The Influence of Conditions of Polycondensation in Acid Medium on the Structure of Oligosilsesquioxanes with a Novel Eugenol-Containing Substituent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
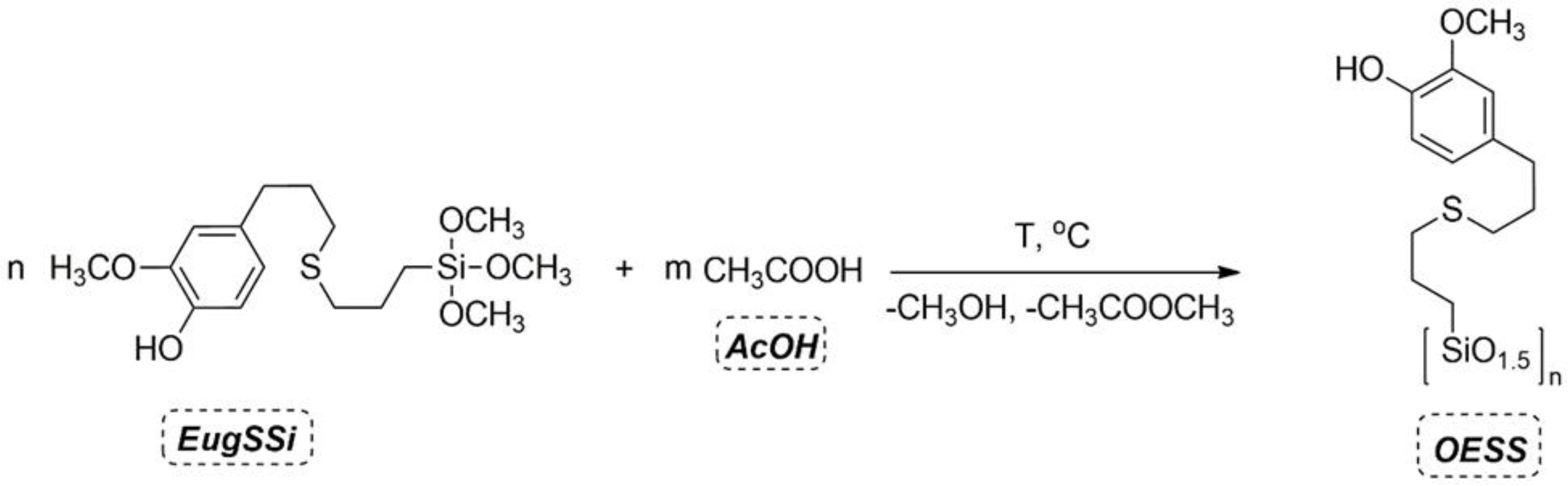
2.2. General Procedure for the Synthesis of Eugenol-Containing Oligosilsesquioxanes (OESSs)
2.2.1. Synthesis of OESS_1.5
2.2.2. Synthesis of OESS_2.0
2.2.3. Synthesis of OESS_3.0
2.2.4. Synthesis of OESS_6
2.2.5. Synthesis of OESS_12
2.2.6. Synthesis of OESS_24
2.2.7. Synthesis of OESS_95
3. Results
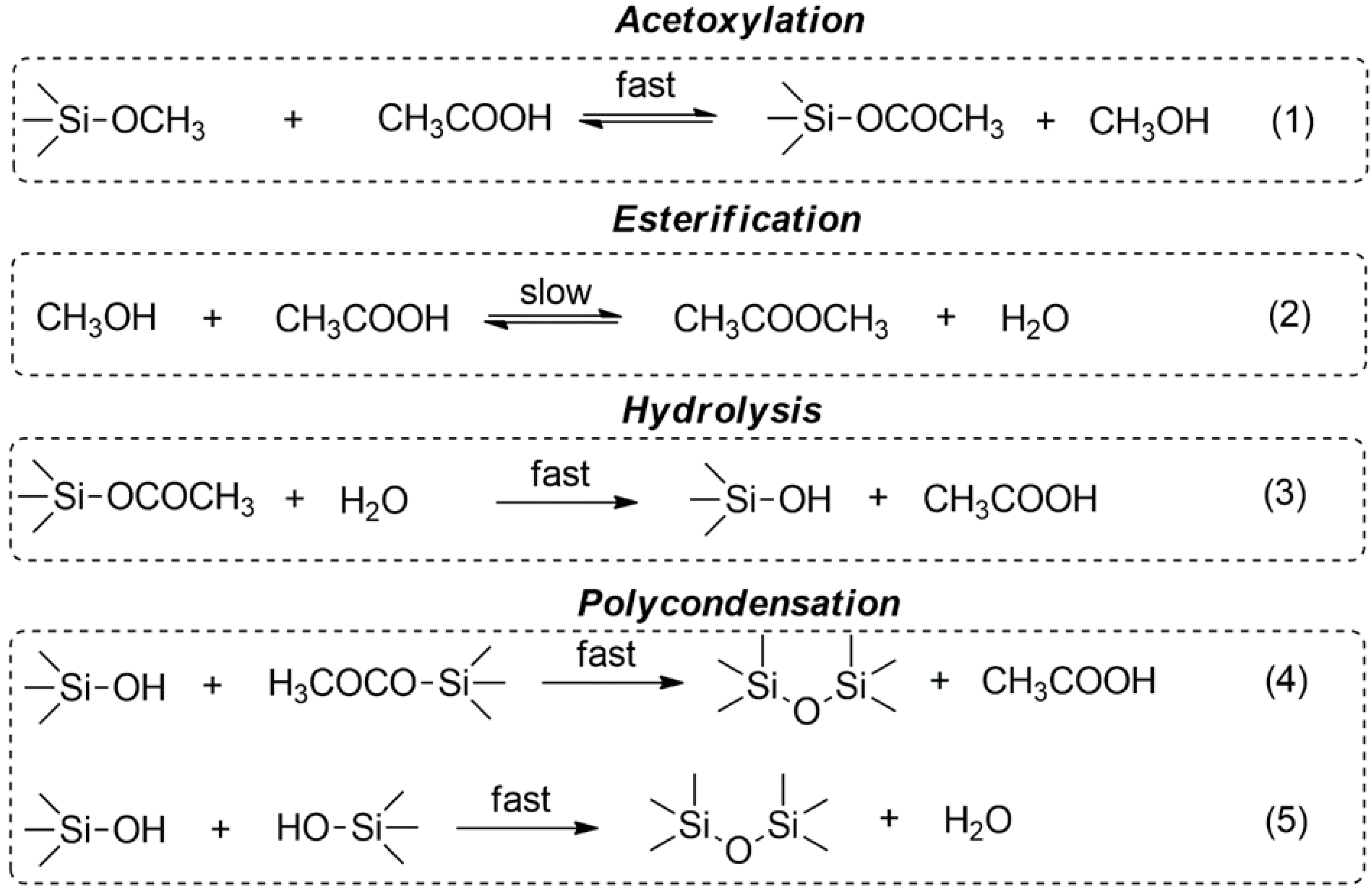
3.1. Effect of EugSSi/AcOH Molar Ratio
3.2. Effect of Reaction Time
3.3. Effect of Reaction Temperature
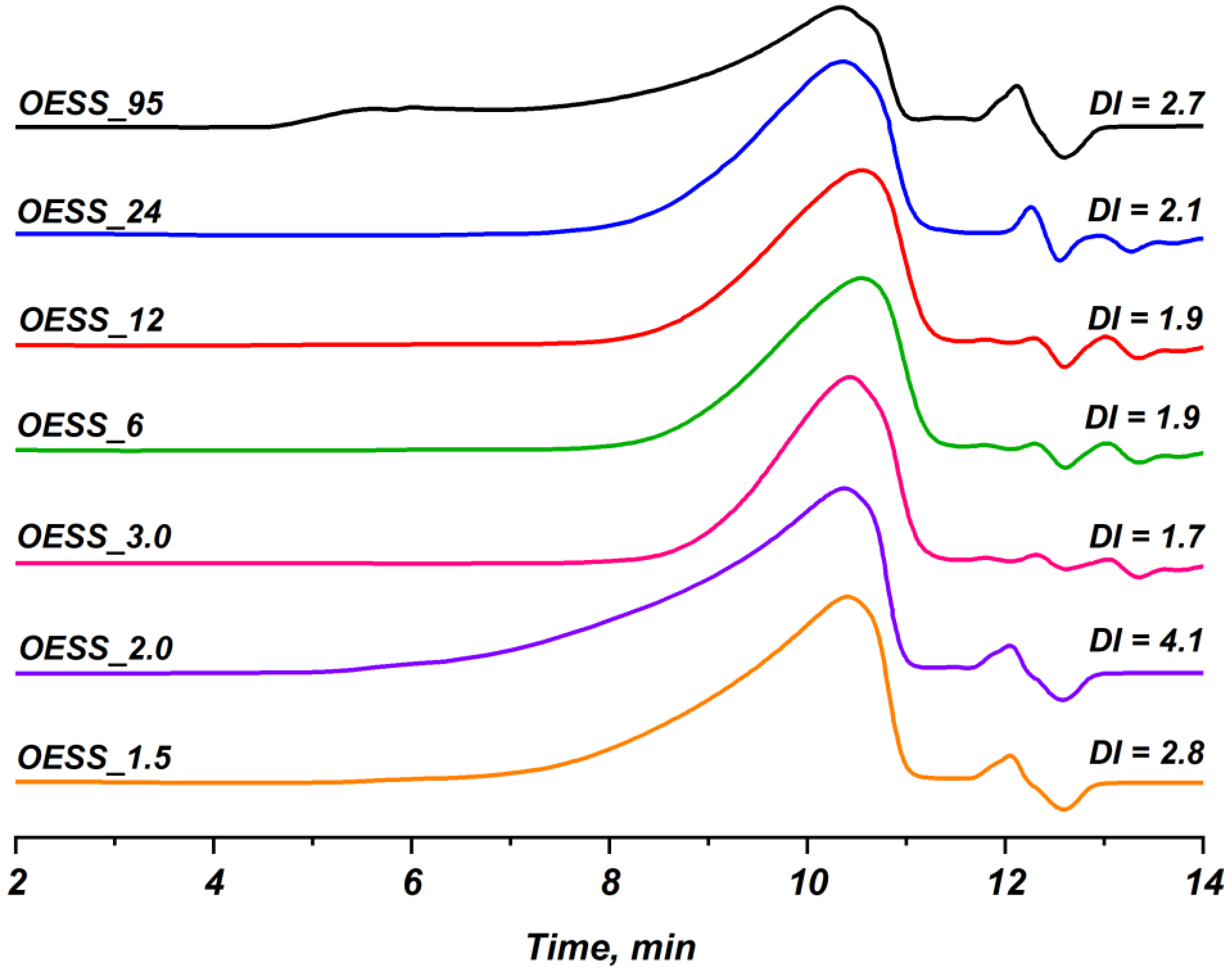
3.4. Study of Molecular Weight Characteristics
3.5. Study of Temperature Transitions and Thermogravimetry
3.6. Effect of Eugenol-Containing Substituent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nowacka, M.; Kowalewska, A. Self-Healing Silsesquioxane-Based Materials. Polymers 2022, 14, 1869. [Google Scholar] [CrossRef]
- Decker, B.; Hartmann-Thompson, C.; Carver, P.I.; Keinath, S.E.; Santurri, P.R. Multilayer Sulfonated Polyhedral Oligosilsesquioxane (S-POSS)-Sulfonated Polyphenylsulfone (S-PPSU) Composite Proton Exchange Membranes. Chem. Mater. 2010, 22, 942–948. [Google Scholar] [CrossRef]
- Bredov, N.S.; Soan, L.P.; Kireev, V.V.; Bykovskaya, A.A.; Sokol’skaya, I.B.; Posokhova, V.F.; Klyukin, B.V.; Chuev, V.P. Methacrylate-Containing Polymer Compounds for Dentistry. Russ. J. Appl. Chem. 2017, 90, 595–601. [Google Scholar] [CrossRef]
- Golubev, A.A.; Baranova, K.S.; Bazhanov, D.A.; Khasbiullin, R.R.; Shcherbina, A.A.; Soldatov, M.A. Preparation and Study of Novel UV-Curable Alkyd-Siloxane Coating Materials. J. Appl. Polym. Sci. 2024, 141, e55838. [Google Scholar] [CrossRef]
- Krutskikh, D.V.; Shapagin, A.V.; Plyusnina, I.O.; Budylin, N.Y.; Shcherbina, A.A.; Soldatov, M.A. Modification of Epoxy Coatings with Fluorocontaining Organosilicon Copolymers. Polymers 2024, 16, 1571. [Google Scholar] [CrossRef]
- Shkinev, P.; Evdokimova, A.; Drozdov, F.V.; Gervits, L.L.; Muzafarov, A.M. Non-Accumulative in the Environment Facile Hydrophobic Coatings Based on Branched Siloxanes with Perfluoroalkyl Substituents. J. Organomet. Chem. 2021, 948, 121910. [Google Scholar] [CrossRef]
- Soldatov, M.A.; Sheremetyeva, N.A.; Serenko, O.A.; Muzafarov, A.M. Synthesis of Fluorine-Containing-Organosilicon Oligomer in Trifluoroacetic Acid as Active Medium. Silicon 2015, 7, 211–216. [Google Scholar] [CrossRef]
