Enhancing Starch Film Properties Using Bacterial Nanocellulose-Stabilized Pickering Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Colloidal Suspensions of Bacterial Cellulose Nanofibrils (BCNFs)
2.3. Preparation of Pickering Emulsions
2.4. Incorporation of Pickering Emulsions into Starch Films
2.5. Conductometric Titration and Zeta Potential of BCNFs
2.6. Transmission Electronic Microscopy (TEM)
2.7. Average Particle Diameter in the Emulsion
2.8. Micrographs of Starch Films
2.9. Water Vapor Permeability (WVP)
2.10. Water Contact Angle
2.11. Fourier-Transform Infrared Spectroscopy (FTIR)
2.12. Tensile Tests of Starch Films
2.13. Statistical Analysis
3. Results and Discussion
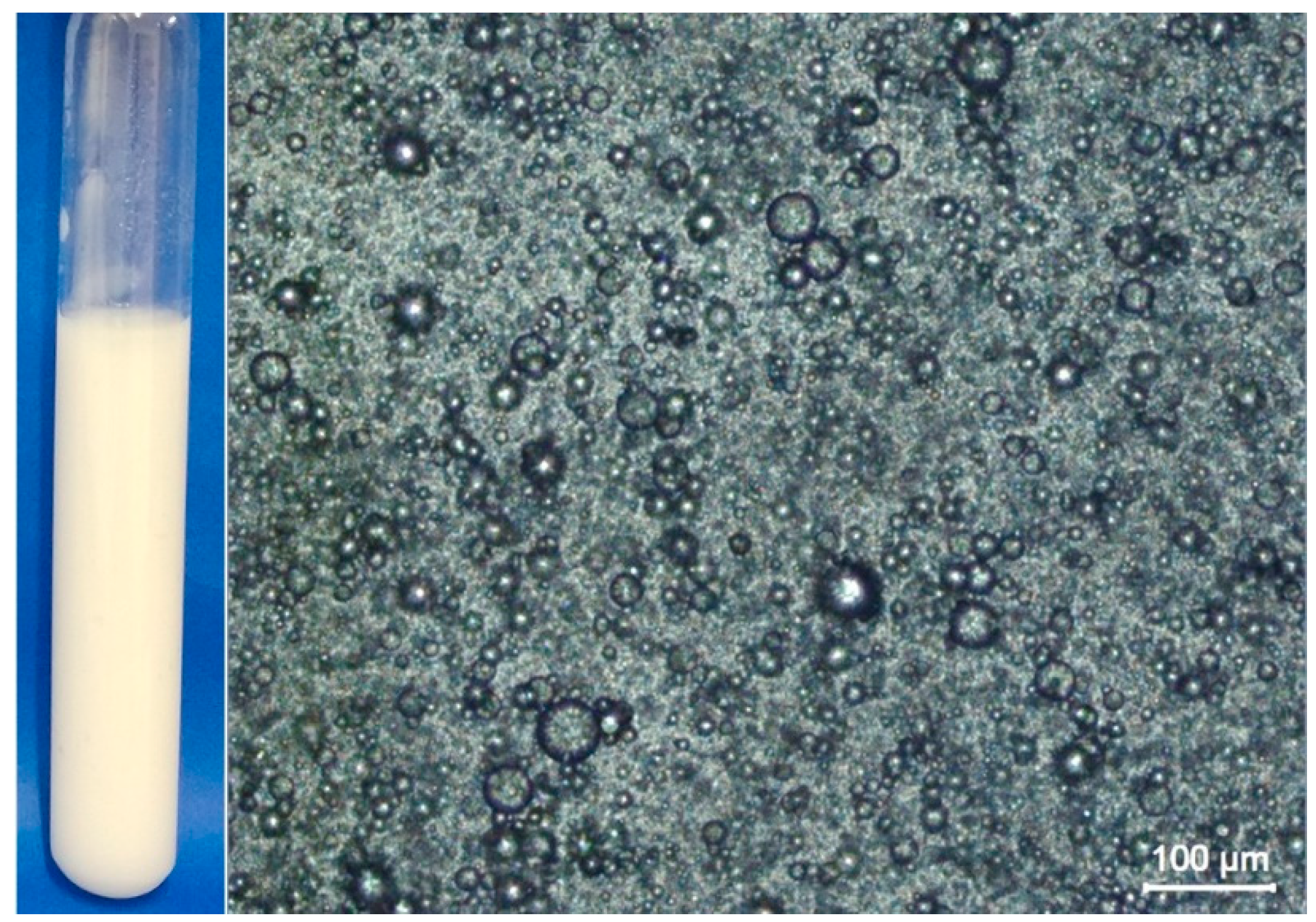
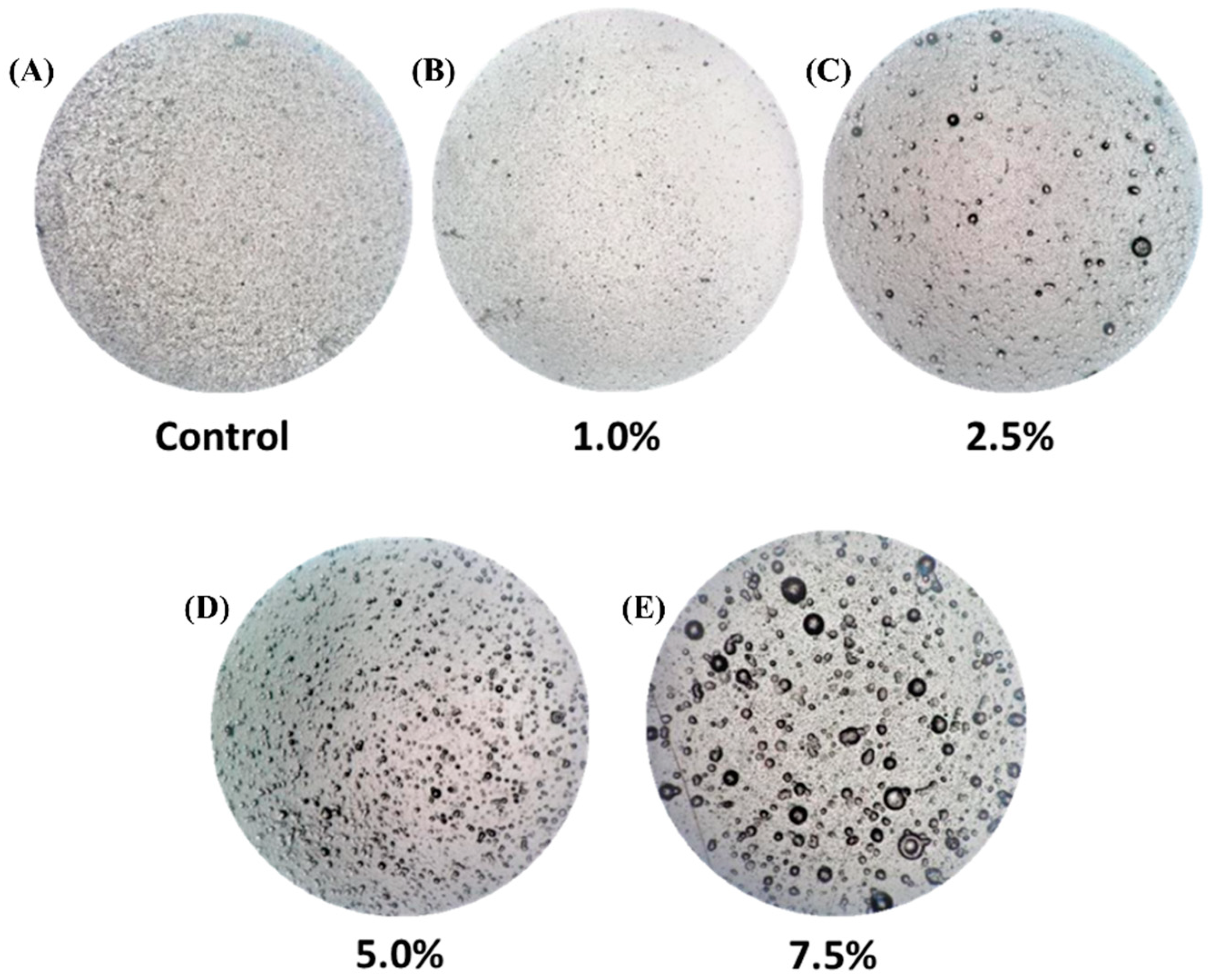
3.1. Microscopic Analysis
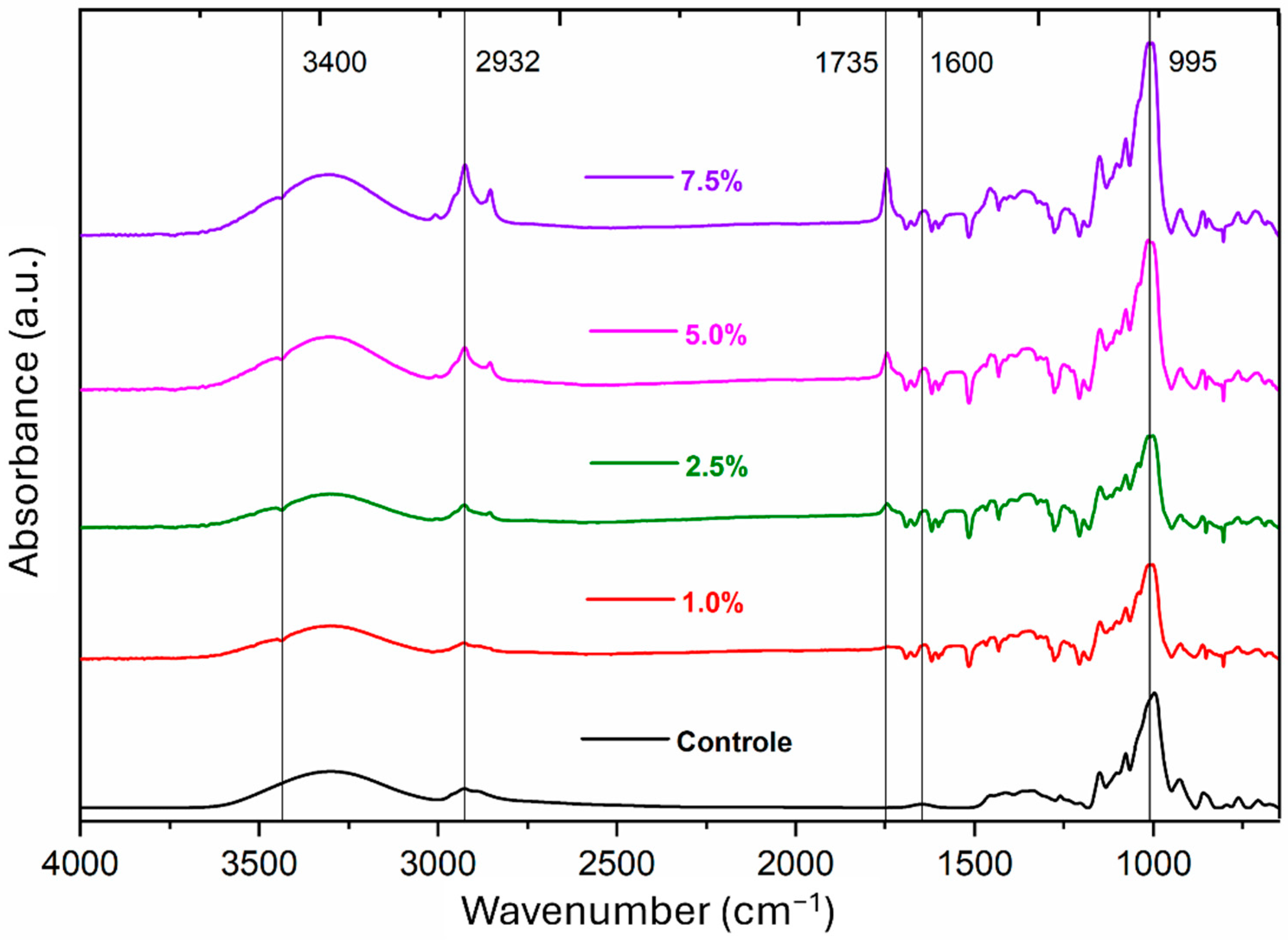
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. Water Vapor Permeability (WVP)
3.4. Hydrophobicity
3.5. Properties of Starch Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its Properties, Polymer Synthesis, and Applications in Starch Based Films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Preparation of a Novel Biodegradable Packaging Film Based on Corn Starch-Chitosan and Poloxamers. Carbohydr. Polym. 