Preparation of Effective NiCrPd-Decorated Carbon Nanofibers Derived from Polyvinylpyrrolidone as a Catalyst for H2 Generation from the Dehydrogenation of NaBH4
Abstract
1. Introduction
2. Experimental
2.1. Materials
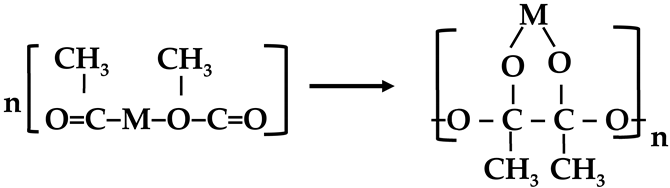
2.2. Preparation of the NiCrPd-Decorated CNF Catalyst
2.3. Characterization
2.4. Dehydrogenation of NaBH4
3. Results and Discussion
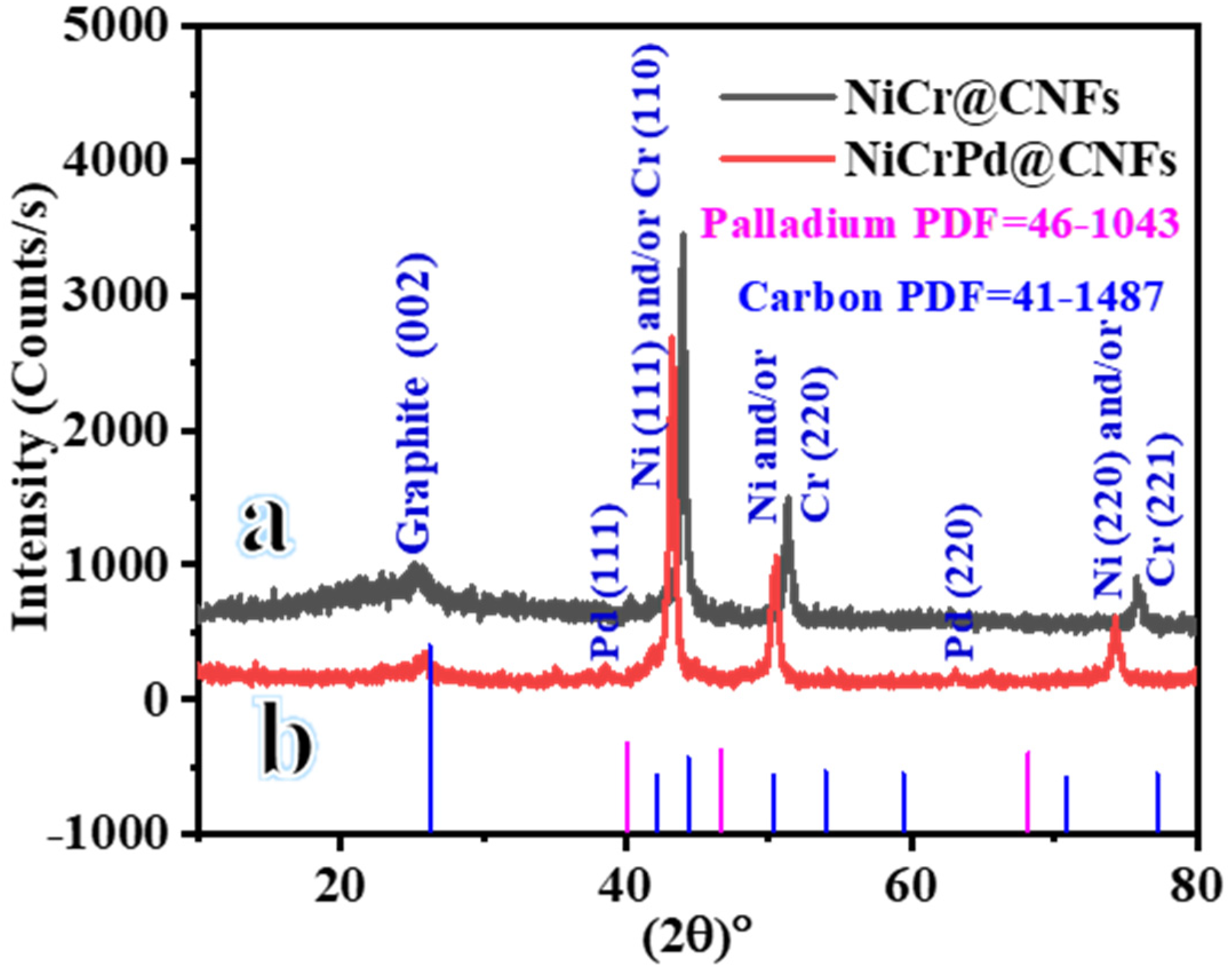
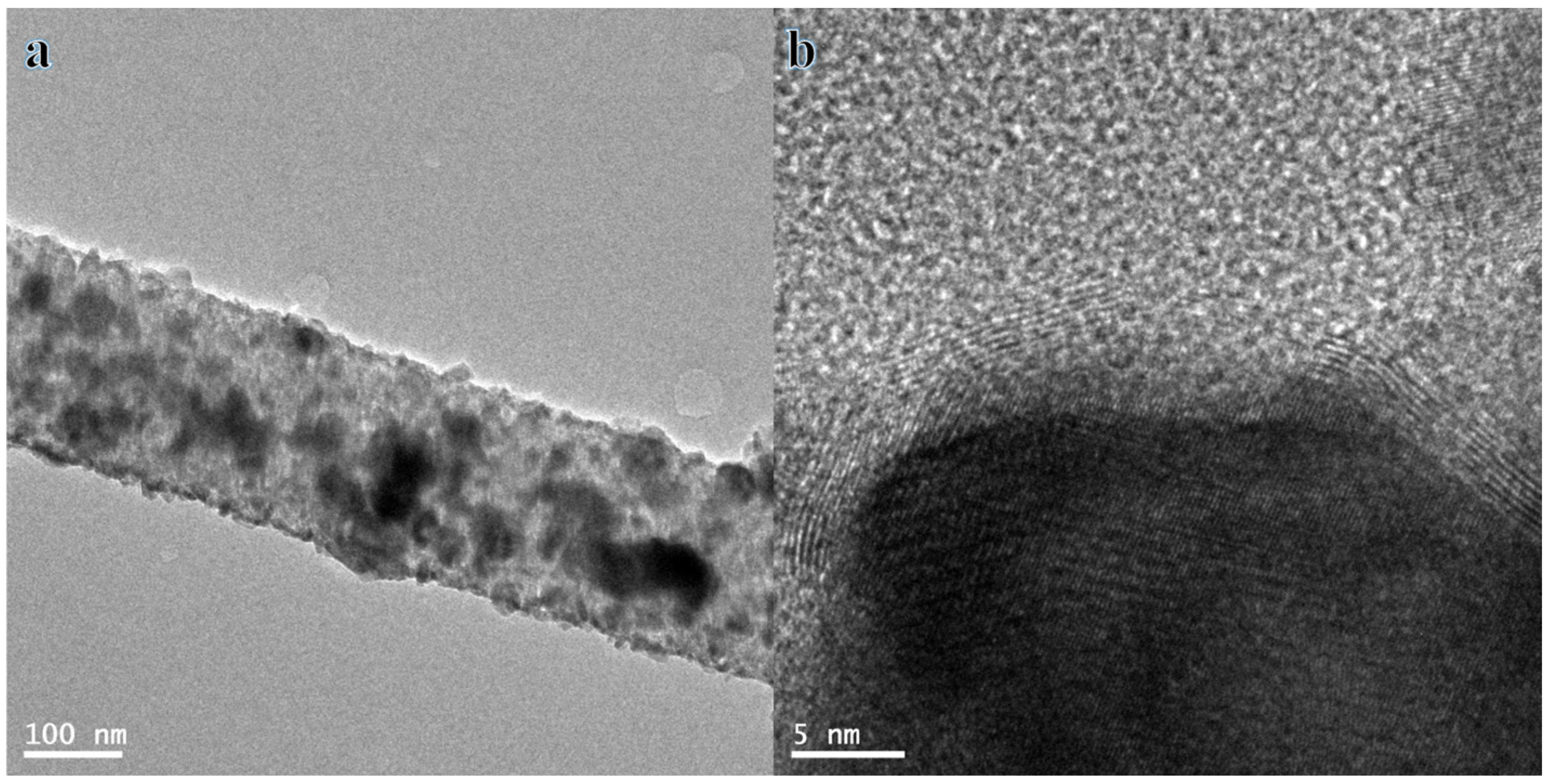
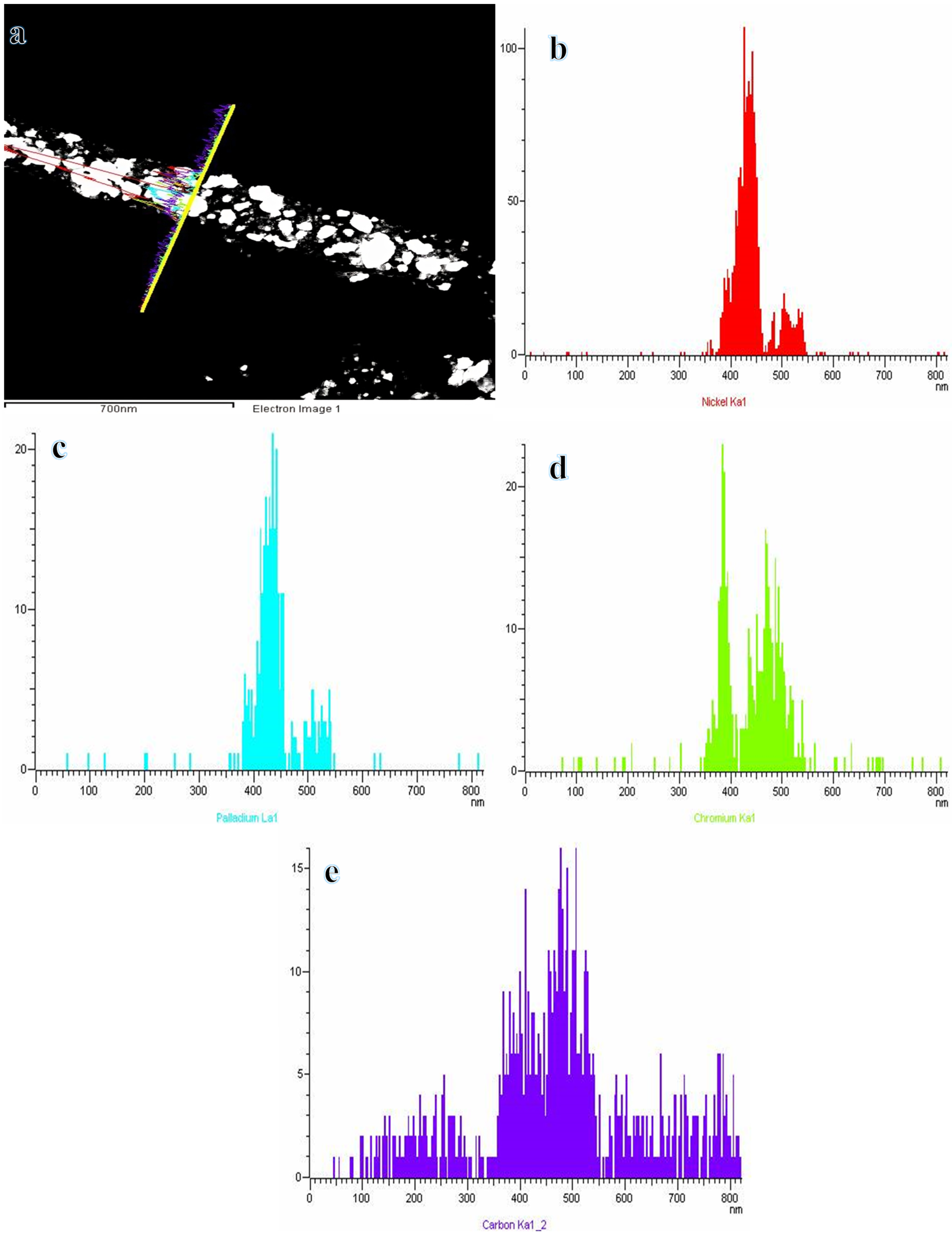
3.1. Characterization of Hybrid Nanofiber Mats
3.2. Dehydrogenation of NaBH4
3.2.1. Catalyst Amount
3.2.2. NaBH4 Concentration
3.2.3. Reaction Temperature
3.2.4. Recyclability Study
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirshafiee, F.; Rezaei, M. Synergistic catalytic performance of Co–Fe/CQD nanocatalyst for rapid hydrogen generation through NaBH4 hydrolysis. Int. J. Hydrogen Energy 2024, 79, 139–149. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Gbadamosi, A.O.; Epelle, E.I.; Abdulrasheed, A.A.; Haq, B.; Patil, S.; Al-Shehri, D.; Kamal, M.S. Hydrogen production, transportation, utilization, and storage: Recent advances towards sustainable energy. J. Energy Storage 2023, 73, 109207. [Google Scholar] [CrossRef]
- Mao, J.; Gregory, D.H. Recent advances in the use of sodium borohydride as a solid state hydrogen store. Energies 2015, 8, 430–453. [Google Scholar] [CrossRef]
- Zouli, N.; Maafa, I.M.; Abutaleb, A.; Yousef, A.; El-Halwany, M.M. Membrane Nanofiber-Supported Cobalt–Nickel Nanoparticles as an Effective and Durable Catalyst for H2 Evolution via Sodium Borohydride Hydrolysis. Polymers 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Zouli, N.; Maafa, I.M.; Abutaleb, A.; Yousef, A.; El-Halwany, M.M. Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation. Polymers 2023, 15, 1083. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, A.; Maafa, I.M.; Zouli, N.; Yousef, A.; El-Halwany, M.J.M. Synthesis of Trimetallic Nanoparticle (NiCoPd)-Supported Carbon Nanofibers as a Catalyst for NaBH4 Hydrolysis. Membranes 2023, 13, 783. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; El-Halwany, M.; Shaikh, S.F.; Pandit, B.; Yousef, A. Electrospun nickel nanoparticles@ poly (vinylidene fluoride-hexafluoropropylene) nanofibers as effective and reusable catalyst for H2 generation from sodium borohydride. Arab. J. Chem. 2022, 15, 104207. [Google Scholar] [CrossRef]
- Alpaydin, C. Investigation of Hydrogen Production from the Catalytic Dehydrogenation of Solid Hydrogen Storage Materials through Experimental and Modeling Studies; Graduate School of Natural and Applied Sciences of Dokuz Eylül University: Izmir, Turkey, 2021. [Google Scholar]
- Kirk, J.; Kim, Y.; Lee, Y.-J.; Kim, M.; Min, D.-S.; Kim, P.S.; Seo, J.H.; Kim, Y.; Lee, J.; Choung, J.W. Pushing the limits of sodium borohydride hydrolysis for on-board hydrogen generation systems. Chem. Eng. J. 2023, 466, 143233. [Google Scholar] [CrossRef]