- Kannan, A.; Muthuraj, C.; Mayavan, A.; Gandhi, S. Multifaceted Applications of Polyhedral Oligomeric Silsesquioxane and Their Composites. Mater. Today Chem. 2023, 30, 101568. [Google Scholar] [CrossRef]
- Ozimek, J.; Łukaszewska, I.; Pielichowski, K. POSS and SSQ Materials in Dental Applications: Recent Advances and Future Outlooks. Int. J. Mol. Sci. 2023, 24, 4493. [Google Scholar] [CrossRef]
- Bredov, N.S.; Bykovskaya, A.A.; Van Tuan, N.; Kireev, V.V.; Tupikov, A.S.; Sokol’skaya, I.B.; Posokhova, V.F.; Chuev, V.P. Oligomeric silsesquioxane–siloxane modifiers for polymer dental compounds. Polym. Sci. Ser. B 2020, 62, 169–176. [Google Scholar] [CrossRef]
- Kireev, V.V.; Bilichenko, Y.V.; Bredov, N.S. Heat-Resistant Binders Based on Oligomeric Organosilsesquioxanes. Plast. Massy 2022, 3–4, 5–10. [Google Scholar] [CrossRef]
- Bredov, N.S.; Kireev, V.V.; Polyakov, V.A.; Sokol’skaya, I.V.; Esin, A.S. Modern Approaches to Obtaining Organofunctional Silsesquioxanes. Vysokomol. Soedin. Ser. C 2023, 65, 193–209. [Google Scholar] [CrossRef]
- Khmelnitskaia, A.G.; Kalinina, A.A.; Meshkov, I.B.; Tukhvatshin, R.S.; Cherkaev, G.V.; Ponomarenko, S.A.; Muzafarov, A.M. Synthesis of Vinyl-Containing Polydimethylsiloxane in An Active Medium. Polymers 2024, 16, 257. [Google Scholar] [CrossRef]
- Andropova, U.S.; Drozdov, F.V.; Shkinev, P.D.; Cherkaev, G.V.; Gervits, L.L.; Serenko, O.A.; Muzafarov, A.M. Functional Silsesquioxane Polymers with Branched Perfluoroalkyl Substituents: Synthesis and Prospect Applications. Eur. Polym. J. 2022, 178, 111523. [Google Scholar] [CrossRef]
- Issa, A.A.; El-Azazy, M.; Luyt, A.S. Polymerization of Organoalkoxysilanes: Kinetics of the Polycondensation Progress and the Effect of Solvent Properties and Salts Addition. Chem. Phys. 2020, 530, 110642. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Egorova, E.V.; Vasilenko, N.G.; Demchenko, N.V.; Tatarinova, E.A.; Muzafarov, A.M. Polycondensation of Alkoxysilanes in an Active Medium as a Versatile Method for the Preparation of Polyorganosiloxanes. Dokl. Chem. 2009, 424, 15–18. [Google Scholar] [CrossRef]
- Lickiss, P.D.; Rataboul, F. Chapter 1 Fully Condensed Polyhedral Oligosilsesquioxanes (POSS): From Synthesis to Application. In Advances in Organometallic Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2008; Volume 57, pp. 1–116. ISBN 9780123744654. [Google Scholar]
- Deetz, J.D.; Faller, R. Reactive Modeling of the Initial Stages of Alkoxysilane Polycondensation: Effects of Precursor Molecule Structure and Solution Composition. Soft Matter 2015, 11, 6780–6789. [Google Scholar] [CrossRef]
- Władyczyn, A.; John, Ł. Silsesquioxane Cages under Solvent Regimen: The Influence of the Solvent on the Hydrolysis and Condensation of Alkoxysilane. Inorg. Chem. 2024, 63, 9145–9155. [Google Scholar] [CrossRef]
