Bio-Inspired Polymeric Solid Lipid Nanoparticles for siRNA Delivery: Cytotoxicity and Cellular Uptake In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection and Preparation
2.3. Synthesis of Solid Lipid Nanoparticles (SLNPs)
2.4. Functionalization of SLNPs with Poly-L-Lysine (PLL)
2.5. Preparation of PLL-SLNP:siRNA Nanocomplexes
2.6. Characterization
2.7. Intercalation Assay
2.8. Band-Shift Assay
2.9. Protection Assay
2.10. Cell Culture
2.11. Cytotoxicity Studies
2.12. Caspase 3/7 Activity
2.13. Cellular Uptake Studies
2.14. Statistical Analysis
3. Results
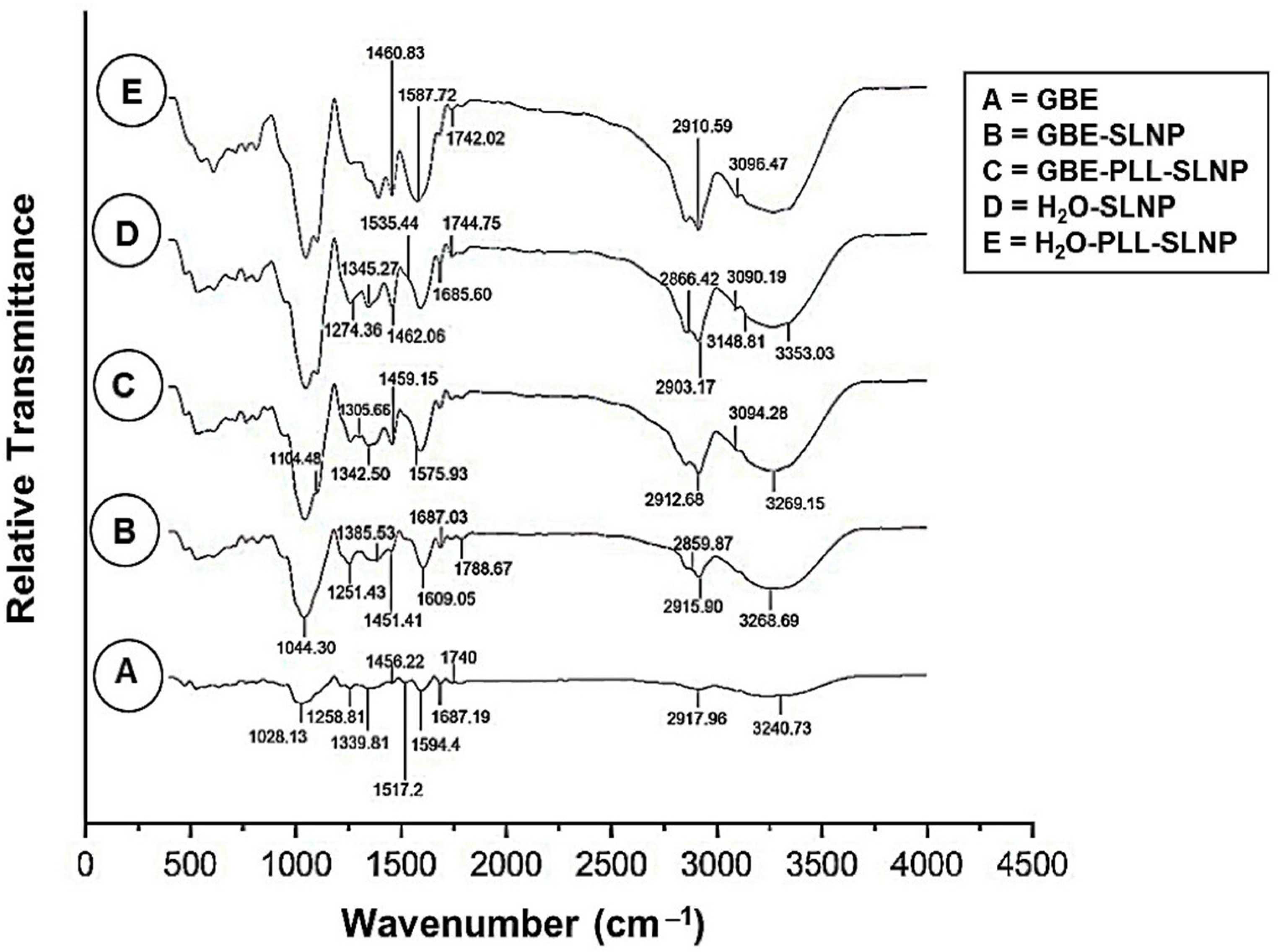
3.1. UV–Vis and FTIR Spectroscopy
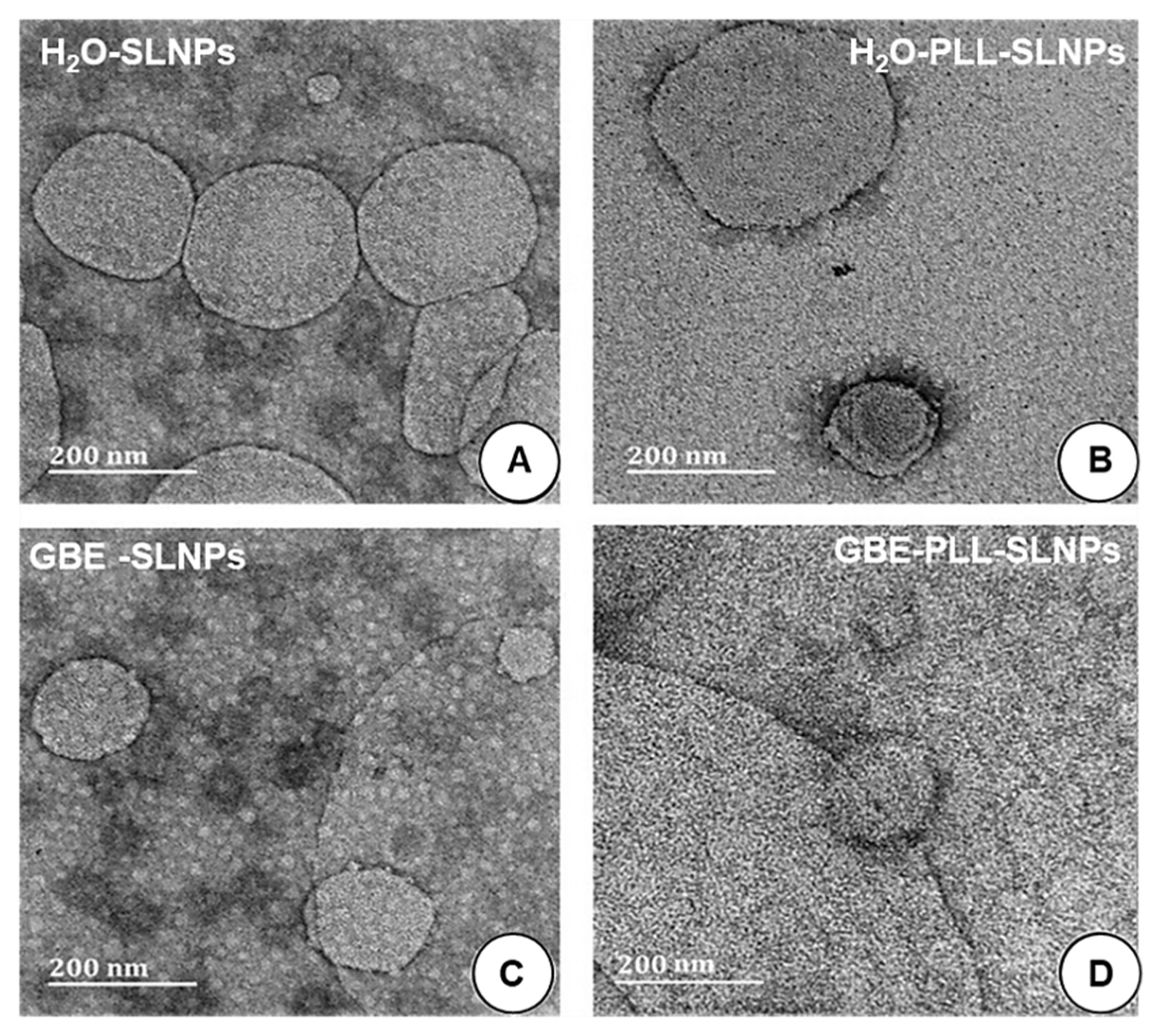
3.2. TEM and DLS
3.3. Intercalation Assay
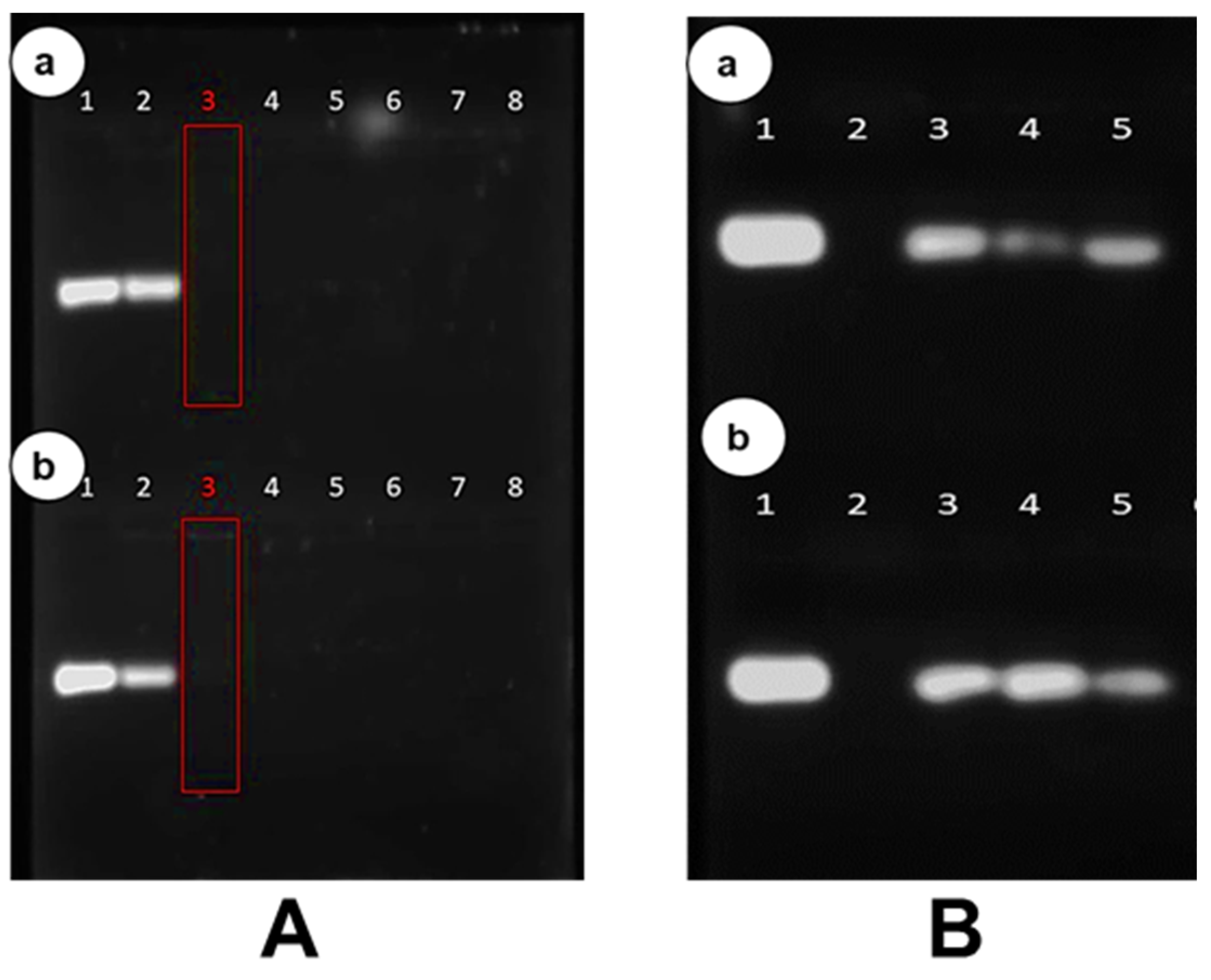
3.4. Band Shift Electrophoresis and Protection Assay
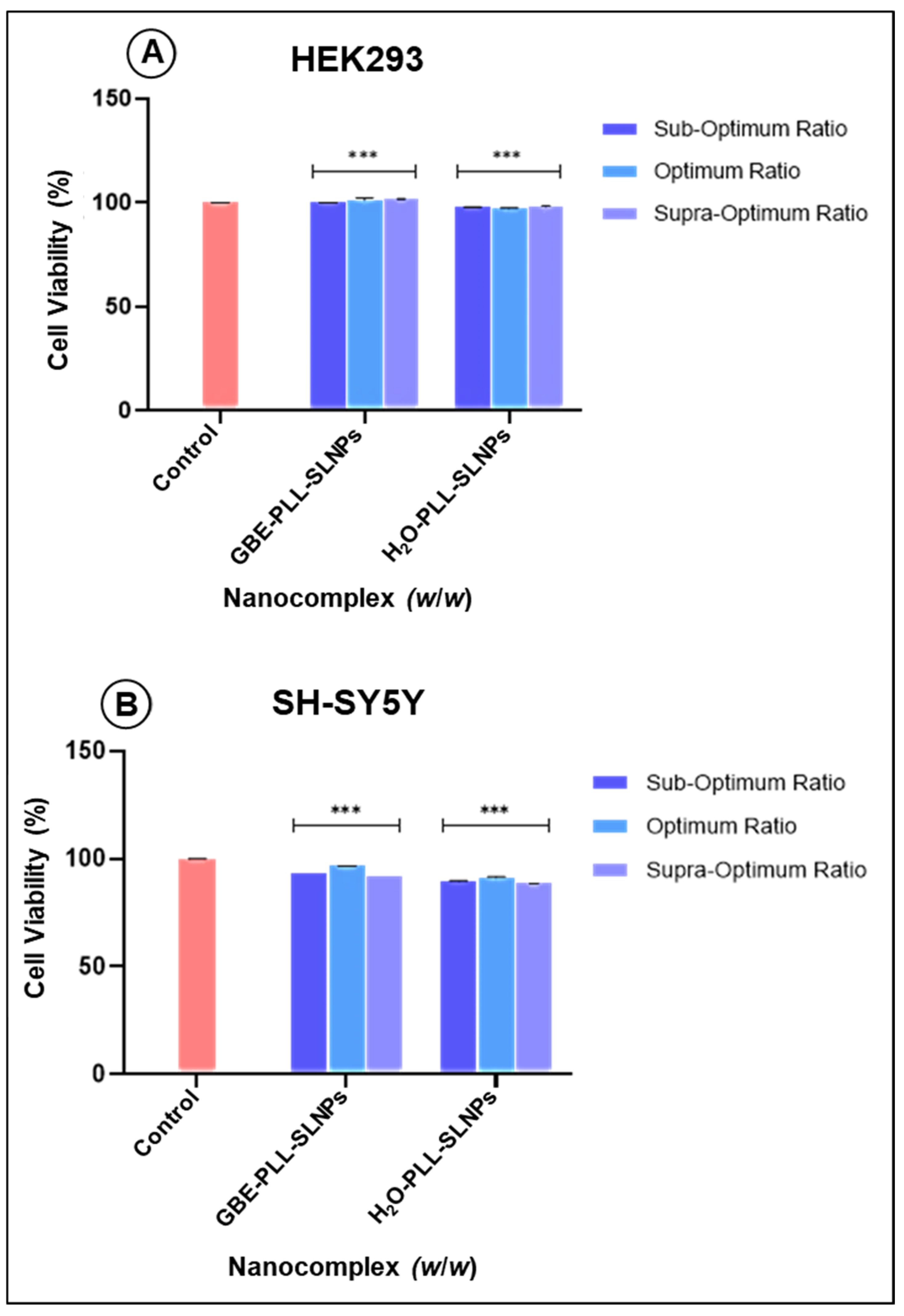
3.5. Cytotoxicity Studies
3.6. Caspase 3/7 Activity
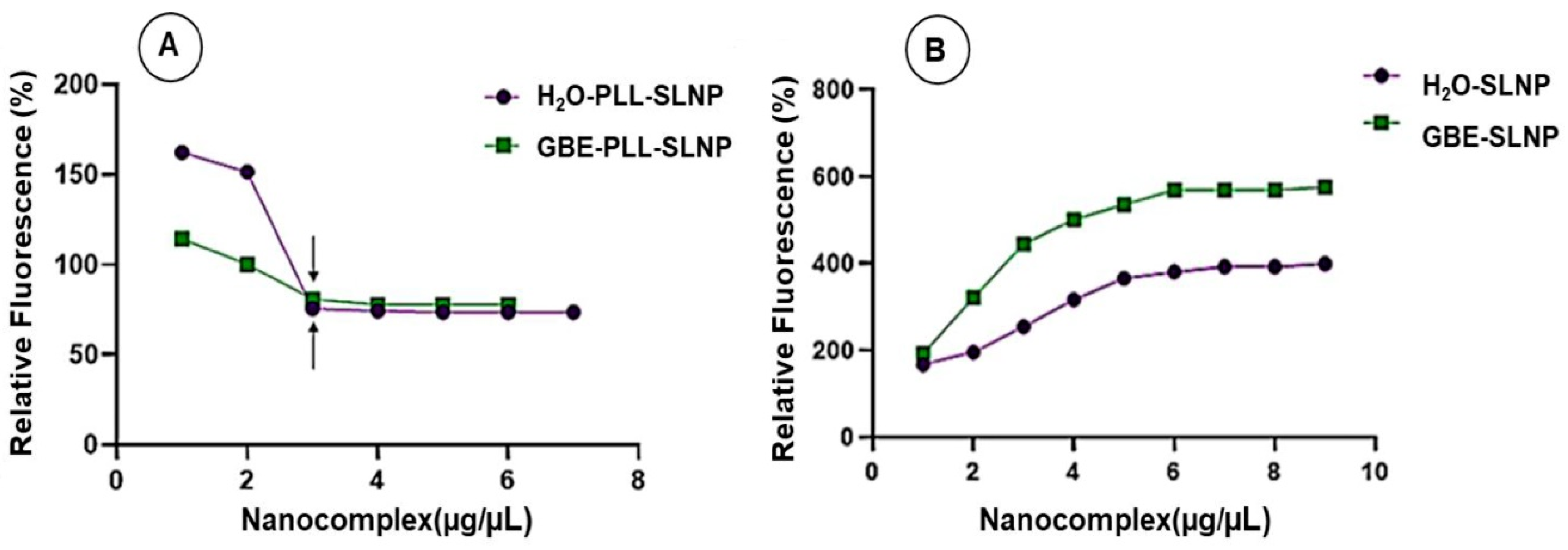
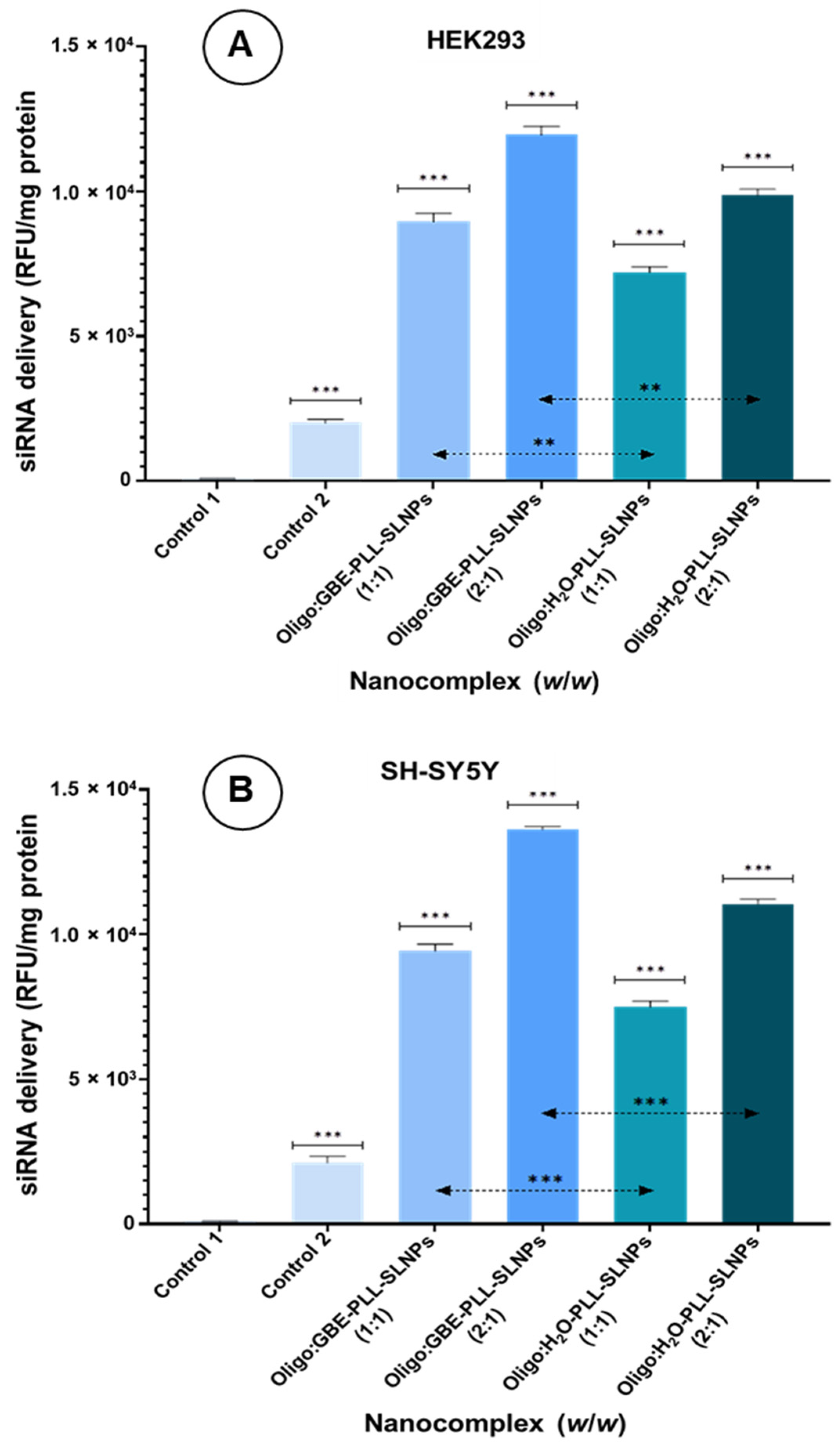
3.7. Cellular Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation characterization and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef] [PubMed]
- Balgobind, A.; Daniels, A.; Ariatti, M.; Singh, M. HER2/neu oncogene silencing in a Breast Cancer Cell Model using Cationic Lipid-Based Delivery Systems. Pharmaceutics 2023, 15, 1190. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Suresh, P. Preparation and characterization of solid lipid nanoparticles-a review. Curr. Drug Disc. Technol. 2012, 9, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Singh, M. Recent advances in lipid-based nanosystems for gemcitabine and gemcitabine–combination therapy. Nanomaterials 2021, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Samani, S.; Ghasemiyeh, P. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid-based nanocarriers: An overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef]
- Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Jafari, F.; Khodabakhshi, S. Mg(HSO4)2/SiO2 as a highly efficient catalyst for the green preparation of 2-Aryl-1,3-dioxalanes/dioxanes and linear acetals. Org. Chem. Int. 2012, 2012, 475301. [Google Scholar] [CrossRef][Green Version]
- Reddy, A.; Parthiban, S.; Vikneswari, A.; Senthilkumar, G. A modern review on solid lipid nanoparticles as novel controlled drug delivery system. Int. J. Res. Pharm. Nano Sci. 2014, 3, 313–325. [Google Scholar]
- Reddy, J.S.; Venkateswarlu, V. Novel delivery systems for drug targeting to the brain. Drug Future 2004, 29, 63–83. [Google Scholar] [CrossRef]
- D’Angelo, G.; Moorthi, S.; Luberto, C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv. Cancer Res. 2018, 140, 61–96. [Google Scholar] [CrossRef] [PubMed]
- Bienias, K.; Fiedorowicz, A.; Sadowska, A.; Prokopiuk, S.; Car, H. Regulation of sphingomyelin metabolism. Pharmacol. Rep. 2016, 68, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signaling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T. The Pathogenic Role of Ganglioside Metabolism in Alzheimer’s Disease-Cholinergic Neuron-Specific Gangliosides and Neurogenesis. Mol. Neurobiol. 2017, 54, 623–638. [Google Scholar] [CrossRef]
- Ward, M.C.; Read, M.L.; Seymour, L.W. Systemic circulation of poly(L-lysine)/DNA vectors is influenced by polycation molecular weight and type of DNA: Differential circulation in mice and rats and the implications for human gene therapy. Blood 2001, 97, 2221–2229. [Google Scholar] [CrossRef]
- Liang, W.; Xu, W.; Zhu, J.; Zhu, Y.; Gu, Q.; Li, Y.; Guo, C.; Huang, Y.; Yu, J.; Wang, W.; et al. Ginkgo biloba extract improves brain uptake of ginsenosides by increasing blood-brain barrier permeability via activating A1 adenosine receptor signaling pathway. J. Ethnopharm. 2020, 246, 112243. [Google Scholar] [CrossRef]
