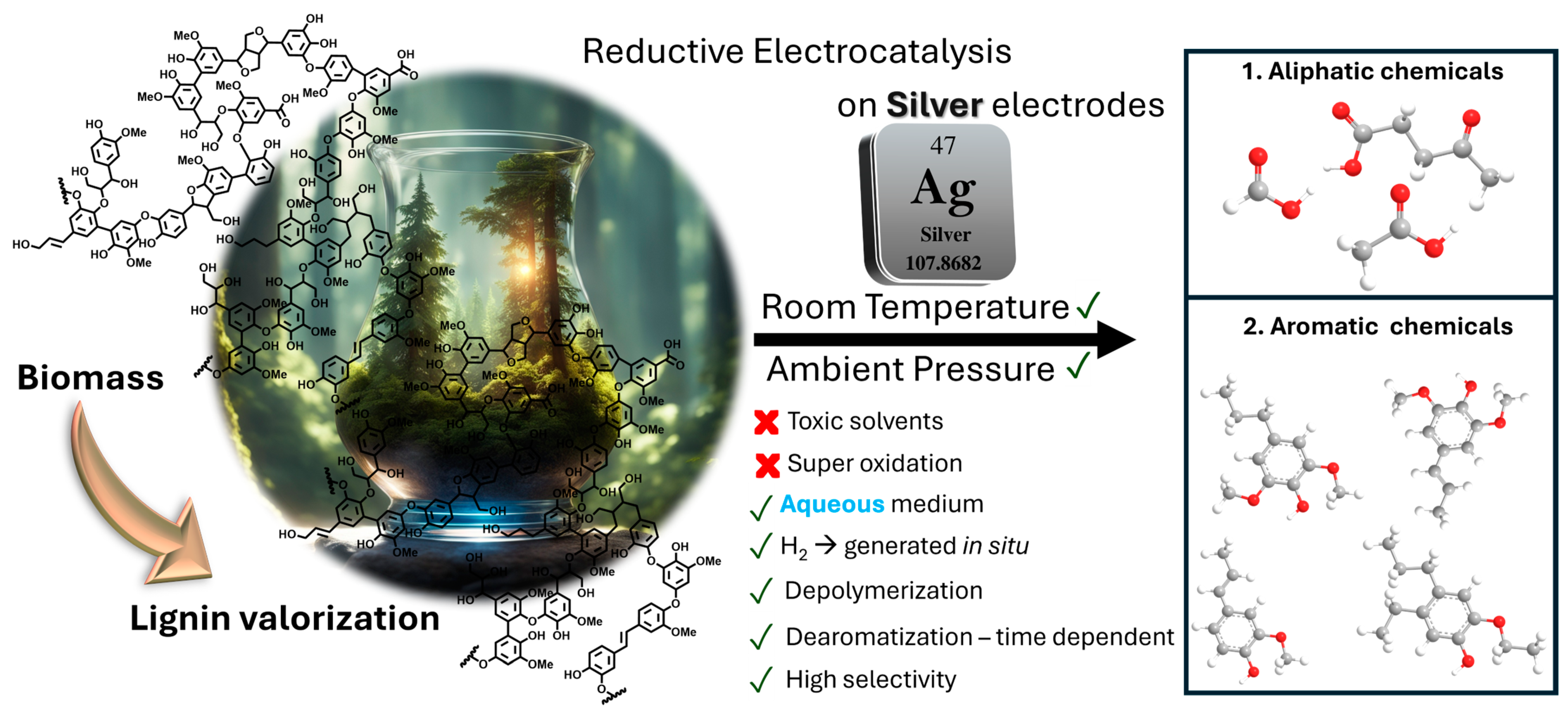
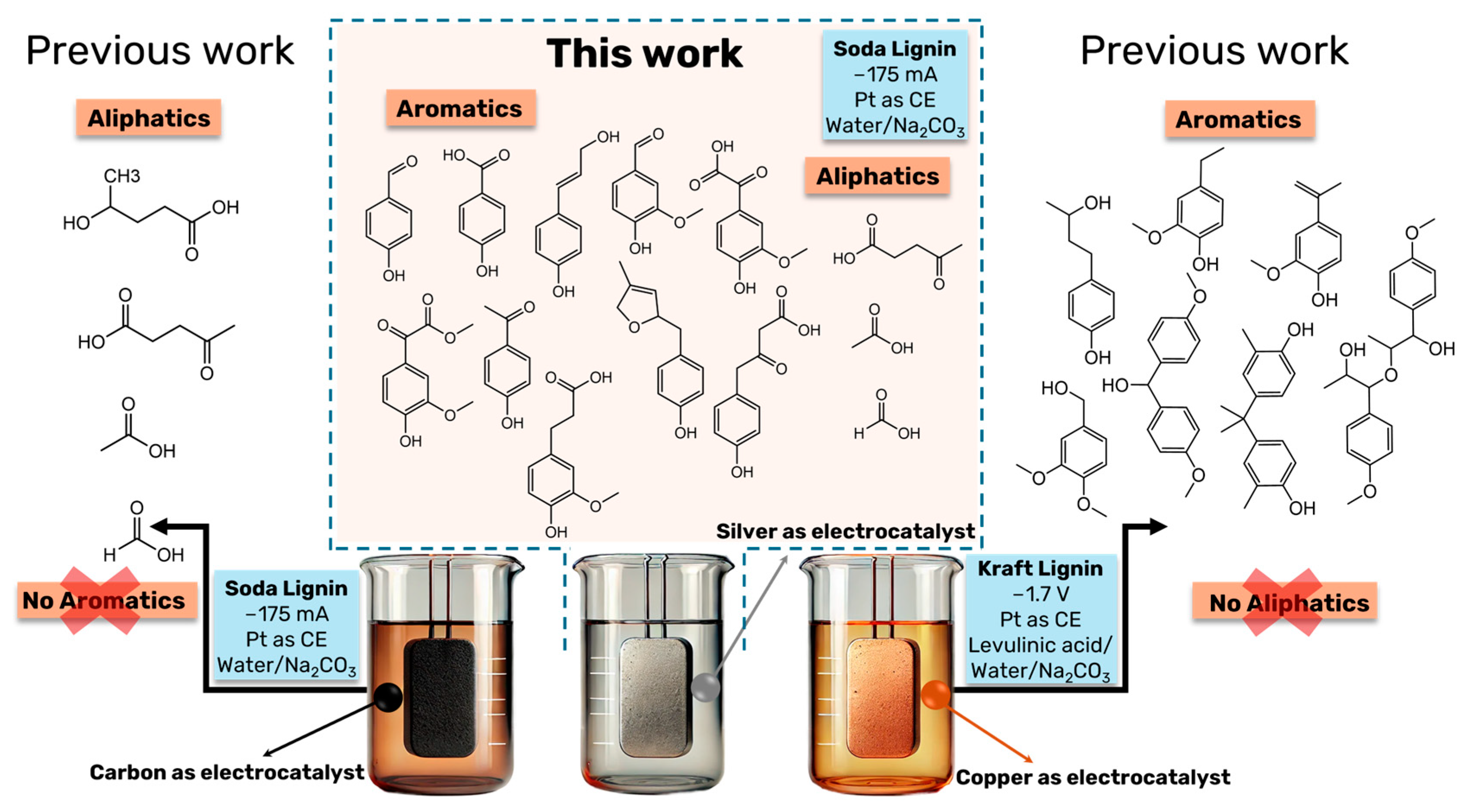
Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Depolymerization
- 0 h: FTIR (Diamond-ATR): ν [cm−1] = 3313 (O-H, w), 2961 (C-H, w), 2918 (C-H, w), 2873 (C-H, w), 2850 (C-H, w), 1717 (C=O, m), 1700 (C=O, m), 1652 (m), 1594 (C=C, m), 1443 (C=C, m), 1346 (s), 1258 (C-O, s), 1015 (C-O-C, C-O-H, s), 877 (m), 866 (m), 835 (C-H, s), 798 (CH, s), 668 (s), 400 (m).
