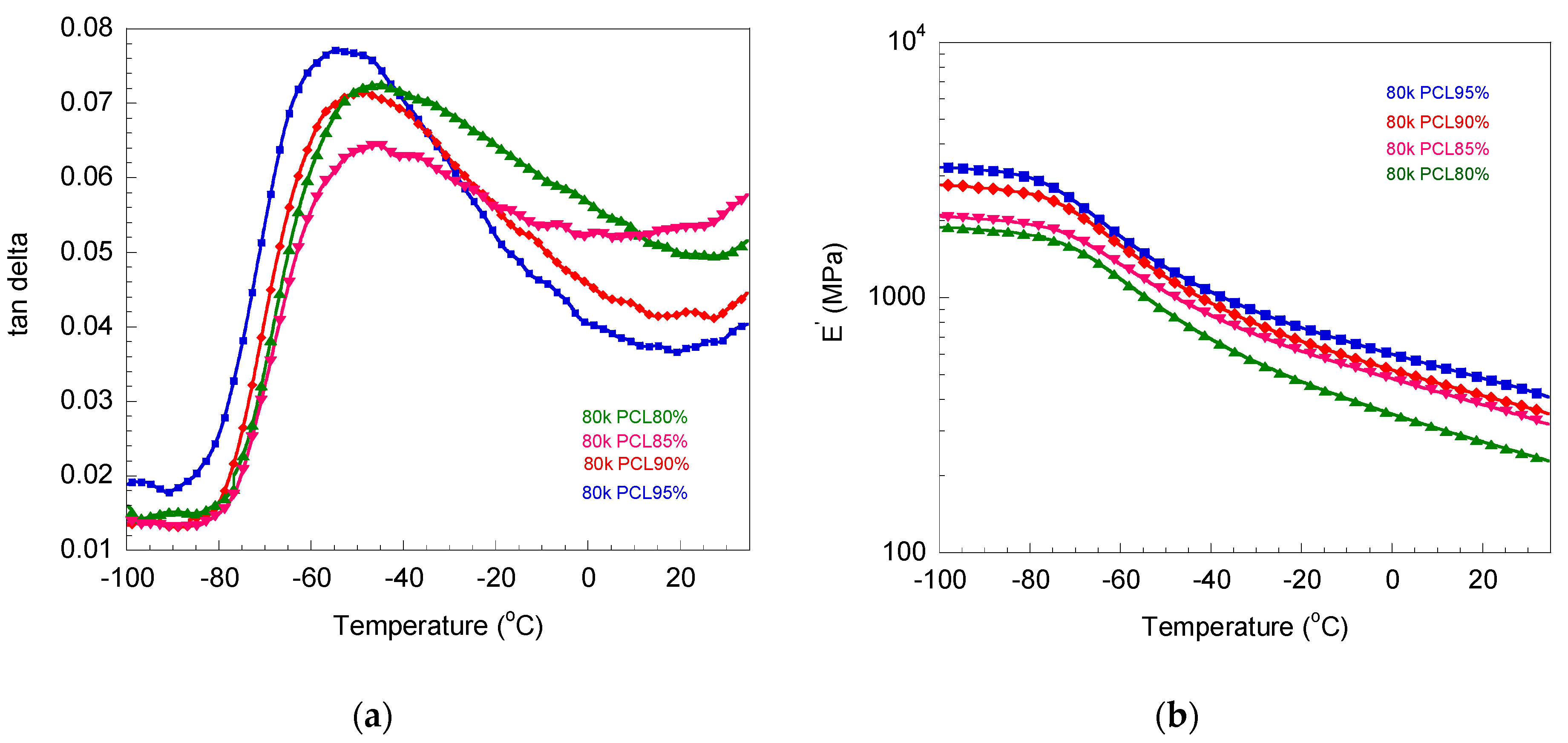
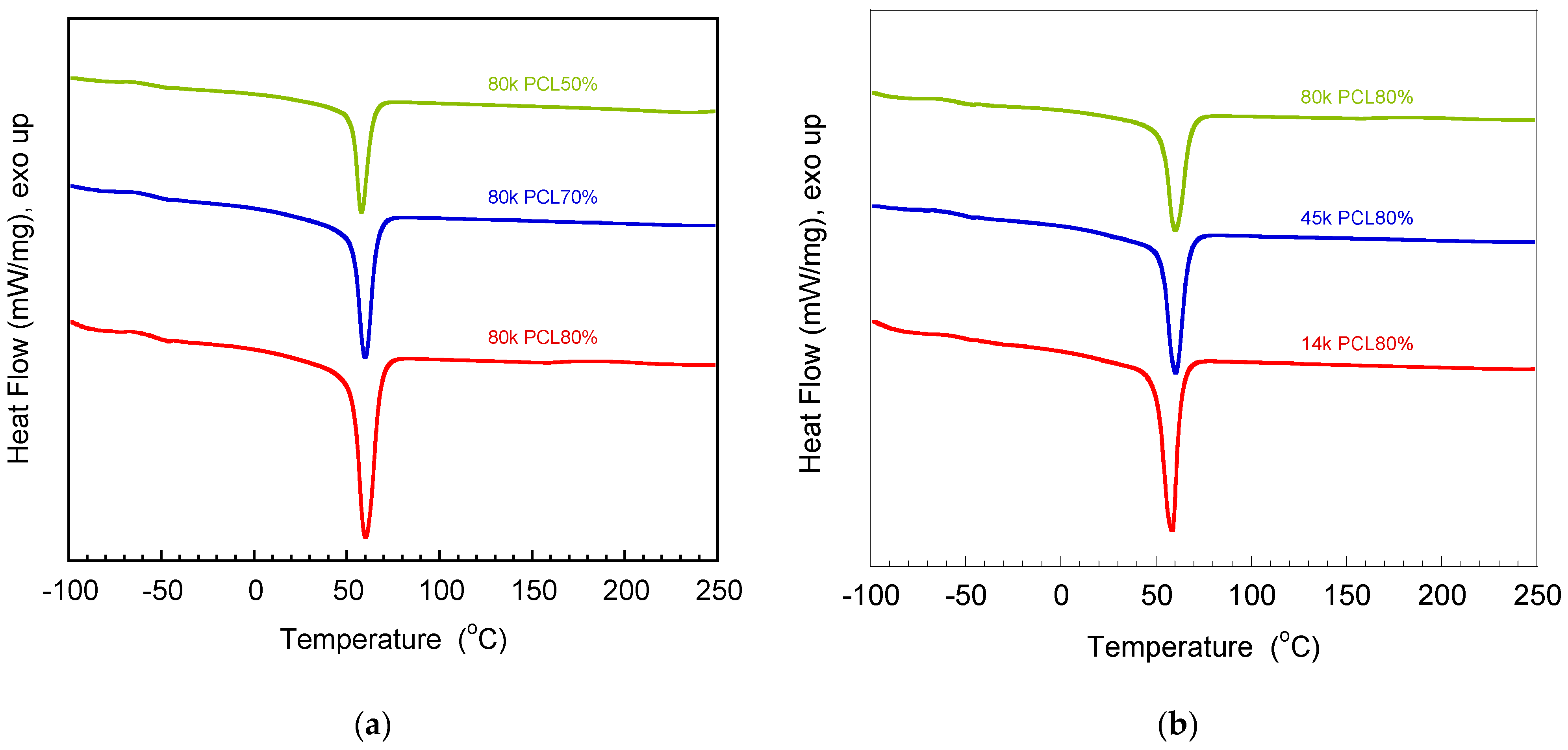
3.2. Determination of Glass Transition Temperature and Melting Temperature
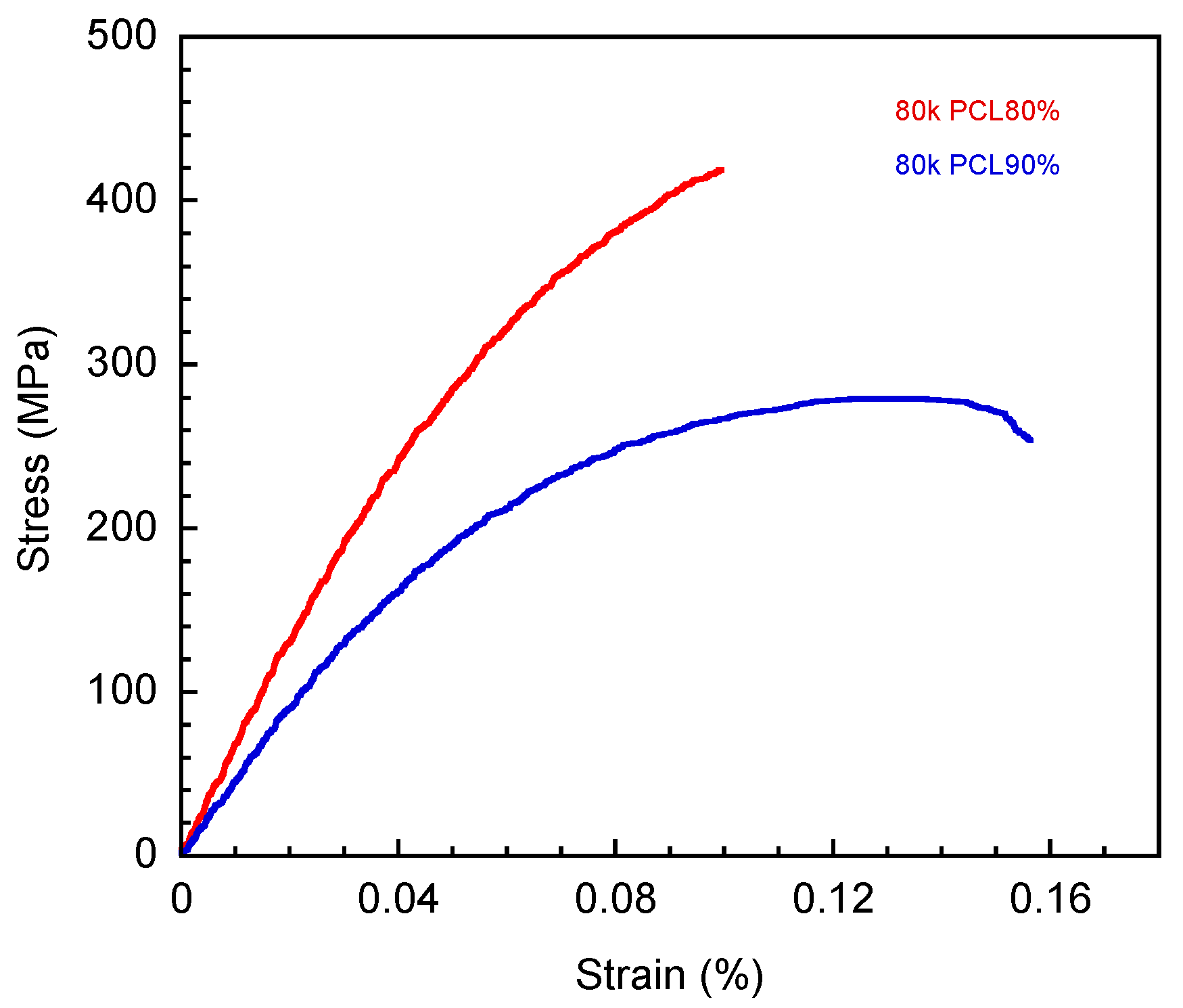
3.3. Mechanical Properties of V-fa/PCL Blends
3.4. Determination of Thermal Stability of V-fa/PCL Blends
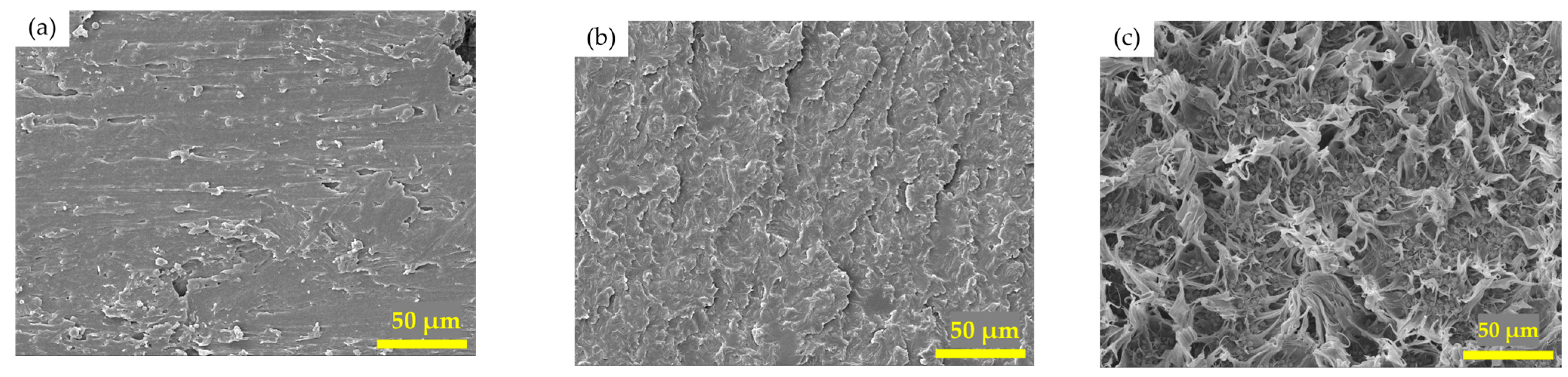
3.5. Morphology of Fracture Surface of V-fa/PCL Blends
3.6. Bendability of V-fa/PCL Blends
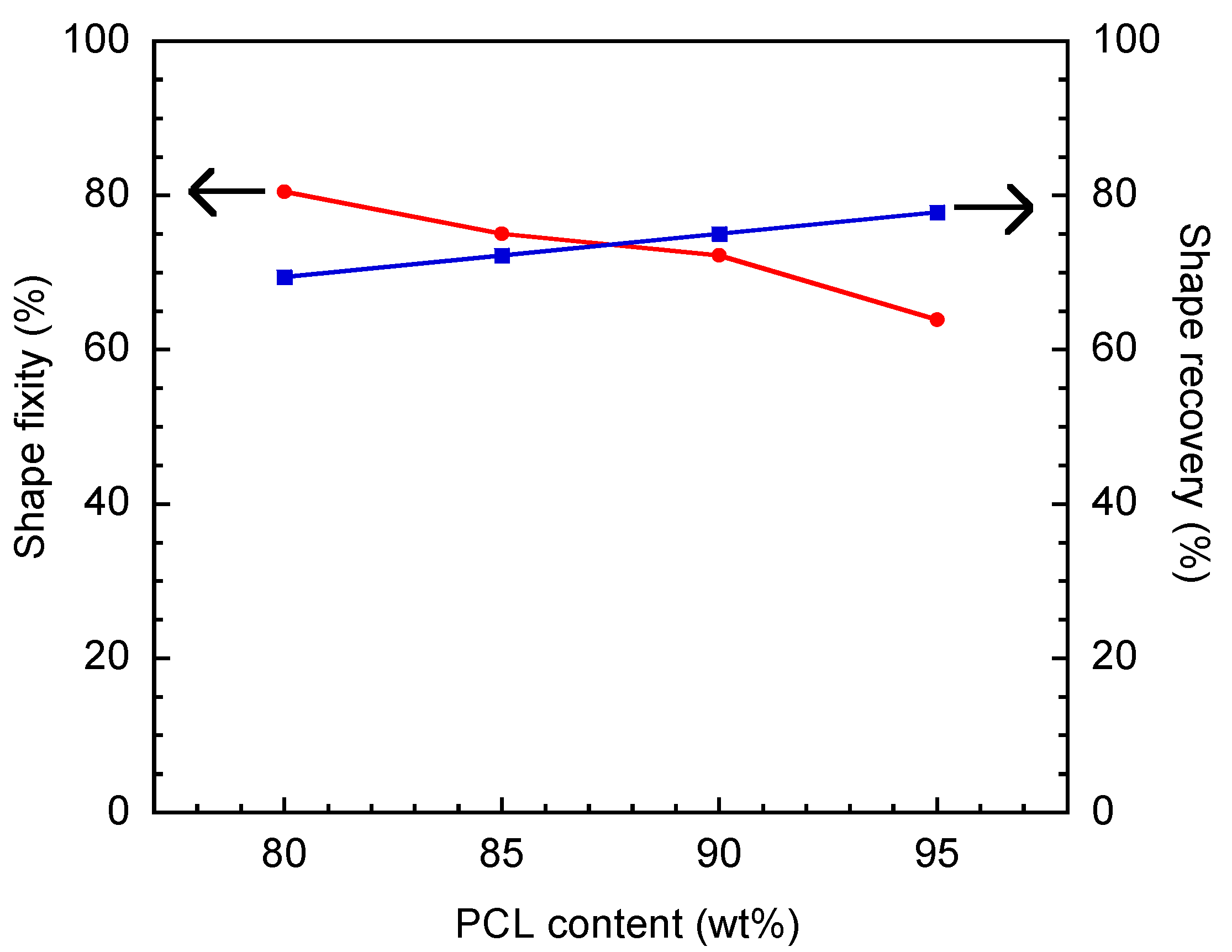
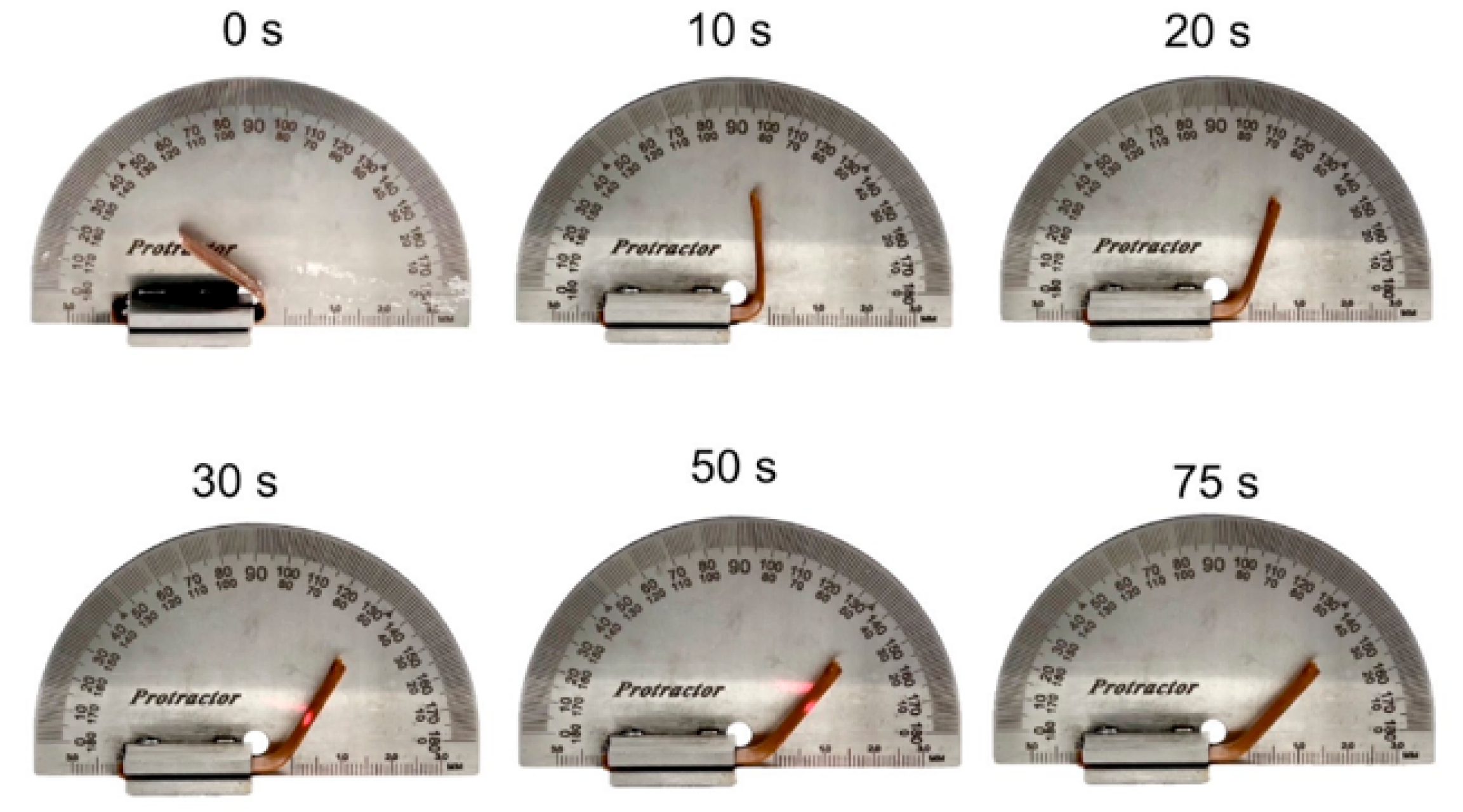
3.7. Shape Memory Behaviors of V-fa/PCL Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishida, H.; Rodriguez, Y. Curing kinetics of a new benzoxazine-based phenolic resin by differential scanning calorimetry. Polymer 1995, 36, 3151–3158. [Google Scholar] [CrossRef]
- Ning, X.; Ishida, H. J Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol-A based benzoxazines and their polymers. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.H. Synergism observed in polybenzoxazine and poly(ε-caprolactone) blends by dynamic mechanical and thermogravimetric analysis. Polymer 2001, 42, 6971. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.H. Study of hydrogen bonding and thermal properties of polybenzoxazine and poly-(e-caprolactone) blends. