Effect of Multivitamins on the Color Stability of Dental Materials Used in Pediatric Dentistry: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
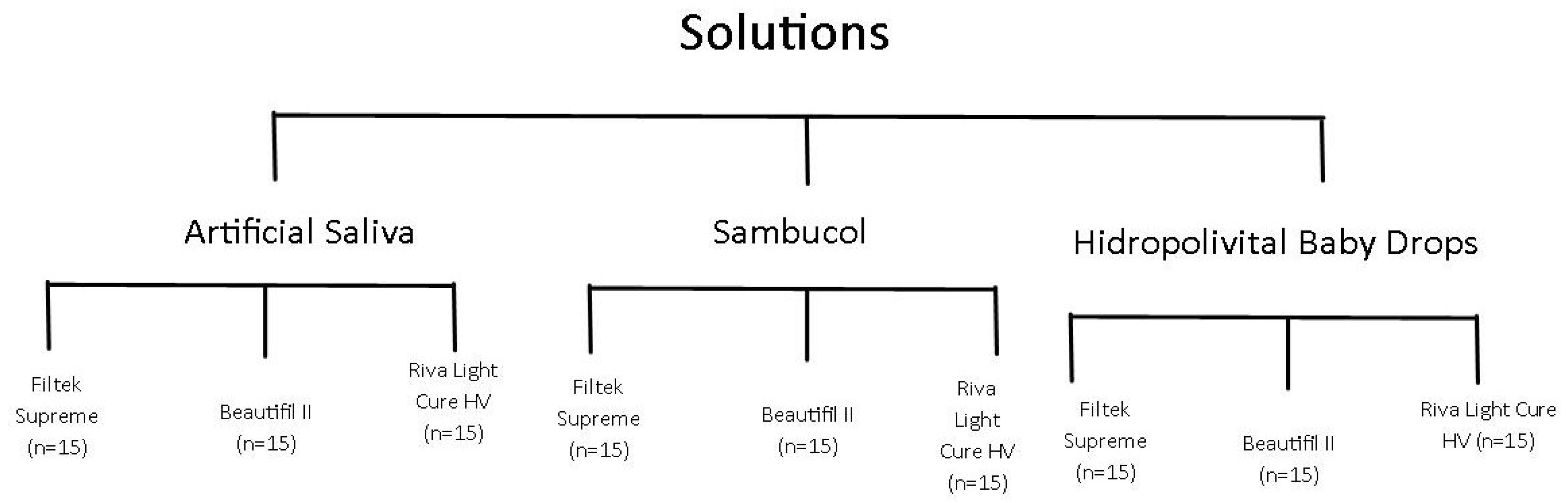
2.1. Study Design
2.2. Specimen Preparation
2.3. Immersion Procedures
2.4. Color Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yildirim, S.; Uslu, Y. Effects of different pediatric drugs and toothbrushing on color change of restorative materials used in pediatric dentistry. Niger. J. Clin. Pract. 2020, 23, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Kathiria, H.P.; Panda, A.K.; Virda, M.; Budakoti, V.; Dave, P.R.; Malge, R. Effect of pediatric drugs on color stability of various esthetic restorations in pediatric dentistry. Int. J. Prev. Clin. Dent. Res. 2021, 8, 35–37. [Google Scholar] [CrossRef]
- Penumatsa, N.V.; Alazmah, A.; Abushanan, A.; Uthman, U.S.; Algahtani, M.; Al Ghwainem, A. Impact of pediatric drugs on color stability of different aesthetic restorative materials used in pediatric dentistry: An in vitro study. World J. Dent. 2022, 13, 316–319. [Google Scholar] [CrossRef]
- Avula, J.S.S.; Adusumilli, H.; Kakarla, P.; Bandi, S.; Mallela, G.M.K.; Vallabhaneni, K. Color stability of esthetic restorative materials used in pediatric dentistry: An in vitro study. Saudi J. Kidney Dis. Transplant. 2016, 34, 233–237. [Google Scholar] [CrossRef]
- Ellakany, P.; Fouda, S.M.; AlGhamdi, M.A.; Aly, N.M. Influence of dental education on esthetics self-perception and shade selection. Int. J. Environ. Res. Public Health 2022, 19, 11547. [Google Scholar] [CrossRef] [PubMed]
- Rusnac, M.E.; Gasparik, C.; Irimie, A.I.; Grecu, A.G.; Mesaros, A.S.; Dudea, D. Giomers in dentistry—At the boundary between dental composites and glass-ionomers. Med. Pharm. Rep. 2019, 92, 123–128. [Google Scholar] [CrossRef]
- Aktas, N.; Akin, Y.; Bal, C.; Bani, M.; Bankoglu Güngor, M. Effect of the different dietary supplements on the average surface roughness and color stability of direct restorative materials used in Pediatric Dentistry. Children 2024, 27, 645. [Google Scholar] [CrossRef]
- Kale, Y.; Nalwade, A.V.; Dahake, P.T.; Dadpe, M.V.; Kendre, S.B. Effect of different pediatric drug formulations on color stability of composite, zirconia-reinforced glass ionomer cement, and glass ionomer cement. Saudi J. Kidney Dis. Transplant. 2019, 37, 151–156. [Google Scholar] [CrossRef]
- Ayaz, E.A.; Bagis, B.; Turgut, S. Effect of antiasthmatic medication on the surface roughness and color stability of dental restorative materials. Med. Princ. Pract. 2014, 23, 24–28. [Google Scholar] [CrossRef]