- 5 h: FTIR (Diamond-ATR): ν [cm−1] = 3566 (O-H, w), 2918 (C-H, w), 2873 (C-H, w), 2851 (C-H, w), 1772 (w), 1733 (C=O, m), 1717 (C=O, m), 1700 (C=O, m), 1652 (m), 1589 (C=C, m), 1521 (C=C, m), 1374 (s), 1349 (s), 1249 (C-O, m), 948 (m), 878 (m), 837 (C-H, s), 767 (m), 700 (C-H, m), 668 (s), 420 (s).
- 10 h: FTIR (Diamond-ATR): ν [cm−1] = 3310 (OH, w), 2917 (CH, m), 2850 (CH, m), 1728 (C=O, m), 1595 (C=C, m), 1565 (m), 1347 (C-H, s), 1260 (C-O, s), 1076 (C-O, s), 1049 (s), 982 (s), 833 (C-H, s), 767 (m), 654 (s), 569 (s).
- 15 h: FTIR (Diamond-ATR): ν [cm−1] = 3350 (O-H, w), 2954 (C-H, w), 2916 (C-H, w), 2872 (C-H, w), 2850 (C-H, w), 1733 (C=O, m), 1717 (C=O, m), 1700 (C=O, m), 1652 (m), 1635 (m), 1564 (C=C, m), 1456 (C-H, m), 1048 (s), 1012 (C-O-C, C-O-H, s), 833 (C-H, s), 781 (m), 668 (s), 400 (m).
- 20 h: FTIR (Diamond-ATR): ν [cm−1] = 3399 (O-H, w), 2962 (C-H, w), 2917 (C-H, w), 1717 (C=O, m), 1700 (C=O, m), 1591 (C=C, m), 1564 (C=C, m), 1441 (C-H, m), 1259 (C-O, s), 1078 (s), 1015 (C-O-C, C-O-H, s), 832 (C-H, s), 798 (C-H, s), 668 (s), 404 (m).
2.3. Characterization
- Nuclear Magnetic Resonance Spectroscopy (NMR): ¹H NMR spectra were obtained using a Bruker Avance III 600 NMR instrument (Bruker Corporation, Billerica, MA, USA) at a temperature of 300 K in deuterium oxide (D2O). The following sample probes were employed: a 5 mm broadband inverse probe with automatic frequency determination, a 5 mm QNP probe, and a 5 mm broadband inverse probe. Chemical shifts were referenced against tetramethylsilane (Me4Si) for ¹H. Unless otherwise specified, 16 scans were recorded with a 1.0 s delay between each scan for the ¹H NMR spectra.
- Vibrational spectroscopy (FTIR): Fourier-transform infrared (FTIR) spectra were measured using a Nicolet iS5 spectrometer (Thermo Scientific, Waltham, MA, USA), equipped with an iD5 diamond attenuated total reflection (ATR) unit.
- Direct infusion (DI) ESI-HRMS: Depolymerized lignin samples were dissolved in methanol, ultrasonicated for 30 min, and centrifuged for 10 min (14,000 rpm). High-resolution mass spectrometry (HRMS) was used as an advanced analytical method to gain the structure information of degradation products of lignin. MS and MSn spectra were obtained using an Orbitrap-IQX high-resolution mass spectrometer (ThermoFisher Scientific, Bremen, Germany), equipped with an ESI source. ESI-MS analyses were carried out in ESI (−) and ESI (+) mode. The solutions were infused into the ESI source via direct infusion (DI) at a rate of 5 µL min−1. Typical spray and ion optics for negative mode conditions were the following: source voltage, 3.0 kV; sheath gas flow rate, 8 arb; capillary temperature, 275 °C; capillary voltage, −50 V; tube lens voltage, −130 V. Fragmentation and interpretation were performed based on negative ionization mode; positive ionization mode was used for additional confirmation. Xcalibur version 2.0.7 and Mass Frontier version 8.0 (ThermoFisher Scientific, Bremen, Germany) software were used for data processing and evaluation.