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 736. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yang, S.-J. Studying the miscibility and thermal behavior of polybenzoxazine/poly(ε caprolactone) blends using DSC, DMA, and solid state 13C NMR spectroscopy. Polymer 2005, 46, 8068. [Google Scholar] [CrossRef]
- Tiptipakorn, S.; Angkanawarangkana, C.; Rimdusit, S.; Hemvichian, K.; Lertsarawut, P. Investigation of Multiple Shape Memory Behaviors, Thermal and Physical Properties of Benzoxazine Blended with Diamino Polysiloxane. Polymers 2023, 15, 3814. [Google Scholar] [CrossRef]
- Morris, B.A. Encyclopedia of Renewable and Sustainable Materials, 2020 in The Science and Technology of Flexible Packaging, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2022. [Google Scholar]
- Prasomsin, W.; Parnklang, T.; Sapcharoenkun, C.; Tiptipakorn, S.; Rimdusit, S. Multiwalled carbon nanotube reinforced bio-based benzoxazine/epoxy composites with NIR-laser stimulated shape memory effects. Nanomaterials 2019, 9, 881. [Google Scholar] [CrossRef]
- Ruenpanya, K.; Mora, P.; Karagiannidis, P.; Bunyanuwat, K.; Rimdusit, S. Magnetic-responsive triple shape memory polymer from bio-based benzoxazine/urethane polymer alloys with iron oxide nanoparticles. Adv. Ind. Eng. Polym. Res. 2024, in press. [Google Scholar] [CrossRef]