- Mori, H. Design and Synthesis of Functional Silsesquioxane-Based Hybrids by Hydrolytic Condensation of Bulky Triethoxysilanes. Int. J. Polym. Sci. 2012, 2012, 173624. [Google Scholar] [CrossRef]
- Meng, L.; Chen, Z.; Feng, S. Synthesis and Properties of Sodium Carboxylate Silicone Surfactant via Thiol-Ene “Click” Reaction. Colloids Interface Sci. Commun. 2022, 49, 100642. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Gorbatsevich, O.B.; Yakhontov, N.G.; Demchenko, N.V.; Vasilenko, N.G.; Kazakova, V.V.; Muzafarov, A.M. Synthesis of Multifunctional Oligomethylsilsesquioxanes by Catalyst-Free Hydrolytic Polycondensation of Methyltrimethoxysilane under Microwave Radiation. Polymers 2023, 15, 291. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, A.A.; Soskov, F.V.; Demchenko, A.I.; Storozhenko, P.A.; Muzafarov, A.M.; Enikolopov, N.S. Condensation of Methylphenylalkoxysilanes in an Active Medium as a Selective Method for Synthesis of Cyclic or Linear Methylphenylsiloxanes*. Russ. Chem. Bull. 2011, 60, 2384–2389. [Google Scholar] [CrossRef]
- Temnikov, M.N.; Muzafarov, A.M. Polyphenylsilsesquioxanes. New Structures-New Properties. RSC Adv. 2020, 10, 43129–43152. [Google Scholar] [CrossRef]
- An, Y.; Lu, C.; You, M.; Liu, X.; Yao, W.; Li, Y. Preparation and Characterization of High Molecular Weight Vinyl-Containing Poly[(3,3,3-Trifluoropropyl)Methylsiloxane. Heliyon 2023, 9, e21707. [Google Scholar] [CrossRef]
- Asmussen, S.V.; Giudicessi, S.L.; Erra-Balsells, R.; Vallo, C.I. Synthesis of Silsesquioxanes Based in (3-Methacryloxypropyl)- Trimethoxysilane Using Methacrylate Monomers as Reactive Solvents. Eur. Polym. J. 2010, 46, 1815–1823. [Google Scholar] [CrossRef]
- Eisenberg, P.; Erra-Balsells, R.; Ishikawa, Y.; Lucas, J.C.; Nonami, H.; Williams, R.J.J. Silsesquioxanes Derived from the Bulk Polycondensation of [3-(Methacryloxy)Propyl]Trimethoxysilane with Concentrated Formic Acid: Evolution of Molar Mass Distributions and Fraction of Intramolecular Cycles. Macromolecules 2002, 35, 1160–1174. [Google Scholar] [CrossRef]
- Pozuelo, J.; Baselga, J. Glass Transition Temperature of Low Molecular Weight Poly(3-Aminopropyl Methyl Siloxane). A Molecular Dynamics Study. Polymer 2002, 43, 6049–6055. [Google Scholar] [CrossRef]
- Ken, I.; Dimzon, D.; Frö, T.; Knepper, T.P. Characterization of 3-Aminopropyl Oligosilsesquioxane. Anal. Chem. 2016, 88, 4894–4902. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Xu, X.; Mo, S.; Peng, D.; Mu, Q.; Zhu, C.; Li, C.; Xu, J. Melt Fluidity and Thermal Property of Thermosetting Siloxane-Containing Polyimide Resins and Their Organic/Inorganic Hybrid Characteristics. Mater. Today Commun. 2020, 25, 101443. [Google Scholar] [CrossRef]
