The Synthesis and Optical Property of a Ternary Hybrid Composed of Aggregation-Induced Luminescent Polyfluorene, Polydimethylsiloxane, and Silica
Abstract
1. Introduction
2. Materials
2.1. Reagents
2.2. Compounds
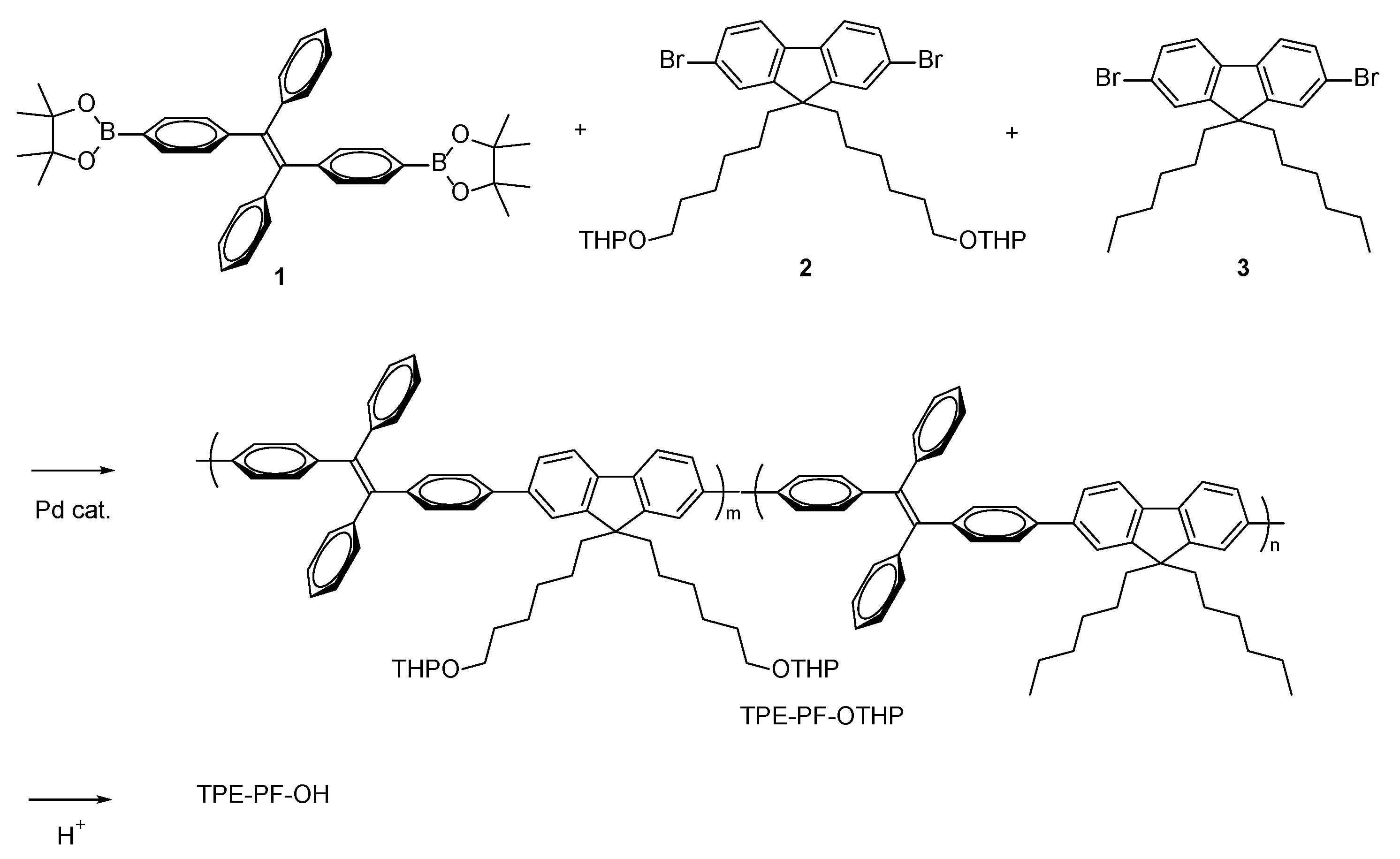
2.2.1. 1,2-Diphenyl-1,2-bis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethene (1)
2.2.2. TPE-PF-OTHP
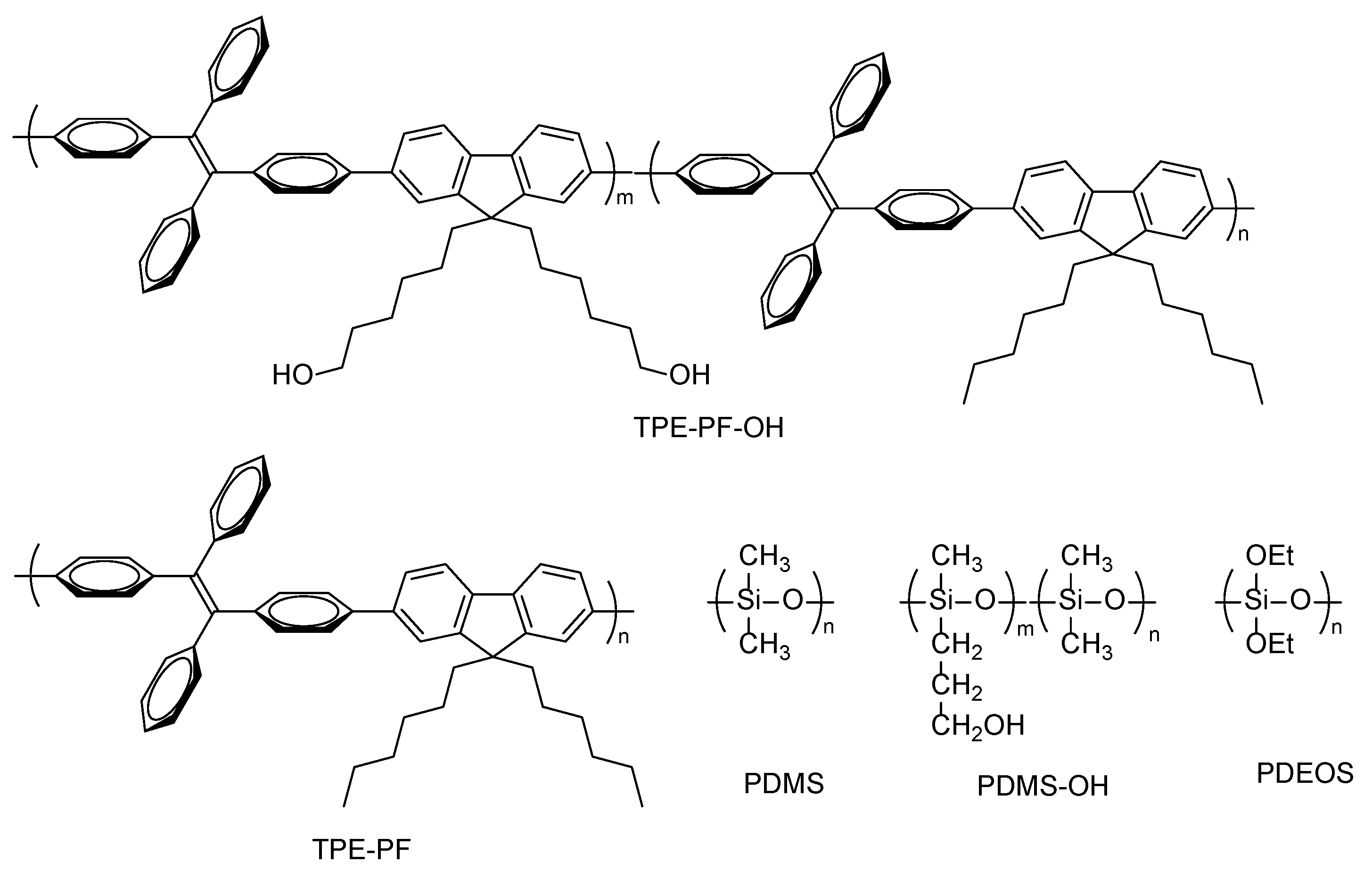
2.2.3. TPE-PF-OH
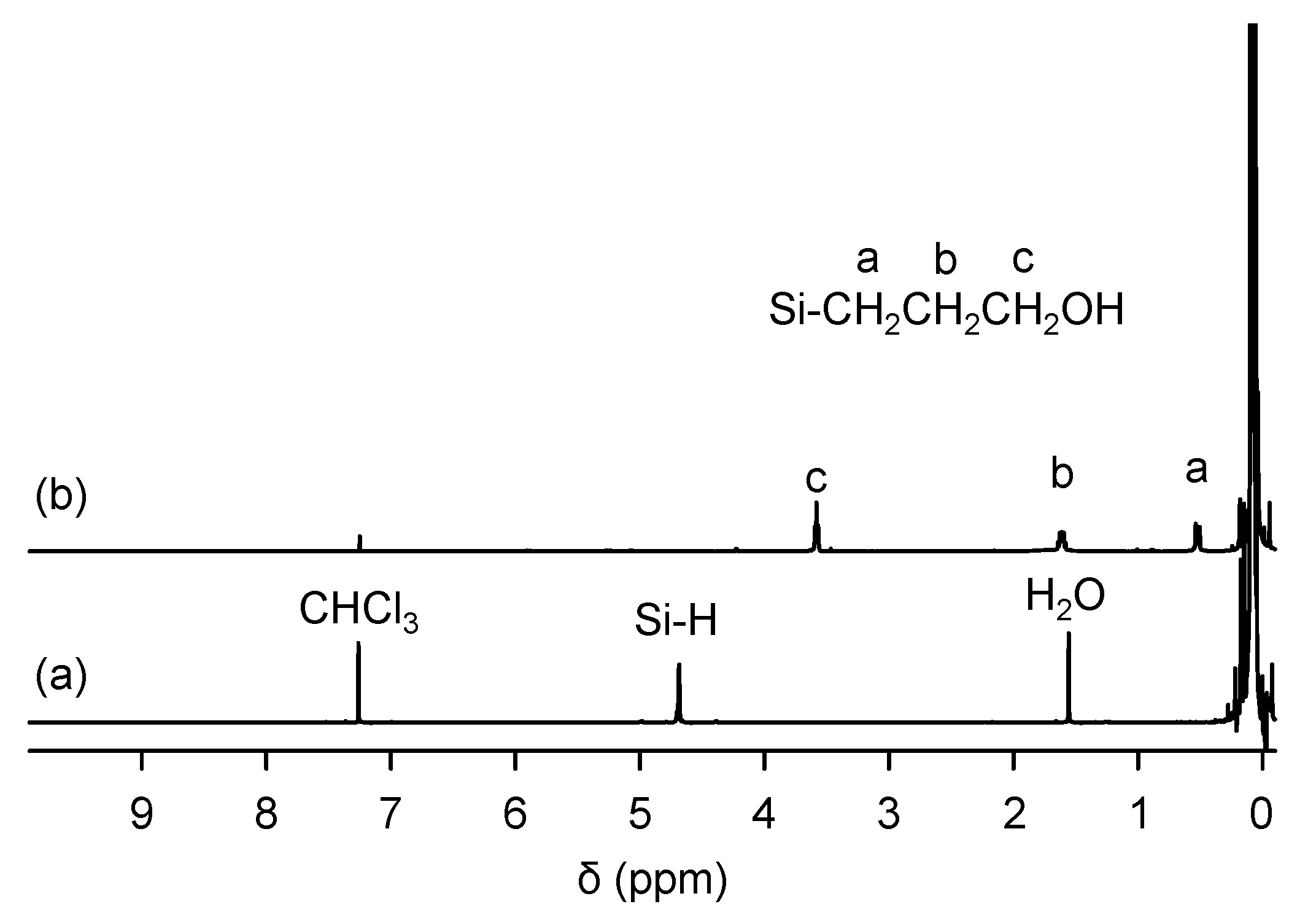
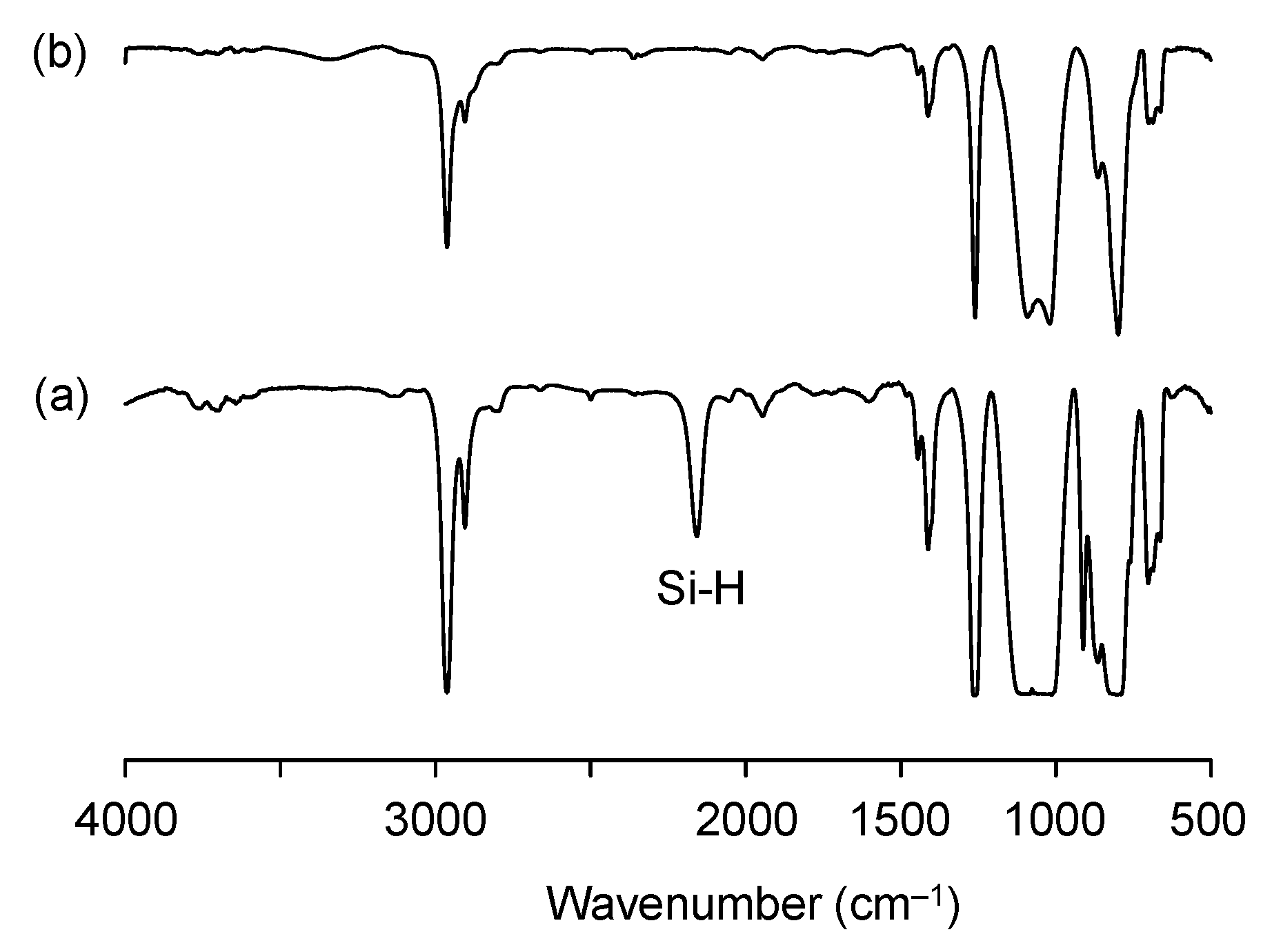
2.2.4. PDMS-OH
2.2.5. TPE-PF
2.2.6. TPE-PF-OH/PDMS-OH/SiO2 Hybrid
2.2.7. TPE-PF/PDMS/SiO2 Composite
2.3. Measurements
3. Results and Discussion
3.1. Preparation of TPE-PF-OH
3.2. Preparation of PDMS-OH
3.3. Preparation of TPE-PF-OH/PDMS-OH/SiO2 and TPE/PDMS/SiO2
3.4. Effect of Silica Content on Emission
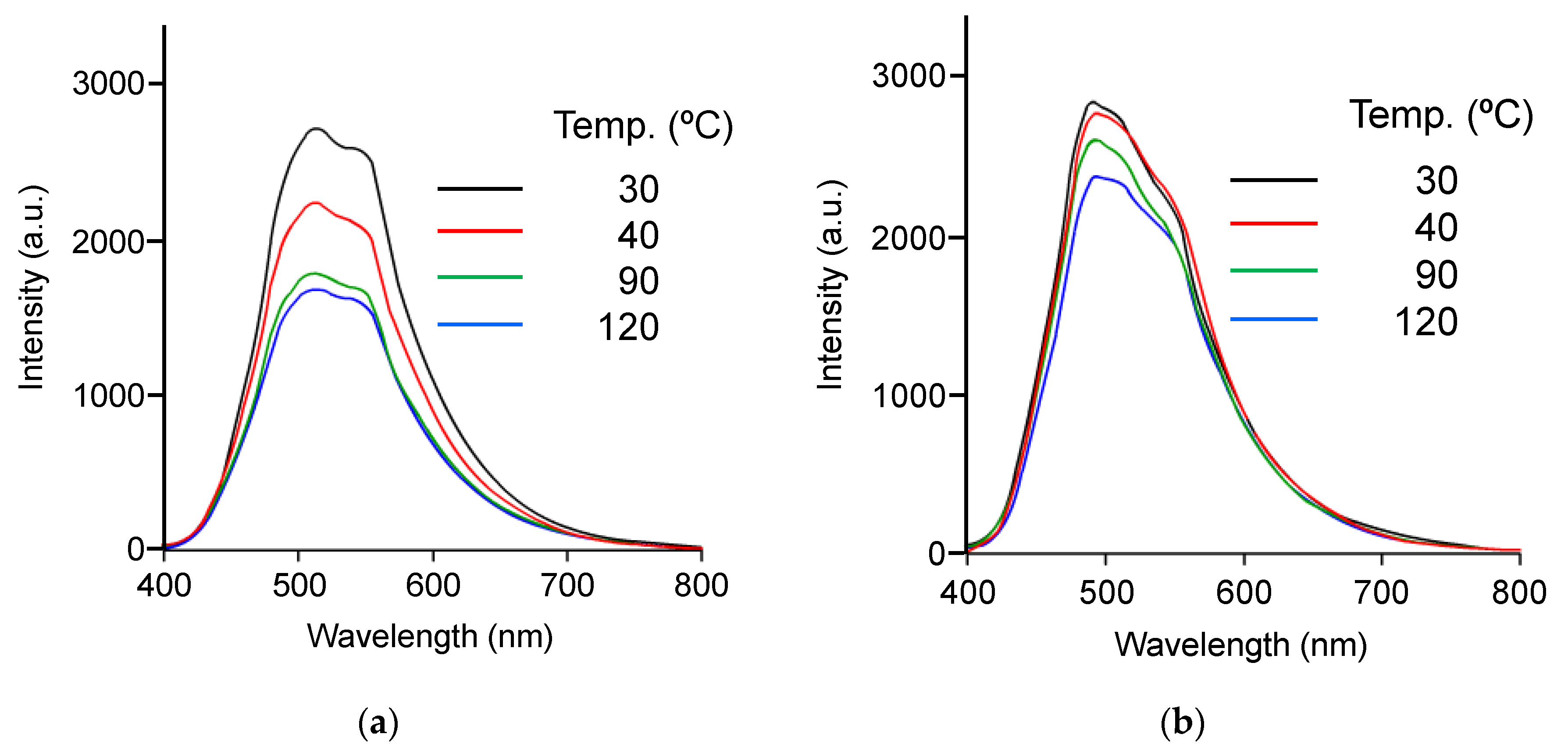
3.5. Effect of Temperature on Emission