- Wang, T.; Xi, J.; Sheng, H.; Zhao, Y. Mechanism of catalytic performance enhancement for hydrolysis of sodium borohydride by modification of cobalt boride with metals: A review. Int. J. Hydrogen Energy 2024, 85, 120–134. [Google Scholar] [CrossRef]
- Demirci, U.B. Exploring the technological maturity of hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2023, 48, 29682–29698. [Google Scholar] [CrossRef]
- Karabiberoğlu, Ş.; Dursun, Z. Au-Pt bimetallic nanoparticles anchored on conducting polymer: An effective electrocatalyst for direct electrooxidation of sodium borohydride in alkaline solution. Mater. Sci. Eng. B 2023, 288, 116158. [Google Scholar] [CrossRef]
- Sperandio, G.H.; de Carvalho, J.P.; de Jesus, C.B.R.; Junior, I.M.; de Oliveira, K.L.A.; Puiatti, G.A.; de Jesus, J.R.; Moreira, R.P.L. Hydrogen evolution from NaBH4 using novel Ni/Pt nanoparticles decorated on a niobium-based composite. Int. J. Hydrogen Energy 2024, 83, 774–783. [Google Scholar] [CrossRef]
- Ababaii, M.A.; Gilani, N.; Pasikhani, J.V. Hydrogen evolution from NaBH4 solution using Cr-doped Ni–B metallic catalyst deposited on rice husk via electroless plating. Int. J. Hydrogen Energy 2024, 51, 648–662. [Google Scholar] [CrossRef]
- Sun, L.; Gao, X.; Ning, X.; Qiu, Z.; Xing, L.; Yang, H.; Li, D.; Dou, J.; Meng, Y. Cobalt-nickel bimetal carbon sphere catalysts for efficient hydrolysis of sodium borohydride: The role of synergy and confine effect. Int. J. Hydrogen Energy 2023, 48, 3413–3428. [Google Scholar] [CrossRef]
- Attia, H.I.; Mahanna, H.; El-Halwany, M.M.; Mahmoud, M.H. Production of hydrogen from sodium borohydride using bimetal catalyst. Mansoura Eng. J. 2024, 49, 16. [Google Scholar] [CrossRef]
- Lu, D.; He, Z.; Liu, W. Hydrogen Generation from NaBH4 Hydrolysis Catalyzed with Efficient and Durable Ni–Co Bimetal Catalyst Supported by ZIF-67/rGO. J. Inorg. Organomet. Polym. Mater. 2024, 34, 1689–1698. [Google Scholar] [CrossRef]
- Yue, C.; Yang, P.; Wang, J.; Zhao, X.; Wang, Y.; Yang, L. Facile synthesis and characterization of nano-Pd loaded NiCo microfibers as stable catalysts for hydrogen generation from sodium borohydride. Chem. Phys. Lett. 2020, 743, 137170. [Google Scholar] [CrossRef]
- Liu, W.; Cai, H.; Lu, P.; Xu, Q.; Zhongfu, Y.; Dong, J. Polymer hydrogel supported Pd–Ni–B nanoclusters as robust catalysts for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2013, 38, 9206–9216. [Google Scholar] [CrossRef]
- Du, Y.; Wang, K.; Zhai, Q.; Chen, A.; Xi, Z.; Yan, J.; Kang, X.; Chen, M.; Yuan, X.; Zhu, M. Alloyed palladium-nickel hollow nanospheres with interatomic charge polarization for improved hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2018, 43, 283–292. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Liu, Q.; Zhou, M.; Mi, G.; Du, X. Hierarchically alloyed Pd–Cu microarchitecture with tunable shapes: Morphological engineering, and catalysis for hydrogen evolution reaction of ammonia borane. Int. J. Hydrogen Energy 2019, 44, 30226–30236. [Google Scholar] [CrossRef]