- Tamaki, R.; Choi, J.; Laine, R.M. A Polyimide Nanocomposite from Octa(Aminophenyl)Silsesquioxane. Chem. Mater. 2003, 15, 793–797. [Google Scholar] [CrossRef]
- Tamaki, R.; Tanaka, Y.; Asuncion, M.Z.; Choi, J.; Laine, R.M. Octa(Aminophenyl)Silsesquioxane as a Nanoconstruction Site. J. Am. Chem. Soc. 2001, 123, 12416–12417. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, R.M.; Loy, D.A.; Wheeler, D.R. Maleimide Functionalized Siloxane Resins. MRS Online Proc. Libr. 1999, 576, 15–20. [Google Scholar] [CrossRef]
- Shi, C.; Lu, X.; Chen, Z.; He, F.; Pan, A.; He, L. Glycidyl Polyhedral Oligomeric Silsesquioxane-Enhanced Flexible Aminosiloxanes to Protect Sandstone Monuments. Prog. Org. Coat. 2024, 196, 108698. [Google Scholar] [CrossRef]
- Lei, Z.; Ji, J.; Wu, Q.; Zhang, J.; Wang, Y.; Jing, X.; Liu, Y. Curing Behavior and Microstructure of Epoxy-POSS Modified Novolac Phenolic Resin with Different Substitution Degree. Polymer 2019, 178, 121587. [Google Scholar] [CrossRef]
- Chapala, P.P.; Bermeshev, M.V.; Korchagina, S.A.; Ashirov, R.V.; Bermesheva, E.V. Synthesis of 3,4-Dihydroxyphenyl-Containing Polymeric Materials from 1,2-Polybutadiene and Eugenol via Thiol-Ene Addition. Izvest. Akad. Nauk Ser. Khim. 2016, 65, 1061–1066. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of New Siloxane or Sulfur Containing Symmetrical Monomers Based on Carvone. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 890–892. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Temnikov, M.N.; Krizhanovskiy, I.; Timoshina, E.I.; Milenin, S.A.; Peregudov, A.S.; Dolgushin, F.M.; Muzafarov, A.M. A Thiol-Ene Click Reaction with Preservation of the Si-H Bond: A New Approach for the Synthesis of Functional Organosilicon Compounds. New J. Chem. 2021, 45, 5764–5769. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.; Anisimov, A.; Krylov, F.; Buzin, M.; Buzin, A.; Peregudov, A.; Shchegolikhina, O.; Muzafarov, A. Synthesis of Functional Derivatives of Stereoregular Organocyclosilsesquioxanes by Thiol-Ene Addition. J. Organomet. Chem. 2021, 954–955, 122072. [Google Scholar] [CrossRef]
- Rubinsztajn, S.; Cella, J.A. A New Polycondensation Process for the Preparation of Polysiloxane Copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Grande, J.B. The Piers-Rubinsztajn Reaction: New Routes to Structured Silicones. Ph.D. Thesis, Mc Master University, Hamilton, ON, Canada, 2013. Available online: http://hdl.handle.net/11375/13435 (accessed on 15 October 2024).
