Application of Chitosan and Its Derivatives Against Plant Viruses
Abstract
1. Introduction
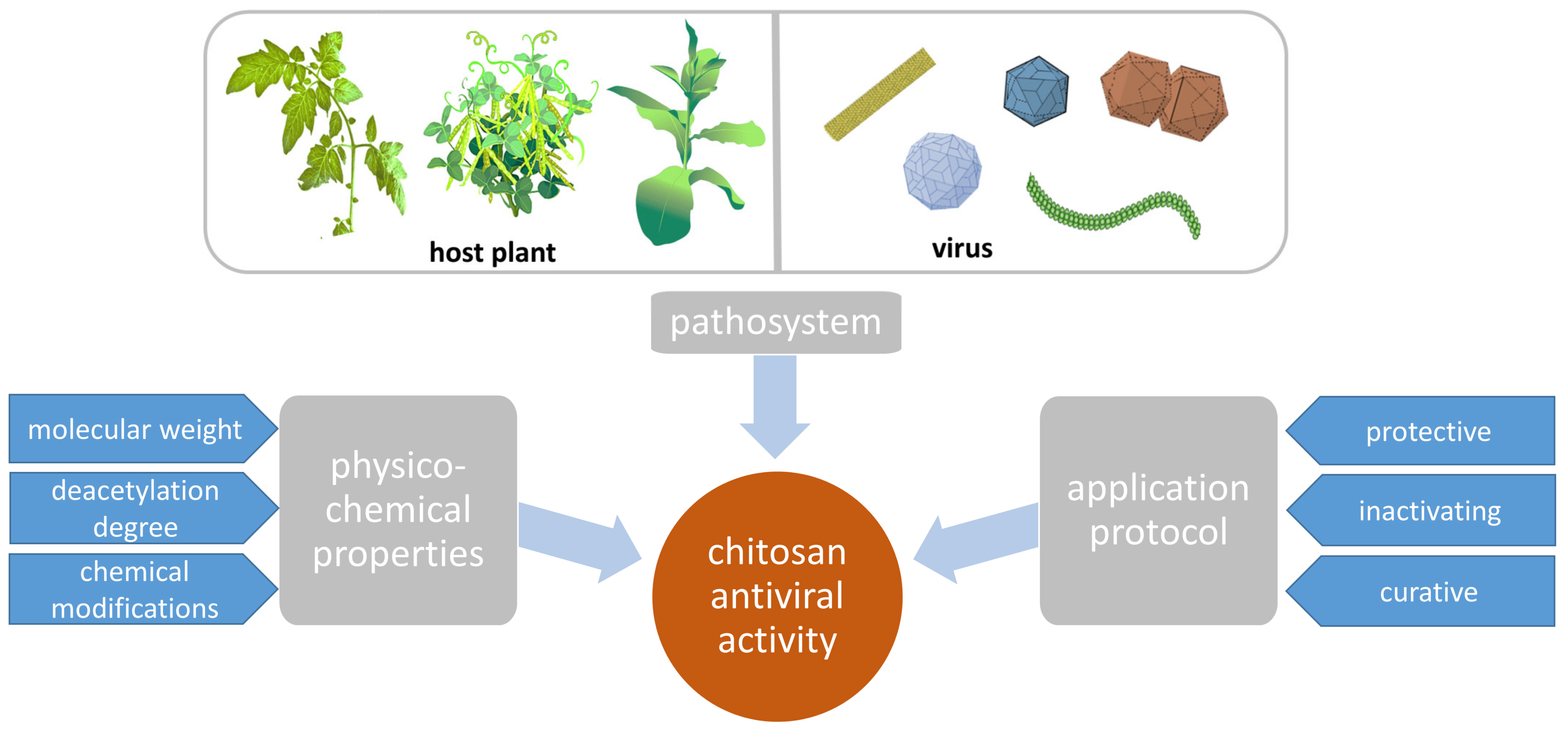
2. Antiviral Activity of CHT
2.1. Pathosystems
| Plant | Virus | Type of Chitosan | Treatment Protocol | Effect | References |
|---|---|---|---|---|---|
| Phaseolus vulgaris (bean) | Tobacco necrosis virus (TNV) | CHT from the Antarctic krill; CHT with a 76 kDa MW and a 85% deacetylation degree (DD) | Plant treatment with 0.1 or 0.15% CHT solution 1 day before inoculation | Reduction in the number of TNV-induced local lesions by 75–100% | [36,39] |
| Alfalfa mosaic virus (AMV) | CHT from krill and crab | (1) 0.1 to 0.00001% CHT 1 day before inoculation (2) Short-time (applied for 5 min and rinsed with water) treatment with 0.01–0.25% CHT 1 day before inoculation (3) 0.1 to 0.00001% CHT mixed with inoculum (4) The lower leaf sprayed with 0.1% CHT solution and non-treated leaf of the same plant was inoculated | The highest efficiency of inhibition was shown for 0.1–0.001% CHT solutions; however, when applied together with inoculum, CHT solution even in 0.00001% concentration inhibited viral infection. Moreover, 40–60% of local lesion reduction was observed in non-treated leaves of the CHT-sprayed plants. | [36,37] | |
| Bean golden mosaic virus (BGMV) | NS | Plant treatment 1 or 3 times weekly with 0.1% CHT solution before inoculation | Protection from infection: no symptoms on CHT-treated plants were detected 3 weeks after inoculation | [40] | |
| Peanut stunt virus (PSV) | CHT from the Antarctic krill | Plant treatment with 0.1% CHT solution 1 day before inoculation | Reduction in the number of systemically infected plants by 50–75% | [36] | |
| Bean mild mosaic virus (BMMV) | Four chitosan fractions with an MW of 1.2, 2.2, 10.1, and 30.3 kDa as well as non-fractionated CHT with an average MW 40.4 | Plant treatment (spray) with 0.001 or 0.01% CHT solution before inoculation | All tested fractions as well as unfractionated CHT suppressed infection development in the inoculated leaves for at least 8 days. Plants treated with the low-molecular -weight CHT (Mw = 2.2 and 1.2 kDa) displayed no systemic infection by the 14th day after inoculation | [38] | |
| Pisum sativum (pea) | AMV, PSV | CHT from the Antarctic krill | Plant treatment with 0.1% CHT solution 1 day before inoculation | Reduction in the number of systemically infected plants by 50–75% | [36] |
| Solanum lycopersicum (tomato) | Potato spindle tuber viroid (PSTV) | CHT from the Antarctic krill | (1) 0.001–0.1% CHT solution was added to inoculum 10 min before plant treatment (2) 0.1% CHT solution was applied as a foliar spray 1 day before inoculation (3) Plants were treated with 0.1% CHT solution 1, 3, 5, 7, or 24 h after inoculation | Inactivating and protective treatment gave the best effect: 85–100% of plants were not infected after 0.01 or 0.1% CHT application; protective treatment resulted in an average of 78% resistant plants. Curative treatment was effective only for first 3 h (60–80% of resistant plants) | [53] |
| Tobacco mosaic virus (TMV), potato virus X (PVX) | CHT from the Antarctic krill | Plant treatment with 0.1% CHT solution 1 day before inoculation | Reduction in the number of systemically infected with PVX plants by 50–75% | [36] | |
| Cucumber mosaic virus (CMV) | CHT with an MW 50–190 kDa and a 75–85% DD | Plants were sprayed with 0.1% CHT solution (10 mL per plant) and inoculated with CMV 24 h later | Significant reduction in CMV accumulation in plants treated with CHT at the 20th (up to 86%) and the 90th (100% virus elimination) day after inoculation | [46] | |
| Tomato leaf curl virus (ToLCV) | NS | Tomato seeds were soaked in 5% CHT solution, and 25 days after sowing, leaves were sprayed with 0.1% CHT | Reduction in disease severity by ~85% and 75% on the 45th and 75th day after inoculation | [54] | |
| Solanum tuberosum (potato) | PVX, PVS, AMV | CHT with an MW of 3 and36 kDa and 85% DD was obtained from crab CHT using enzymatic digestion; 120 kDa CHT with 69% DD was obtained from krill | Non-infected potato plants were sprayed with CHT solution (0.1%), and 1, 2, 3, or 4 days later, leaves were (1) detached and inoculated with PVX; after that, 1 cm disks were cut off from these leaves and incubated for 6 days in Petri dishes on the surface of distilled water (2) The treated leaves (still attached to the plant) were inoculated with PVX, and the efficiency of systemic infection was assessed 3 weeks later in the upper non-inoculated leaves (3) Cuttings from plants infected with PVX or PVS were put into the liquid Murashige and Skoog medium with or without CHT; virus accumulation levels were assessed after a month (curative treatment) | (1) The disks from treated leaves (120 kDa CHT had the best effect) accumulated a significantly lowerconcentration of the virus than the control (2) The whole plants sprayed with CHT and infected with PVX demonstrated resistance to PVX (120 kDa CHT treatment gave the best effect) (3) No reduction in PVS or PVX levels in the infected plants was observed (no curative effect) | [55] |