- Ramirez, O.; Bonardd, S.; Saldias, C.; Kroff, M.; O’Shea, J.N.; Diaz, D.D.; Leiva, A. Bimetallic NiPt nanoparticles-enhanced catalyst supported on alginate-based biohydrogels for sustainable hydrogen production. Int. J. Biol. Macromol. 2023, 225, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Darabi, R.; Gu, Q.; Mehrizi, A.A.; Altuner, E.E.; Alhrasishawi, W.; Gulbagca, F.; Tiri, R.N.E.; Tekeli, Y.; Seyrankaya, A.; Kaynak, I. Synthesis, characterization, and enhanced hydrogen generation from NaBH4 methanolysis of highly dispersed bimetallic PdNi nanoparticles supported on Vulcan carbon. Mol. Catal. 2023, 547, 113245. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, D.; Lim, S.H.; Liu, Y.; Chu, W. Confined PtNi catalysts for enhanced catalytic performances in one-pot cellobiose conversion to hexitols: A combined experimental and DFT study. Green Chem. 2019, 21, 5999–6011. [Google Scholar] [CrossRef]
- Garcıa-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Rh-Ni nanocatalysts for the CO2 and CO2+H2O reforming of methane. Catal Today 2011, 172, e42. [Google Scholar] [CrossRef]
- Pan, C.; Guo, Z.; Dai, H.; Ren, R.; Chu, W. Anti-sintering mesoporous Ni–Pd bimetallic catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 16133–16143. [Google Scholar] [CrossRef]
- Cai, H.-K.; Jiang, Z.-Y.; Xu, S.; Xu, Y.; Lu, P.; Dong, J. Polymer Hydrogel Supported Ni/Pd Alloys for Hydrogen Gas Production from Hydrolysis of Dimethylamine Borane with a Long Recyclable Lifetime. Polymers 2022, 14, 4647. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Li, W.; Xiong, X.; Wang, Y.; Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. In-situ confinement of ultrasmall palladium nanoparticles in silicalite-1 for methane combustion with excellent activity and hydrothermal stability. Appl. Catal. B Environ. 2020, 276, 119142. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Peng, X.; Jing, C.; Hu, W.; Tian, S.; Tian, J. In situ synthesis of cobalt-based tri-metallic nanosheets as highly efficient catalysts for sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2016, 41, 219–226. [Google Scholar] [CrossRef]
- Patil, K.N.; Prasad, D.; Manoorkar, V.K.; Nabgan, W.; Nagaraja, B.M.; Jadhav, A.H. Engineered nano-foam of tri-metallic (FeCuCo) oxide catalyst for enhanced hydrogen generation via NaBH4 hydrolysis. Chemosphere 2021, 281, 130988. [Google Scholar] [CrossRef]
- Jiao, C.; Huang, Z.; Wang, X.; Zhang, H.; Lu, L.; Zhang, S. Synthesis of Ni/Au/Co trimetallic nanoparticles and their catalytic activity for hydrogen generation from alkaline sodium borohydride aqueous solution. RSC Adv. 2015, 5, 34364–34371. [Google Scholar] [CrossRef]