- Joseph, A.; Lawan, I.; Charoensuk, K.; Luengrojanakul, P.; Mora, P.; Ahn, C.-H.; Rimdusit, S. Development of a magnified sunlight responsive shape memory bio-composite: Effects of titanium nitride (TiN) nanoparticles on a bio-based benzoxazine/epoxy copolymer. Nanoscale Adv. 2024, 6, 4407. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634. [Google Scholar] [CrossRef]
- Schäfer, H.; Hartwig, A.; Kascheck, K. The nature of bonding matters: Benzoxazine based shape memory polymers. Polymers 2018, 135, 285. [Google Scholar] [CrossRef]
- Takeichi, T.; Kawauchi, T. Molecular Design of polybenzoxazine: A Novel Phenolic Resin. J. Syn. Org. Chem. Jpn. 2010, 68, 136–142. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jubsilp, C.; Tiptipakorn, S. Alloys and Composites of Polybenzoxazines: Properties and Applications; Springer: New York, NY, USA, 2013. [Google Scholar]
- Tiptipakorn, S.; Keungputpong, N.; Phothiphiphit, S.; Rimdusit, S. Effects of polycaprolactone molecular weights on thermal and mechanical properties of polybenzoxazine. J. Appl. Polym. Sci. 2015, 132, 41915. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems: Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743. [Google Scholar] [CrossRef]