2021, 251, 117009. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J. Understanding Starch Structure and Functionality. Starch Food 2024, 10, 55–77. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Goiana, M.L.; Mattos, A.L.A.; de Azeredo, H.M.C.; de Freitas Rosa, M.; Fernandes, F.A.N. Influence of Dielectric Barrier Discharge Cold Plasma Treatment on Starch, Gelatin, and Bacterial Cellulose Biodegradable Polymeric Films. Polymers 2022, 14, 5215. [Google Scholar] [CrossRef]
- Hafizulhaq, F.; Abral, H.; Kasim, A.; Arief, S. Enhancing Functional Properties of Low Amylose Bengkoang (Pachyrhizuserosus) Starch Film by Ultrasonication. Food Sci. Technol. Res. 2020, 26, 159–166. [Google Scholar] [CrossRef]
- Silva, A.P.; Oliveira, A.V.; Pontes, S.M.; Pereira, A.L.; Rosa, M.F.; Azeredo, H.M. Mango Kernel Starch Films as Affected by Starch Nanocrystals and Cellulose Nanocrystals. Carbohydr. Polym. 2019, 211, 209–216. [Google Scholar] [CrossRef]
- Liang, X.; Hu, W.; Zhong, J.J. Use of Bacterial Cellulose (BC) from a Mutated Strain for BC-Starch Composite Film Preparation. Process Biochem. 2023, 131, 1–12. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, Z.; Cheung, K.M.; Ngai, T. Robust, Flexible, and High-Barrier Films from Bacterial Cellulose Modified by Long-Chain Alkenyl Succinic Anhydrides. ACS Sustain. Chem. Eng. 2023, 11, 2486–2498. [Google Scholar] [CrossRef]
- Tavassoli, M.; Khezerlou, A.; Punia Bangar, S.; Bakhshizadeh, M.; Haghi, P.B.; Moghaddam, T.N.; Ehsani, A. Functionality Developments of Pickering Emulsion in Food Packaging: Principles, Applications, and Future Perspectives. Trends Food Sci. Technol. 2023, 132, 171–187. [Google Scholar] [CrossRef]
- Niro, C.M.; Medeiros, J.A.; Freitas, J.A.M.; Azeredo, H.M.C. Advantages and Challenges of Pickering Emulsions Applied to Bio-Based Films: A Mini-Review. J. Sci. Food Agric. 2021, 101, 3535–3540. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-Based Pickering Emulsions: Formation, Stabilization and Applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, Y.; He, K.; Li, Y.; Luo, X.; Li, B.; Wang, C.; Liu, S. Flexible Cellulose Nanofibrils as Novel Pickering Stabilizers: The Emulsifying Property and Packing Behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Pinto, N.O.F.; Bourbon, A.I.; Martins, D.; Pereira, A.; Cerqueira, M.A.; Pastrana, L.; Gama, M.; Azeredo, H.M.C.; Rosa, M.F.; Gonçalves, C. Bacterial Cellulose Nanocrystals or Nanofibrils as Pickering Stabilizers in Low-Oil Emulsions: A Comparative Study. Food Hydrocoll. 2024, 157, 110427. [Google Scholar] [CrossRef]
- Pereira, A.L.; Feitosa, J.P.; Morais, J.P.; de Freitas Rosa, M. Bacterial Cellulose Aerogels: Influence of Oxidation and Silanization on Mechanical and Absorption Properties. Carbohydr. Polym. 2020, 250, 116927. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Mechanical and Water Vapour Barrier Properties of Edible Gellan Films. Food Res. Int. 2000, 33, 563–570. [Google Scholar] [CrossRef]
- Fennema, O.; Donhowe, I.G.; Kester, J.J. Lipid Type and Location of the Relative Humidity Gradient Influence on the Barrier Properties of Lipids to Water Vapor. In Water in Foods; Elsevier: Amsterdam, The Netherlands, 1994; pp. 225–239. [Google Scholar]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated Cellulose from TEMPO-Oxidized Eucalyptus Fibres: Effect of the Carboxyl Content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- E96-00 Method. Available online: https://www.astm.org/e0096-00.html (accessed on 1 July 2024).