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barford, W. Electronic and Optical Properties of Conjugated Polymers, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Müllen, K.; Scherf, U. Organic Light Emitting Devices; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2012, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens: Emission Brightened by Aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef]
- Hu, R.; Kang, Y.; Tang, B.Z. Recent advances in AIE polymers. Polym. J. 2016, 48, 359–370. [Google Scholar] [CrossRef]
- Wu, W.; Ye, S.; Tang, R.; Huang, L.; Li, Q.; Yu, G.; Liu, Y.; Qin, J.; Li, Z. New tetraphenylethylene-containing conjugated polymers: Facile synthesis, aggregation-induced emission enhanced characteristics and application as explosive chemsensors and PLEDs. Polymer 2012, 53, 3163–3171. [Google Scholar] [CrossRef]
- Kubo, M.; Takimoto, C.; Minami, T.; Uno, T.; Itoh, T.; Shoyama, M. Incorporation of π-Conjugated Polymer into Silica: Preparation of Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene]/Silica and Poly(3-hexylthiophene)/Silica Composites. Macromolecules 2005, 38, 7314–7320. [Google Scholar] [CrossRef]
- Sugiura, Y.; Shoyama, M.; Inoue, K.; Uno, T.; Itoh, T.; Kubo, M. Emission from Silica Hybrid Containing RGB Fluorescent Conjugated Polymers. Polym. Bull. 2006, 57, 865–871. [Google Scholar] [CrossRef]
- Kumazawa, N.; Towatari, M.; Uno, T.; Itoh, T.; Kubo, M. Preparation of Self-Doped Conducting Polycyclopentadithiophene and Its Incorporation into Silica. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1376–1380. [Google Scholar] [CrossRef]
- Miyao, A.; Mori, Y.; Uno, T.; Itoh, T.; Yamasaki, T.; Koshio, A.; Kubo, M. Incorporation of Fluorene-Based Emitting Polymers into Silica. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5322–5328. [Google Scholar] [CrossRef]
- Nishikawa, S.; Kami, S.; Nurul, A.; Badrul, H.; Uno, T.; Itoh, T.; Kubo, M. Hybridization of Emitting Polyfluorene with Silicone. J. Polym. Sci. Part A Polym. Chem. 2016, 53, 622–628. [Google Scholar] [CrossRef]
- Yamashita, T.; Deguchi, K.; Nagotani, S.; Abe, K. Vascular Protection and Restoractive Therapy in Ischemic Stroke. Cell Transpl. 2011, 20, 95–97. [Google Scholar] [CrossRef]