- Tian, F.; Yap, A.U.J.; Wang, X.; Gao, X. Effect of staining solutions on color of pre-reacted glass-ionomer containing composites. Dent. Mater. J. 2012, 31, 384–388. [Google Scholar] [CrossRef]
- Pássaro, A.L.; Olegário, I.C.; Laus, C.M.; Oliveira, R.C.; Tedesco, T.K.; Raggio, D.P. Giomer composite compared to glass ionomer in occlusoproximal ART restorations of primary molars: 24-month RCT. Aust. Dent. J. 2022, 67, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Paganini, A.; Attin, T.; Tauböck, T.T. Margin integrity of Bulk-Fill composite restorations in primary teeth. Materials 2020, 13, 3802. [Google Scholar] [CrossRef] [PubMed]
- Tauböck, T.T.; Buchalla, W.; Hiltebrand, U.; Roos, M.; Krejci, I.; Attin, T. Influence of the interaction of light- and self-polymerization on subsurface hardening of a dual-cured core build-up resin composite. Acta Odontol. Scand. 2011, 69, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, T.; Melian, K.; Krejci, I. Effect of dual-cure composite resin as restorative material on marginal adaptation of Class 2 restorations. Quintessence Int. 2013, 44, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Fouquet, V.; Attal, J.P.; Dursun, E. Commercially available fluoride-releasing restorative materials: A review and a proposal for classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef]
- López-García, S.; Pecci-LLoret, M.P.; Pecci-LLoret, M.R.; Oñate-Sánchez, R.E.; García-Bernal, D.; Castelo-Baz, P.; Rodríguez-Lozano, F.J.; Guerrero-Gironés, J. In Vitro evaluation of the biological effects of ACTIVA Kids BioACTIVE Restorative, Ionolux, and Riva Light Cure on human dental pulp stem cells. Materials 2019, 12, 3694. [Google Scholar] [CrossRef]
- da Silva Martins, D.; Mosella Pegatin, G.; Tozzi Portaluppe Bergantin, B.; Lira Di Leone, C.C.; Boteon, A.P.; Wang, L.; Rios, D.; Hónorio, H.M. Are S-PRG composites to able to resist against erosive and abrasive challenges and protect surrounding enamel in situ? J. Dent. 2024, 142, 104874. [Google Scholar] [CrossRef]
- Faghihi, T.; Heidarzadeh, Z.; Jafari, K.; Farhoudi, I.; Hekmatfar, S. An experimental study on the effect of four pediatric drug types on color stability in different tooth-colored restorative materials. Dent. Res. J. 2021, 18, 75. [Google Scholar] [CrossRef]
- Almutairi, M.; Moussa, I.; Alsaeri, N.; Alqahtani, A.; Alsulaiman, S.; Alhajri, M. The Effects of Different Pediatric Drugs and Brushing on the Color Stability of Esthetic Restorative Materials Used in Pediatric Dentistry: An In Vitro Study. Children 2022, 9, 1026. [Google Scholar] [CrossRef]