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, T.L. Electrochemical Valorization of Lignin: Status, Challenges, and Prospects. J. Bioresour. Bioprod. 2023, 8, 1–14. [Google Scholar] [CrossRef]
- Lindenbeck, L.M.; Barra, V.C.; Dahlhaus, S.; Brand, S.; Wende, L.M.; Beele, B.B.; Schebb, N.H.; Rodrigues, B.V.M.; Slabon, A. Organic Chemicals from Wood: Selective Depolymerization and Dearomatization of Lignin via Aqueous Electrocatalysis. ChemSusChem 2024, 17, e202301617. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, M.G.A.; Gueret, R.; Chen, J.; Piątek, J.; Beele, B.; Sipponen, M.H.; Frauscher, M.; Budnyk, S.; Rodrigues, B.V.M.; Slabon, A. Electrochemical Depolymerization of Lignin in a Biomass-Based Solvent. ChemSusChem 2022, 15, e202200718. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Maldonado, S.; Stephenson, C.R.J. Electrocatalytic Lignin Oxidation. ACS Catal. 2021, 11, 10104–10114. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Duan, H. Recent Progress in Electrocatalytic Conversion of Lignin: From Monomers, Dimers, to Raw Lignin. Precis. Chem. 2024, 2, 428–446. [Google Scholar] [CrossRef]
- Margellou, A.G.; Pappa, C.P.; Psochia, E.A.; Petala, M.D.; Triantafyllidis, K.S. Mild Isolation and Characterization of Surface Lignin from Hydrothermally Pretreated Lignocellulosic Forestry and Agro-Industrial Waste Biomass. Sustain. Chem. Pharm. 2023, 33, 101056. [Google Scholar] [CrossRef]
- Sapouna, I.; van Erven, G.; Heidling, E.; Lawoko, M.; McKee, L.S. Impact of Extraction Method on the Structure of Lignin from Ball-Milled Hardwood. ACS Sustain. Chem. Eng. 2023, 11, 15533–15543. [Google Scholar] [CrossRef]
- Wijaya, Y.; Smith, K.; Kim, C.; Gyenge, E. Electrocatalytic Hydrogenation and Depolymerization Pathways for Lignin Valorization: Toward Mild Synthesis of Chemicals and Fuels from Biomass. Green Chem. 2020, 22, 7233–7264. [Google Scholar] [CrossRef]
- Hinsch, J.J.; Liu, J.; White, J.J.; Wang, Y. The Role of Steps on Silver Nanoparticles in Electrocatalytic Oxygen Reduction. Catalysts 2022, 12, 576. [Google Scholar] [CrossRef]
- Ma, M.; Trześniewski, B.J.; Xie, J.; Smith, W.A. Selective and Efficient Reduction of Carbon Dioxide to Carbon Monoxide on Oxide-Derived Nanostructured Silver Electrocatalysts. Angew. Chem. Int. Ed. 2016, 55, 9748–9752. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Yadav, S.; Dutta, S.; Kale, H.B.; Warkad, I.R.; Zbořil, R.; Varma, R.S.; Gawande, M.B. Silver Nanomaterials: Synthesis and (Electro/Photo) Catalytic Applications. Chem. Soc. Rev. 2021, 50, 11293–11380. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.B.; Tsai, M.-C.; Chala, S.A.; Berihun, M.K.; Kahsay, A.W.; Berhe, T.A.; Su, W.-N.; Hwang, B.-J. A Review of Transition Metal-Based Bifunctional Oxygen Electrocatalysts. J. Chin. Chem. Soc. 2019, 66, 829–865. [Google Scholar] [CrossRef]