- Wang, X.; Sun, S.; Huang, Z.; Zhang, H.; Zhang, S. Preparation and catalytic activity of PVP-protected Au/Ni bimetallic nanoparticles for hydrogen generation from hydrolysis of basic NaBH4 solution. Int. J. Hydrogen Energy 2014, 39, 905–916. [Google Scholar] [CrossRef]
- Al-Msrhad, T.M.H.; Devrim, Y.; Uzundurukan, A.; Budak, Y. Investigation of hydrogen production from sodium borohydride by carbon nano tube-graphene supported PdRu bimetallic catalyst for PEM fuel cell application. Int. J. Energy Res. 2022, 46, 4156–4173. [Google Scholar] [CrossRef]
- Hansu, T.A. A novel and active ruthenium based supported multiwalled carbon nanotube tungsten nanoalloy catalyst for sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2022, 48, 6788–6797. [Google Scholar] [CrossRef]
- Ivanenko, I.; Ruda, A. Cobalt, nitrogen-doped carbons as catalysts for sodium borohydride hydrolysis: Role of surface chemistry. J. Mater. Sci. 2022, 57, 1994–2011. [Google Scholar] [CrossRef]
- Saka, C. Phosphorus decorated g-C3N4-TiO2 particles as efficient metal-free catalysts for hydrogen release by NaBH4 methanolysis. Fuel 2022, 322, 124196. [Google Scholar] [CrossRef]
- Luo, X.; Sun, L.; Xu, F.; Cao, Z.; Zeng, J.; Bu, Y.; Zhang, C.; Xia, Y.; Zou, Y.; Zhang, K. Metal boride-decorated CoNi layered double hydroxides supported on muti-walled carbon nanotubes as efficient hydrolysis catalysts for sodium borohydride. J. Alloys Compd. 2023, 930, 167339. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Q.; Wu, G.; Qiu, S.; Zou, Y.; Xia, Y.; Xu, F.; Sun, L.; Chu, H. Zn-MOF-74-derived graphene nanosheets supporting CoB alloys for promoting hydrolytic dehydrogenation of sodium borohydride. J. Alloys Compd. 2023, 930, 167486. [Google Scholar] [CrossRef]
- Zouli, N.; Hameed, R.M.A.; Abutaleb, A.; Maafa, I.M.; Yousef, A. Calcined Nickel Oxide Nanostructures at Different Temperatures Onto Graphite for Efficient Electro-Oxidation of Ethylene Glycol in Basic Electrolyte. Appl. Organomet. Chem. 2024, e7729. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Brooks, R.M.; Ahmad, M.; El-Halwany, M.; El-Newehy, M.H.; Yousef, A. In-situ synthesis of amorphous co nanoparticles supported onto TiO2 nanofibers as a catalyst for hydrogen generation from the hydrolysis of ammonia borane. J. Nanosci. Nanotechnol. 2018, 18, 4714–4719. [Google Scholar] [CrossRef]
- Wang, H.; Zou, W.; Liu, C.; Sun, Y.; Xu, Y.; Sun, W.; Wang, Y. β-Ketoenamine-Linked Covalent Organic Framework with Co Intercalation: Improved Lithium-Storage Properties and Mechanism for High-Performance Lithium-Organic Batteries. Batter. Supercaps 2023, 6, e202200434. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Qin, Y.; Xie, Y.; Ling, Y.; Ye, H.; Zhang, Y. Facile construction of BiOBr/CoAl-LDH heterojunctions with suppressed Z-axis growth for efficient photoreduction of CO2. Sep. Purif. Technol. 2022, 302, 122090. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Guo, C.; Wang, Y. Molybdenum carbide-based photocatalysts: Synthesis, functionalization, and applications. Langmuir 2022, 38, 12739–12756. [Google Scholar] [CrossRef] [PubMed]