- Cui, Y.; Lai, X.; Liu, K.; Liang, B.; Ma, G.; Wang, L. Ginkgo biloba leaf polysaccharide stabilized palladium nanoparticles with enhanced peroxidase-like property for the colorimetric detection of glucose. RSC Adv. 2020, 10, 7012–7018. [Google Scholar] [CrossRef]
- Zauner, W.; Ogris, M.; Wagner, E. Polylysine-based transfection systems utilising receptor-mediated delivery. Adv. Drug Deliv. Rev. 1998, 30, 97–113. [Google Scholar] [CrossRef]
- Kadlecova, Z.; Rajendra, Y.; Matasci, M.; Baldi, L.; Hacker, D.L.; Wurm, F.M.; Klok, H.-A. DNA delivery with hyperbranched polylysine: A comparative study with linear and dendritic polylysine. J. Control Release 2013, 169, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.; Daniels, A.; Habib, S.; Singh, M. Poly-L-Lysine–Lactobionic Acid-Capped Selenium Nanoparticles for Liver-Targeted Gene Delivery. Int. J. Mol. Sci. 2022, 23, 1492. [Google Scholar] [CrossRef] [PubMed]
- Venkatas, J.; Daniels, A. The optimization of curcumin-capped Gold Nanoparticle synthesis for FLuc-mRNA Delivery to Cervical Cancer Cells in vitro. Biointerface Res. Appl. Chem. 2023, 13, 484. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Kato, S.; Cho, K.Y.; Tiwari, R.K. Overcoming Barriers for siRNA Therapeutics: From Bench to Bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Lai, Y.; Chern, G.; Huang, S.; Tsai, C.; Sung, Y.; Chiang, C.-C.; Hwang, P.-B.; Ho, T.-L.; Huang, R.-L.; et al. Galactose derivative-modified nanoparticles for efficient siRNA delivery to hepatocellular carcinoma. Biomacromolecules 2018, 19, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Yang, Y.L.; Liao, K.H.; Lai, T.W. Adenosine receptor agonist NECA increases cerebral extravasation of fluorescein and low molecular weight dextran independent of blood–brain barrier modulation. Sci. Rep. 2016, 6, 23882. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Folate-Targeted mRNA Delivery Using Chitosan-Functionalized Selenium Nanoparticles: Potential in Cancer Immunotherapy. Pharmaceuticals 2019, 12, 164. [Google Scholar] [CrossRef]