- Wolfsgruber, M.; Patil, P.; Pichler, C.M.; Bischof, R.H.; Budnyk, S.; Paulik, C.; Rodrigues, B.V.M.; Slabon, A. Potential Dependence of Gluconic Acid to Glucose Electroreduction on Silver. Catal. Sci. Technol. 2023, 13, 5998–6005. [Google Scholar] [CrossRef]
- Wolfsgruber, M.; Bischof, R.H.; Paulik, C.; Slabon, A.; Rodrigues, B.V.M. Revisiting the Electrocatalytic Hydrogenation of Furfural to Furfuryl Alcohol Using Biomass-Derived Electrolytes. RSC Sustain. 2024, 2, 1142–1153. [Google Scholar] [CrossRef]
- Wang, D.; Yang, H.; Yang, J.; Wang, B.; Wasnik, P.; Xu, B.B.; Shi, Z. Efficient Visible Light-Induced Photodegradation of Industrial Lignin Using Silver-CuO Catalysts Derived from Cu-Metal Organic Framework. Adv. Compos. Hybrid Mater. 2023, 6, 138. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, M.-W.; Lee, S.; Lee, J.; Cho, S.; Lee, H.; Cha, H.G.; Kim, H.S. Enhancing Photocatalytic β-O-4 Bond Cleavage in Lignin Model Compounds by Silver-Exchanged Cadmium Sulfide. ACS Catal. 2020, 10, 8465–8475. [Google Scholar] [CrossRef]
- Linge, J.M.; Briega-Martos, V.; Hutzler, A.; Fritsch, B.; Erikson, H.; Tammeveski, K.; Cherevko, S. Stability of Carbon Supported Silver Electrocatalysts for Alkaline Oxygen Reduction and Evolution Reactions. ACS Appl. Energy Mater. 2023, 6, 11497–11509. [Google Scholar] [CrossRef]
- Khaksar, Z.; Habibi, M.F.; Arvand, M.; Rezapour, R. Improving Oxygen Reduction Reaction of Microbial Fuel Cell by Silver Vanadate Blended Functionalized Multiwall Carbon Nanotubes as Cathode. Fuel 2024, 373, 132367. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Mooste, M.; Piirsoo, H.-M.; Kaljuvee, T.; Kikas, A.; Aruväli, J.; Kisand, V.; Tamm, A.; Kannan, A.M.; et al. Ag Nanoparticles on Mesoporous Carbon Support as Cathode Catalyst for Anion Exchange Membrane Fuel Cell. Int. J. Hydrog. Energy 2023, 48, 11058–11070. [Google Scholar] [CrossRef]
- Ding, J.; Wei, T.; Hou, T.; Liu, W.; Liu, Q.; Zhang, H.; Luo, J.; Liu, X. Easily Constructed Porous Silver Films for Efficient Catalytic CO2 Reduction and Zn–CO2 Batteries. Nanoscale 2024, 16, 10628–10636. [Google Scholar] [CrossRef] [PubMed]
- Poolnapol, L.; Kao-ian, W.; Somwangthanaroj, A.; Mahlendorf, F.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Silver Decorated Reduced Graphene Oxide as Electrocatalyst for Zinc–Air Batteries. Energies 2020, 13, 462. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A Selective and Efficient Electrocatalyst for Carbon Dioxide Reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef]