- Al-Enizi, A.M.; Nafady, A.; El-Halwany, M.; Brooks, R.M.; Abutaleb, A.; Yousef, A. Electrospun carbon nanofiber-encapsulated NiS nanoparticles as an efficient catalyst for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 21716–21725. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; El-Newehy, M.H.; Ahmed, M.; Kim, H.Y.J.C.; Physicochemical, S.A.; Aspects, E. Catalytic hydrolysis of ammonia borane for hydrogen generation using Cu (0) nanoparticles supported on TiO2 nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 194–201. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Khalil, K.A.; Unnithan, A.R.; Panthi, G.; Pant, B.; Kim, H.Y.J.C.; Physicochemical, S.A.; Aspects, E. Photocatalytic release of hydrogen from ammonia borane-complex using Ni (0)-doped TiO2/C electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 59–65. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.M.; Kim, H.Y. Electrospun Cu-doped titania nanofibers for photocatalytic hydrolysis of ammonia borane. Appl. Catal. A Gen. 2013, 467, 98–106. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; Abutaleb, A.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun CoCr7C3-supported C nanofibers: Effective, durable, and chemically stable catalyst for H2 gas generation from ammonia borane. Mol. Catal. 2017, 434, 32–38. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; EL-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A.M. Cu0/S-doped TiO2 nanoparticles-decorated carbon nanofibers as novel and efficient photocatalyst for hydrogen generation from ammonia borane. Ceram. Int. 2016, 42, 1507–1512. [Google Scholar] [CrossRef]
- Abbas, M.; Hameed, R.A.; Al-Enizi, A.M.; Thamer, B.M.; Yousef, A.; El-Newehy, M.H. Decorated carbon nanofibers with mixed nickel− manganese carbides for methanol electro-oxidation in alkaline solution. Int. J. Hydrogen Energy 2021, 46, 6494–6512. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Amna, T.; Unnithan, A.R.; Al-Deyab, S.S.; Kim, H.Y. Influence of CdO-doping on the photoluminescence properties of ZnO nanofibers: Effective visible light photocatalyst for waste water treatment. J. Lumin. 2012, 132, 1668–1677. [Google Scholar] [CrossRef]
- Pittala, R.K.; Sharma, P.; Anne, G.; Patil, S.; Varghese, V.; Das, S.R.; Kumar, C.S.; Fernandes, F. Development and Mechanical Characterization of Ni-Cr Alloy Foam Using Ultrasonic-Assisted Electroplating Coating Technique. Coatings 2023, 13, 1002. [Google Scholar] [CrossRef]