- Rimdusit, S.; Lohwerathama, M.; Hemvichian, K.; Kasemsiri, P.; Dueramae, I. Shape memory polymers from benzoxazine-modified epoxy. Smart Mater. Struct. 2013, 22, 75033. [Google Scholar] [CrossRef]
- Prathumrat, P.; Tiptipakorn, S.; Rimdusit, S. Multiple-shape memory polymers from benzoxazine–urethane copolymers. Smart Mater. Struct. 2017, 26, 65025. [Google Scholar] [CrossRef]
- Likitaporn, C.; Mora, P.; Tiptipakorn, S.; Rimdusit, S. Recovery stress enhancement in shape memory composites from silicon carbide whisker-filled benzoxazine-epoxy polymer alloy. J. Intell. Mater. Syst. Struct. 2018, 29, 388–396. [Google Scholar] [CrossRef]
- Akabori, S.; Habata, Y.; Nakazawa, M.; Yamada, Y.; Shindo, Y.; Sugimura, T.; Sato, S. The novel syntheses of photoreversible cyclobutanocrown ethers by the intramolecular photoaddition of α, ω-dicinnamoyl polyethylene glycol derivatives. Chem. Chem. Ind. Bull. Chem. Soc. Jpn. 1987, 60, 3453. [Google Scholar] [CrossRef]
- Camacho-Lopez, M.; Finkelmann, H.; Palffy-Muhoray, P.; Shelley, M. Fast liquid-crystal elastomer swims into the dark. Nat. Mater. 2004, 3, 307. [Google Scholar] [CrossRef]
- Finkelmann, H.; Nishikawa, E.; Pereira, G.G.; Warner, M. A New Opto-Mechanical Effect in Solids. Phys. Rev. Lett. 2001, 87, 015501. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.; Jünger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Honda, K. Photoreversible reactions of polymers containing cinnamylidene acetate derivatives and the model compounds. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 2685. [Google Scholar] [CrossRef]
- Han, X.J.; Dong, Z.Q.; Fan, M.M.; Liu, Y.; Li, J.H.; Wang, Y.F.; Yuan, Q.J.; Li, B.J.; Zhang, S. pH-Induced Shape-Memory Polymers. Macromol. Rapid Commun. 2012, 33, 1055. [Google Scholar] [CrossRef] [PubMed]
- Yabe, H.; Fujii, R.; Nishi, Y.; Oguri, K.; Uchida, H.-H.; Matsumura, Y.; Uchida, H. Magnetic field induced shape memory effect of Fe-Pd alloy thin film. J. Adv. Sci. 2000, 12, 44. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Sanada, M.; Matsumoto, M.; Itagaki, K. Magnetic-field induced shape memory effect in Ni2MnGa sputtered films. Mater. Sci. Eng. A 2004, 378, 377. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1661. [Google Scholar] [CrossRef]
- Basit, A.; L’Hostis, G.; Durand, B. Multi-shape memory effect in shape memory polymer composites. Mater. Lett. 2012, 74, 220. [Google Scholar] [CrossRef]
- Tiptipakorn, S.; Rimdusit, S. Shape Memory Polymers from Polybenzoxazine-Modified Polymers. Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; p. 1029. [Google Scholar]