| Nicotiana tabacum (tobacco) | TMV | CHT from the Antarctic krill; CHT preparations of high MW (130–500 kDa) or low MW (from 2 to 17.0 kDa) and different DD | Plant treatment with 0.1% CHT solution 1 day before inoculation; inactivating treatment (CHT mixed with inoculum); protoplasts incubation with 0.1 or 0.01% CHT | Reduction in the number of TMV-induced local lesions by ~20–50% depending on experimental set-up. Low-MW CHT (2–17 kDa) was shown to be the most effective (up to 90% reduction in the number of TMV-induced local lesions). Tobacco protoplasts’ incubation with CHT led to their partial resistance to TMV | [15,36,42,44,45] |
| TNV | CHT with a 85% DD and a 2500–3000 polymerization degree (MW ~ 400–500 kDa) | Plant treatment with 0.1% CHT solution 2 days before inoculation | Reduction in the number of TNV local lesions by a range from 32% to 83%; BY-2 cells incubated with CHT (from 0.01 to 0.1%) demonstrated typical morphological features of apoptosis | [43] | |
| CMV | CHT with an MW of 50–190 kDa and a DD of 75–85% | Plants were sprayed with 0.1% CHT solution and inoculated with CMV 24 h later | 11 days after inoculation, CHT-treated plants showed no symptoms while untreated plants showed mosaic | [46] | |
| Nicotiana glutinosa | CMV, pepper mild mottle virus (PMMoV) | 600 kDa CHT, 80–95% DD, and a compound obtained from CHT and ammonium polyphosphate (P-CHT) | Foliar application of 0.01%, 0.05%, and 0.1% solution of CHT or P-CHT daily 3 times before inoculation | The number of PMMoV local lesions reduced depending on CHT concentration: the best effect was obtained for plants treated with 0.1% CHT (~75% decrease), while P-CHT appeared to be less effective | [47] |
| Capsicum annum (chili pepper) | CMV | 600 kDa CHT, 80–95% DD, and a compound obtained from CHT and ammonium polyphosphate (P-CHT) | Foliar application of 0.01%, 0.05%, and 0.1% solution of CHT or P-CHT daily 3 times before inoculation | The accumulation of CMV (as detected via ELISA) in the plants treated with 0.1% CHT- or P-CHT was lower by ~30% compared to the untreated plants; moreover, the symptoms of infection in the treated plants were markedly less severe | [47] |
| Datura stramonium (stramony) | TMV, figwort mosaic virus (FMV) | NS | Plant treatment with 0.2% CHT solution weekly 3 times before inoculation | The percent of plants with FMV symptoms decreased 3-fold compared to untreated plants. Local lesion production caused by TMV was moderately inhibited on CHT-treated young leaves | [40] |
| Arabidopsis thaliana | TMV | CHT oligosaccharides | Plants were treated with 0.005% CHT oligosaccharides and 1 day later challenged with TMV | TMV coat protein content was 4-fold reduced in CHT-treated plants compared to the control infected group. In addition, pre-treatment with CHT resulted in a ~25–30% decrease in the disease index and amount of necrotic cells in the TMV-inoculated leaves | [48] |
| Brassica campestris (turnip) * | Cauliflower mosaic virus (CaMV) | NS | Plant treatment with 0.1 or 0.3% CHT or its chemically modified polyanionic form solution a day before inoculation | 2–3 weeks after infection, no protective effect of CHT was observed (symptoms developed in the same manner—time and severity—as for non-treated plants); virus accumulation was confirmed via ELISA | [40] |
| Turnip mosaic virus (TuMV), radish mosaic virus (RaMV) | NS | Plant treatment with 0.1 or 0.3% CHT | 2–3 weeks after infection, no protective effect of CHT was observed (symptoms developed in the same manner—time and severity—as for non-treated plants); virus accumulation was confirmed via ELISA for TuMV or double immunodiffusion test in agar gel for RMV | [40] | |
| Cucumis sativus (cucumber) | Squash mosaic virus (SqMV) | NS | Seeds treatment with 0.9% CHT solution for 1 h. 2, 4, and 6 weeks after planting, leaves were sprayed with the same solution | CHT application significantly delayed appearance of symptoms and reduced disease severity as well as virus titer, especially in the generative phase | [56] |