- Morsin, M.; Nafisah, S.; Sanudin, R.; Razali, N.L.; Mahmud, F.; Soon, C.F. The role of positively charge poly-L-lysine in the formation of high yield gold nanoplates on the surface for plasmonic sensing application. PLoS ONE 2021, 16, e0259730. [Google Scholar] [CrossRef]
- Venkatas, J.; Singh, M. Curcumin-reduced gold nanoparticles facilitate IL-12 delivery to a cervical cancer in vitro cell model. Nanomedicine 2023, 18, 945–960. [Google Scholar] [CrossRef]
- Singh, M. Assessing nucleic acid: Cationic nanoparticle interaction for gene delivery. In Bio-Carrier Vectors; Narayanan, K., Ed.; Springer: New York, NY, USA, 2021; Volume 2211, pp. 43–55. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Tripathi, M.; Lal, R.K. FTIR and Raman spectra analysis of polyherbal formulation used in treatment of neurodegenerative disorders. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 119–126. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.L.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Zhong, C. Characterization and bioactivity analysis of Ginkgo biloba leaf extract nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 2307–2315. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.S. FTIR spectroscopy study of sphingomyelin-cholesterol solid lipid nanoparticles for drug delivery applications. Int. J. Pharm. 2020, 587, 119693. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Phenolic component profiles of mustard greens, Yu Choy, and 15 other Brassica vegetables. J. Agric. Food Chem. 2007, 55, 2833–2840. [Google Scholar] [CrossRef]
- David, L.L.; Daniels, A.; Habib, S.; Singh, M. Gold Nanoparticles in Transferrin-targeted dual-drug delivery in vitro. J. Drug Deliv. Sci. Technol. 2023, 90, 105168. [Google Scholar] [CrossRef]