- Madsen, F.B.; Javakhishvili, I.; Jensen, R.E.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. Synthesis of Telechelic Vinyl/Allyl Functional Siloxane Copolymers with Structural Control. Polym. Chem. 2014, 5, 7054–7061. [Google Scholar] [CrossRef]
- Krizhanovskiy, I.; Temnikov, M.; Kononevich, Y.; Anisimov, A.; Drozdov, F.; Muzafarov, A. The Use of the Thiol-Ene Addition Click Reaction in the Chemistry of Organosilicon Compounds: An Alternative or a Supplement to the Classical Hydrosilylation? Polymers 2022, 14, 3079. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, T.B.; Grath, A.; Wang, A.; Xu, C.; Hong, Y.; Dai, G. Evaluation of Photochemistry Reaction Kinetics to Pattern Bioactive Proteins on Hydrogels for Biological Applications. Bioact. Mater. 2018, 3, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Long, K.F.; Bongiardina, N.J.; Mayordomo, P.; Olin, M.J.; Ortega, A.D.; Bowman, C.N. Effects of 1°, 2°, and 3° Thiols on Thiol-Ene Reactions: Polymerization Kinetics and Mechanical Behavior. Macromolecules 2020, 53, 5805–5815. [Google Scholar] [CrossRef]
- Rissing, C.; Son, D.Y. Thiol-Ene Reaction for the Synthesis of Multifunctional Branched Organosilanes. Organometallics 2008, 27, 5394–5397. [Google Scholar] [CrossRef]
- Xue, L.; Li, L.; Feng, S.; Liu, H. A Facile Route to Multifunctional Cage Silsesquioxanes via the Photochemical Thiol-Ene Reaction We Dedicate This Paper to Prof. Hongzhi Liu’s Mother, Who Is Dearly Missed. J. Organomet. Chem. 2015, 783, 49–54. [Google Scholar] [CrossRef]
- Rózga-Wijas, K.; Chojnowski, J. Synthesis of New Polyfunctional Cage Oligosilsesquioxanes and Cyclic Siloxanes by Thiol-Ene Addition. J. Inorg. Organomet. Polym. Mater. 2012, 22, 588–594. [Google Scholar] [CrossRef]
- Tucker-Schwartz, A.K.; Farrell, R.A.; Garrell, R.L. Thiol-Ene Click Reaction as a General Route to Functional Trialkoxysilanes for Surface Coating Applications. J. Am. Chem. Soc. 2011, 133, 11026–11029. [Google Scholar] [CrossRef]
- Sierke, J.K.; Ellis, A.V. High Purity Synthesis of a Polyhedral Oligomeric Silsesquioxane Modified with an Antibacterial. Inorg. Chem. Commun. 2015, 60, 41–43. [Google Scholar] [CrossRef]
- Wang, B.; Shi, M.; Ding, J.; Huang, Z. Polyhedral Oligomeric Silsesquioxane (POSS)-Modified Phenolic Resin: Synthesis and Anti-Oxidation Properties. E-Polymers 2021, 21, 316–326. [Google Scholar] [CrossRef]