| Carica papaya (papaya) | Papaya ringspot virus (PRSV) | NS | Root irrigation andfoliar spraying of papaya plants with 200 µL of 0.5% CHT solution 1 day before virus inoculation; treatment was performed at seedling stage or at fruiting stage | The disease index (based on evaluation of symptoms severity) was reduced in CHT-treated plans more than two-fold compared to control untreated plants; the difference was registered up to the 42nd day after inoculation | [57] |
| Passiflora spp. (passiflora) | CMV | CHT oligosaccharides | Passiflora seedlings were sprayed with ~0.007% CHT solution daily 3 times and then inoculated with CMV | CMV virulence halved in plants treated with COS, and the CMV RNA level reduced both in the laboratory and field experiments | [58] |
| Chenopodium quinoa (quinoa) | TNV, CMV | CHT from the Antarctic krill | Plant treatment with 0.1% CHT solution 1 day before inoculation | Reduction in the number of TNV-induced local lesions by 25–50% and CMV-induced lesions by 50–75% | [36] |
| CHT-based nanoparticles (NPs) | |||||
| Nicotiana benthamiana | Potato virus Y (PVY) | CHT quaternary ammonium salt NP | Plant treatment with CQAS NPs via root soaking, foliar spraying, or infiltration | More than a 25-fold decrease in viral CP RNA accumulation in plants pre-treated with CQAS NPs,; however, Western blot analysis of CP level demonstrates only a moderate reduction in CP accumulation | [59] |
| Nicotiana glutinosa | AMV | CHT-dextran-NPs were made of 100–300 kDa CHT and dextran sulfate using the ionic gelation method. Hydrodynamic diameter range of the CHT-dextran-NPs was between 20 and 160 nm, with an average diameter of 91.68 nm | Protective (before inoculation), curative (after inoculation), or inactivating (mixed with inoculum) treatment of plants with 100 µg/mL of CHT-dextran NPs | Protective and inactivating treatment gave better results than curative treatment; virus accumulation was assessed via ELISA in extracts from systemic leaves 22 days after inoculation | [60] |
| Capsicum annum (chili pepper) | AMV | CHT-based NPs were prepared from 50 to 190 kDa CHT (75–85% DD). CHT was cross-linked with TPP; CHT-NPs were spherical, with a hydrodynamic diameter of 37.8 nm and a zeta potential of +48.4 mV. CHT-silver NPs (CHT-Ag-NPs) were obtained via chitosan reduction of silver nitrate and were spherical, with a hydrodynamic diameter of 12.55 nm and a zeta potential of +65.1 mV. | Pepper seedlings were treated with 0.1–0.4 mg/mL CHT NPs or 0.05–0.2 mg/mL CHT-Ag NPs 1 day before or 1 day after AMV inoculation or immediately after inoculation | The most prominent effect (90% of inhibition) of CHT-NPs and CHT-Ag-NPs was obtained when they were applied 1 day after virus inoculation (curative effect). Virus accumulation was assessed 21 days after inoculation in extracts of systemic leaves via ELISA | [61] |
2.2. Dependency of CHT Effect on Its Molecular Weight, Deacetylation Degree, and Charge
2.3. CHT-Based Nanoparticles to Fight Plant Virus Infection
2.4. Protective, Inactivating, and Curative Activity of CHT
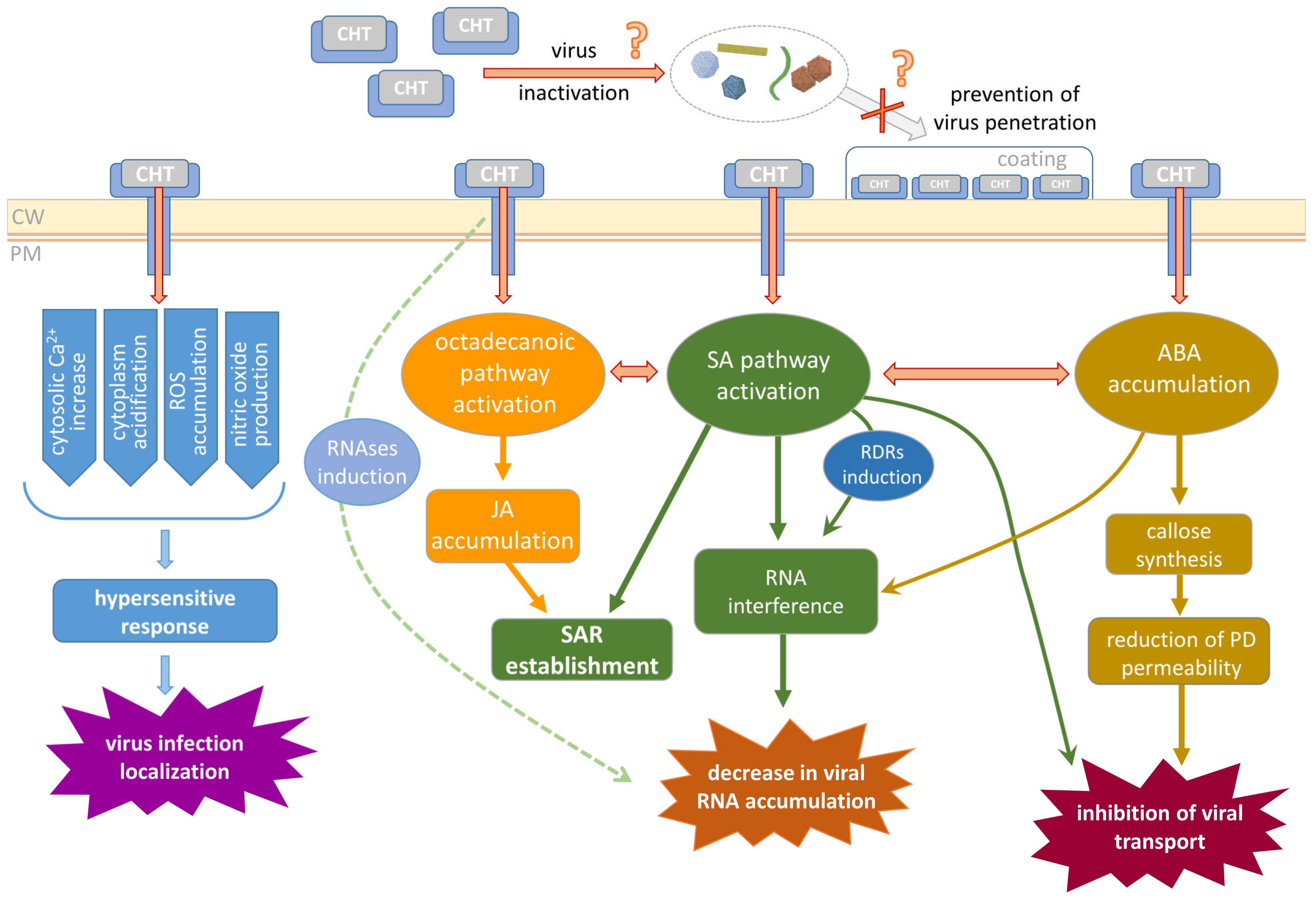
3. Mechanisms Underlying CHT Biological Effects
3.1. CHT Perception and Early Events
3.2. CHT-Induced Activation of Hormone-Dependent Defense Reactions
3.3. The Role of Callose in CHT-Mediated Plant Defense
3.4. Putative Mechanisms of CHT Antiviral Activity
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munir, S.; Azeem, A.; Sikandar Zaman, M.; Zia Ul Haq, M. From Field to Table: Ensuring Food Safety by Reducing Pesticide Residues in Food. Sci. Total Environ. 2024, 922, 171382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, W. Reproductive and Developmental Toxicity of Plant Growth Regulators in Humans and Animals. Pestic. Biochem. Physiol. 2023, 196, 105640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Arcot, Y.; Medina, R.F.; Bernal, J.; Cisneros-Zevallos, L.; Akbulut, M.E.S. Integrated Pest Management: An Update on the Sustainability Approach to Crop Protection. ACS Omega 2024, 9, 41130–41147. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers 2023, 15, 3150. [Google Scholar] [CrossRef]
- Shrestha, R.; Thenissery, A.; Khupse, R.; Rajashekara, G. Strategies for the Preparation of Chitosan Derivatives for Antimicrobial, Drug Delivery, and Agricultural Applications: A Review. Molecules 2023, 28, 7659. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Mu, L.; Wu, L.; Wu, S.; Ye, Q.; Zhong, Z. Progress in Chitin/Chitosan and Their Derivatives for Biomedical Applications: Where We Stand. Carbohydr. Polym. 2024, 343, 122233. [Google Scholar] [CrossRef]
- Perelygin, V.V.; Zharikov, M.V.; Zmitrovich, I.V.; Nekrasova, T.A. Chitin and Its Derivative Chitosan: Distribution in Nature, Applications, and Technology Research (A Review). Int. J. Med. Mushrooms 2024, 26, 69–81. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The Versatile Biopolymer Chitosan: Potential Sources, Evaluation of Extraction Methods and Applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef]
