Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering
Abstract
1. Introduction
2. Background on Electrospinning
2.1. Tunable Electrospun PCL-Based Nanofiber Properties
2.2. Electrospun PCL-Based Nanofibrous Scaffolds
2.3. Biomaterials Blend-Electrospun with PCL for Enhanced Scaffold Properties
2.3.1. Gelatin
2.3.2. Collagen
2.3.3. Silk Fibroin
2.3.4. Other Natural Products
2.3.5. Synthetic Polymers
2.3.6. Nanoparticles
3. Techniques for Enhancement of PCL-Based Scaffold Properties
3.1. Multilayered Scaffold Fabrication
3.2. Surface Modification or Functionalization
3.3. Surface Coating and Hydrogel Incorporation
3.4. 3D Printing
3.5. Advanced PCL-Based Scaffold Functionality
4. Applications in Tissue Engineering
4.1. Skin Tissue Engineering
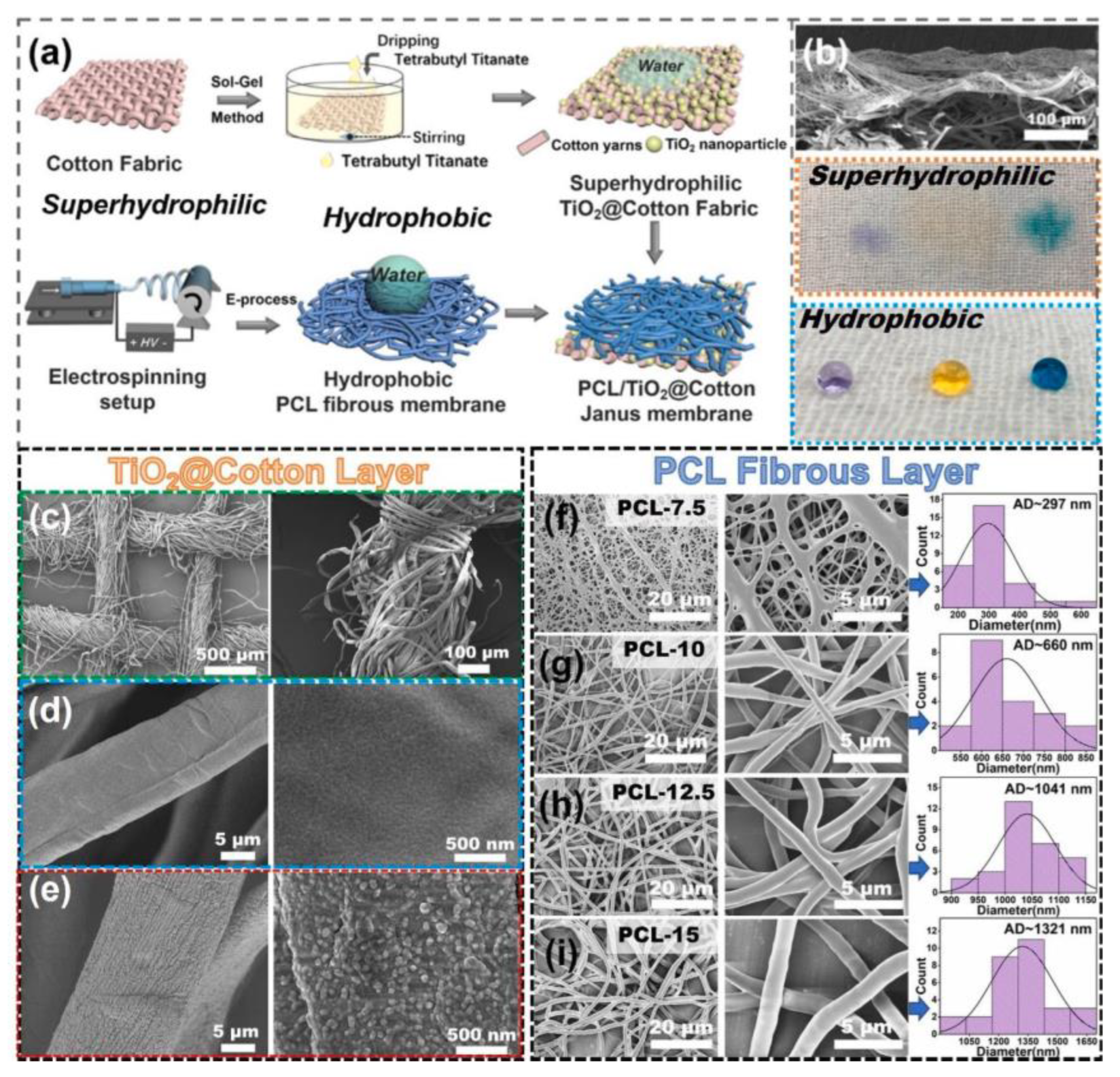
4.2. Bone Regeneration
5. Conclusions
5.1. Challenges and Limitations
5.2. Future Work
Funding
Acknowledgments
Conflicts of Interest
References
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Xu, P.; Kankala, R.K.; Wang, S.; Chen, A. Decellularized Extracellular Matrix-Based Composite Scaffolds for Tissue Engineering and Regenerative Medicine. Regen. Biomater. 2024, 11, rbad107. [Google Scholar] [CrossRef]
- Mu, J.; Luo, D.; Li, W.; Ding, Y. Multiscale Polymeric Fibers for Drug Delivery and Tissue Engineering. Biomed. Technol. 2024, 5, 60–72. [Google Scholar] [CrossRef]
- Azhari Rad, R.; Naghdi, Y.; Majidi Jamalabadi, M.; Masoumi, S.; Rezakhani, L.; Alizadeh, M. Tissue Engineering Scaffolds Loaded With a Variety of Plant Extracts: Novel Model in Breast Cancer Therapy. Breast Cancer 2024, 18, 11782234241236358. [Google Scholar] [CrossRef]
- Diedkova, K.; Pogrebnjak, A.D.; Kyrylenko, S.; Smyrnova, K.; Buranich, V.V.; Horodek, P.; Zukowski, P.; Koltunowicz, T.N.; Galaszkiewicz, P.; Makashina, K.; et al. Polycaprolactone-Mxene Nanofibrous Scaffolds for Tissue Engineering. ACS Appl. Mater. Interfaces 2023, 15, 14033–14047. [Google Scholar] [CrossRef]
- Howard, C.J.; Paul, A.; Duruanyanwu, J.; Sackho, K.; Campagnolo, P.; Stolojan, V. The Manufacturing Conditions for the Direct and Reproducible Formation of Electrospun PCL/Gelatine 3D Structures for Tissue Regeneration. Nanomaterials 2023, 13, 3107. [Google Scholar] [CrossRef]
- Kimura, V.T.; Zanin, M.H.A.; Wang, S.H. Influence of Thickness on the Properties of Electrospun PCL/Gelatin Nanofiber Scaffolds. Polym. Bull. 2024, 81, 9347–9361. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral. Biol. Craniofac Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Pang, L.; Sun, P.; Dong, X.; Tang, T.; Chen, Y.; Liu, Q.; Qi, M. Shear Viscoelasticity of Electrospinning PCL Nanofibers Reinforced Alginate Hydrogels. Mater. Res. Express 2021, 8, 55402. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-Caprolactone)-Based Electrospun Nano-Featured Substrate for Tissue Engineering Applications: A Review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP Blends for Tissue Engineering Scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Leonés Gil, A.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef]
- Prabha, R.D.; Kraft, D.C.E.; Harkness, L.; Melsen, B.; Varma, H.; Nair, P.D.; Kjems, J.; Kassem, M. Bioactive Nano-Fibrous Scaffold for Vascularized Craniofacial Bone Regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1537–e1548. [Google Scholar] [CrossRef]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of Type I Collagen and PCL Nanofibers Using Acetic Acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of Aligned and Random Fibers with Different Diameter on Cell Behaviors. Colloids Surf. B Biointerfaces 2018, 171, 461–467. [Google Scholar] [CrossRef]
- Fowler, M.J.; Riley, C.O.; Tomasson, E.; Mehta, S.; Grande-Allen, J.; Ballester, L.; Sandberg, D.I.; Janssen, C.F.; Sirianni, R.W. Engineering Subarachnoid Trabeculae with Electrospun Poly(Caprolactone) (PCL) Scaffolds to Study Leptomeningeal Metastasis in Medulloblastoma. Biomater. Adv. 2023, 155, 213646. [Google Scholar] [CrossRef]
- Yu, L.; Wang, F.; Huang, S. Fabrication and Properties of Polycaprolactone/Poly(Butylene Succinate) Blends Based on Electrospinning. Int. J. Polym. Sci. 2023, 2023, 9471371. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Markatos, D.N.; Dimopoulos, A.; Marazioti, A.; Mikelis, C.M.; Mavrilas, D. Development and Evaluation of Biodegradable Core-Shell Microfibrous and Nanofibrous Scaffolds for Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2024, 35, 10. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y. Sacrificial Biomaterials in 3D Fabrication of Scaffolds for Tissue Engineering Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35312. [Google Scholar] [CrossRef]