- Wang, D.; Ding, J.; Wang, B.; Zhuang, Y.; Huang, Z. Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (Poss) Modified Phenolic Resin. Polymers 2021, 13, 1182. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-Modified Polysiloxanes as Effective Anticorrosion Additives for Epoxy Resin Coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, S.; Li, W.S.J.; Perrin, R.; Ladmiral, V.; Bongiovanni, R.; Caillol, S.; Lacroix-Desmazes, P. Radical Aqueous Emulsion Copolymerization of Eugenol-Derived Monomers for Adhesive Applications. Biomacromolecules 2020, 21, 4514–4521. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas Méndez, L.Y. Synthesis of Eugenol-Based Monomers for Sustainable Epoxy Thermoplastic Polymers. J. Appl. Polym. Sci. 2022, 139, 52237. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, W.; Shao, W.; Pei, Y.; Wang, J. Partially Bio-Based and Fluorinated Polysiloxane with High Transparency and Low Dielectric Constant. Eur. Polym. J. 2023, 194, 112136. [Google Scholar] [CrossRef]
- Chen, P.; Ge, X.; Liang, W.; Lv, J.; Zhang, Z.; Yin, S.; Li, C.; Chen, Y.; Liu, W.; Ge, J. Eugenol-Containing Siloxane Synthesized Via Heterogeneous Catalytic Hydrosilylation and Its Application in Preparation of Superb Viscoelastic Low Modulus Gels. Silicon 2023, 15, 1123–1131. [Google Scholar] [CrossRef]
- Ageenkov, A.D.; Rozhkov, I.M.; Piskarev, M.S.; Soldatov, M.A. Synthesis of Novel Eugenol-Containing Polysilsesquioxanes with a Flexible Spacer and Their Use for Functional Anticorrosive Coatings. ChemistrySelect 2024, 9, e202401623. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Elsevier/BH: Amsterdam, The Netherlands, 2009; ISBN 9781856175678. [Google Scholar]
- Kalinina, A.; Strizhiver, N.; Vasilenko, N.; Perov, N.; Demchenko, N.; Muzafarov, A. Polycondensation of Diethoxydimethylsilane in Active Medium. Silicon 2015, 7, 95–106. [Google Scholar] [CrossRef]
- Borovin, E.; Callone, E.; Ribot, F.; Diré, S. Mechanism and Kinetics of Oligosilsesquioxane Growth in the in Situ Water Production Sol-Gel Route: Dependence on Water Availability. Eur. J. Inorg. Chem. 2016, 2016, 2166–2174. [Google Scholar] [CrossRef]
- Chimjarn, S.; Kunthom, R.; Chancharone, P.; Sodkhomkhum, R.; Sangtrirutnugul, P.; Ervithayasuporn, V. Synthesis of Aromatic Functionalized Cage-Rearranged Silsesquioxanes (T8, T10, and T12) via Nucleophilic Substitution Reactions. Dalton Trans. 2014, 44, 916–919. [Google Scholar] [CrossRef]