- Davydova, V.N.; Nagorskaya, V.P.; Gorbach, V.I.; Kalitnik, A.A.; Reunov, A.V.; Solov’eva, T.F.; Ermak, I.M. Chitosan Antiviral Activity: Dependence on Structure and Depolymerization Method. Appl. Biochem. Microbiol. 2011, 47, 103–108. [Google Scholar] [CrossRef]
- Lamarque, G.; Lucas, J.-M.; Viton, C.; Domard, A. Physicochemical Behavior of Homogeneous Series of Acetylated Chitosans in Aqueous Solution: Role of Various Structural Parameters. Biomacromolecules 2005, 6, 131–142. [Google Scholar] [CrossRef]
- Berth, G.; Dautzenberg, H. The Degree of Acetylation of Chitosans and Its Effect on the Chain Conformation in Aqueous Solution. Carbohydr. Polym. 2002, 47, 39–51. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan Functionalization: Covalent and Non-Covalent Interactions and Their Characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef]
- Komarova, T.; Ilina, I.; Taliansky, M.; Ershova, N. Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells. Int. J. Mol. Sci. 2023, 24, 16665. [Google Scholar] [CrossRef] [PubMed]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Varma, R.S. A Review of Chitosan Nanoparticles: Nature’s Gift for Transforming Agriculture through Smart and Effective Delivery Mechanisms. Int. J. Biol. Macromol. 2024, 260, 129522. [Google Scholar] [CrossRef] [PubMed]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Faoro, F.; Gozzo, F. Is Modulating Virus Virulence by Induced Systemic Resistance Realistic? Plant Sci. Int. J. Exp. Plant Biol. 2015, 234, 1–13. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Chitosan-Induced Antiviral Activity and Innate Immunity in Plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 2935–2944. [Google Scholar] [CrossRef]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Li, B.; Liu, B.; Shan, C.; Ibrahim, M.; Lou, Y.; Wang, Y.; Xie, G.; Li, H.; Sun, G. Antibacterial Activity of Two Chitosan Solutions and Their Effect on Rice Bacterial Leaf Blight and Leaf Streak. Pest Manag. Sci. 2013, 69, 312–320. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Francis, J.; Bowrin, V.; Ramjegathesh, R.; Ramsubhag, A.; Jayaraman, J. Bio-Efficacy of a Chitosan Based Elicitor on Alternaria Solani and Xanthomonas Vesicatoria Infections in Tomato under Tropical Conditions. Ann. Appl. Biol. 2016, 169, 274–283. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Han, X.; Du, Y. Antifungal Activity of Oligochitosan against Phytophthora Capsici and Other Plant Pathogenic Fungi in Vitro. Pestic. Biochem. Physiol. 2007, 87, 220–228. [Google Scholar] [CrossRef]
- Falcón, A.B.; Cabrera, J.C.; Costales, D.; Ramírez, M.A.; Cabrera, G.; Toledo, V.; Martínez-Téllez, M.A. The Effect of Size and Acetylation Degree of Chitosan Derivatives on Tobacco Plant Protection against Phytophthora Parasitica Nicotianae. World J. Microbiol. Biotechnol. 2008, 24, 103–112. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Akila, G.; Einstein Charles, R. Chitosan-Induced Defence Responses in Tomato Plants against Early Blight Disease Caused by Alternaria Solani (Ellis and Martin) Sorauer. Arch. Phytopathol. Plant Prot. 2014, 47, 1777–1787. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Steurbaut, W.; Rogge, T.M.; Stevens, C.V.; Smagghe, G.; Höfte, M. Insecticidal and Fungicidal Activity of New N,O-Acyl Chitosan Derivatives. Commun. Agric. Appl. Biol. Sci. 2004, 69, 793–797. [Google Scholar] [PubMed]
- Rabea, E.I.; Badawy, M.E.I.; Rogge, T.M.; Stevens, C.V.; Höfte, M.; Steurbaut, W.; Smagghe, G. Insecticidal and Fungicidal Activity of New Synthesized Chitosan Derivatives. Pest Manag. Sci. 2005, 61, 951–960. [Google Scholar] [CrossRef]
- Haas, J.; Lozano, E.R.; Haida, K.S.; Mazaro, S.M.; de Souza Vismara, E.; Poppy, G.M. Getting Ready for Battle: Do Cabbage Seeds Treated with Jasmonic Acid and Chitosan Affect Chewing and Sap-Feeding Insects? Entomol. Exp. Appl. 2018, 166, 412–419. [Google Scholar] [CrossRef]
- Pospieszny, H.; Chirkov, S.; Atabekov, J. Induction of Antiviral Resistance in Plants by Chitosan. Plant Sci. 1991, 79, 63–68. [Google Scholar] [CrossRef]
- Chirkov, S.N.; Surgucheva, N.A.; Gamzazade, A.I.; Abdulabekov, I.M.; Pospieszny, H. Comparative Efficiency of Chitosan Derivatives at Inhibition of Plants Virus Infection. Dokl. Akad. Nauk SSSR 1998, 360, 271–273. [Google Scholar]
- Kulikov, S.N.; Chirkov, S.N.; Il’ina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Prikl. Biokhim. Mikrobiol. 2006, 42, 224–228. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Abscisic Acid Is Involved in Chitosan-Induced Resistance to Tobacco Necrosis Virus (TNV). Plant Physiol. Biochem. PPB 2008, 46, 1106–1111. [Google Scholar] [CrossRef]