- Tüzüner, T.; Turgut, S.; Baygin, O.; Yilmaz, N.; Tuna, E.B.; Ozen, B. Effects of Different Pediatric Drugs on the Color Stability of Various Restorative Materials Applicable in Pediatric Dentistry. BioMed Res. Int. 2017, 2017, 9684193. [Google Scholar] [CrossRef]
- Valera, B.; Bhatt, R.; Patel, M.; Patel, C.; Makwani, D.; Goyal, S. Effect of different pediatric medications on various tooth colored restorative materials used in pediatric dentistry. Int. J. Health Sci. 2022, 31, 508–521. [Google Scholar] [CrossRef]
- Chandana Krishna Shree, C.; Pallavi Urs, G.; Pooja, H.; Mascarenhas, A.; Jenny, A.; Nagar, P. Gauging the upshot of liquid medicaments on surface roughness and stability of color in pit and fissure sealant—In vitro study. SRM J. Res. Dent. Sci. 2022, 13, 96–100. [Google Scholar] [CrossRef]
- Çinar, B.; Eren, D.; Akin, S. Effect of low pH dietary supplements on discoloration of resin composites. Niger. J. Clin. Pract. 2023, 26, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Alberton Da Silva, V.; Alberton Da Silva, S.; Pecho, O.E.; Bacchi, A. Influence of composite type and light irradiance on color stability after immersion in different beverages. J. Esthet. Restor. Dent. 2018, 30, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Pecho, O.E.; Ghinea, R.; Pulgar, R.; Della Bona, A. Recent Advances in Color and Whiteness Evaluations in Dentistry. Curr. Dent. 2019, 1, 23–29. [Google Scholar] [CrossRef]
- Taşin, S.; Ismatullaev, A. Effect of coffee thermocycling on the color and translucency of milled and 3D printed definitive restoration materials. J. Prosthet. Dent. 2024, 131, 969.e1–969.e7. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Della Bona, A.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Pérez, M.M. Color differences thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Casado, M.; Duveiller, V.; Ghinea, R.; Gautheron, A.; Clerc, R.; Salomon, J.-P.; del Mar Pérez, M.; Hébert, M.; Herrera, L.J. Comparative analysis of optical and numerical models for reflectance and color prediction of monolithic dental resin composites with varying thicknesses. Dent. Mater. 2024, 40, 1677–1684. [Google Scholar] [CrossRef]
- Bethapudy, D.R.; Bhat, C.; Lakade, L.; Chaudhary, S.; Kunte, S.; Patil, S. Comparative evaluation of water sorption, solubility, and microhardness of Zirconia-reinforced glass ionomer, resin-modified glass ionomer, and type IX glass ionomer restorative materials: An in vitro study. Int. J. Clin. Pediatr. Dent. 2022, 15, 175–181. [Google Scholar] [CrossRef]