- Zenze, M.; Singh, M. Receptor targeting using copolymer-modified gold nanoparticles for pCMV-Luc gene delivery to liver cancer cells in vitro. Int. J. Mol. Sci. 2024, 25, 5016. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Deepalakshmi, K.; Somasundaram, S.T. Protective effect of Ginkgo biloba extract against oxidative stress-induced neurotoxicity in SH-SY5Y cells. J. Neurochem. 2021, 158, 569–582. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, H.; Wang, T.; Wang, C. Green synthesis and antimicrobial activity of monodisperse silver nanoparticles synthesized using Ginkgo Biloba leaf extract. Phys. Lett. A 2016, 380, 3773–3777. [Google Scholar] [CrossRef]
- Gallego-Urrea, J.A.; Tuoriniemi, J.; Hassellöv, M. Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples. Trends Anal. Chem. 2011, 30, 473–483. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Y.; Xu, L.; Li, J.; Yang, W. Conformational change induced reversible assembly/disassembly of poly-L-lysine-functionalized gold nanoparticles. J. Phys. Chem. 2007, 111, 9172–9176. [Google Scholar] [CrossRef]
- Zha, J.; Dong, C.; Wang, X.; Zhang, X.; Xiao, X.; Yang, X. Green synthesis and characterization of monodisperse gold nanoparticles using Ginkgo Biloba leaf extract. Optik 2017, 144, 511–521. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of copper nanoparticles using Ginkgo biloba L. leaf extract and their catalytic activity for the Huisgen [3 + 2] cycloaddition of azides and alkynes at room temperature. Colloid Interface Sci. 2015, 457, 141–147. [Google Scholar] [CrossRef]