- Quan, C.; He, Y. Properties of nanocrystalline Cr coatings prepared by cathode plasma electrolytic deposition from trivalent chromium electrolyte. Surf. Coat. Technol. 2015, 269, 319–323. [Google Scholar] [CrossRef]
- Lopez-Baez, I.; Martinez-Franco, E.; Zoz, H.; Trapaga-Martinez, L.G. Structural evolution of Ni-20Cr alloy during ball milling of elemental powders. Rev. Mex. De Física 2011, 57, 176–183. [Google Scholar]
- Puri, R. Metallurgical Characterization of New Palladium-Containing CoCr and NiCr Alloys; Marquette University: Milwaukee, WI, USA, 2011. [Google Scholar]
- Yao, Q.; Shi, Y.; Zhang, X.; Chen, X.; Lu, Z.H. Facile synthesis of platinum–cerium (IV) oxide hybrids arched on reduced graphene oxide catalyst in reverse micelles with high activity and durability for hydrolysis of ammonia borane. Chem. Asian J. 2016, 11, 3251–3257. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P. A review: Hydrogen generation from borohydride hydrolysis reaction. J. Power Sources 2009, 187, 527–534. [Google Scholar] [CrossRef]
- Yao, Q.; Ding, Y.; Lu, Z.-H. Noble-metal-free nanocatalysts for hydrogen generation from boron-and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Heyman, A.; Abdel-Fattah, T.M. Palladium nanoparticle multiwalled carbon nanotube composite as catalyst for hydrogen production by the hydrolysis of sodium borohydride. ACS Appl. Energy Mater. 2018, 1, 4635–4640. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Zou, Y.; Xiang, C.; Zhang, H.; Xu, F.; Sun, L.; He, K. Modulating valence band to enhance the catalytic activity of Co-Cr-B/NG for hydrolysis of sodium borohydride. J. Alloys Compd. 2022, 924, 166556. [Google Scholar] [CrossRef]
- Falconer, J.L.; Magrini-Bair, K.A. Photocatalytic and thermal catalytic oxidation of acetaldehyde on Pt/TiO2. J. Catal. 1998, 179, 171–178. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H. Fabrication of amorphous Co–Cr–B and catalytic sodium borohydride hydrolysis for hydrogen generation. J. Mater. Res. 2020, 35, 281–288. [Google Scholar] [CrossRef]
- Prasad, D.; Patil, K.N.; Sandhya, N.; Chaitra, C.R.; Bhanushali, J.T.; Samal, A.K.; Keri, R.S.; Jadhav, A.H.; Nagaraja, B.M. Highly efficient hydrogen production by hydrolysis of NaBH4 using eminently competent recyclable Fe2O3 decorated oxidized MWCNTs robust catalyst. Appl. Surf. Sci. 2019, 489, 538–551. [Google Scholar] [CrossRef]
- Prasad, D.; Patil, K.N.; Chaitra, C.R.; Sandhya, N.; Bhanushali, J.T.; Gosavi, S.W.; Jadhav, A.H.; Nagaraja, B.M. Sulfonic acid functionalized PVA/PVDF composite hollow microcapsules: Highly phenomenal & recyclable catalysts for sustainable hydrogen production. Appl. Surf. Sci. 2019, 488, 714–727. [Google Scholar]