- Bassindale, A.R.; Parker, D.J.; Taylor, P.G.; Watt, A.C. Structural Elucidations of T8 and Q8 Silsesquioxane Cages Containing Two Types of Pendant Group Using 29Si NMR Spectroscopy. Can. J. Chem. 2003, 81, 1341–1349. [Google Scholar] [CrossRef]
- Hook, R.J. A 29Si NMR Study of the Sol-Gel Polymerisation Rates of Substituted Ethoxysilanes. J. Non-Cryst. Solids 1996, 195, 1–15. [Google Scholar] [CrossRef]
- Pohl, S.; Kickelbick, G. Influence of Alkyl Groups on the Formation of Softenable Polysilsesquioxanes. J. Solgel Sci. Technol. 2023, 107, 329–346. [Google Scholar] [CrossRef]
- Brochier Salon, M.C.; Belgacem, M.N. Hydrolysis-Condensation Kinetics of Different Silane Coupling Agents. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 240–254. [Google Scholar] [CrossRef]
- Dong, H.; Brook, M.A.; Brennan, J.D. A New Route to Monolithic Methylsilsesquioxanes: Gelation Behavior of Methyltrimethoxysilane and Morphology of Resulting Methylsilsesquioxanes under One-Step and Two-Step Processing. Chem. Mater. 2005, 17, 2807–2816. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Yang, H.; Kanamori, K.; Zhu, Y.; Nakanishi, K. Gelation Behavior and Phase Separation of Macroporous Methylsilsesquioxane Monoliths Prepared by in Situ Two-Step Processing. J. Solgel Sci. Technol. 2013, 67, 406–413. [Google Scholar] [CrossRef]
- Tajikawa, R.; Tokuami, I.; Nagao, M.; Okada, A.; Imoto, H.; Naka, K. Phenyl-Substituted Cage Silsesquioxane-Based Star-Shaped Giant Molecular Clusters: Synthesis, Properties, and Surface Segregation Behavior. Langmuir 2024, 40, 11795–11805. [Google Scholar] [CrossRef]
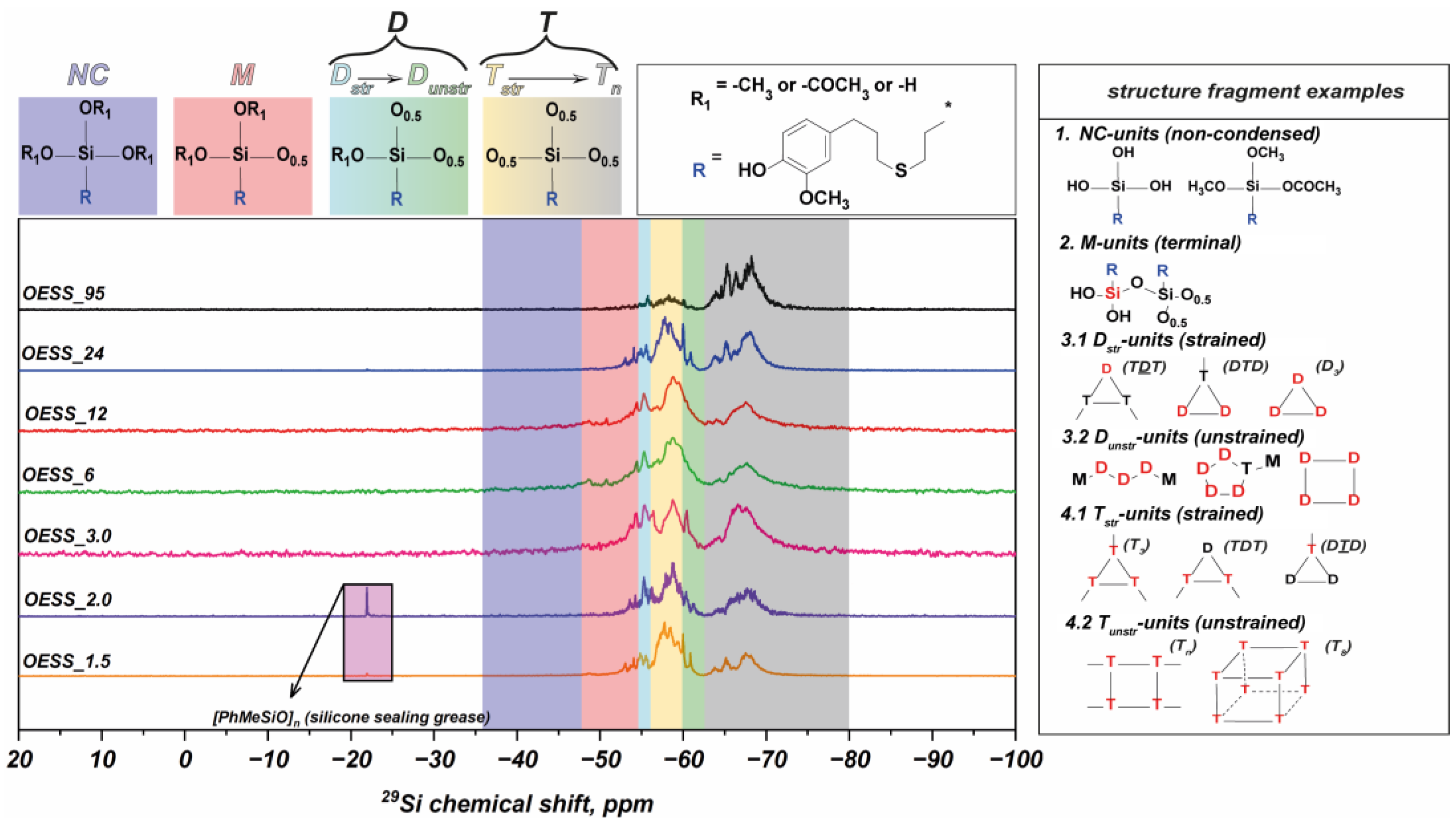
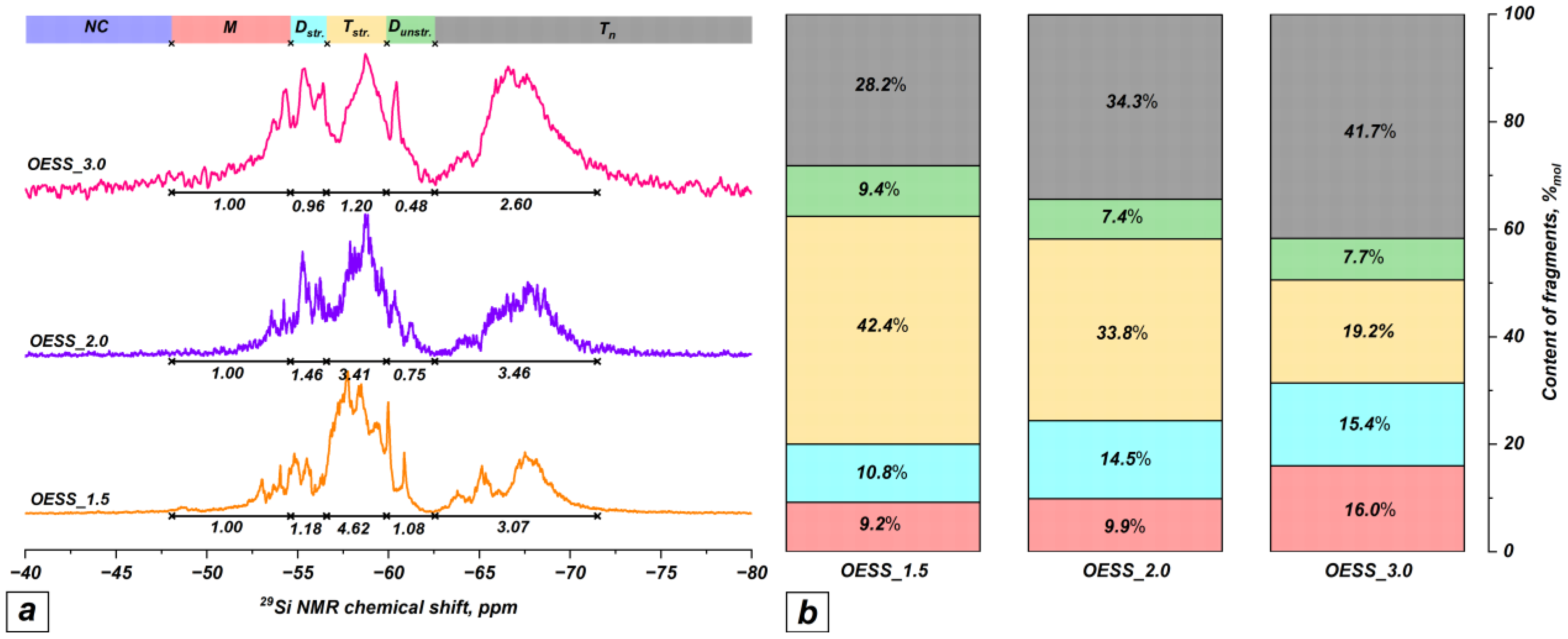
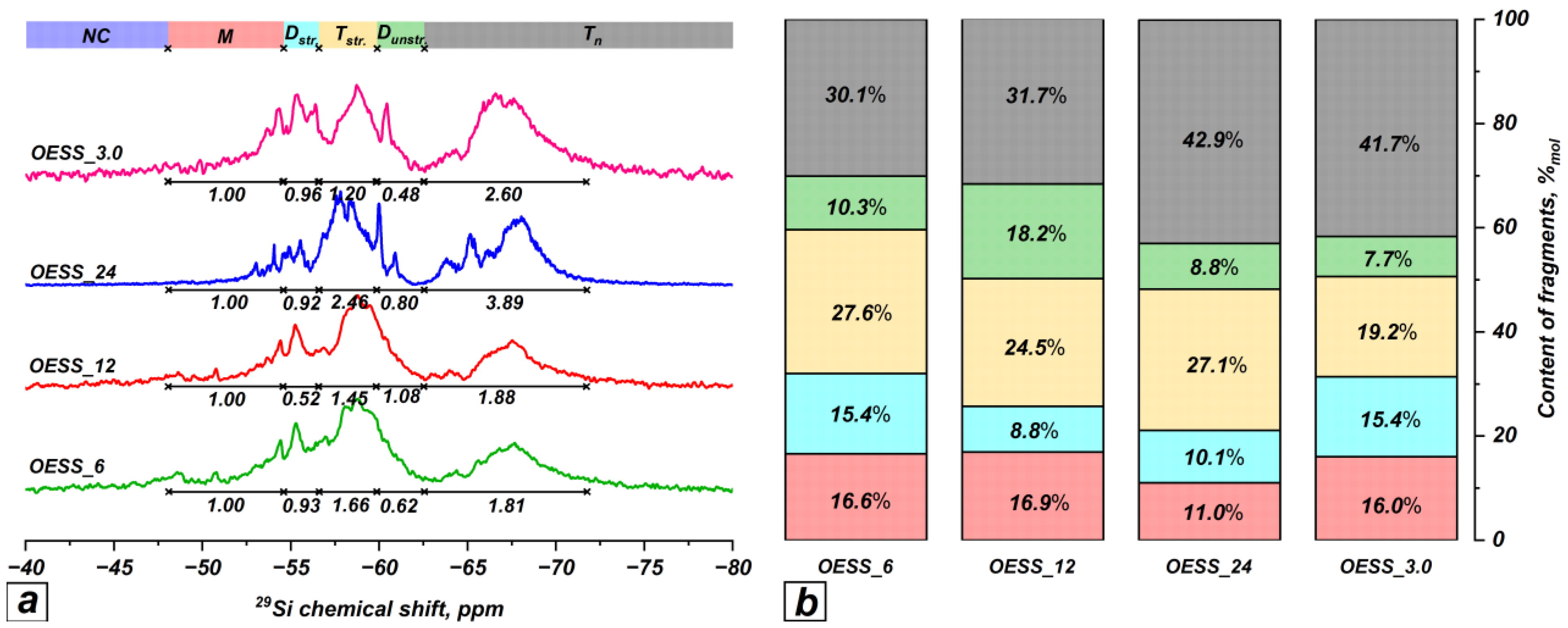
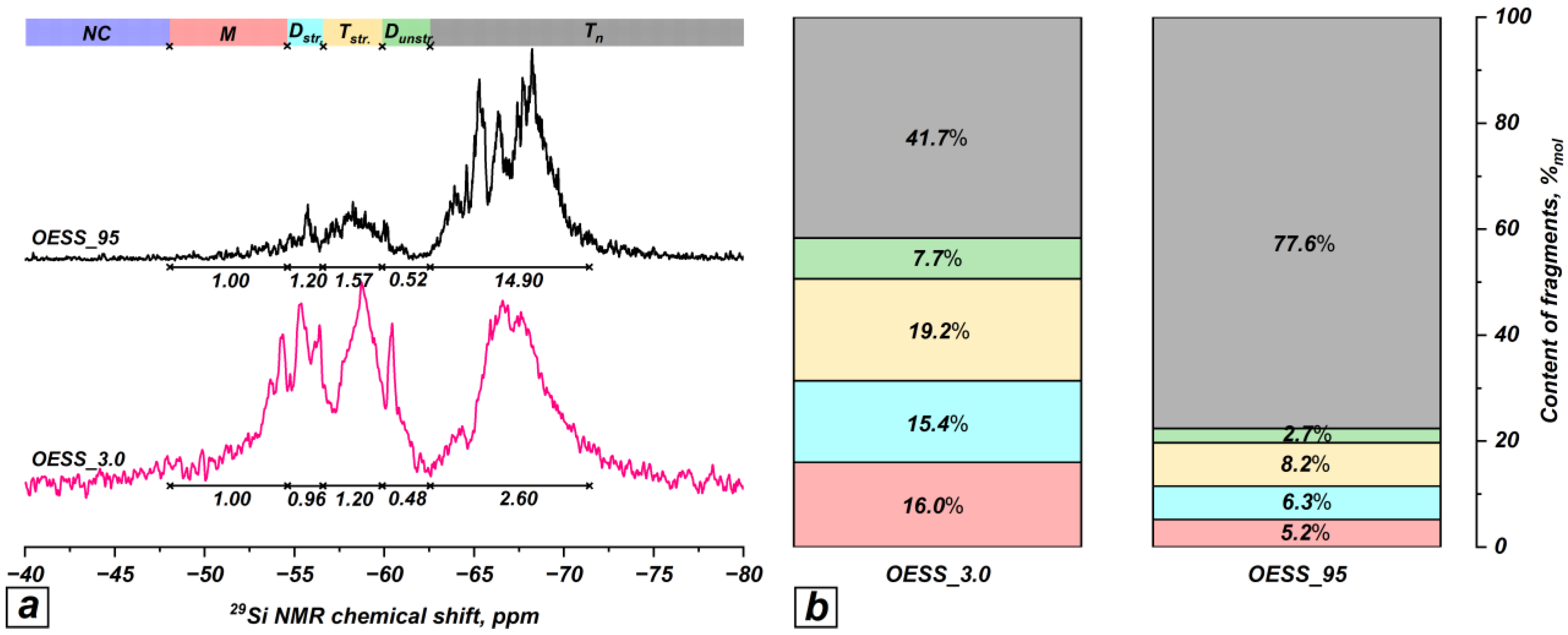



| Product | Molar Ratio of EugSSi/AcOH | Reaction Time, h | Content of Structural Units in Oligoorganosiloxanes, % mol | |||||
|---|---|---|---|---|---|---|---|---|
| NC | M | Dstr. | Tstr. | Dunstr. | Tn | |||
| OESS_1.5 | 1:1.5 | 48 | − | 9.2 | 10.8 | 42.4 | 9.4 | 28.2 |
| OESS_2.0 | 1:2.0 | 48 | − | 9.9 | 14.5 | 33.8 | 7.4 | 34.3 |
| OESS_3.0 | 1:3.0 | 48 | − | 16.0 | 15.4 | 19.2 | 7.7 | 41.7 |