- Akinyelu, J.; Singh, M. Chitosan stabilized Gold-Folate-Poly(lactide-co-glycolide) Nanoplexes Facilitate Efficient Gene Delivery in Hepatic and Breast Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 4478–4486. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, X.; Hu, X. Ginkgo biloba extract promotes cell proliferation and reduces UV-induced cytotoxicity in human dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2019, 35, 239–246. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, F.; Ayaz, M. Ginkgo biloba extract: An overview of its biological potentials. Clin. Phytosci. 2020, 6, 12–18. [Google Scholar]
- Wang, J.; Li, M.; Zhu, L. Enhanced viability of A549 cells by Ginkgo biloba extract-loaded nanoparticles. J. Biomed. Nanotechnol. 2021, 17, 465–472. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Zhang, J. Ginkgo biloba extract-loaded polymeric nanoparticles reduce oxidative stress and apoptosis in PC12 cells. Neurochem. Int. 2020, 137, 104748. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Taatjes, D.J.; Sobel, B.E.; Budd, R.C. Morphological and cytochemical determination of cell death by apoptosis. Histochem. Cell Biol. 2008, 129, 33–43. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Krieglstein, J. Neuroprotective effects of Ginkgo biloba extract. Cell. Mol. Life Sci. 2003, 60, 1779–1792. [Google Scholar] [CrossRef]
- Fisher, T.L.; Terhorst, T.; Cao, X.; Wagner, R.W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993, 21, 3857–3865. [Google Scholar] [CrossRef]
- Pozzi, D.; Marchini, C.; Cardarelli, F.; Amenitsch, H.; Garulli, C.; Bifone, A.; Caracciolo, G. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim. Biophys. Acta 2012, 1818, 2335–2343. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638. [Google Scholar] [CrossRef]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Rogers, J.; Xie, J. Ginkgo biloba extract in Alzheimer’s disease: From action mechanisms to medical practice. Int. J. Mol. Sci. 2011, 12, 764–797. [Google Scholar]