- Kaliaperumal, C.; Thulasisingh, A. Electrospun Polycaprolactone/Chitosan/Pectin Composite Nanofibre: A Novel Wound Dressing Scaffold. Bull. Mater. Sci. 2023, 46, 23. [Google Scholar] [CrossRef]
- Cotrim, M.; Oréfice, R. Tailoring Polycaprolactone/Silk Electrospun Nanofiber Yarns by Varying Compositional and Processing Parameters. Polym. Bull. 2024, 81, 593–610. [Google Scholar] [CrossRef]
- Oztemur, J.; Ozdemir, S.; Tezcan-Unlu, H.; Cecener, G.; Sezgin, H.; Yalcin-Enis, I. Investigation of Biodegradability and Cellular Activity of PCL/PLA and PCL/PLLA Electrospun Webs for Tissue Engineering Applications. Biopolymers 2023, 114, e23564. [Google Scholar] [CrossRef]
- Han, S.; Nie, K.; Li, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. 3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications. Stem Cells Int. 2021, 2021, 8790143. [Google Scholar] [CrossRef]
- Maji, K.; Pramanik, K. Electrospun Scaffold for Bone Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1915784. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning Nanofiber Scaffolds for Soft and Hard Tissue Regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Clement Mokhena, T.; Brian Chabalala, M.; Mapukata, S.; Mtibe, A.; Hlekelele, L.; Cele, Z.; Jonas Mochane, M.; Ntsendwana, B.; Amos Nhlapo, T.; Patrick Mokoena, T.; et al. Electrospun PCL-Based Materials for Health-Care Applications: An Overview. Macromol. Mater. Eng. 2024, 309, 2300388. [Google Scholar] [CrossRef]
- Kaliaperumal, C.; Thulasisingh, A. In-Vitro and in-Vivo Assessment of Polycaprolactone-Chitosan-Pectin Imbibed Nanofiber Potentials as a Wound Healing Biomaterial. J. Polym. Res. 2023, 30, 160. [Google Scholar] [CrossRef]
- Hejazi, F.; Mirzadeh, H.; Shojaei, S. PCL-based 3D nanofibrous structure with well-designed morphology and enhanced specific surface area for tissue engineering application. Prog. Biomater. 2023, 12, 113–122. [Google Scholar] [CrossRef]
- Sharifi, M.; Sadati, S.A.; Bahrami, S.H.; Haramshahi, S.M.A. Modeling and Optimization of Poly(Lactic Acid)/Poly(ℇ-Caprolactone)/Nigella Sativa Extract Nanofibers Production for Skin Wounds Healing by Artificial Neural Network and Response Surface Methodology Models. Int. J. Biol. Macromol. 2023, 253, 127227. [Google Scholar] [CrossRef]
- Gkouti, E.; Czekanski, A.; AlAttar, A. Simulating and Predicting the Mechanical Behavior of Electrospun Scaffolds for Cardiac Patches Fabrication. Materials 2023, 16, 7095. [Google Scholar] [CrossRef]
- Sameti, M.; Shojaee, M.; Saleh, B.M.; Moore, L.K.; Bashur, C.A. Peritoneal Pre-Conditioning Impacts Long-Term Vascular Graft Patency and Remodeling. Biomater. Adv. 2023, 148, 213386. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Chen, C.A.; Martinez, J.; Tran, P.; Komvopoulos, K. Novel Electrospun Polycaprolactone/Calcium Alginate Scaffolds for Skin Tissue Engineering. Materials 2023, 16, 136. [Google Scholar] [CrossRef]
- Jadidi Kouhbanani, M.A.; Mosleh-Shirazi, S.; Beheshtkhoo, N.; Kasaee, S.R.; Nekouian, S.; Alshehery, S.; Kamyab, H.; Chelliapan, S.; Ali, M.A.; Amani, A.M. Investigation through the Antimicrobial Activity of Electrospun PCL Nanofiber Mats with Green Synthesized Ag–Fe Nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 85, 104541. [Google Scholar] [CrossRef]
- Pisani, S.; Mauri, V.; Negrello, E.; Friuli, V.; Genta, I.; Dorati, R.; Bruni, G.; Marconi, S.; Auricchio, F.; Pietrabissa, A.; et al. Hybrid 3D-Printed and Electrospun Scaffolds Loaded with Dexamethasone for Soft Tissue Applications. Pharmaceutics 2023, 15, 2478. [Google Scholar] [CrossRef]
- Erdoğmuş, S.F.; Altıntaş, Ö.E.; Çelik, S. Production of Fungal Chitosan and Fabrication of Fungal Chitosan/Polycaprolactone Electrospun Nanofibers for Tissue Engineering. Microsc. Res. Tech. 2023, 86, 1309–1321. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mohammadi, A.A.; Rajabi, S.S.; Sanati, P.; Rafati, A.; Kian, M.; Zarei, Z. Preparation and Evaluation of a Polycaprolactone/Chitosan/Propolis Fibrous Nanocomposite Scaffold as a Tissue Engineering Skin Substitute. BioImpacts 2023, 13, 275–287. [Google Scholar] [CrossRef]
- Osanloo, M.; Noori, F.; Varaa, N.; Tavassoli, A.; Goodarzi, A.; Moghaddam, M.T.; Ebrahimi, L.; Abpeikar, Z.; Farmani, A.R.; Safaei, M.; et al. The Wound Healing Effect of Polycaprolactone-Chitosan Scaffold Coated with a Gel Containing Zataria Multiflora Boiss. Volatile Oil Nanoemulsions. BMC Complement. Med. Ther. 2024, 24, 56. [Google Scholar] [CrossRef]
- Ediz, E.F.; Güneş, C.; Demirel Kars, M.; Avcı, A. In Vitro Assessment of Momordica Charantia/Hypericum Perforatum Oils Loaded PCL/Collagen Fibers: Novel Scaffold for Tissue Engineering. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000231221067. [Google Scholar] [CrossRef]