- Czarnobaj, K. Sol-gel-processed silica/polydimethylsiloxane/calcium xerogels aspolymeric matrices for Metronidazole delivery system. Polym. Bull. 2011, 66, 223–237. [Google Scholar] [CrossRef][Green Version]
- Prokopowicz, M.; Zeglinski, J.; Gandhi, A.; Sawicki, W.; Tofail, S.A. Bioactive silica-based drug delivery systems containing doxorubicin hydrochloride: In vitro studies. Colloids Surf. B Biointerfaces 2012, 93, 249–259. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Xia, B.-B.; Ye, H.-P.; Zhang, Y.-L.; Xiao, B.; Yan, L.-H.; Lv, H.-B.; Jiang, B. One-step sol-gel preparation of PDMS-silica ORMOSILs as environment-resistant and crack-free thick antireflective coatings. J. Mater. Chem. 2012, 22, 13132–13140. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Wang, W.; Mao, L.-H.; Wang, C.-F.; Chen, S. Versatile superhydrophobic and photocatalytic films generated from TiO2-SiO2@PDMS and their applications on fabrics. J. Mater. Chem. A 2014, 2, 4178–4184. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Elvira, M.R.; Mazo, M.A.; Tamayo, A.; Rubio, F.; Rubio, J. Study and Characterization of Organically Modified Silica-Zirconia Anti-Graffiti Coatings Obtained by Sol-Gel. J. Chem. Chem. Eng. 2013, 7, 120–131. [Google Scholar]
- Kim, N.H.; Kim, D. Identification of the donor-substitution effect of tetraphenylethylene AIEgen: Synthesis, photophysical property analysis, and bioimaging applications. Dyes Pigments 2022, 199, 110098. [Google Scholar] [CrossRef]
- Ranger, M.; Rondeau, D.; Leclerc, M. New Well-Defined Poly(2,7-fluorene) Derivatives: Photoluminescence and Base Doping. Macromolecules 1997, 30, 7686–7691. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Huang, Q.; Iwamoto, T. Mechanical Properties of Ormosils. J. Sol-Gel Sci. Technol. 1996, 7, 151–161. [Google Scholar] [CrossRef]
- Chemtob, A.; Peter, N.; Belon, C.; Dietlin, C.; Croutxé-Barghorn, C.; Vidal, L.; Rigolet, S. Macroporous organosilica films via a template-free photoinduced sol-gel process. J. Mater. Chem. 2010, 20, 9104–9112. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Lu, H.; Huang, M.; Shen, Z.; Yang, J. Thermally responsive AIE-active polyurethanes based on a tetraaniline derivative. RSC Adv. 2020, 10, 41424–41429. [Google Scholar] [CrossRef]
- Ma, C.; Han, T.; Niu, N.; Al-Shok, L.; Efstathiou, S.; Lester, D.; Huband, S.; Haddleton, D. Well-defined polyacrylamides with AIE properties via rapid Cu-mediated living radical polymerization in aqueous solution: Thermoresponsive nanoparticles for bioimaging. Polym. Chem. 2022, 13, 58–68. [Google Scholar] [CrossRef]





| Entry | PDMS-OH, g | PDEOS, g | SiO2 Content, wt% |
|---|---|---|---|
| 1 | 0.60 | 0.37 | 20 |
| 2 | 0.45 | 0.73 | 40 |
| 3 | 0.30 | 1.1 | 60 |
| Entry | PDMS, g | PDEOS, g | SiO2 Content, wt% |
|---|---|---|---|
| 1 | 0.60 | 0.37 | 20 |
| 2 | 0.45 | 0.73 | 40 |
| 3 | 0.30 | 1.1 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainuddin, N.A.S.; Suizu, Y.; Uno, T.; Kubo, M. The Synthesis and Optical Property of a