- Chirkov, S.N.; Surguchova, N.; Atabekov, J.G. Chitosan Inhibits Systemic Infections Caused by DNA-containing Plant Viruses. Arch. Phytopathol. Plant Prot. 1994, 29, 21–24. [Google Scholar] [CrossRef]
- Faoro, F.; Iriti, M. Callose Synthesis as a Tool to Screen Chitosan Efficacy in Inducing Plant Resistance to Pathogens. Caryologia 2007, 60, 121–124. [Google Scholar] [CrossRef]
- Chirkov, S.N.; Surgucheva, N.A.; Atabekov, J.G. Stimulation of the Synthesis of Cell Proteins and Inhibition of Viral Infections by Chitosan in Isolated Tobacco Protoplast. Dokl. Akad. Nauk SSSR 1995, 341, 836–838. [Google Scholar]
- Iriti, M.; Sironi, M.; Gomarasca, S.; Casazza, A.P.; Soave, C.; Faoro, F. Cell Death-Mediated Antiviral Effect of Chitosan in Tobacco. Plant Physiol. Biochem. 2006, 44, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Nagorskaya, V.P.; Reunov, A.V.; Lapshina, L.A.; Davydova, V.N.; Yermak, I.M. Electron Microscopic Study of Chitosan Action on Intracellular Accumulation and the State of Tobacco Mosaic Virus Particles in Tobacco Leaves. Cell Tissue Biol. 2011, 5, 171–177. [Google Scholar] [CrossRef]
- Nagorskaya, V.; Reunov, A.; Lapshina, L.; Davydova, V.; Yermak, I. Effect of Chitosan on Tobacco Mosaic Virus (TMV) Accumulation, Hydrolase Activity, and Morphological Abnormalities of the Viral Particles in Leaves of N. tabacum L. Cv. Samsun. Virol. Sin. 2014, 29, 250–256. [Google Scholar] [CrossRef]
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-Elicited Defense Responses in Cucumber Mosaic Virus (CMV)-Infected Tomato Plants. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef]
- Gangireddygari, V.S.R.; Chung, B.N.; Cho, I.-S.; Yoon, J.-Y. Inhibitory Effect of Chitosan and Phosphate Cross-Linked Chitosan against Cucumber Mosaic Virus and Pepper Mild Mottle Virus. Plant Pathol. J. 2021, 37, 632–640. [Google Scholar] [CrossRef]
- Jia, X.; Meng, Q.; Zeng, H.; Wang, W.; Yin, H. Chitosan Oligosaccharide Induces Resistance to Tobacco Mosaic Virus in Arabidopsis via the Salicylic Acid-Mediated Signalling Pathway. Sci. Rep. 2016, 6, 26144. [Google Scholar] [CrossRef]
- Chen, H.-P.; Xu, L.-L. Isolation and Characterization of a Novel Chitosan-Binding Protein from Non-Heading Chinese Cabbage Leaves. J. Integr. Plant Biol. 2005, 47, 452–456. [Google Scholar] [CrossRef]
- Yin, H.; Li, S.; Zhao, X.; Du, Y.; Ma, X. cDNA Microarray Analysis of Gene Expression in Brassica Napus Treated with Oligochitosan Elicitor. Plant Physiol. Biochem. PPB 2006, 44, 910–916. [Google Scholar] [CrossRef]
- Kocięcka, J.; Liberacki, D. The Potential of Using Chitosan on Cereal Crops in the Face of Climate Change. Plants 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, B.M.; Chakraborty, S.; Dalvi, S.G.; Satpute, S.K. Chitosan and Its Derivatives: Promising Biomaterial in Averting Fungal Diseases of Sugarcane and Other Crops. J. Basic Microbiol. 2022, 62, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Pospieszny, H. Antiviroid Activity of Chitosan. Crop Prot. 1997, 16, 105–106. [Google Scholar] [CrossRef]
- Mishra, S.; Jagadeesh, K.S.; Krishnaraj, P.U.; Prem, S. Biocontrol of Tomato Leaf Curl Virus (ToLCV) in Tomato with Chitosan Supplemented Formulations of Pseudomonas sp. under Field Conditions. Aust. J. Crop Sci. 2014, 8, 347–355. [Google Scholar]
- Chirkov, S.N.; Il’ina, A.V.; Surgucheva, N.A.; Letunova, E.V.; Varitsev, Y.A.; Tatarinova, N.Y.; Varlamov, V.P. Effect of Chitosan on Systemic Viral Infection and Some Defense Responses in Potato Plants. Russ. J. Plant Physiol. 2001, 48, 774–779. [Google Scholar] [CrossRef]
- Firmansyah, D.; Widodo; Hidayat, S.H. Chitosan and Plant Growth Promoting Rhizobacteria Application to Control Squash Mosaic Virus on Cucumber Plants. Asian J. Plant Pathol. 2017, 11, 148–155. [Google Scholar] [CrossRef]
- Fan, H.; Yan, X.; Fu, M.; Liu, D.; Awan, A.W.; Chen, P.; Rasheed, S.M.; Gao, L.; Zhang, R. Interactive Effect of Biological Agents Chitosan, Lentinan and Ningnanmycin on Papaya Ringspot Virus Resistance in Papaya (Carica papaya L.). Molecules 2022, 27, 7474. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L.; Zhao, Z.; Li, P.; Tan, S. Chitosan Oligosaccharide as a Plant Immune Inducer on the Passiflora spp. (Passion Fruit) CMV Disease. Front. Plant Sci. 2023, 14, 1131766. [Google Scholar] [CrossRef]