- Pamu, D.; Garikapati, K.K.; Kuppusamy, G.; Krishnamurthy, P.T.; Ganesan, S.; Naik, M.R.; Karri, V.V.S.R.; Ponpandian, N.; Alexiou, A.; Antoniou, S.; et al. Electrospun Statin-Loaded Nanofibers for Treating Diabetic Wounds. Polym. Eng. Sci. 2024, 64, 1715–1730. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Aldubaisi, Y.; Swami, V.; Swami, V.; Xu, G.; Vaughan, M.B.; Wolf, R.F.; Khandaker, M. Polycaprolactone Electrospun Nanofiber Membrane with Skin Graft Containing Collagen and Bandage Containing MgO Nanoparticles for Wound Healing Applications. Polymers 2023, 15, 2014. [Google Scholar] [CrossRef]
- Yildiz, G.; Arslan, Y.E.; Derkus, B.; Sezgin, B.; Menceloglu, Y.Z.; Bayar, G.R. Development and Characterization of Skin Substitutes from Electrospun Polycaprolactone/Silk Fibroin. J. Bioact. Compat. Polym. 2024, 39, 46–62. [Google Scholar] [CrossRef]
- Feng, K.; Tang, J.; Qiu, R.; Wang, B.; Wang, J.; Hu, W. Fabrication of a Core-Shell Nanofibrous Wound Dressing with an Antioxidant Effect on Skin Injury. J. Mater. Chem. B 2024, 12, 2384–2393. [Google Scholar] [CrossRef]
- Sadeghi, M.; Falahi, F.; Akbari-Birgani, S.; Nikfarjam, N. Trilayer Tubular Scaffold to Mimic Ductal Carcinoma Breast Cancer for the Study of Chemo-Photothermal Therapy. ACS Appl. Polym. Mater. 2023, 5, 2394–2407. [Google Scholar] [CrossRef]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA Fibers for Drug Delivery: A Review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Zia, S.; Djalali-Cuevas, A.; Pflaum, M.; Hegermann, J.; Dipresa, D.; Kalozoumis, P.; Kouvaka, A.; Burgwitz, K.; Andriopoulou, S.; Repanas, A.; et al. Development of a Dual-Component Infection-Resistant Arterial Replacement for Small-Caliber Reconstructions: A Proof-of-Concept Study. Front. Bioeng. Biotechnol. 2023, 11, 957458. [Google Scholar] [CrossRef]
- Jarrell, D.K.; Jacot, J.G. An in Vitro Characterization of a PCL-Fibrin Scaffold for Myocardial Repair. Mater. Today Commun. 2023, 37, 107596. [Google Scholar] [CrossRef]
- Almasi-Jaf, A.; Shamloo, A.; Shaygani, H.; Seifi, S. Fabrication of Heparinized Bi-Layered Vascular Graft with PCL/PU/Gelatin Co-Electrospun and Chitosan/Silk Fibroin/Gelatin Freeze-Dried Hydrogel for Improved Endothelialization and Enhanced Mechanical Properties. Int. J. Biol. Macromol. 2023, 253, 126807. [Google Scholar] [CrossRef]
- Gholipour Choubar, E.; Nasirtabrizi, M.H.; Salimi, F.; Sadeghianmaryan, A. Improving Bone Regeneration with Electrospun Antibacterial Polycaprolactone/Collagen/Polyvinyl Pyrrolidone Scaffolds Coated with Hydroxyapatite and Cephalexin Delivery Capability. J. Biomater. Sci. Polym. Ed. 2024, 35, 127–145. [Google Scholar] [CrossRef]
- Tang, T.N.; Nguyen, T.H.A.; Tran, C.M.; Doan, V.K.; Nguyen, N.T.T.; Vu, B.T.; Dang, N.N.T.; Duong, T.T.; Pham, V.H.; Tran, L.D.; et al. Fabrication of Silver Nanoparticle-Containing Electrospun Polycaprolactone Membrane Coated with Chitosan Oligosaccharides for Skin Wound Care. J. Sci. Adv. Mater. Devices 2023, 8, 100582. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, Y.; Huang, Y.; Wang, F.; Qian, Y.; Wang, Y. Preparation and Properties of (Sc2O3-MgO)/Pcl/Pvp Electrospun Nanofiber Membranes for the Inhibition of Escherichia Coli Infections. Int. J. Mol. Sci. 2023, 24, 7649. [Google Scholar] [CrossRef]
- Soliman, S.; Sant, S.; Nichol, J.W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the Porosity of Fibrous Scaffolds by Modulating the Fiber Diameter and Packing Density. J. Biomed. Mater. Res. A 2011, 96A, 566–574. [Google Scholar] [CrossRef]
- Liu, Y.X.; Chaparro, F.J.; Tian, Z.; Jia, Y.; Gosser, J.; Gaumer, J.; Ross, L.; Tafreshi, H.; Lannutti, J.J. Visualization of Porosity and Pore Size Gradients in Electrospun Scaffolds Using Laser Metrology. PLoS ONE 2023, 18, e0282903. [Google Scholar] [CrossRef]
- Ege, D.; Pourshahrestani, S.; Iorio, F.; Reinfelder, H.; de Ligny, D.; Boccaccini, A.R. Processing and Characterization of Aligned Electrospun Gelatin/Polycaprolactone Nanofiber Mats Incorporating Borate Glass (13-93B3) Microparticles. Biomed. Mater. 2023, 18, 055030. [Google Scholar] [CrossRef]
- Rezaei Kolarijani, N.; Cheraghali, D.; Khastar, H.; Ehterami, A.; Alizade, M.; Vaez, A.; Amini, S.M.; Salehi, M. Nanofibrous Polycaprolactone/Gelatin Scaffold Containing Gold Nanoparticles: Physicochemical and Biological Characterization for Wound Healing. Wound Repair. Regen. 2023, 31, 804–815. [Google Scholar] [CrossRef]
- Mohammadi, S.S.; Shafiei, S.S. Electrospun Biodegradable Scaffolds Based on Poly (ε-Caprolactone)/Gelatin Containing Titanium Dioxide for Bone Tissue Engineering Application; in Vitro Study. J. Macromol. Sci. Part. A Pure Appl. Chem. 2023, 60, 270–281. [Google Scholar] [CrossRef]