- Wang, Q.; Li, X.; Li, Y.; Chen, B.; Zhang, T. Role of flavonoids in enhancing receptor-mediated endocytosis for targeted drug delivery. J. Nanomed. Nanotechnol. 2019, 10, 500–512. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Jiang, Y.; Wang, L. Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, Y.; Li, Y.; Yang, Z.; Wang, L. Ginkgo biloba extract attenuates the disruption of pro- and anti-inflammatory balance of peripheral blood in arsenism patients by decreasing hypermethylation of the Foxp3 promoter region. Biol. Trace Elem. Res. 2022, 200, 4967–4976. [Google Scholar] [CrossRef]
- Allmann, S.; Li, J.; Hou, Z.; Fan, Z. Ginkgo biloba extract enhances nanoparticle uptake in hepatocytes through oxidative stress modulation. Nucleic Acids Res. 2020, 48, 12085–12101. [Google Scholar] [CrossRef]
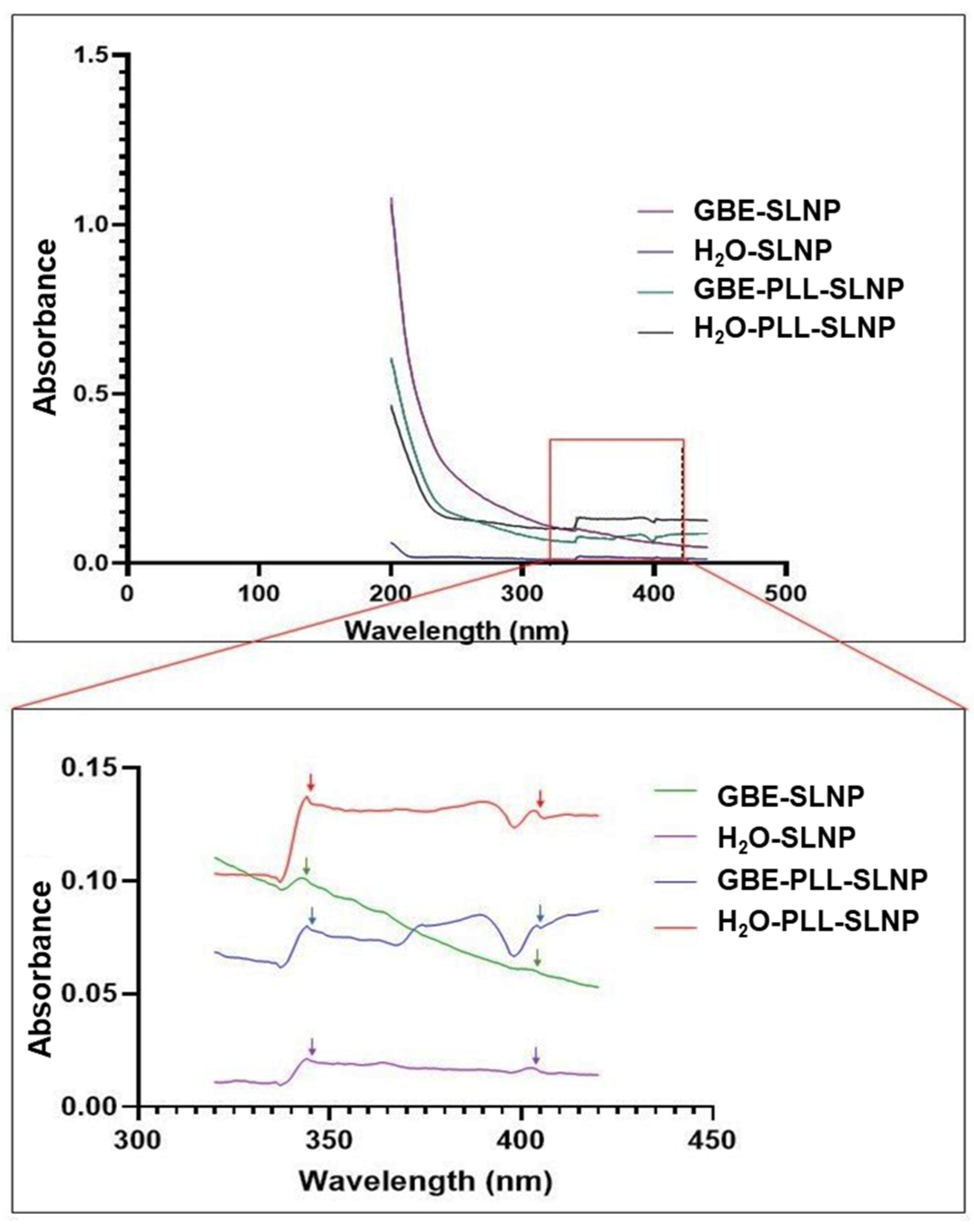

| Nanoparticles | Peak 1 | Peak 2 | Trough |
|---|---|---|---|
| SLNPS | 343 nm | 401 nm | - |
| PLL-SLNPS | 345 nm | 402 nm | 400 nm |
| GBE-SLNPS | 345 nm | 401 nm | - |
| GBE-PLL-SLNPS | 341 nm | 404 nm | 397 nm |
| Functional Group | Wavenumber (cm−1) | Interpretation |
|---|---|---|
| O-H | 3297.30 | Hydroxyl groups in phenolic compounds |
| C-H | 2918.90 | Methyl and methylene groups of lipids |
| C=O | 1789.10 | Carbonyl groups |
| C=C | 1674.00 | Alkene groups |
| N-H | 1595.40 | Amide groups in proteins or peptides |
| C-O-C | 1258.00 | Ester and ether linkages |
| O-H | 3268.69 | Incorporation of phenolic compounds from GBE |
| C-H | 2915.90, 2859.87 | Aliphatic chains in sphingomyelin and cholesterol |
| C=O | 1788.67 | Hydrogen bonding, successful encapsulation |
| C-O | 1251.53 | Presence of esters and ethers from GBE |
| N-H | 3094.28 | Conjugation of PLL to SLNPs |
| C-H | 2912.68 | Aliphatic chains in lipids and PLL |
| C=O | 1742.15 | Conjugation of PLL to GBE |
| C-N | 1305.66 | Amine groups from PLL |
| O-H | 3353.03 | Hydration of lipid components |