- Xu, X.; Yu, T.; Zhang, D.; Song, H.; Huang, K.; Wang, Y.; Shen, L.; Li, Y.; Wang, F.; Zhang, S.; et al. Evaluation of the Anti-Viral Efficacy of Three Different dsRNA Nanoparticles against Potato Virus Y Using Various Delivery Methods. Ecotoxicol. Environ. Saf. 2023, 255, 114775. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.-R.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana Glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Soliman, A.M.; Ismail, A.M.; Sattar, M.N.; Farroh, K.Y.; Shafie, R.M. Antiviral Activity of Chitosan Nanoparticles and Chitosan Silver Nanocomposites against Alfalfa Mosaic Virus. Polymers 2023, 15, 2961. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. A Biopolymer Chitosan and Its Derivatives as Promising Antimicrobial Agents against Plant Pathogens and Their Applications in Crop Protection. Int. J. Carbohydr. Chem. 2011, 2011, 460381. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of Acetylation Degree and Molecular Weight of Homogeneous Chitosans on Antibacterial and Antifungal Activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Ali, J.; Dadkhah-Aghdash, H.; Kurjak, D.; Kačík, F.; Ďurkovič, J. Chitosan-Induced Biotic Stress Tolerance and Crosstalk with Phytohormones, Antioxidants, and Other Signalling Molecules. Front. Plant Sci. 2023, 14, 1217822. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; de Barros-Alexandrino, T.T.; Assis, O.B.G.; Junior, A.C.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Chitosan and Crosslinked Chitosan Nanoparticles: Synthesis, Characterization and Their Role as Pickering Emulsifiers. Carbohydr. Polym. 2020, 250, 116878. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan Nanoparticles: A Positive Modulator of Innate Immune Responses in Plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of Chitosan and Chitosan Nanoparticles for the Control of Fusarium Head Blight of Wheat (Fusarium graminearum) in Vitro and Greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Chun, S.-C.; Chandrasekaran, M. Chitosan and Chitosan Nanoparticles Induced Expression of Pathogenesis-Related Proteins Genes Enhances Biotic Stress Tolerance in Tomato. Int. J. Biol. Macromol. 2019, 125, 948–954. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, P.; Gowda, S.; Chethana, C.S.; Madhura, S. Antifungal Activity of Chitosan-Silver Nanoparticle Composite against Colletotrichum Gloeosporioides Associated with Mango Anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar]
- Sarkar, A.; Chakraborty, N.; Acharya, K. Chitosan Nanoparticles Mitigate Alternaria Leaf Spot Disease of Chilli in Nitric Oxide Dependent Way. Plant Physiol. Biochem. PPB 2022, 180, 64–73. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Lopez-Llorca, L.V. Omics for Investigating Chitosan as an Antifungal and Gene Modulator. J. Fungi 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Faoro, F. Chitosan as a MAMP, Searching for a PRR. Plant Signal. Behav. 2009, 4, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM Receptor Kinase, Is Essential for Chitin Elicitor Signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef]
- Petutschnig, E.K.; Jones, A.M.E.; Serazetdinova, L.; Lipka, U.; Lipka, V. The Lysin Motif Receptor-like Kinase (LysM-RLK) CERK1 Is a Major Chitin-Binding Protein in Arabidopsis Thaliana and Subject to Chitin-Induced Phosphorylation. J. Biol. Chem. 2010, 285, 28902–28911. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, Y.; Tanaka, K.; Nguyen, C.T.; Jedrzejczak, R.P.; Joachimiak, A.; Stacey, G. The Kinase LYK5 Is a Major Chitin Receptor in Arabidopsis and Forms a Chitin-Induced Complex with Related Kinase CERK1. eLife 2014, 3, e03766. [Google Scholar] [CrossRef]
- Xue, D.-X.; Li, C.-L.; Xie, Z.-P.; Staehelin, C. LYK4 Is a Component of a Tripartite Chitin Receptor Complex in Arabidopsis Thaliana. J. Exp. Bot. 2019, 70, 5507–5516. [Google Scholar] [CrossRef]
- Kaku, H.; Nishizawa, Y.; Ishii-Minami, N.; Akimoto-Tomiyama, C.; Dohmae, N.; Takio, K.; Minami, E.; Shibuya, N. Plant Cells Recognize Chitin Fragments for Defense Signaling through a Plasma Membrane Receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 11086–11091. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nakano, T.; Takamizawa, D.; Desaki, Y.; Ishii-Minami, N.; Nishizawa, Y.; Minami, E.; Okada, K.; Yamane, H.; Kaku, H.; et al. Two LysM Receptor Molecules, CEBiP and OsCERK1, Cooperatively Regulate Chitin Elicitor Signaling in Rice. Plant J. Cell Mol. Biol. 2010, 64, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiao, S.; Cheng, G.; Li, X.; Pei, Z.; Pei, Y.; Yin, H.; Du, Y. Identification of Chitosan Oligosaccharides Binding Proteins from the Plasma Membrane of Wheat Leaf Cell. Int. J. Biol. Macromol. 2018, 111, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Leppyanen, I.V.; Pavlova, O.A.; Vashurina, M.A.; Bovin, A.D.; Dolgikh, A.V.; Shtark, O.Y.; Sendersky, I.V.; Dolgikh, V.V.; Tikhonovich, I.A.; Dolgikh, E.A. LysM Receptor-Like Kinase LYK9 of Pisum sativum L. May Regulate Plant Responses to Chitooligosaccharides Differing in Structure. Int. J. Mol. Sci. 2021, 22, 711. [Google Scholar] [CrossRef]