- Gautam, S.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Sharma, C.; Chou, C.F.; Mishra, N.C. Surface Modification of PCL-Gelatin-Chitosan Electrospun Scaffold by Nano-Hydroxyapatite for Bone Tissue Engineering. Mater. Today Commun. 2023, 34, 105237. [Google Scholar] [CrossRef]
- Loyo, C.; Cordoba, A.; Palza, H.; Canales, D.; Melo, F.; Vivanco, J.F.; Baier, R.V.; Millán, C.; Corrales, T.; Zapata, P.A. Effect of Gelatin Coating and GO Incorporation on the Properties and Degradability of Electrospun PCL Scaffolds for Bone Tissue Regeneration. Polymers 2024, 16, 129. [Google Scholar] [CrossRef]
- Lashkari, M.; Rahmani, M.; Yousefpoor, Y.; Ahmadi-Zeidabadi, M.; Faridi-Majidi, R.; Ameri, Z.; Salary, M.; Azizi, S.; Shahabi, A.; Rahi, A.; et al. Cell-Based Wound Dressing: Bilayered PCL/Gelatin Nanofibers-Alginate/Collagen Hydrogel Scaffold Loaded with Mesenchymal Stem Cells. Int. J. Biol. Macromol. 2023, 239, 124099. [Google Scholar] [CrossRef]
- Esmaeilneia, S.; Amiri Dehkharghani, R.; Zamanlui Benisi, S. Architecture of a Dual Biocompatible Platform to Immobilize Genistin: Fabrication with Physio-Chemical and in Vitro Evaluation. Sci. Rep. 2023, 13, 22439. [Google Scholar] [CrossRef]
- Xu, H.; Gao, Z.; Wang, Z.; Wu, W.; Li, H.; Liu, Y.; Jia, S.; Hao, D.; Zhu, L. Electrospun PCL Nerve Conduit Filled with GelMA Gel for CNTF and IGF-1 Delivery in Promoting Sciatic Nerve Regeneration in Rat. ACS Biomater. Sci. Eng. 2023, 9, 6309–6321. [Google Scholar] [CrossRef]
- Lacy, H.A.; Nealy, S.L.; Stanishevsky, A. Degradation and Mechanical Behavior of Fish Gelatin/Polycaprolactone AC Electrospun Nanofibrous Meshes. Macromol. Mater. Eng. 2023, 308, 2300106. [Google Scholar] [CrossRef]
- Rosalia, M.; Giacomini, M.; Tottoli, E.M.; Dorati, R.; Bruni, G.; Genta, I.; Chiesa, E.; Pisani, S.; Sampaolesi, M.; Conti, B. Investigation on Electrospun and Solvent-Casted PCL-PLGA Blends Scaffolds Embedded with Induced Pluripotent Stem Cells for Tissue Engineering. Pharmaceutics 2023, 15, 2736. [Google Scholar] [CrossRef]
- Ustundag, C.R.; Piskin, M.B. Investigation of Electrospun Poly (ε-Caprolactone) Fiber Mats Loaded with Calophyllum Inophyllum Essential Oil for Wound Dressing Applications: Morphology, Drug Release and in Vitro Evaluation. Mater. Technol. 2023, 38, 2223018. [Google Scholar] [CrossRef]
- Du, M.; Liu, S.; Lan, N.; Liang, R.; Liang, S.; Lan, M.; Feng, D.; Zheng, L.; Wei, Q.; Ma, K. Electrospun PCL/Gelatin/Arbutin Nanofiber Membranes as Potent Reactive Oxygen Species Scavengers to Accelerate Cutaneous Wound Healing. Regen. Biomater. 2024, 11, rbad114. [Google Scholar] [CrossRef]
- Shafizadeh, S.; Heydari, P.; Zargar Kharazi, A.; Shariati, L. Coaxial Electrospun PGS/PCL and PGS/PGS-PCL Nanofibrous Membrane Containing Platelet-Rich Plasma for Skin Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2024, 35, 482–500. [Google Scholar] [CrossRef]
- Afrashi, M.; Semnani, D.; Hashemibeni, B.; Shokrgozar, M.A. Degradable and Biocompatible Nanofibrous Scaffold Incorporating a Natural Cell Culture Medium for Skin Tissue Engineering. Phys. Scr. 2024, 99, 035029. [Google Scholar] [CrossRef]
- Mirbagheri, M.S.; Akhavan-Mahdavi, S.; Hasan, A.; Kharazmi, M.S.; Jafari, S.M. Propolis-Loaded Nanofiber Scaffolds Based on Polyvinyl Alcohol and Polycaprolactone. Int. J. Pharm. 2023, 642, 123186. [Google Scholar] [CrossRef]
- Cárdenas, V.; Fernández, D.; Romero-Araya, P.; Werlinger, F.; Martínez, J.; Moreno-Villoslada, I.; Flores, M.E. Tuning the Properties of Polycaprolactone-Based Fibers by Using Polyethylene Oxide / Polycaprolactone Block Copolymers. J. Polym. Res. 2024, 31, 60. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Zhou, A.; Zhao, Y.; Tian, Z.; Zhang, Y.; Chen, K.; Ning, X.; Xu, Y. Light-Activated Chemically Reactive Fibrous Patch Revolutionizes Wound Repair Through the Prevention of Postoperative Adhesion. Nano Lett. 2023, 23, 1435–1444. [Google Scholar] [CrossRef]
- Tran, C.M.; Nguyen, N.T.T.; Ho, M.H.; Doan, V.K.; Ly, K.L.; Dang, N.N.T.; Tran, N.M.P.; Nguyen, H.T.T.; Truong, L.P.; Do, T.M.; et al. One-Pot Preparation of Antibacterial Electrospun Polycaprolactone Membrane Embedded with Gamma Irradiation-Induced Silver Nanoparticles. Fibers Polym. 2023, 24, 29–43. [Google Scholar] [CrossRef]
- Asadian, M.; Tomasina, C.; Onyshchenko, Y.; Chan, K.V.; Norouzi, M.; Zonderland, J.; Camarero-Espinosa, S.; Morent, R.; De Geyter, N.; Moroni, L. The Role of Plasma-Induced Surface Chemistry on Polycaprolactone Nanofibers to Direct Chondrogenic Differentiation of Human Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2024, 112, 210–230. [Google Scholar] [CrossRef]