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of Food-Grade Pickering Emulsions Stabilized by a Mixture of Cellulose Nanofibrils and Nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Pértile, R.A.N.; Rambo, C.R.; Porto, L.M. Enriched Glucose and Dextrin Mannitol-Based Media Modulates Fibroblast Behavior on Bacterial Cellulose Membranes. Mater. Sci. Eng. C 2013, 33, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, Q.; Shi, Z.; Ye, J. Interactions between Wheat Starch and Cellulose Derivatives in Short-Term Retrogradation: Rheology and FTIR Study. Food Res. Int. 2017, 100, 858–863. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Pickering Emulsions with Chitosan and Macroalgal Polyphenols Stabilized by Layer-by-Layer Electrostatic Deposition. Carbohydr. Polym. 2023, 300, 120256. [Google Scholar] [CrossRef]
- Baran, N.; Singh, V.K.; Pal, K.; Anis, A.; Pradhan, D.K.; Pramanik, K. Development and Characterization of Soy Lecithin and Palm Oil-Based Organogels. Polym. Plast. Technol. Eng. 2014, 53, 865–879. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade, F.K.; Vieira, L.D.; Vieira, R.S.; Vaz, J.M.; Chevallier, P.; Mantovani, D.; Borges, M.D.; Rosa, M.D. Oxidized Bacterial Cellulose Membrane as Support for Enzyme Immobilization: Properties and Morphological Features. Cellulose 2020, 27, 3055–3083. [Google Scholar] [CrossRef]
- de Oliveira Barros, M.; Mattos, A.L.; de Almeida, J.S.; de Freitas Rosa, M.; de Brito, E.S. Effect of Ball-Milling on Starch Crystalline Structure, Gelatinization Temperature, and Rheological Properties: Towards Enhanced Utilization in Thermosensitive Systems. Foods 2023, 12, 2924. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the Structural Order of Native Starch Granules Using Combined FTIR and XRD Analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Liu, J.; Song, F.; Chen, R.; Deng, G.; Chao, Y.; Yang, Z.; Wu, H.; Bai, M.; Zhang, P.; Hu, Y. Effect of Cellulose Nanocrystal-Stabilized Cinnamon Essential Oil Pickering Emulsions on Structure and Properties of Chitosan Composite Films. Carbohydr. Polym. 2022, 275, 118704. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Mohammad, M. Recent Advances in Cellulose-Based Hydrophobic Food Packaging. Emergent Mater. 2022, 5, 703–718. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.A.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccoli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Correlating Emulsion Characteristics with the Properties of Active Starch Films Loaded with Lemongrass Essential Oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-Based Films Enriched with Nanocellulose-Stabilized Pickering Emulsions Containing Different Essential Oils for Possible Applications in Food Packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]