- Zuppini, A.; Baldan, B.; Millioni, R.; Favaron, F.; Navazio, L.; Mariani, P. Chitosan Induces Ca2+-Mediated Programmed Cell Death in Soybean Cells. New Phytol. 2004, 161, 557–568. [Google Scholar] [CrossRef]
- Amborabé, B.-E.; Bonmort, J.; Fleurat-Lessard, P.; Roblin, G. Early Events Induced by Chitosan on Plant Cells. J. Exp. Bot. 2008, 59, 2317–2324. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Yang, J.; Yin, H.; Wang, W.; Lu, H.; Du, Y. Nitric Oxide Production and Its Functional Link with OIPK in Tobacco Defense Response Elicited by Chitooligosaccharide. Plant Cell Rep. 2011, 30, 1153–1162. [Google Scholar] [CrossRef]
- Vasil’ev, L.A.; Dzyubinskaya, E.V.; Zinovkin, R.A.; Kiselevsky, D.B.; Lobysheva, N.V.; Samuilov, V.D. Chitosan-Induced Programmed Cell Death in Plants. Biochem. Biokhimiia 2009, 74, 1035–1043. [Google Scholar] [CrossRef]
- Kiselevsky, D.B.; Frolova, O.Y.; Solovyev, A.G.; Dorokhov, Y.L.; Morozov, S.Y.; Samuilov, V.D. Plant Cell Death Caused by Fungal, Bacterial, and Viral Elicitors: Protective Effect of Mitochondria-Targeted Quinones. Biochem. Biokhimiia 2014, 79, 1322–1332. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Spoel, S.H. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol. Stuttg. Ger. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.M.; Zhou, T.; Carr, J.P. An Update on Salicylic Acid Biosynthesis, Its Induction and Potential Exploitation by Plant Viruses. Curr. Opin. Virol. 2020, 42, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kuyyogsuy, A.; Deenamo, N.; Khompatara, K.; Ekchaweng, K.; Churngchow, N. Chitosan Enhances Resistance in Rubber Tree (Hevea brasiliensis), through the Induction of Abscisic Acid (ABA). Physiol. Mol. Plant Pathol. 2018, 102, 67–78. [Google Scholar] [CrossRef]
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and Chitosan Activate Plant Defensive Genes through the Octadecanoid Pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098. [Google Scholar] [CrossRef]
- Rakwal, R.; Tamogami, S.; Agrawal, G.K.; Iwahashi, H. Octadecanoid Signaling Component “Burst” in Rice (Oryza sativa L.) Seedling Leaves upon Wounding by Cut and Treatment with Fungal Elicitor Chitosan. Biochem. Biophys. Res. Commun. 2002, 295, 1041–1045. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, H.; Gong, P.; Sadeghnezhad, E.; Pang, Q.; Dong, T.; Li, T.; Jin, H.; Fand, J. Chitosan Induces Jasmonic Acid Production Leading to Resistance of Ripened Fruit against Botrytis Cinerea Infection. Food Chem. 2021, 337, 127772. [Google Scholar] [CrossRef]
- Jia, X.; Zeng, H.; Wang, W.; Zhang, F.; Yin, H. Chitosan Oligosaccharide Induces Resistance to Pseudomonas Syringae Pv. Tomato DC3000 in Arabidopsis Thaliana by Activating Both Salicylic Acid- and Jasmonic Acid-Mediated Pathways. Mol. Plant-Microbe Interact. MPMI 2018, 31, 1271–1279. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.-S. Antiviral Roles of Abscisic Acid in Plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Deng, X.-G.; Zhu, T.; Zou, L.-J.; Han, X.-Y.; Zhou, X.; Xi, D.-H.; Zhang, D.-W.; Lin, H.-H. Orchestration of Hydrogen Peroxide and Nitric Oxide in Brassinosteroid-Mediated Systemic Virus Resistance in Nicotiana Benthamiana. Plant J. Cell Mol. Biol. 2016, 85, 478–493. [Google Scholar] [CrossRef]
- Iriti, M.; Castorina, G.; Vitalini, S.; Mignani, I.; Soave, C.; Fico, G.; Faoro, F. Chitosan-Induced Ethylene-Independent Resistance Does Not Reduce Crop Yield in Bean. Biol. Control 2010, 54, 241–247. [Google Scholar] [CrossRef]
- Czékus, Z.; Iqbal, N.; Pollák, B.; Martics, A.; Ördög, A.; Poór, P. Role of Ethylene and Light in Chitosan-Induced Local and Systemic Defence Responses of Tomato Plants. J. Plant Physiol. 2021, 263, 153461. [Google Scholar] [CrossRef]
- Köhle, H.; Young, D.H.; Kauss, H. Physiological Changes in Suspension-Cultured Soybean Cells Elicited by Treatment with Chitosan. Plant Sci. Lett. 1984, 33, 221–230. [Google Scholar] [CrossRef]
- Kauss, H.; Jeblick, W.; Domard, A. The Degrees of Polymerization and N-Acetylation of Chitosan Determine Its Ability to Elicit Callose Formation in Suspension Cells and Protoplasts of Catharanthus Roseus. Planta 1989, 178, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A Novel Role of PR2 in Abscisic Acid (ABA) Mediated, Pathogen-Induced Callose Deposition in Arabidopsis Thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Kumar, R.; Iswanto, A.B.B.; Kim, J.-Y. Callose Balancing at Plasmodesmata. J. Exp. Bot. 2018, 69, 5325–5339. [Google Scholar] [CrossRef]