- Tian, J.; Paterson, T.E.; Zhang, J.; Li, Y.; Ouyang, H.; Asencio, I.O.; Hatton, P.V.; Zhao, Y.; Li, Z. Enhanced Antibacterial Ability of Electrospun PCL Scaffolds Incorporating ZnO Nanowires. Int. J. Mol. Sci. 2023, 24, 14420. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, H.; Sun, Y.; Nan, J.; Liu, W.; Lei, P.; Hu, Y. The Bio-Functionalized Membrane Loaded with Ta/WH Nanoparticles Promote Bone Regeneration through Neurovascular Coupling. Colloids Surf. B Biointerfaces 2023, 230, 113506. [Google Scholar] [CrossRef]
- Choi, S.; Raja, I.S.; Selvaraj, A.R.; Kang, M.S.; Park, T.E.; Kim, K.S.; Hyon, S.H.; Han, D.W.; Park, J.C. Activated Carbon Nanofiber Nanoparticles Incorporated Electrospun Polycaprolactone Scaffolds to Promote Fibroblast Behaviors for Application to Skin Tissue Engineering. Adv. Compos. Hybrid. Mater. 2023, 6, 24. [Google Scholar] [CrossRef]
- Azari, A.; Rahimi, A.; Rajabibazl, M.; Abbaszadeh, H.A.; Hosseinzadeh, S.; Rahimpour, A. Evaluation of in Vitro Coculture of Keratinocytes Derived from Foreskin and Adipose-Derived Mesenchymal Stem Cells (AMSCs) on a Multilayer Oxygen-Releasing Electrospun Scaffold Based on PU/PCL.Sodium Percarbonate (SPC)-Gelatine/PU. Cell Biochem. Funct. 2023, 41, 434–449. [Google Scholar] [CrossRef]
- Meng, C.; Tang, D.; Liu, X.; Meng, J.; Wei, W.; Gong, R.H.; Li, J. Heterogeneous Porous PLLA/PCL Fibrous Scaffold for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2023, 235, 123781. [Google Scholar] [CrossRef]
- Xu, C.; Cheong, J.Y.; Mo, X.; Jérôme, V.; Freitag, R.; Agarwal, S.; Gharibi, R.; Greiner, A. Thoroughly Hydrophilized Electrospun Poly(L-Lactide)/Poly(ε-Caprolactone) Sponges for Tissue Engineering Application. Macromol. Biosci. 2023, 23, e2300143. [Google Scholar] [CrossRef]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface Modification of Polycaprolactone Nanofibers through Hydrolysis and Aminolysis: A Comparative Study on Structural Characteristics, Mechanical Properties, and Cellular Performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef]
- Ruocco, G.; Zoso, A.; Mortati, L.; Carmagnola, I.; Chiono, V. Biomimetic Electrospun Scaffold-Based In Vitro Model Resembling the Hallmarks of Human Myocardial Fibrotic Tissue. ACS Biomater. Sci. Eng. 2023, 9, 4368–4380. [Google Scholar] [CrossRef]
- Gao, C.; Lu, C.; Liu, H.; Zhang, Y.; Qiao, H.; Jin, A.; Dai, Q.; Liu, Y. Biofabrication of Biomimetic Undulating Microtopography at the Dermal-Epidermal Junction and Its Effects on the Growth and Differentiation of Epidermal Cells. Biofabrication 2024, 16, 025018. [Google Scholar] [CrossRef]
- El-Okaily, M.S.; Mostafa, A.A.; Dulnik, J.; Denis, P.; Sajkiewicz, P.; Mahmoud, A.A.; Dawood, R.; Maged, A. Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization. Pharmaceutics 2023, 15, 1990. [Google Scholar] [CrossRef]
- Fiaschini, N.; Carnevali, F.; Van der Esch, S.A.; Vitali, R.; Mancuso, M.; Sulli, M.; Diretto, G.; Negroni, A.; Rinaldi, A. Innovative Multilayer Electrospun Patches for the Slow Release of Natural Oily Extracts as Dressings to Boost Wound Healing. Pharmaceutics 2024, 16, 159. [Google Scholar] [CrossRef]
- Behtaj, S.; Karamali, F.; Najafian, S.; Masaeli, E.; Rybachuk, M. Ciliary Neurotrophic Factor Mediated Growth of Retinal Ganglion Cell Axons on PGS/PCL Scaffolds. Biomed. Mater. 2024, 19, 025001. [Google Scholar] [CrossRef]
- Yadav, P.; Beniwal, G.; Saxena, K.K. A Review on Pore and Porosity in Tissue Engineering. Proc. Mater. Today Proc. 2021, 44, 2623–2628. [Google Scholar] [CrossRef]
- Belgheisi, G.; Nazarpak, M.H.; Solati-Hashjin, M. Microstructure, Mechanical, and In Vitro Performance of a Novel Combination of Layered Double Hydroxides and Polycaprolactone Electrospun/3D Printed Scaffolds for Bone Tissue Engineering. Fibers Polym. 2023, 24, 3085–3099. [Google Scholar] [CrossRef]
- de Siqueira, L.; dos Santos, V.R.; de Araújo, J.C.R.; de Oliveira Filho, H.G.P.; de Vasconcellos, L.M.R.; de Sousa Trichês, E.; Borges, A.L.S. Ultrathin Polymer Fibers Coated with an Amorphous SiO2–CaO–P2O5 Bioactive Powders for Biomedical Applications. Fibers Polym. 2023, 24, 3139–3150. [Google Scholar] [CrossRef]
- Saghafi, Y.; Baharifar, H.; Najmoddin, N.; Asefnejad, A.; Maleki, H.; Sajjadi-Jazi, S.M.; Bonkdar, A.; Shams, F.; Khoshnevisan, K. Bromelain- and Silver Nanoparticle-Loaded Polycaprolactone/Chitosan Nanofibrous Dressings for Skin Wound Healing. Gels 2023, 9, 672. [Google Scholar] [CrossRef]
- Osanloo, M.; Noori, F.; Tavassoli, A.; Ataollahi, M.R.; Davoodi, A.; Seifalah-Zade, M.; Taghinezhad, A.; Fereydouni, N.; Goodarzi, A. Effect of PCL Nanofiber Mats Coated with Chitosan Microcapsules Containing Cinnamon Essential Oil for Wound Healing. BMC Complement. Med. Ther. 2023, 23, 84. [Google Scholar] [CrossRef]