- Paolone, G.; Mandurino, M.; De Palma, F.; Mazzitelli, C.; Scotti, N.; Breschi, L.; Gherlone, E.; Cantatore, G.; Vichi, A. Color stability of polymer-based composite CAD/CAM blocks: A systematic review. Polymers 2023, 15, 464. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Bogucki, M.; Szkutnik, J.; Rózyło-Kalinowska, I. The influence of popular beverages on mechanical properties of composite resins. Materials 2021, 14, 3097. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Raszewski, Z.; Alamoush, R.A.; Chojnacka, K.; Mikulewicz, M.; Haider, J. Influence of storing composite filling materials in a low-pH artificial saliva on their mechanical properties—An in vitro study. J. Funct. Biomater. 2023, 14, 328. [Google Scholar] [CrossRef] [PubMed]

| Brand Name | Material Type | Batch #LOT | Manufacturer | Composition * |
|---|---|---|---|---|
| Filtek Supreme XTE Universal | Nanihybrid Composite | 100262213 | 3M ESPE, Congers, NY, USA | Matrix: Bis-GMA, UDMA, TEGDMA Fillers:72.5 wt%, 55.6 vol% silica, zirconia fillers |
| Beautifil II | Giomer | 022352 | Shofu, Kyoto, Japan | Matrix: Bis-GMA, TEGDMA, Fillers: multifunctional filler, S-PRG filler based on fluoboroaluminiosilicate glass |
| Riva Light Cure High Viscosity | Reinforced Glass Ionomer | 1212859 | SDI, Victoria, Australia | Liquid: polyacrlic acid, tartaric acid, HEMA. Powder: Fluoroaluminiosilicate glass |
| Solution | Composition | Manufacturer |
|---|---|---|
| Artificial saliva (control group) | Purified water, glycerol, lemon essence, nipagin sodium, saccharosse | Farmacia Xalabarder, Barcelona, Spain |
| Sambucol Kids | Glucose syrup, purified water, black elderberry juice (Sambucus nigra), Vitamin C (ascorbic acid), citric acid, potassium sorbate | Pharma Care Europe, West Sussex, UK |
| Hidropolivital Baby drops | Bottle: water, sorbitol, fructose, zinc gluconate, honey, pantothenic acid (vitamin B5), citric acid, E-433 emulgent, potassium sorbate, pyroxidine hydrochloride (vitamin B6), sodium fluoride. Plug: L-ascorbic acid (vitamin C), lactose, retinyl acetate (vitamin A), cholecalciferol (vitamin D), silicon dioxide, riboflavin 5′ sodium phosphate (vitamin B2), thiamine hydrochloride (vitamin B1), phylloquinone (vitamin K), cyanocobalamin (vitamin B12). | Menarini, Badalona, Spain |
| Solution | Material | T1 | T2 | T3 | T4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | ||