- Jogaiah, S.; Satapute, P.; De Britto, S.; Konappa, N.; Udayashankar, A.C. Exogenous Priming of Chitosan Induces Upregulation of Phytohormones and Resistance against Cucumber Powdery Mildew Disease Is Correlated with Localized Biosynthesis of Defense Enzymes. Int. J. Biol. Macromol. 2020, 162, 1825–1838. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Monir, G.A.; Hassan, M.S.S.; Ahmed, Y.; Refaat, M.H.; Ismail, I.A.; El-Garhy, H.A.S. Exogenously Applied Chitosan and Chitosan Nanoparticles Improved Apple Fruit Resistance to Blue Mold, Upregulated Defense-Related Genes Expression, and Maintained Fruit Quality. Horticulturae 2021, 7, 224. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Foliar Application of Chitosan Nanoparticle Improves Yield, Mineral Content and Boost Innate Immunity in Finger Millet Plants. Carbohydr. Polym. 2021, 258, 117691. [Google Scholar] [CrossRef]
- Narasimhamurthy, K.; Udayashankar, A.C.; De Britto, S.; Lavanya, S.N.; Abdelrahman, M.; Soumya, K.; Shetty, H.S.; Srinivas, C.; Jogaiah, S. Chitosan and Chitosan-Derived Nanoparticles Modulate Enhanced Immune Response in Tomato against Bacterial Wilt Disease. Int. J. Biol. Macromol. 2022, 220, 223–237. [Google Scholar] [CrossRef]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging Roles of β-Glucanases in Plant Development and Adaptative Responses. Plants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, S.; Roggero, P. Systemic Acquired Resistance Against Plant Virus Infections: A Reality? J. Plant Pathol. 1998, 80, 179–186. [Google Scholar]
- Chivasa, S.; Murphy, A.M.; Naylor, M.; Carr, J.P. Salicylic Acid Interferes with Tobacco Mosaic Virus Replication via a Novel Salicylhydroxamic Acid-Sensitive Mechanism. Plant Cell 1997, 9, 547–557. [Google Scholar] [CrossRef]
- Murphy, A.M.; Carr, J.P. Salicylic Acid Has Cell-Specific Effects on Tobacco Mosaic Virus Replication and Cell-to-Cell Movement. Plant Physiol. 2002, 128, 552–563. [Google Scholar] [CrossRef]
- Wong, C.E.; Carson, R.A.J.; Carr, J.P. Chemically Induced Virus Resistance in Arabidopsis Thaliana Is Independent of Pathogenesis-Related Protein Expression and the NPR1 Gene. Mol. Plant-Microbe Interact. MPMI 2002, 15, 75–81. [Google Scholar] [CrossRef]
- Mayers, C.N.; Lee, K.-C.; Moore, C.A.; Wong, S.-M.; Carr, J.P. Salicylic Acid-Induced Resistance to Cucumber Mosaic Virus in Squash and Arabidopsis Thaliana: Contrasting Mechanisms of Induction and Antiviral Action. Mol. Plant-Microbe Interact. MPMI 2005, 18, 428–434. [Google Scholar] [CrossRef]
- Yu, D.; Fan, B.; MacFarlane, S.A.; Chen, Z. Analysis of the Involvement of an Inducible Arabidopsis RNA-Dependent RNA Polymerase in Antiviral Defense. Mol. Plant-Microbe Interact. 2003, 16, 206–216. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Q.; Wu, B.; Ai, T.; Guo, X. NgRDR1, an RNA-Dependent RNA Polymerase Isolated from Nicotiana Glutinosa, Was Involved in Biotic and Abiotic Stresses. Plant Physiol. Biochem. PPB 2009, 47, 359–368. [Google Scholar] [CrossRef]
- Li, S.; Yu, F.; Wang, M.; Guo, X.; Li, H. Molecular Characterization of a Nicotiana tabacum NtRDR6 Gene. Plant Mol. Biol. Report. 2012, 30, 1375–1384. [Google Scholar] [CrossRef]
- Feng, B.; Chen, Y.; Zhao, C.; Zhao, X.; Bai, X.; Du, Y. Isolation of a Novel Ser/Thr Protein Kinase Gene from Oligochitosan-Induced Tobacco and Its Role in Resistance against Tobacco Mosaic Virus. Plant Physiol. Biochem. PPB 2006, 44, 596–603. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhao, X.; Guo, P.; An, H.; Du, Y.; Han, Y.; Liu, H.; Zhang, Y. Functions of Oligochitosan Induced Protein Kinase in Tobacco Mosaic Virus Resistance and Pathogenesis Related Proteins in Tobacco. Plant Physiol. Biochem. PPB 2009, 47, 724–731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarova, T.; Shipounova, I.; Kalinina, N.; Taliansky, M. Application of Chitosan and Its Derivatives Against Plant Viruses. Polymers 2024, 16, 3122. https://doi.org/10.3390/polym16223122
Komarova T, Shipounova I, Kalinina N, Taliansky M. Application of Chitosan and Its Derivatives Against Plant Viruses. Polymers. 2024; 16(22):3122. https://doi.org/10.3390/polym16223122
Chicago/Turabian StyleKomarova, Tatiana, Irina Shipounova, Natalia Kalinina, and Michael Taliansky. 2024. "Application of Chitosan and Its Derivatives Against Plant Viruses" Polymers 16, no. 22: 3122. https://doi.org/10.3390/polym16223122
APA StyleKomarova, T., Shipounova, I., Kalinina, N., & Taliansky, M. (2024). Application of Chitosan and Its Derivatives Against Plant Viruses. Polymers, 16(22), 3122. https://doi.org/10.3390/polym16223122