- Táborská, J.; Blanquer, A.; Brynda, E.; Filová, E.; Jenčová, V.; Havlíčková, K.; Riedelová, Z.; Riedel, T.; Stiborová, L. PLCL/PCL Dressings with Platelet Lysate and Growth Factors Embedded in Fibrin for Chronic Wound Regeneration. Int. J. Nanomed. 2023, 18, 595–610. [Google Scholar] [CrossRef]
- Noori, F.; Osanloo, M.; Moradi, H.R.; Ghaderi Jafarbeigloo, H.; Jirehnezhadyan, M.; Kouhpayeh, S.A.; Tirgare, M.; Bozorgi, A.; Goodarzi, A. Fabrication, Characterization, and in Vivo Implantation of Eugenol-Loaded Nanogels and PCL/Cs Electrospun Nanofibers for Wound Healing Applications. J. Bioact. Compat. Polym. 2023, 38, 480–492. [Google Scholar] [CrossRef]
- Azarsa, S.; Pezeshki-Modaress, M.; Yazdian, F.; Bagher, Z.; Chahsetareh, H.; Simorgh, S.; Heidari, M.K.; Davachi, S.M. Nanofiber/Hydrogel Composite Scaffolds Based on Alginate Sulfate and Extracellular Matrix for Cartilage Tissue Engineering Applications. Process Biochem. 2024, 136, 60–71. [Google Scholar] [CrossRef]
- Kalakonda, P.; Thudumu, S.; Mynepally, S.L.; Mandal, P.; Banne, S.; Kalakonda, P.B.; Podili, B.B. Engineering Micro/Nano-Fibrous Scaffolds with Silver Coating for Tailored Wound Repair Applications. J. Nanoparticle Res. 2023, 25, 254. [Google Scholar] [CrossRef]
- Huner, K. Poly(ε-Caprolactone)/Poly(m-Anthranilic Acid) and Poly(ε-Caprolactone)/Poly(3,4-Ethylenedioxythiophene)-Poly(Styrenesulfonate) Electrospun Nanofibers: Characterization, Antioxidant, and Electrochemical Properties. Polym. Eng. Sci. 2023, 63, 605–614. [Google Scholar] [CrossRef]
- González Rodríguez, O.A.; Ramírez Guerrero, N.C.; Casañas Pimentel, R.G.; Jaime Fonseca, M.R.; San Martín Martínez, E. Polycaprolactone, Polylactic Acid, and Nanohydroxyapatite Scaffolds Obtained by Electrospinning and 3D Printing for Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 73, 1279–1290. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, C.; Yu, B.; Wang, P.; Tang, X.; Shi, D.; Xia, Y.; Hu, Y.; Li, S.; Zhou, W. Well-Ordered and Visual Poly(ε-Caprolactone) Composite Fibrous Membranes for the Treatment of Skin Wounds. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131940. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Sadeghzadeh, H.; Asl, A.N.; Kaamyabi, S.; Akbarzadeh, A. Nanocomposite Electrospun Scaffold Based on Polyurethane/Polycaprolactone Incorporating Gold Nanoparticles and Soybean Oil for Tissue Engineering Applications. J. Bionic Eng. 2023, 20, 1712–1722. [Google Scholar] [CrossRef]
- Bagherzadeh, E.; Sherafat, Z.; Zebarjad, S.M.; Khodaei, A.; Amin Yavari, S. Stimuli-Responsive Piezoelectricity in Electrospun Polycaprolactone (PCL)/Polyvinylidene Fluoride (PVDF) Fibrous Scaffolds for Bone Regeneration. J. Mater. Res. Technol. 2023, 23, 379–390. [Google Scholar] [CrossRef]
- Fallah-Darrehchi, M.; Zahedi, P. Improvement of Intracellular Interactions through Liquid Crystalline Elastomer Scaffolds by the Alteration of Topology. ACS Omega 2023, 8, 46878–46891. [Google Scholar] [CrossRef]
- Lotfi, Z.; Khakbiz, M.; Davari, N.; Bonakdar, S.; Mohammadi, J.; Shokrgozar, M.A.; Derhambakhsh, S. Fabrication and Multiscale Modeling of Polycaprolactone/Amniotic Membrane Electrospun Nanofiber Scaffolds for Wound Healing. Artif. Organs 2023, 47, 1267–1284. [Google Scholar] [CrossRef]
- Augustine, R.; Nikolopoulos, V.K.; Camci-Unal, G. Hydrogel-Impregnated Self-Oxygenating Electrospun Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 854. [Google Scholar] [CrossRef]
- Sadia, M.; Mohd Zaki, M.A.; Jaganathan, S.K.; Md Shakhih, M.F.; Kamarozaman, A.S.; Ab’lah, N.N.; Saidin, S. Blending of Moringa Oleifera into Biodegradable Polycaprolactone/Silver Electrospun Membrane for Hemocompatibility Improvement. Arab. J. Sci. Eng. 2023, 48, 7323–7336. [Google Scholar] [CrossRef]
- Handley, E.L.; Callanan, A. Effects of Electrospun Fibers Containing Ascorbic Acid on Oxidative Stress Reduction for Cardiac Tissue Engineering. J. Appl. Polym. Sci. 2023, 140, e54242. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Chai, G.; Sun, S.; Ding, Q.; Cheng, Z.; Liu, X.; Zhao, Y.; Zhao, T.; Wang, Y.; et al. Antibacterial, Anti-Inflammatory, Rapid Hemostasis, and Accelerated Repair by Multifunctional Metal–Organic Frameworks Fibrous Scaffolds for Diabetic Wounds. Chem. Eng. J. 2023, 477, 147262. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, X.; He, W.; Li, H.; Huang, Y.; Wu, G. Autocatalytically Hydroxyl-Producing Composite Wound Dressing for Bacteria-Infected Wound Healing. Nanomedicine 2023, 51, 102683. [Google Scholar] [CrossRef]