- Patil, K.N.; Prasad, D.; Bhanushali, J.T.; Kim, H.; Atar, A.B.; Nagaraja, B.M.; Jadhav, A.H. Sustainable hydrogen generation by catalytic hydrolysis of NaBH 4 using tailored nanostructured urchin-like CuCo 2 O 4 spinel catalyst. Catal. Lett. 2020, 150, 586–604. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, C.-F.; Guo, C.-Q.; Lu, Z.-X.; Shi, Y.; Wang, Z.-D. Synthesis of GO-modified Cu2O nanosphere and the photocatalytic mechanism of water splitting for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 4007–4016. [Google Scholar] [CrossRef]
- Du, X.; Liu, C.; Du, C.; Cai, P.; Cheng, G.; Luo, W. Nitrogen-doped graphene hydrogel-supported NiPt-CeO x nanocomposites and their superior catalysis for hydrogen generation from hydrazine at room temperature. Nano Res. 2017, 10, 2856–2865. [Google Scholar] [CrossRef]
- Song, X.; Yang, P.; Wang, J.; Zhao, X.; Zhou, Y.; Li, Y.; Yang, L. NiFePd/UiO-66 nanocomposites as highly efficient catalysts to accelerate hydrogen evolution from hydrous hydrazine. Inorg. Chem. Front. 2019, 6, 2727–2735. [Google Scholar] [CrossRef]
- Sen, B.; Demirkan, B.; Şavk, A.; Gülbay, S.K.; Sen, F. Trimetallic PdRuNi nanocomposites decorated on graphene oxide: A superior catalyst for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 17984–17992. [Google Scholar] [CrossRef]
- Wu, Y.; Tiri, R.N.E.; Bekmezci, M.; Altuner, E.E.; Aygun, A.; Mei, C.; Yuan, Y.; Xia, C.; Dragoi, E.-N.; Sen, F. Synthesis of novel activated carbon-supported trimetallic Pt–Ru–Ni nanoparticles using wood chips as efficient catalysts for the hydrogen generation from NaBH4 and enhanced photodegradation on methylene blue. Int. J. Hydrogen Energy 2022, 48, 21055–21065. [Google Scholar] [CrossRef]
- Wen, M.; Sun, Y.; Li, X.; Wu, Q.; Wu, Q.; Wang, C. Ru-capped/FeCo nanoflowers with high catalytic efficiency towards hydrolytic dehydrogenation. J. Power Sources 2013, 243, 299–305. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Sukackienė, Z.; Antanavičiūtė, K.; Vaičiūnienė, J.; Naujokaitis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Investigation of hydrogen generation from sodium borohydride using different cobalt catalysts. Int. J. Hydrogen Energy 2021, 46, 1989–1996. [Google Scholar] [CrossRef]
- Groven, L.J.; Pfeil, T.L.; Pourpoint, T.L. Solution combustion synthesized cobalt oxide catalyst precursor for NaBH4 hydrolysis. Int. J. Hydrogen Energy 2013, 38, 6377–6380. [Google Scholar] [CrossRef]
- Baydaroglu, F.O.; Özdemir, E.; Gürek, A.G. Polypyrrole supported Co–W–B nanoparticles as an efficient catalyst for improved hydrogen generation from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2022, 47, 9643–9652. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Alharbi, H.Y.; Paterson, R.; Wills, C.; Dixon, C.; Šiller, L.; Chamberlain, T.W.; Griffiths, A.; Collins, S.M. Efficient hydrolytic hydrogen evolution from sodium borohydride catalyzed by polymer immobilized ionic liquid-stabilized platinum nanoparticles. ChemCatChem 2022, 14, e202101752. [Google Scholar] [CrossRef]