| N-H | 3148.81, 3090.19 | Presence of sphingomyelin within SLNPs |
| C-H | 2903.17 | Aliphatic chains in cholesterol and sphingomyelin |
| C=O | 1744.75 | Ester groups in the SLNP matrix |
| N-H | 3096.43 | Interaction between PLL and the lipid matrix |
| C=O | 1742.01 | Electrostatic interactions between lipids and PLL |
| NPs | TEM | DLS | |||||
|---|---|---|---|---|---|---|---|
| Nanoparticle | Nanocomplex | ||||||
| Size (nm ± SD) | Size (nm ± SD) | Zeta Potential (mV) Mean ± SD (n = 3) | PDI | Size (nm ± SD) | Zeta Potential (mV) Mean ± SD (n = 3) | PDI | |
| H2O-SLNPs | 180.2 ± 25.3 | 190.5 ± 13.9 | 13.1 ± 1.1 | 0.003 | - | - | - |
| H2O-PLL-SLNPs | 140.0 ± 1.0 | 120.4 ± 7.1 | 35.6 ± 0.3 | 0.013 | 139.5 ± 35.2 | 43.3 ± 0.1 | 0.037 |
| GBE-SLNPs | 118.3 ± 15.6 | 122.4 ± 12.7 | 24.4 ± 1.3 | 0.012 | - | - | - |
| GBE-PLL-SLNPs | 136.3 ± 1.0 | 115.3 ± 10.7 | 36.8 ± 1.3 | 0.010 | 128.3 ± 20.3 | 45.4 ± 0.8 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagaran, K.; Habib, S.; Singh, M. Bio-Inspired Polymeric Solid Lipid Nanoparticles for siRNA Delivery: Cytotoxicity and Cellular Uptake In Vitro. Polymers 2024, 16, 3265. https://doi.org/10.3390/polym16233265
Jagaran K, Habib S, Singh M. Bio-Inspired Polymeric Solid Lipid Nanoparticles for siRNA Delivery: Cytotoxicity and Cellular Uptake In Vitro. Polymers. 2024; 16(23):3265. https://doi.org/10.3390/polym16233265
Chicago/Turabian StyleJagaran, Keelan, Saffiya Habib, and Moganavelli Singh. 2024. "Bio-Inspired Polymeric Solid Lipid Nanoparticles for siRNA Delivery: Cytotoxicity and Cellular Uptake In Vitro" Polymers 16, no. 23: 3265. https://doi.org/10.3390/polym16233265
APA StyleJagaran, K., Habib, S., & Singh, M. (2024). Bio-Inspired Polymeric Solid Lipid Nanoparticles for siRNA Delivery: Cytotoxicity and Cellular Uptake In Vitro. Polymers, 16(23), 3265. https://doi.org/10.3390/polym16233265