- Lanno, G.M.; Ramos, C.; Preem, L.; Putrins, M.; Laidmaë, I.; Tenson, T.; Kogermann, K. Antibacterial Porous Electrospun Fibers as Skin Scaffolds for Wound Healing Applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Mo, X.; Wang, H. Fabrication of Scaffold Based on Gelatin and Polycaprolactone (PCL) for Wound Dressing Application. J. Drug Deliv. Sci. Technol. 2021, 63, 102501. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-Based Materials in Wound Healing Applications. Polym. Bull. 2022, 79, 7041–7063. [Google Scholar] [CrossRef]
- Longo, R.; Catauro, M.; Vertuccio, L.; Guadagno, L. Comparison between Morphological and Mechanical Properties of Membranes Produced via Coaxial and Monoaxial Electrospinning. Macromol. Symp. 2023, 409, 4–7. [Google Scholar] [CrossRef]
- Hajati Ziabari, A.; Ebrahimi, S.; Jafari, K.; Doodmani, S.M.; Natouri, O.; Nobakht, A.; Mouseli, S. Bilayer Nanofibers Loaded with Malva Sylvestris Extract for Enhanced Wound Healing Applications. J. Drug Deliv. Sci. Technol. 2024, 93, 105373. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. Fiber Density of Electrospun Gelatin Scaffolds Regulates Morphogenesis of Dermal-Epidermal Skin Substitutes. J. Biomed. Mater. Res. A 2008, 84, 1078–1086. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Chellappan, V.; Neisiany, R.E.; Dubey, N.; Amuthavalli, K.; Verma, N.K.; Lakshminarayanan, R.; Ramakrishna, S. An Innovative Tunable Bimodal Porous PCL/Gelatin Dressing Fabricated by Electrospinning and 3D Printing for Efficient Wound Healing and Scalable Production. Compos. Sci. Technol. 2024, 247, 110402. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Yuan, X.F.; Bayat, A. Electrospun Silk Fibroin Fiber Diameter Influences in Vitro Dermal Fibroblast Behavior and Promotes Healing of Ex Vivo Wound Models. J. Tissue Eng. 2014, 5, 2041731414551661. [Google Scholar] [CrossRef]
- Song, Z.; Wang, J.; Tan, S.; Gao, J.; Wang, L. Conductive Biomimetic Bilayer Fibrous Scaffold for Skin Regeneration. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130211. [Google Scholar] [CrossRef]
- Hamdan, N.; Khodir, W.K.W.A.; Hamid, S.A.; Nasir, M.H.M.; Hamzah, A.S.; Cruz-Maya, I.; Guarino, V. PCL/Gelatin/Graphene Oxide Electrospun Nanofibers: Effect of Surface Functionalization on In Vitro and Antibacterial Response. Nanomaterials 2023, 13, 488. [Google Scholar] [CrossRef]
- Davis, K.A.; Gottipatti, A.; Peng, H.; Donahue, R.; Chelvarajan, L.; Cahall, C.; Tripathi, H.; Al-Darraji, A.; Ye, S.; Abdel-Latif, A.; et al. Gelatin Coating Enhances Therapeutic Cell Adhesion to the Infarcted Myocardium via ECM Binding. PLoS ONE 2022, 17, e0277561. [Google Scholar] [CrossRef]
- Manjit, M.; Kumar, K.; Kumar, M.; Jha, A.; Bharti, K.; Tiwari, P.; Tilak, R.; Singh, V.; Koch, B.; Mishra, B. Fabrication of Gelatin Coated Polycaprolactone Nanofiber Scaffolds Co-Loaded with Luliconazole and Naringenin for Treatment of Candida Infected Diabetic Wounds. Int. J. Biol. Macromol. 2024, 261, 129621. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Hayatdavoodi, Z.; Kian, M.; Nemati, N.H.; Mehrabani, D.; Mohammadi, A.A.; Rafati, A.; Ghaedi, M.; Ghafari, B.; Naini, A.A. Evaluation of a Polycaprolactone/Gelatin/Lucilia Sericata Larva Extract Nanofibrous Mat for Burn-Wound Healing. Fibers Polym. 2023, 24, 3809–3820. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Wang, Y.; Meng, J.; Wu, M.; Xu, H.; Du, L.; Yang, X. Fabrication and Characterization of Electrospun Poly(Caprolactone)/Tannic Acid Scaffold as an Antibacterial Wound Dressing. Polymers 2023, 15, 593. [Google Scholar] [CrossRef]
- Hu, Q.; Wan, X.; Wang, S.; Huang, T.; Zhao, X.; Tang, C.; Zheng, M.; Wang, X.; Li, L. Ultrathin, Flexible, and Piezoelectric Janus Nanofibrous Dressing for Wound Healing. Sci. China Mater. 2023, 66, 3347–3360. [Google Scholar] [CrossRef]
- Pi, H.; Xi, Y.; Wu, J.; Hu, M.; Tian, B.; Yang, Y.; Wang, R.; Zhang, X. Janus Fibrous Membrane with Directional Liquid Transport Capacity for Wound Healing Promotion. Chem. Eng. J. 2023, 455, 140853. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Xiang, J.; Wu, M.; Chen, Z.; Yang, Z.; Wei, R.; Cai, L. Deferoxamine-Loaded Janus Electrospun Nanofiber Dressing with Spatially Designed Structure for Diabetic Wound Healing. Mater. Des. 2023, 233, 112166. [Google Scholar] [CrossRef]
- Yang, L.; Lou, Y.; Zhang, G.; Sun, Y.; Yang, Y.; Wu, J.; Ye, Y.; Chu, X.; Du, L.; Jiang, Z.; et al. Hybrid Manufacturing of Highly Stretchable Functionalized Membrane for Joint Wound Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132655. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent Advances in 3D Printing for Wound Healing: A Systematic Review. J. Drug Deliv. Sci. Technol. 2022, 74. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D Printed Hydrogel/PCL Core/Shell Fiber Scaffolds with NIR-Triggered Drug Release for Cancer Therapy and Wound Healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Oliveira, S.R.P.; Lima, G.G.; de Azevedo, M.M.F.; Furtado, A.S.A.; Gusmão soares, S.B.; da Silva Nascimento, E.; de França Bento, R.R.; Santo, F.E.P.; Pinto, L.S.S.; Stocco, T.D. Controlled Drug Release from Policaprolactone-Piperine Electrospun Scaffold for Bone Tissue Engineering. J. Drug Deliv. Sci. Technol. 2024, 91, 105188. [Google Scholar] [CrossRef]