- Gu, S.; Jana, S. Effects of polybenzoxazine on shape memory properties of polyurethane with amorphous and crystalline with segments. Polymer 2014, 6, 1008. [Google Scholar] [CrossRef]
- Luo, L.; Niu, Z.; Hu, R.; Zhang, R.; Zhang, F.; Liu, Y.; Leng, J. Triple-shape memory polybenzoxazine resins and their composites. Compos. A Appl. Sci. Manuf. 2024, 177, 107910. [Google Scholar] [CrossRef]
- Sini, N.K.; Bijwe, J.; Varma, I.K. Renewable Benzoxazine Monomer from Vanillin: Synthesis, Characterization, and Studies on Curing Behavior. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 7–11. [Google Scholar] [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344. [Google Scholar] [CrossRef]
- Petrakova, V.V.; Kireev, V.V.; Onuchin, D.V.; Sarychev, I.A.; Shutov, V.V.; Kuzmich, A.A.; Bornosuz, N.V.; Gorlov, M.V.; Pavov, N.V.; Shapagin, A.V.; et al. Benzoxazine Monomers and Polymers Based on 3,3’-Dichloro-4,4’-Diaminodiphenylmethane: Synthesis and Characterization. Polymers 2021, 13, 1421. [Google Scholar] [CrossRef] [PubMed]
- Thirukumaran, P.; Parveen, A.-S.; Sarojadevi, M. Synthesis and copolymerization of fully biobased benzoxazines from renewable resources. ACS Sustain. Chem. Eng. 2014, 2, 2790. [Google Scholar] [CrossRef]