| Artificial Saliva | Beautifil | 0.28 ± 0.15 | 0.31 (0.10–0.47) | 0.31 ± 0.16 | 0.31 (0.08–0.52) | 0.37 ± 0.20 | 0.42 (0.11–0.85) | 0.33 ± 0.12 | 0.34 (0.11–0.59) |
| Filtek | 0.28 ± 0.13 | 0.26 (0.12–0.54) | 0.30 ± 0.22 | 0.22 (0.08–0.99) | 0.27 ± 0.15 | 0.23 (0.08–0.71) | 0.50 ± 0.24 | 0.57 (0.12–0.96) | |
| Riva | 2.03 ± 0.52 | 1.99 (1.14–3.24) | 2.08 ± 0.53 | 2.06 (1.23–3.41) | 2.32 ± 0.56 | 2.22 (1.39–3.42) | 2.08 ± 0.56 | 2.17 (1.12–3.43) | |
| Sambucol | Beautifil | 0.93 ± 0.23 | 0.99 (0.55–1.23) | 0.67 ± 0.22 | 0.68 (0.37–1.12) | 0.63 ± 0.17 | 0.63 (0.30–1.03) | 0.89 ± 0.29 | 0.79 (0.60–1.03) |
| Filtek | 0.82 ± 0.23 | 0.82 (0.49–1.36) | 0.81 ± 0.263 | 0.79 (0.40–1.34) | 0.70 ± 0.27 | 0.72 (0.30–1.18) | 1.07 ± 0.41 | 0.96 (0.46–1.91) | |
| Riva | 1.71 ± 0.46 | 1.58 (1.02–2.71) | 1.92 ± 0.73 | 1.75 (1.01–3.28) | 2.15 ± 0.80 | 1.93 (1.10–3.47) | 1.63 ± 0.65 | 1.43 (0.75–3.24) | |
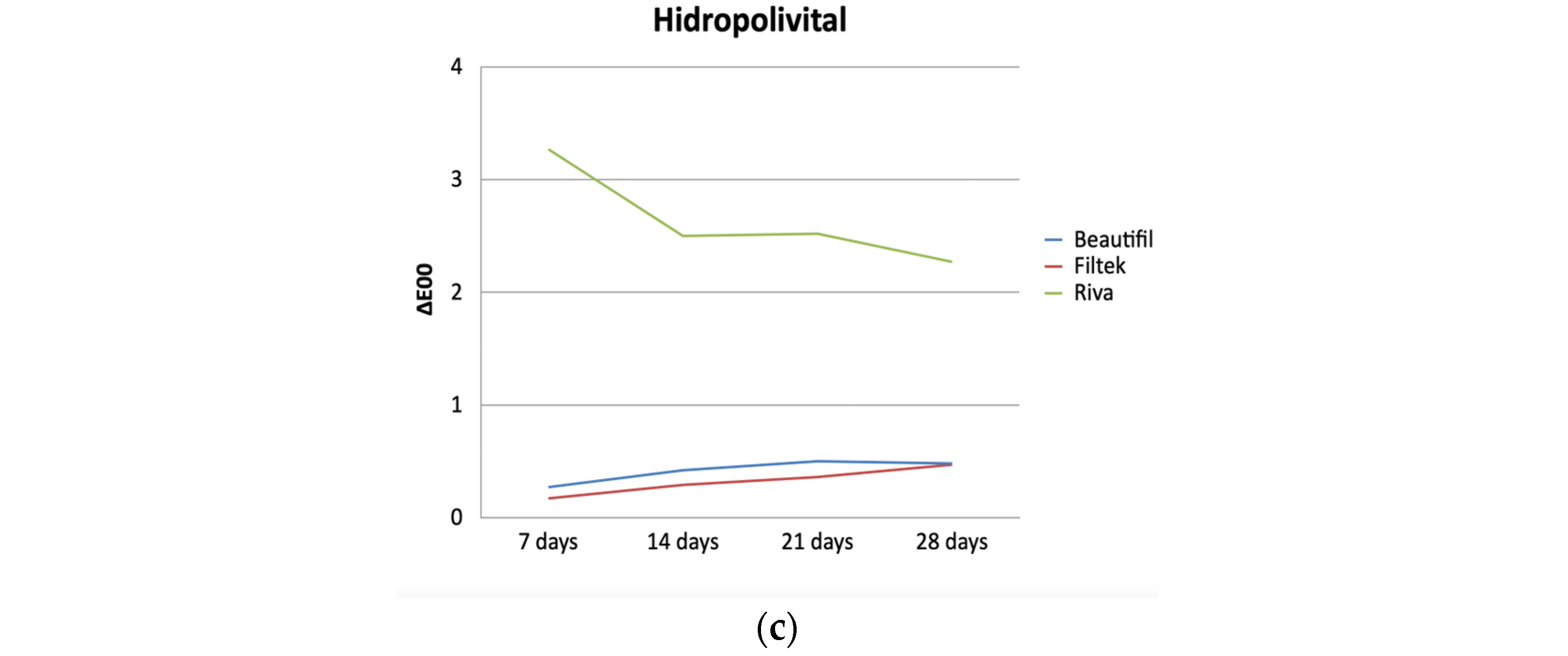
| Hidropoli- vital | Beautifil | 0.35 ± 0.25 | 0.27 (0.09–0.91) | 0.45 ± 0.20 | 0.42 (0.09–0.85) | 0.49 ± 0.28 | 0.50 (0.19–1.31) | 0.55 ± 0.37 | 0.48 (0.03–1.67) |
| Filtek | 0.23 ± 0.11 | 0.17 (0.09–0.43) | 0.31 ± 0.13 | 0.29 (0.14–0.64) | 0.38 ± 0.16 | 0.36 (0.19–0.76) | 0.59 ± 0.40 | 0.47 (0.21–1.55) | |
| Riva | 3.19 ± 0.70 | 3.27 (2.38–4.59) | 2.56 ± 0.79 | 2.50 (1.37–4.45) | 2.78 ± 0.72 | 2.52 (1.94–4.59) | 2.54 ± 0.86 | 2.27 (1.19–4.14) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arregui, M.; Contreras Arellano, J.d.P.; Veloso Durán, A.; Guinot Jimeno, F. Effect of Multivitamins on the Color Stability of Dental Materials Used in Pediatric Dentistry: An In Vitro Study. Polymers 2024, 16, 2948. https://doi.org/10.3390/polym16202948
Arregui M, Contreras Arellano JdP, Veloso Durán A, Guinot Jimeno F. Effect of Multivitamins on the Color Stability of Dental Materials Used in Pediatric Dentistry: An In Vitro Study. Polymers. 2024; 16(20):2948. https://doi.org/10.3390/polym16202948
Chicago/Turabian StyleArregui, María, Josefina del Pilar Contreras Arellano, Ana Veloso Durán, and Francisco Guinot Jimeno. 2024. "Effect of Multivitamins on the Color Stability of Dental Materials Used in Pediatric Dentistry: An In Vitro Study" Polymers 16, no. 20: 2948. https://doi.org/10.3390/polym16202948
APA StyleArregui, M., Contreras Arellano, J. d. P., Veloso Durán, A., & Guinot Jimeno, F. (2024). Effect of Multivitamins on the Color Stability of Dental Materials Used in Pediatric Dentistry: An In Vitro Study. Polymers, 16(20), 2948. https://doi.org/10.3390/polym16202948