| OESS_6 | 1:3.0 | 6 | − | 16.6 | 15.4 | 27.6 | 10.3 | 30.1 |
| OESS_12 | 1:3.0 | 12 | − | 16.9 | 8.8 | 24.5 | 18.2 | 31.7 |
| OESS_24 | 1:3.0 | 24 | − | 11.0 | 10.1 | 27.1 | 8.8 | 42.9 |
| OESS_95 * | 1:3.0 | 48 | − | 5.2 | 6.3 | 8.2 | 2.7 | 77.6 |
| Product | Mw | Mn | DI |
|---|---|---|---|
| OESS_1.5 | 10,700 | 3800 | 2.8 |
| OESS_2.0 | 15,300 | 3700 | 4.1 |
| OESS_3.0 | 6300 | 3700 | 1.7 |
| OESS_6 | 7000 | 3600 | 1.9 |
| OESS_12 | 7000 | 3600 | 1.9 |
| OESS_24 | 8900 | 4200 | 2.1 |
| OESS_95 | 10,400 | 3800 | 2.7 |
| Product | Glass Transition Tg (°C) | Crosslink Density * (1/Mc)∙10−2 (mol/kg) | Tn-Unit Content According to 29Si NMR (%mol) | |
|---|---|---|---|---|
| 1st Run | 2nd Run | |||
| OESS_1.5 | −6.7 | −6.5 | 0.513 | 28.2 |
| OESS_2.0 | −6.4 | −6.1 | 0.749 | 34.3 |
| OESS_3.0 | −8.0 | −6.7 | 3.333 | 41.7 |
| OESS_6 | −10.6 | −7.7 | 7.436 | 30,1 |
| OESS_12 | −7.1 | −5.0 | 5.385 | 31.7 |
| OESS_24 | −10.6 | −9.7 | 2.308 | 42.9 |
| OESS_95 | −10.7 | −4.6 | 15.641 | 77.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageenkov, A.D.; Bredov, N.S.; Shcherbina, A.A.; Khasbiullin, R.R.; Tupikov, A.S.; Soldatov, M.A. The Influence of Conditions of Polycondensation in Acid Medium on the Structure of Oligosilsesquioxanes with a Novel Eugenol-Containing Substituent. Polymers 2024, 16, 2951. https://doi.org/10.3390/polym16202951
Ageenkov AD, Bredov NS, Shcherbina AA, Khasbiullin RR, Tupikov AS, Soldatov MA. The Influence of Conditions of Polycondensation in Acid Medium on the Structure of Oligosilsesquioxanes with a Novel Eugenol-Containing Substituent. Polymers. 2024; 16(20):2951. https://doi.org/10.3390/polym16202951
Chicago/Turabian StyleAgeenkov, Alexander D., Nikolay S. Bredov, Anna A. Shcherbina, Ramil R. Khasbiullin, Anton S. Tupikov, and Mikhail A. Soldatov. 2024. "The Influence of Conditions of Polycondensation in Acid Medium on the Structure of Oligosilsesquioxanes with a Novel Eugenol-Containing Substituent" Polymers 16, no. 20: 2951. https://doi.org/10.3390/polym16202951
APA StyleAgeenkov, A. D., Bredov, N. S., Shcherbina, A. A., Khasbiullin, R. R., Tupikov, A. S., & Soldatov, M. A. (2024). The Influence of Conditions of Polycondensation in Acid Medium on the Structure of Oligosilsesquioxanes with a Novel Eugenol-Containing Substituent. Polymers, 16(20), 2951. https://doi.org/10.3390/polym16202951