- Lindenbeck, L.M.; Dahlhaus, S.; Wende, L.M.; Beele, B.B.; Frauscher, M.; Schebb, N.H.; Lehmann, C.W.; Bornhorst, J.; Slabon, A.; Rodrigues, B.V.M. Breaking down Lignin in Gamma-Valerolactone: Advances into a Bioelectrorefinery. Green Chem. Lett. Rev. 2024, 17, 2390867. [Google Scholar] [CrossRef]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical Depolymerisation of Lignin in a Deep Eutectic Solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Wang, T.; Tao, L.; Zhu, X.; Chen, C.; Chen, W.; Du, S.; Zhou, Y.; Zhou, B.; Wang, D.; Xie, C.; et al. Combined Anodic and Cathodic Hydrogen Production from Aldehyde Oxidation and Hydrogen Evolution Reaction. Nat. Catal. 2022, 5, 66–73. [Google Scholar] [CrossRef]
- Kozmelj, T.R.; Bartolomei, E.; Dufour, A.; Leclerc, S.; Arnoux, P.; Likozar, B.; Jasiukaitytė-Grojzdek, E.; Grilc, M.; Le Brech, Y. Oligomeric Fragments Distribution, Structure and Functionalities upon Ruthenium-Catalyzed Technical Lignin Depolymerization. Biomass Bioenergy 2024, 181, 107056. [Google Scholar] [CrossRef]
- Vuković, J.P.; Tišma, M. The Role of NMR Spectroscopy in Lignocellulosic Biomass Characterisation: A Mini Review. Food Chem. Mol. Sci. 2024, 9, 100219. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Guo, T.; Zhao, H.; Jin, Y. Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy. Polymers 2018, 10, 736. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Zirbes, M.; Graßl, T.; Neuber, R.; Waldvogel, S.R. Peroxodicarbonate as a Green Oxidizer for the Selective Degradation of Kraft Lignin into Vanillin. Angew. Chem. Int. Ed. 2023, 62, e202219217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Liu, S.; Karlen, S.D.; Kim, H.; Lu, F.; Ralph, J.; Vázquez Ramos, L.M.; Huber, G.W.; Dumesic, J.A. Poplar Lignin Structural Changes during Extraction in γ-Valerolactone (GVL). Green Chem. 2023, 25, 336–347. [Google Scholar] [CrossRef]
- Wang, C.; Liu, K.; Jin, Y.; Huang, S.; Chun-Ho Lam, J. Amorphous RuO2 Catalyst for Medium Size Carboxylic Acid to Alkane Dimer Selective Kolbe Electrolysis in an Aqueous Environment. ChemSusChem 2023, 16, e202300222. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.; Khan, S.A.; Shahid, S.; Javed, M.; Qamar, M.A.; Chinnathambi, A.; Almoallim, H.S. Synthesis of Novel Ternary Hybrid G-C3N4@Ag-ZnO Nanocomposite with Z-Scheme Enhanced Solar Light-driven Methylene Blue Degradation and Antibacterial Activities. J. Environ. Chem. Eng. 2021, 9, 105366. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Riddell, L.A.; de Peinder, P.; Polizzi, V.; Vanbroekhoven, K.; Meirer, F.; Bruijnincx, P.C.A. Predicting Molecular Weight Characteristics of Reductively Depolymerized Lignins by ATR-FTIR and Chemometrics. ACS Sustain. Chem. Eng. 2024, 12, 8968–8977. [Google Scholar] [CrossRef]