Ternary Hybrid Composed of Aggregation-Induced Luminescent Polyfluorene, Polydimethylsiloxane, and Silica. Polymers 2024, 16, 3331. https://doi.org/10.3390/polym16233331
Zainuddin NAS, Suizu Y, Uno T, Kubo M. The Synthesis and Optical Property of a Ternary Hybrid Composed of Aggregation-Induced Luminescent Polyfluorene, Polydimethylsiloxane, and Silica. Polymers. 2024; 16(23):3331. https://doi.org/10.3390/polym16233331
Chicago/Turabian StyleZainuddin, Nurul Amira Shazwani, Yusuke Suizu, Takahiro Uno, and Masataka Kubo. 2024. "The Synthesis and Optical Property of a Ternary Hybrid Composed of Aggregation-Induced Luminescent Polyfluorene, Polydimethylsiloxane, and Silica" Polymers 16, no. 23: 3331. https://doi.org/10.3390/polym16233331
APA StyleZainuddin, N. A. S., Suizu, Y., Uno, T., & Kubo, M. (2024). The Synthesis and Optical Property of a Ternary Hybrid Composed of Aggregation-Induced Luminescent Polyfluorene, Polydimethylsiloxane, and Silica. Polymers, 16(23), 3331. https://doi.org/10.3390/polym16233331