- Kahri, H.; Flaud, V.; Touati, R.; Miele, P.; Demirci, U.B. Reaction intermediate/product-induced segregation in cobalt–copper as the catalyst for hydrogen generation from the hydrolysis of sodium borohydride. RSC Adv. 2016, 6, 102498–102503. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Intrazeolite cobalt (0) nanoclusters as low-cost and reusable catalyst for hydrogen generation from the hydrolysis of sodium borohydride. Appl. Catal. B Environ. 2009, 91, 21–29. [Google Scholar] [CrossRef]
- Akkus, M.S. Examination of the catalytic effect of Ni, NiCr, and NiV catalysts prepared as thin films by magnetron sputtering process in the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2023, 48, 23055–23066. [Google Scholar] [CrossRef]

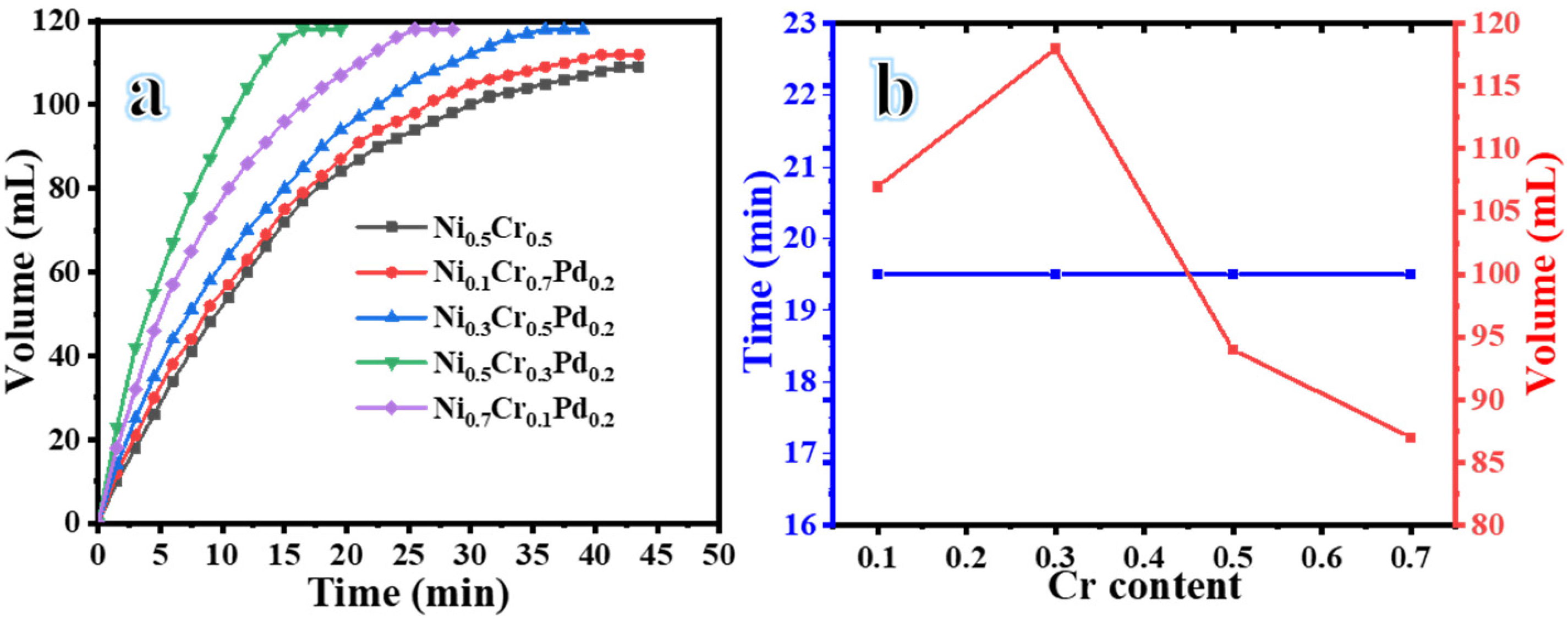
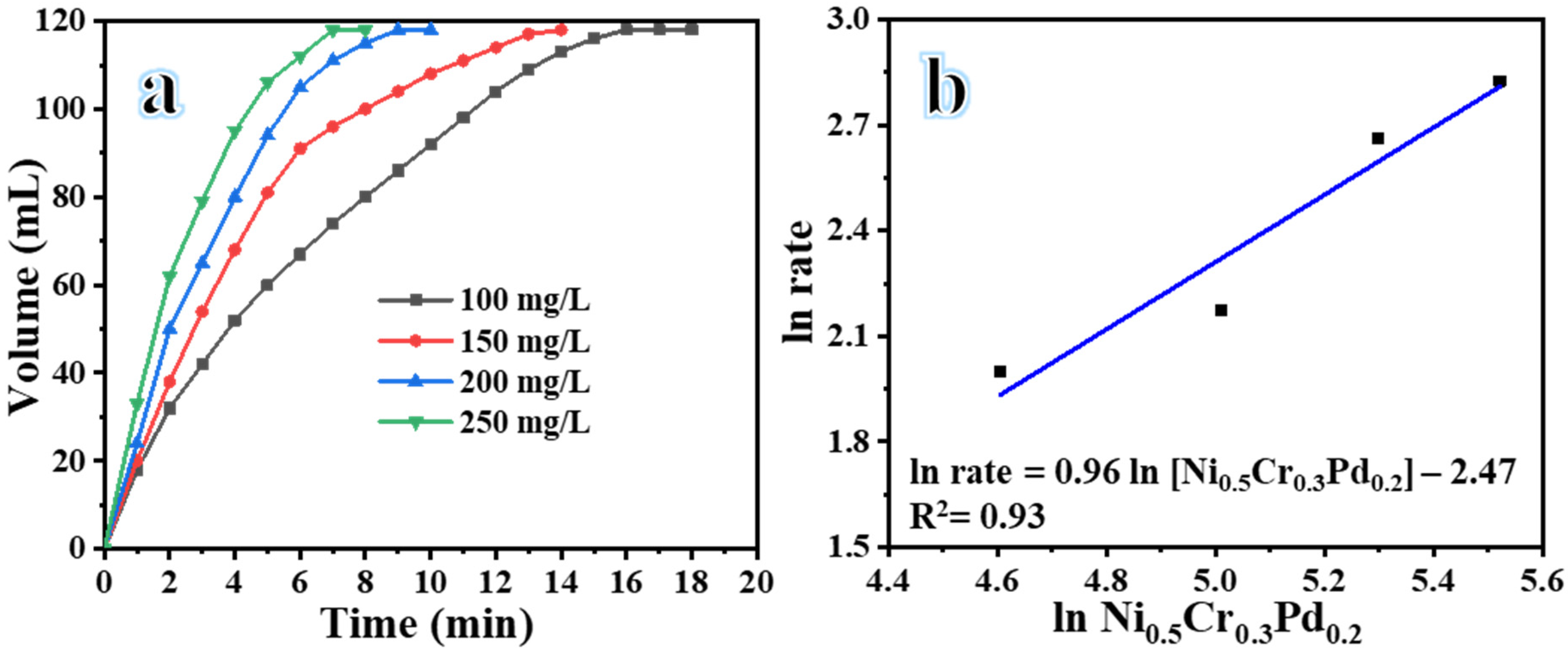
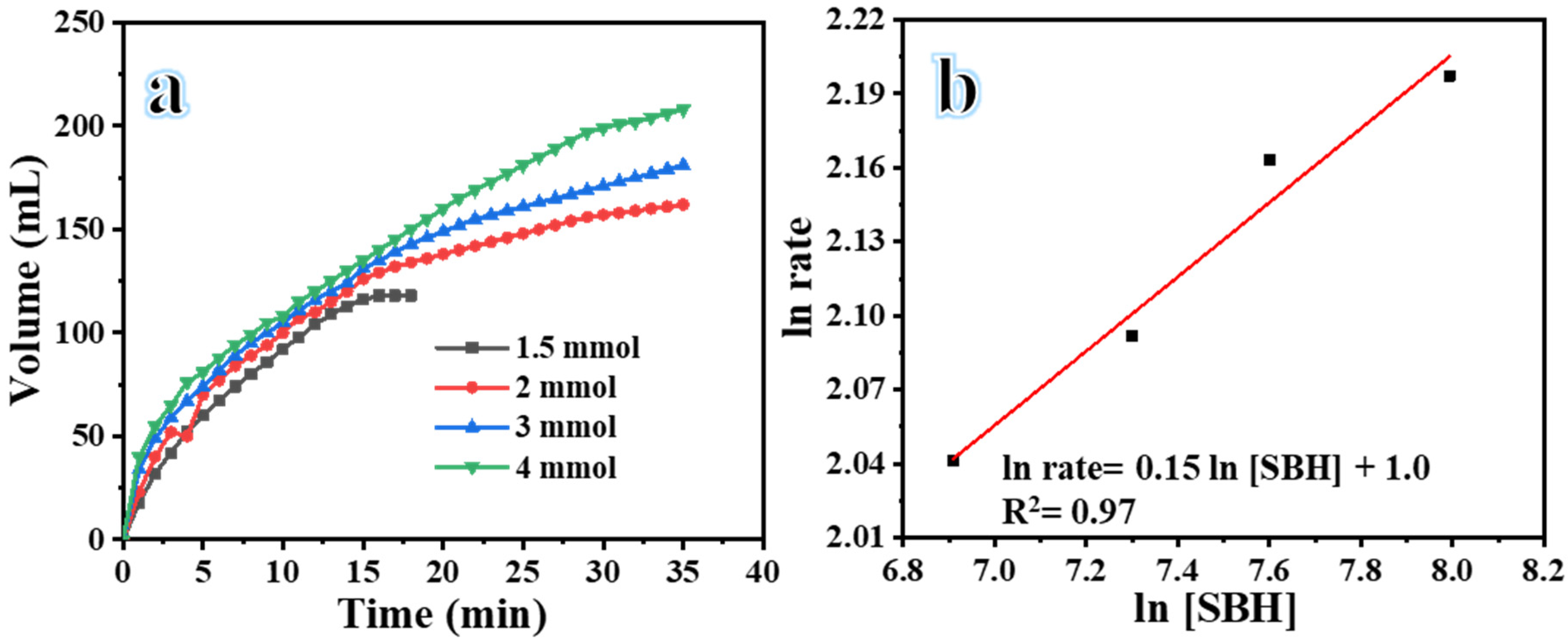

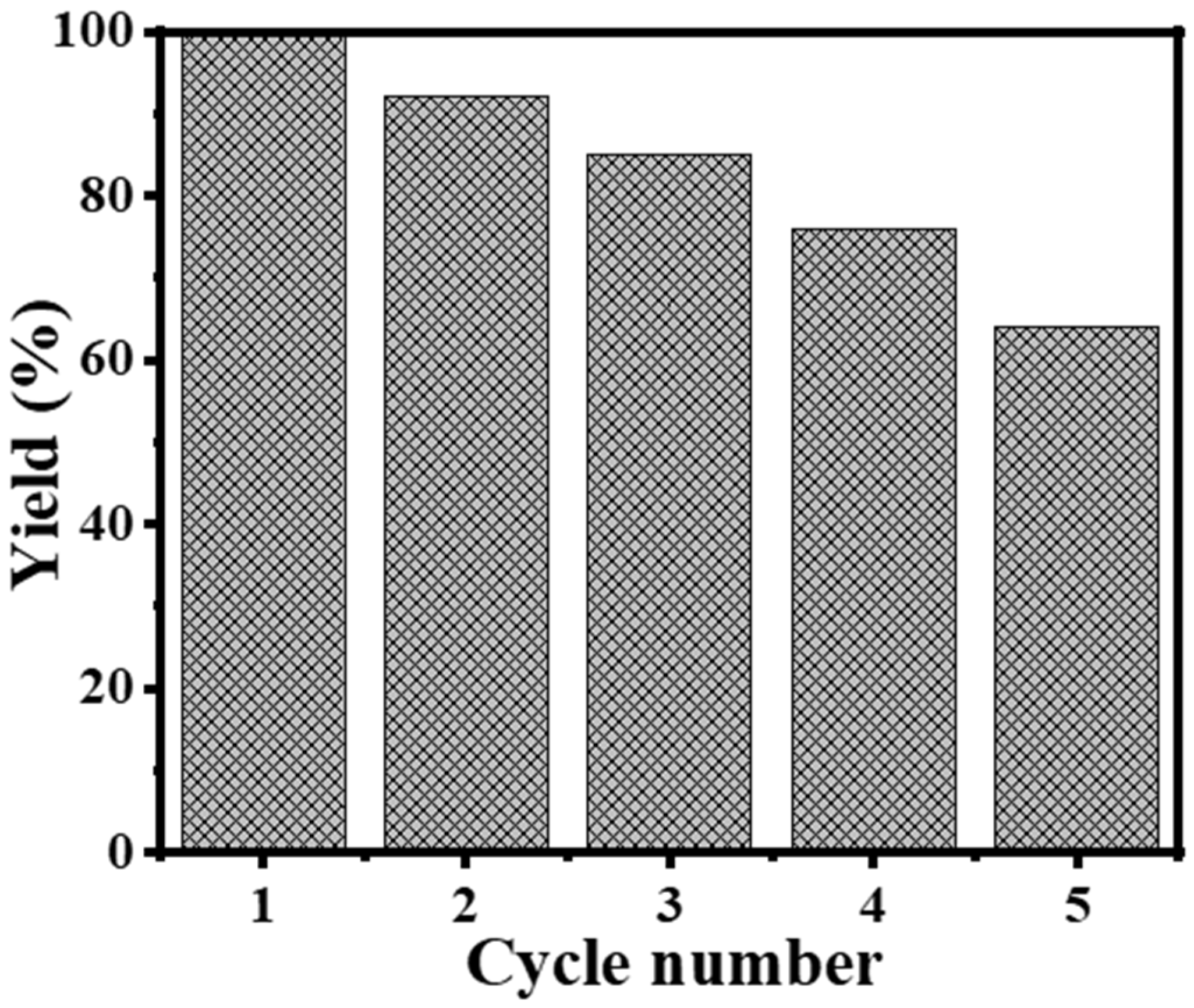
| Catalyst | Volume (mL) | Time (min) | Yield (%) | Rate (mL H2/min) | Rate (mol H2/h. mol Metal) |
|---|---|---|---|---|---|
| NiCr | 109 | 46 | 90.8 | 2.51 | 353.3 |
| Ni0.7Cr0.1Pd0.2 | 118 | 25 | 98.3 | 4.83 | 652.4 |
| Ni0.5Cr0.3Pd0.2 | 118 | 16 | 98.3 | 7.15 | 1008.2 |
| Ni0.3Cr0.5Pd0.2 | 118 | 36 | 98.3 | 3.27 | 462.1 |
| Ni0.1Cr0.7Pd0.2 | 112 | 40 | 93.3 | 2.74 | 375.9 |
| Catalyst Loading (g) | Volume (mL) | Time (min) | Yield (%) | Rate (mL H2/min) |
|---|---|---|---|---|
| 0.1 | 118 | 16 | 98.3 | 7.38 |
| 0.15 | 118 | 14 | 98.3 | 8.8 |
| 0.2 | 118 | 11 | 98.3 | 14.3 |
| 0.25 | 118 | 8 | 98.3 | 16.86 |
| Catalyst | Ea (KJ/mol) | Ref. |
|---|---|---|
| (Ni5Pt5)1-(CeOx)0.3/NGH | 38.66 | [68] |
| Ni0.25Fe0.25Pd0.5/UiO-66 | 43.5 | [69] |
| Ni45Au45Co10 | 18.8 | [32] |
| PdRuNi@GO | 55.47 | [70] |
| AC@Pt-Ru-Ni | 24.29 | [71] |
| Cu0.04Co0.864Ni0.096 | 40 | [30] |
| Ru-capped/FeCo | 42.9 | [72] |
| Ni0.5Cr0.3Pd0.2 | 26.55 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, A. Preparation of Effective NiCrPd-Decorated Carbon Nanofibers Derived from Polyvinylpyrrolidone as a Catalyst for H2 Generation from the Dehydrogenation of NaBH4. Polymers 2024, 16, 2908. https://doi.org/10.3390/polym16202908
Yousef A. Preparation of Effective NiCrPd-Decorated Carbon Nanofibers Derived from Polyvinylpyrrolidone as a Catalyst for H2 Generation from the Dehydrogenation of NaBH4. Polymers. 2024; 16(20):2908. https://doi.org/10.3390/polym16202908
Chicago/Turabian StyleYousef, Ayman. 2024. "Preparation of Effective NiCrPd-Decorated Carbon Nanofibers Derived from Polyvinylpyrrolidone as a Catalyst for H2 Generation from the Dehydrogenation of NaBH4" Polymers 16, no. 20: 2908. https://doi.org/10.3390/polym16202908
APA StyleYousef, A. (2024). Preparation of Effective NiCrPd-Decorated Carbon Nanofibers Derived from Polyvinylpyrrolidone as a Catalyst for H2 Generation from the Dehydrogenation of NaBH4. Polymers, 16(20), 2908. https://doi.org/10.3390/polym16202908