- Riddell, L.A.; Lindner, J.-P.B.; de Peinder, P.; Meirer, F.; Bruijnincx, P.C.A. Rapid Lignin Thermal Property Prediction through Attenuated Total Reflectance-Infrared Spectroscopy and Chemometrics. ChemSusChem 2024, 17, e202301464. [Google Scholar] [CrossRef]
- Jennings, K.R. Spectrometric Identification of Organic Compounds (Fifth Edition) R. M. SILVERSTEIN, G. C. BASSLER AND T. C. MORRILL. Wiley, New York, 1991. No. of Pages: 430. ISBN 0471 63404 2. Price: £50.25, $76.10. Org. Mass Spectrom. 1991, 26, 813. [Google Scholar] [CrossRef]
- Lindenbeck, L.M.; Beele, B.B.; Lützenkirchen-Hecht, D.F.; Rodrigues, B.V.M.; Slabon, A. Electrochemical Biomass Depolymerization: Will Complex Catalysts Trigger High Product Selectivity? Chem. Mater. 2024, 36, 9173–9188. [Google Scholar] [CrossRef]
- Lucas, F.; Grim, R.; Tacey, S.; Downes, C.; Hasse, J.; Roman, A.; Farberow, C.; Schaidle, J.; Holewinski, A. Electrochemical Routes for the Valorization of Biomass-Derived Feedstocks: From Chemistry to Application. ACS Energy Lett. 2021, 6, 1205–1270. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolai, T.; Van Aelst, K.; Maesen, M.; et al. A Sustainable Wood Biorefinery for Low-Carbon Footprint Chemicals Production. Science 2020, 367, 1385. [Google Scholar] [CrossRef]
- Supriyanto; Usino, D.; Ylitervo, P.; Dou, J.; Sipponen, M.; Richards, T. Identifying the Primary Reactions and Products of Fast Pyrolysis of Alkali Lignin. J. Anal. Appl. Pyrolysis 2020, 151, 104917. [Google Scholar] [CrossRef]
- Curet, L.; Lafargue dit-Hauret, W.; Benet-Buchholz, J.; Martínez-Belmonte, M.; Foix, D.; Palomares, E.; Billon, L.; Begué, D.; Viterisi, A. Self-Assembled Infinite Silver Cluster with Atomic Precision as a Scalable Catalyst for CO2-Electroreduction under Industry-Relevant Reaction Rates. EES. Catal. 2024. [Google Scholar] [CrossRef]
- Deng, X.; Alfonso, D.; Nguyen-Phan, T.-D.; Kauffman, D.R. Breaking the Limit of Size-Dependent CO2RR Selectivity in Silver Nanoparticle Electrocatalysts through Electronic Metal–Carbon Interactions. ACS Catal. 2023, 13, 15301–15309. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Kisielewska, A.; Ginter, J.; Bałuszyńska, K.; Piwoński, I. Elucidation of the Function of Oxygen Moieties on Graphene Oxide and Reduced Graphene Oxide in the Nucleation and Growth of Silver Nanoparticles. RSC Adv. 2016, 6, 60056–60067. [Google Scholar] [CrossRef]