| Sample | H2O (mL) | Emulsion | Starch (g) | Glycerol (g) | |
|---|---|---|---|---|---|
| Volume (mL) | BCNF (g) | ||||
| Control | 50.00 | - | - | 2.50 | 0.625 |
| 1.0% | 49.50 | 0.50 | 0.0050 | 2.50 | 0.625 |
| 2.5% | 48.75 | 1.25 | 0.0125 | 2.50 | 0.625 |
| 5.0% | 47.50 | 2.50 | 0.0250 | 2.50 | 0.625 |
| 7.5% | 46.25 | 3.75 | 0.0375 | 2.50 | 0.625 |
| Bands (cm−1) | Control | 1.0% | 2.5% | 5.0% | 7.5% |
|---|---|---|---|---|---|
| 1600 | --- | 0.07 | 0.01 | 0.05 | 0.03 |
| 1735 | --- | 0.05 | 0.10 | 0.16 | 0.28 |
| 2932 | 0.11 | 0.09 | 0.08 | 0.20 | 0.30 |
| 3400 | 0.28 | 0.29 | 0.23 | 0.28 | 0.24 |
| Properties | Emulsion Concentration (% v/v) | ||||
|---|---|---|---|---|---|
| Control | 1.0 | 2.5 | 5.0 | 7.5 | |
| WVP (g∙mm/h∙m2∙kPa) | 0.085 ± 0.04 a | 0.018 ± 0.01 c | 0.046 ± 0.02 b | 0.016 ± 0.01 c | 0.066 ± 0.03 b |
| Contact angle (°) | 49.7 ± 1.5 b | 66.7 ± 1.9 a | 64.5 ± 3.1 a | 70.0 ± 3.9 a | 71.0 ± 1.4 a |
| Tensile Strength (MPa) | 15.2 ± 0.8 a | 9.97 ± 0.45 b | 14.2 ± 2.0 ab | 10.9 ± 0.9 ab | 14.8 ± 1.5 ab |
| Elongation at Break (%) | 23.3 ± 2.4 a | 22.2 ± 1.6 a | 20.9 ± 3.3 a | 10.2 ± 0.6 b | 4.83 ± 1.1 b |
| Young’s Modulus (MPa) | 589 ± 32 ab | 346 ± 16 a | 522 ± 87 ab | 431 ± 38 ab | 648 ± 79 b |
| Thickness (µm) | 54.8 ± 5.3 a | 46.4 ± 2.2 a | 33.6 ± 3.2 b | 43.4 ± 1.0 ab | 38.0 ± 1.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, N.T.; Pereira, A.L.S.; de Oliveira Barros, M.; Mattos, A.L.A.; Rosa, M.d.F. Enhancing Starch Film Properties Using Bacterial Nanocellulose-Stabilized Pickering Emulsions. Polymers 2024, 16, 3346. https://doi.org/10.3390/polym16233346
de Almeida NT, Pereira ALS, de Oliveira Barros M, Mattos ALA, Rosa MdF. Enhancing Starch Film Properties Using Bacterial Nanocellulose-Stabilized Pickering Emulsions. Polymers. 2024; 16(23):3346. https://doi.org/10.3390/polym16233346
Chicago/Turabian Stylede Almeida, Natália Tavares, André Luís Sousa Pereira, Matheus de Oliveira Barros, Adriano Lincoln Albuquerque Mattos, and Morsyleide de Freitas Rosa. 2024. "Enhancing Starch Film Properties Using Bacterial Nanocellulose-Stabilized Pickering Emulsions" Polymers 16, no. 23: 3346. https://doi.org/10.3390/polym16233346
APA Stylede Almeida, N. T., Pereira, A. L. S., de Oliveira Barros, M., Mattos, A. L. A., & Rosa, M. d. F. (2024). Enhancing Starch Film Properties Using Bacterial Nanocellulose-Stabilized Pickering Emulsions. Polymers, 16(23), 3346. https://doi.org/10.3390/polym16233346