- Razavi, M.; Wang, S.-Q. Why is crystalline poly(lactic acid) brittle at room temperature? Macromolecules 2019, 52, 5429. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Tan, H.; Du, X. Thermal, mechanical and shape memory properties of shape memory epoxy resin. Mater. Sci. Eng. A. 2010, 527, 2510. [Google Scholar] [CrossRef]
- Tiptipakorn, S.; Rimdusit, S. Miscellaneous Studies on Epoxy/Synthetic Fiber Composites. Handbook of Epoxy/Fiber Composites; Springer Nature: Singapore, 2022; p. 253. [Google Scholar]



| Sample | Flexural Modulus (MPa) | Flexural Strength (MPa) |
|---|---|---|
| 14 k PCL 90% | 1629 ± 482 | 8.9 ± 1.9 |
| 45 k PCL 85% | 1327 ± 176 | 16.0 ± 4.3 |
| 45 k PCL 90% | 1312 ± 185 | 14.4 ± 1.9 |
| 45 k PCL 100% | 1249 ± 266 | 10.7 ± 0.4 |
| 80 k PCL 90% | 1005 ± 122 | 53.2 ± 5.1 |
| 80 k PCL 100% | 870 ± 162 | 38.4 ± 6.3 |
| Cycle Number | Shape Recovery Ratio (%) | Recovery Time (Seconds) | ||||||
|---|---|---|---|---|---|---|---|---|
| Polycaprolactone Content (%) | Polycaprolactone Content (%) | |||||||
| 80 | 85 | 90 | 95 | 80 | 85 | 90 | 95 | |
| 1 | 69 | 72 | 75 | 78 | 180 | 175 | 150 | 75 |
| 2 | 64 | 67 | 67 | 67 | 185 | 180 | 160 | 90 |
| 3 | 61 | 61 | 64 | 67 | 195 | 180 | 175 | 160 |
| 4 | 61 | 61 | 64 | 67 | 220 | 185 | 180 | 160 |