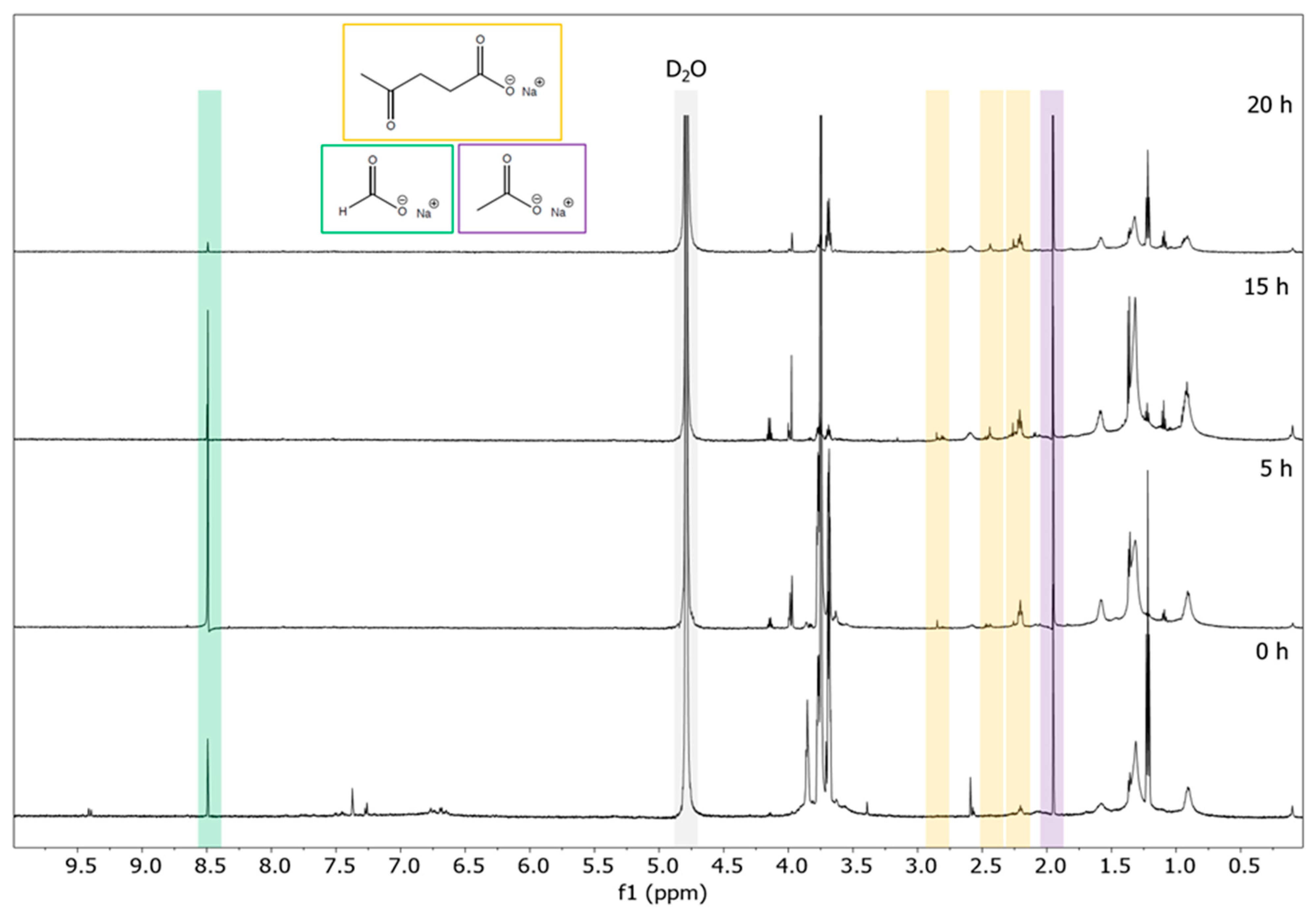


| Duration | Soda Lignin | 0 h | 5 h | 10 h | 15 h | 20 h |
|---|---|---|---|---|---|---|
| Yield [wt%] | 7 | 6 | 13.4 | 21.8 | 18.33 | 18.35 |
| m/z | 121.0292 | 137.0272 | 149.0605 | 151.0400 | 165.0193 |
| Chemical Structure | 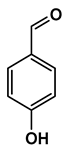 |  | 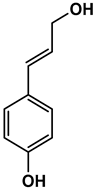 |  | 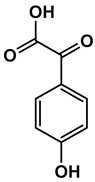 |
| 4-hydroxybenzaldehyde | 4-hydroxybenzoic acid | (E)-4-(3-hydroxyprop-1-en-1-yl)phenol | 4-hydroxy-3-methoxybenzaldehyde | 2-(4-hydroxy-3-methoxyphenyl)-2-oxoacetic acid | |
| m/z | 165.0552 | 189.0915 | 193.0504 | 195.0297 | 195.0661 |
| Chemical Structure | 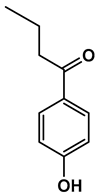 | 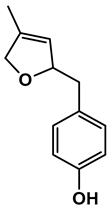 | 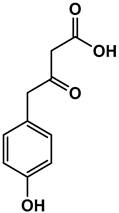 | 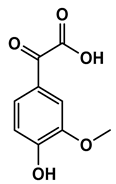 | 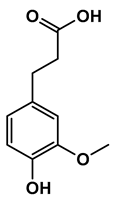 |
| 1-(4-hydroxyphenyl)butan-1-one | 4-((4-methyl-2,5-dihydrofuran-2-yl)methyl)phenol | 4-(4-hydroxyphenyl)-3-oxobutanoic acid | 2-(4-hydroxy-3-methoxyphenyl)-2-oxoacetic acid | 3-(4-hydroxy-3-methoxyphenyl)propanoic acid |
| m/z | 0 h | 5 h | 10 h | 15 h | 20 h |
| 121.0292 | |||||
| 137.0272 | |||||
| 149.0605 | |||||
| 151.0400 | |||||
| 165.0193 | |||||
| 165.0552 | |||||
| 189.0915 | |||||
| 193.0504 | |||||
| 195.0297 | |||||
| 195.0661 | -- | -- | -- | -- | |
| Intensity | maximum | medium | low | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenbeck, L.; Brand, S.; Stallmann, F.; Barra, V.; Frauscher, M.; Beele, B.B.; Slabon, A.; Rodrigues, B.V.M. Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics. Polymers 2024, 16, 3325. https://doi.org/10.3390/polym16233325
Lindenbeck L, Brand S, Stallmann F, Barra V, Frauscher M, Beele BB, Slabon A, Rodrigues BVM. Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics. Polymers. 2024; 16(23):3325. https://doi.org/10.3390/polym16233325
Chicago/Turabian StyleLindenbeck, Lucie, Silas Brand, Franka Stallmann, Vanessa Barra, Marcella Frauscher, Björn B. Beele, Adam Slabon, and Bruno V. Manzolli Rodrigues. 2024. "Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics" Polymers 16, no. 23: 3325. https://doi.org/10.3390/polym16233325
APA StyleLindenbeck, L., Brand, S., Stallmann, F., Barra, V., Frauscher, M., Beele, B. B., Slabon, A., & Rodrigues, B. V. M. (2024). Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics. Polymers, 16(23), 3325. https://doi.org/10.3390/polym16233325