| 5 | 61 | 61 | 61 | 67 | 225 | 185 | 180 | 170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiptipakorn, S.; Chaipakdee, N.; Rimdusit, S.; Hemvichian, K.; Lertsarawut, P. Study on the Mechanical and Thermal Properties and Shape Memory Behaviors of Blends of Bio-Based Polybenzoxazine and Polycaprolactone with Different Molecular Weights. Polymers 2024, 16, 3391. https://doi.org/10.3390/polym16233391
Tiptipakorn S, Chaipakdee N, Rimdusit S, Hemvichian K, Lertsarawut P. Study on the Mechanical and Thermal Properties and Shape Memory Behaviors of Blends of Bio-Based Polybenzoxazine and Polycaprolactone with Different Molecular Weights. Polymers. 2024; 16(23):3391. https://doi.org/10.3390/polym16233391
Chicago/Turabian StyleTiptipakorn, Sunan, Naritsara Chaipakdee, Sarawut Rimdusit, Kasinee Hemvichian, and Pattra Lertsarawut. 2024. "Study on the Mechanical and Thermal Properties and Shape Memory Behaviors of Blends of Bio-Based Polybenzoxazine and Polycaprolactone with Different Molecular Weights" Polymers 16, no. 23: 3391. https://doi.org/10.3390/polym16233391
APA StyleTiptipakorn, S., Chaipakdee, N., Rimdusit, S., Hemvichian, K., & Lertsarawut, P. (2024). Study on the Mechanical and Thermal Properties and Shape Memory Behaviors of Blends of Bio-Based Polybenzoxazine and Polycaprolactone with Different Molecular Weights. Polymers, 16(23), 3391. https://doi.org/10.3390/polym16233391