- Zhao, X.; Zhuang, Y.; Cao, Y.; Cai, F.; Lv, Y.; Zheng, Y.; Yang, J.; Shi, X. Electrospun Biomimetic Periosteum Capable of Controlled Release of Multiple Agents for Programmed Promoting Bone Regeneration. Adv. Healthc. Mater. 2024, 13, e2303134. [Google Scholar] [CrossRef]
- Xu, J.; Xia, Y.; Song, H.; Wang, L.; Zhang, X.; Lian, J.; Zhang, Y.; Li, X.; Li, Y.; Kang, J.; et al. Electrospun the Oriented Silk Fibroin/ Bioactive Glass @ Silk Fibroin/ Polycaprolactone Composite Bi-Layered Membranes for Guided Bone Regeneration. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132224. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, S.; Xu, D.; Cheng, G.; Shi, B. Resveratrol-Loaded Co-Axial Electrospun Poly(ε-Caprolactone)/Chitosan/Polyvinyl Alcohol Membranes for Promotion of Cells Osteogenesis and Bone Regeneration. Int. J. Biol. Macromol. 2023, 249, 126085. [Google Scholar] [CrossRef]
- Hua, W.; Xiang, J.; Wu, Y.; Yang, W.; Zhao, L. Growth Factor-Encapsulated Triphasic Scaffolds of Electrospun Polylactic Acid-Polycaprolactone (PLA-PCL) Nanofibrous Mats Combined with a Directionally Freeze-Dried Chitosan Hydrogel for Periodontal Tissue Regeneration. Mater. Adv. 2023, 4, 4798–4811. [Google Scholar] [CrossRef]
- Liang, C.; Wang, G.; Liang, C.; Li, M.; Sun, Y.; Tian, W.; Liao, L. Hierarchically Patterned Triple-Layered Gelatin-Based Electrospun Membrane Functionalized by Cell-Specific Extracellular Matrix for Periodontal Regeneration. Dent. Mater. 2024, 40, 90–101. [Google Scholar] [CrossRef]
- Prado-Prone, G.; Silva-Bermudez, P.; Rodil, S.E.; Ganjkhani, Y.; Moradi, A.R.; Méndez, F.J.; García-Macedo, J.A.; Bazzar, M.; Almaguer-Flores, A. ZnO Nanoparticles-Modified Polycaprolactone-Gelatin Membranes for Guided/Bone Tissue Regeneration, Antibacterial and Osteogenic Differentiation Properties. Biomed. Phys. Eng. Express 2023, 9, 035011. [Google Scholar] [CrossRef]
- Niknam, Z.; Fathi Azarbayjani, A.; Rafiaei, S.M.; Rasmi, Y.; Tayebi, L. Polycaprolactone/Graphene Oxide/Magnesium Oxide as a Novel Composite Scaffold for Bone Tissue Engineering: Preparation and Physical/Biological Assessment. J. Drug Deliv. Sci. Technol. 2024, 95, 105531. [Google Scholar] [CrossRef]
- Qian, F.; Huang, Z.; Liu, W.; Liu, Y.; He, X. Functional β-TCP/MnO2/PCL Artificial Periosteum Promoting Osteogenic Differentiation of BMSCs by Reducing Locally Reactive Oxygen Species Level. J. Biomed. Mater. Res. A 2023, 111, 1678–1691. [Google Scholar] [CrossRef]
- Tariq, S.; Shah, S.A.; Hameed, F.; Mutahir, Z.; Khalid, H.; Tufail, A.; Akhtar, H.; Chaudhry, A.A.; Khan, A.F. Tissue Engineered Periosteum: Fabrication of a Gelatin Basedtrilayer Composite Scaffold with Biomimetic Properties for Enhanced Bone Healing. Int. J. Biol. Macromol. 2024, 263, 130371. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Nan, J.; Lei, P.; Sun, Y.; Hu, Y. Nano Artificial Periosteum PCL/Ta/ZnO Accelerates Repair of Periosteum via Antibacterial, Promoting Vascularization and Osteogenesis. Biomater. Adv. 2023, 154, 213624. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, X.; Ding, H.; Kang, M.; Liang, S.; Wei, Y.; Huang, D. Multilayer Functional Bionic Fabricated Polycaprolactone Based Fibrous Membranes for Osteochondral Integrated Repair. Colloids Surf. B Biointerfaces 2023, 225, 113279. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chou, Y.C.; Chen, C.L.; Yu, Y.H.; Lu, C.J.; Liu, S.J. Development of Novel Hybrid 3D-Printed Degradable Artificial Joints Incorporating Electrospun Pharmaceutical- and Growth Factor-Loaded Nanofibers for Small Joint Reconstruction. Biomater. Adv. 2024, 159, 213821. [Google Scholar] [CrossRef]
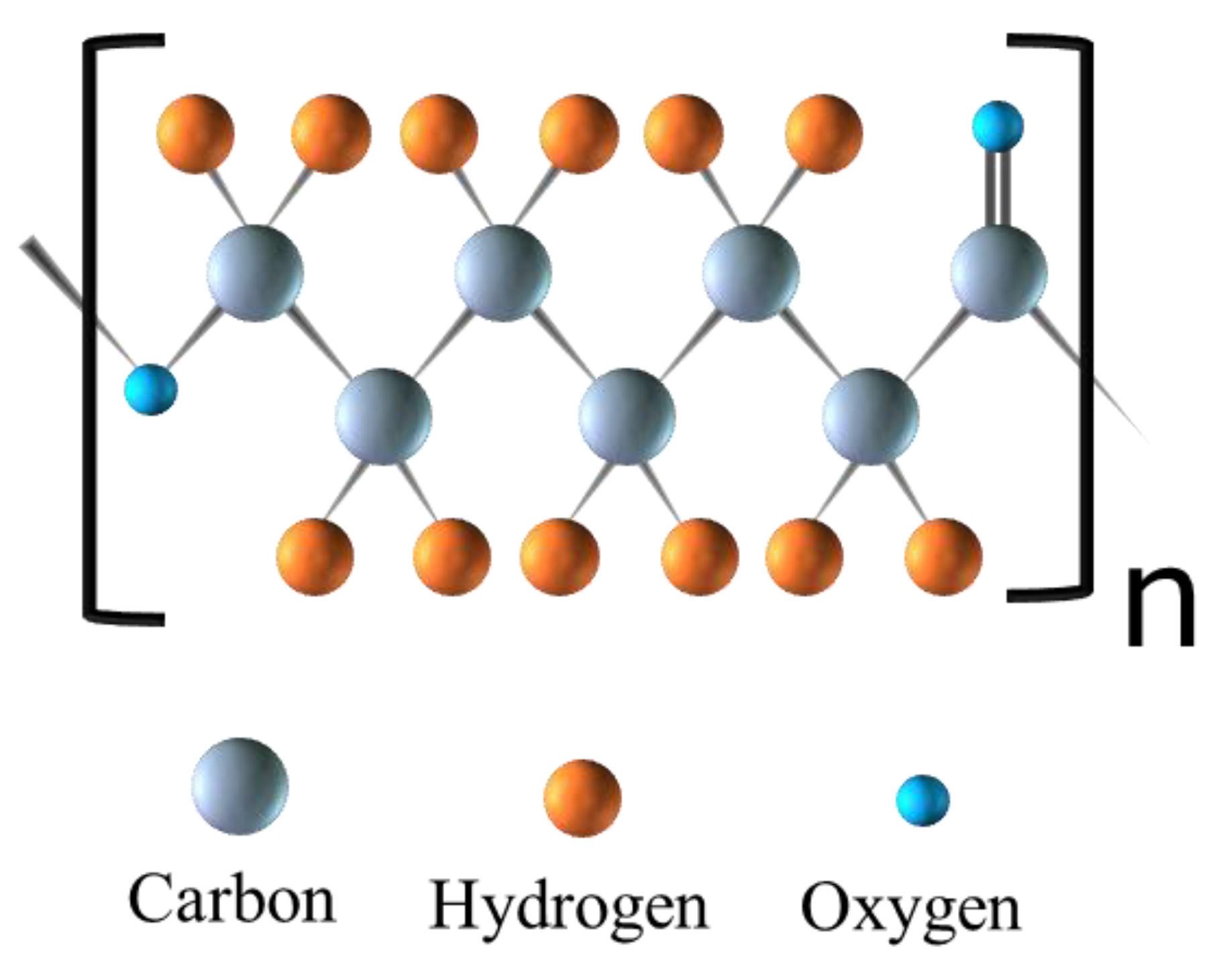

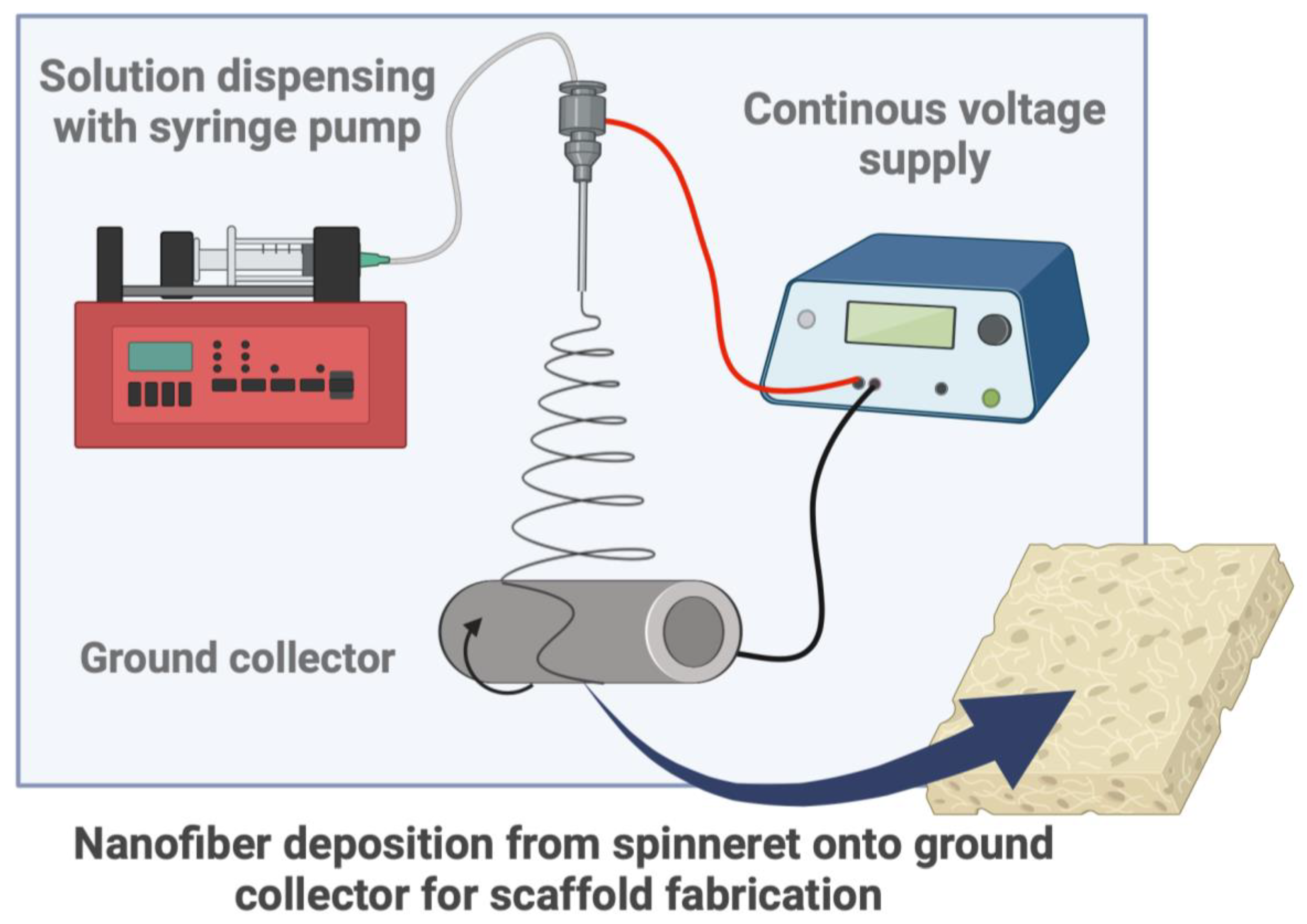

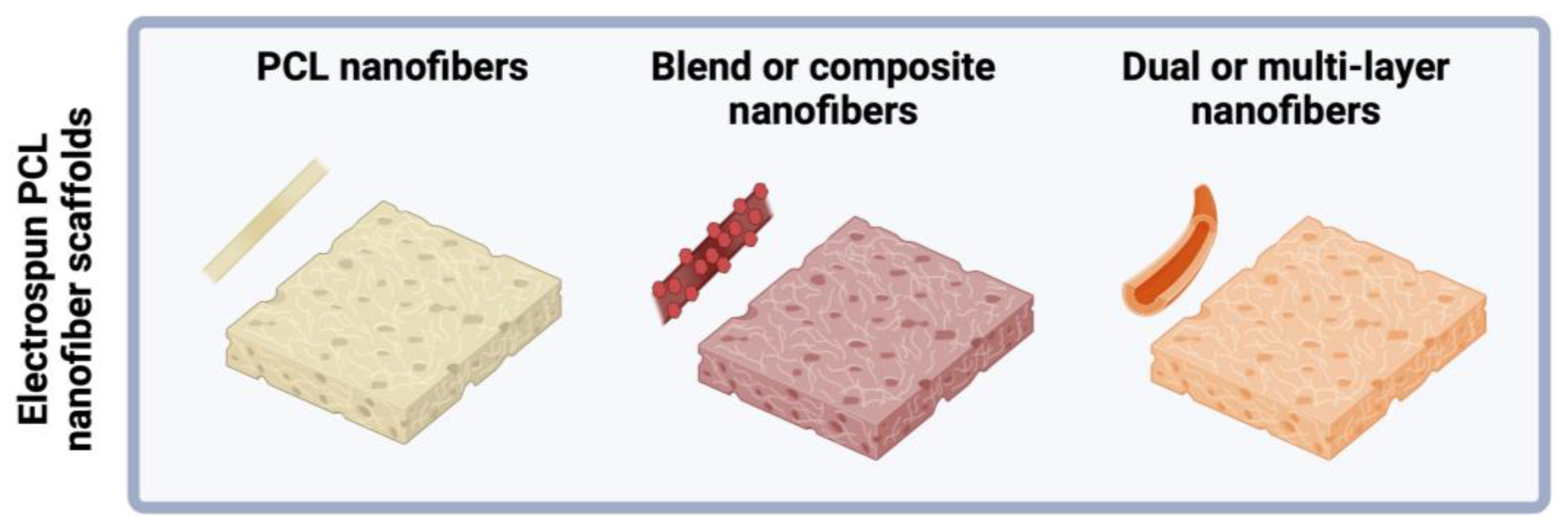

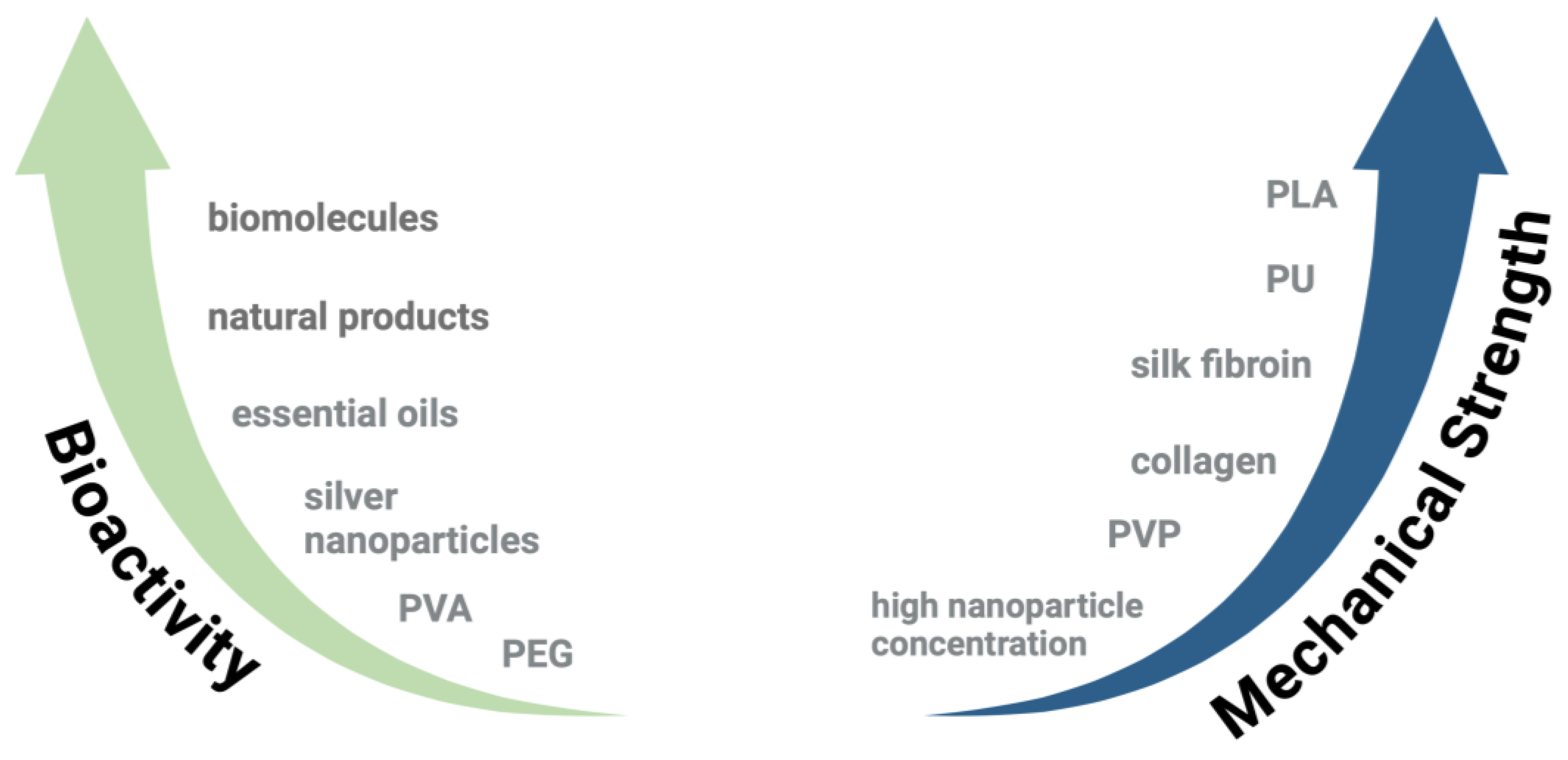
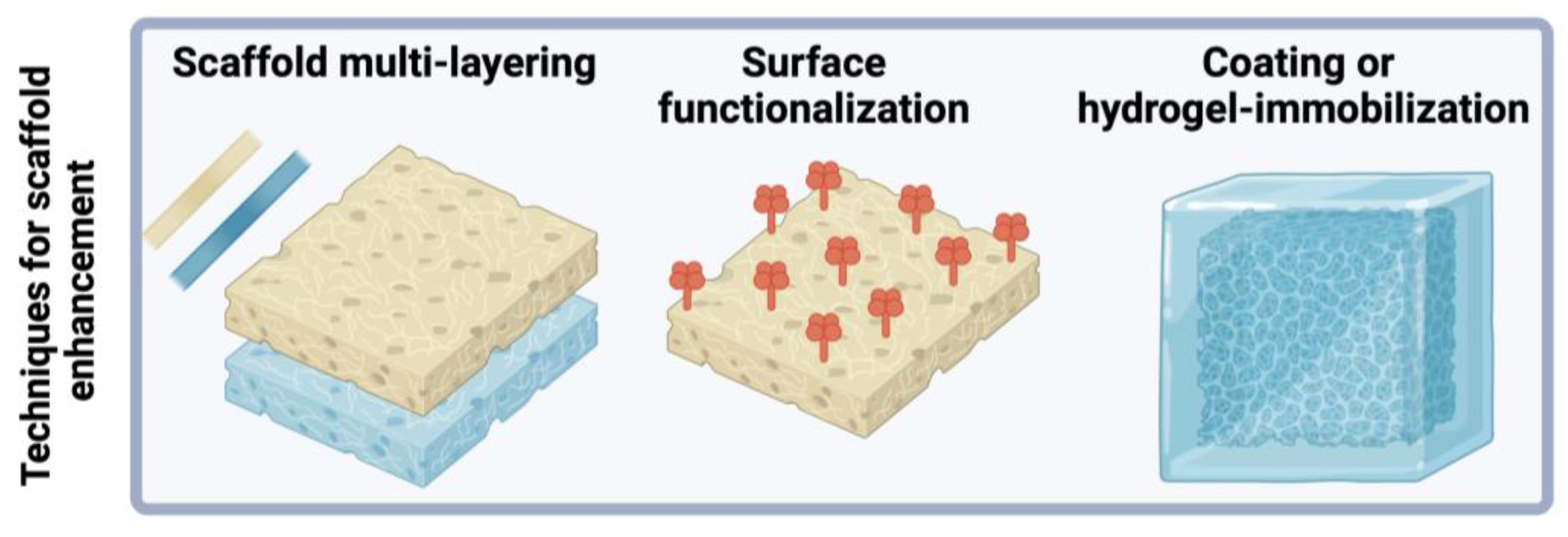

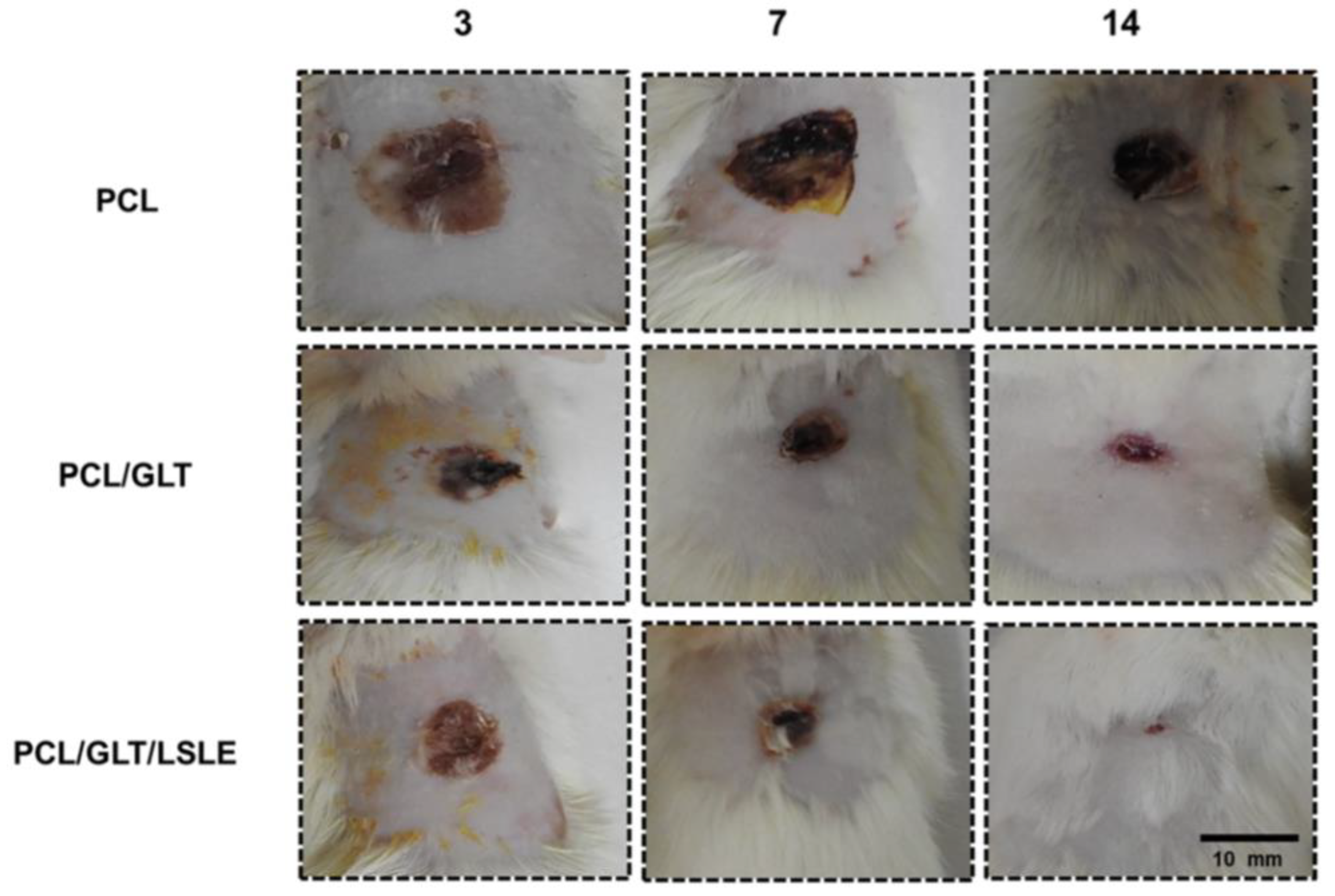
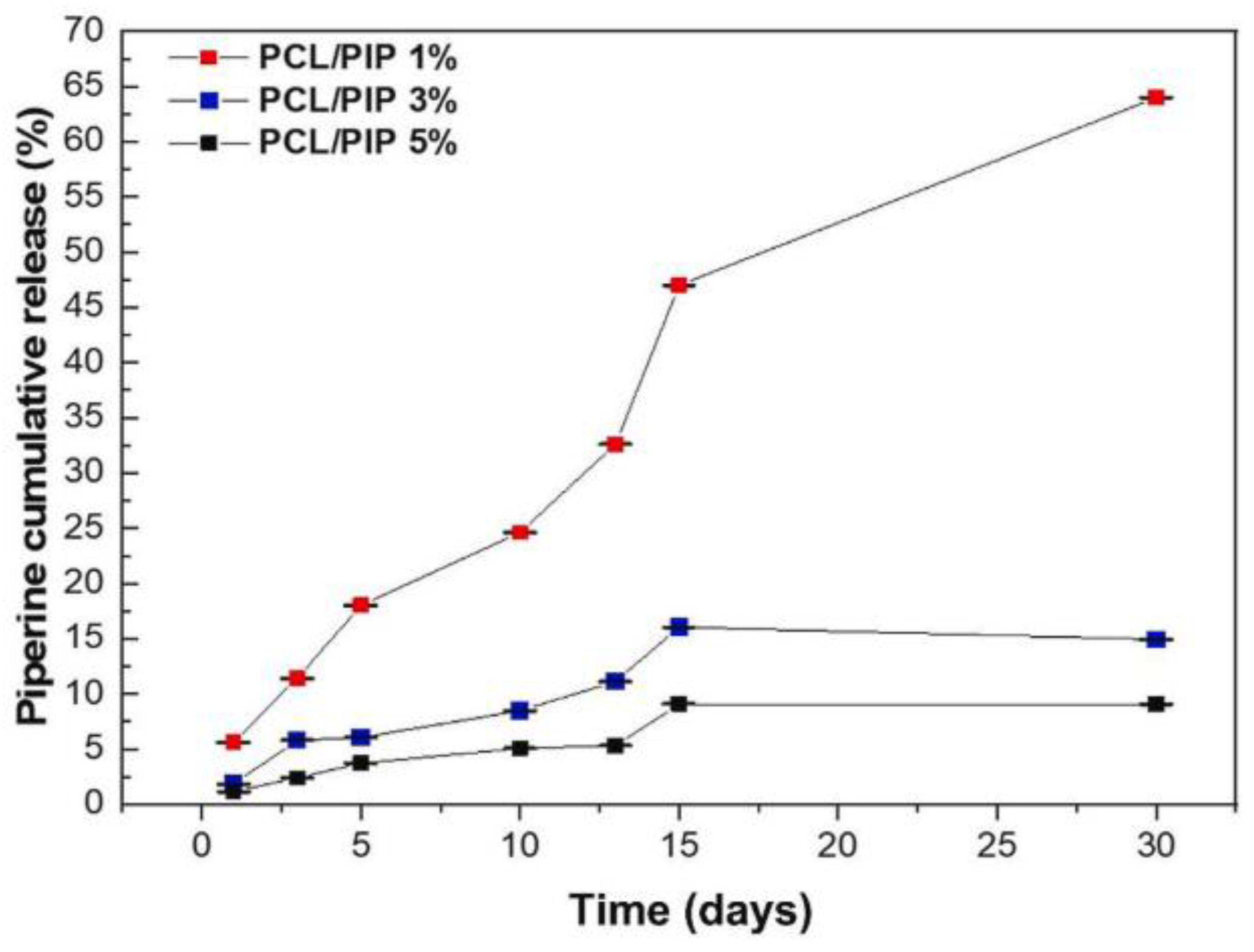

| Biomaterial | Type | Benefits | Disadvantages | Reference |
|---|---|---|---|---|
| Gelatin | Biopolymer | Promotes cell attachment and proliferation, supports wound healing, naturally degrades | Globular protein structure causes low spinnability, degrades rapidly, lacks mechanical strength, batch-to-batch variability | [7,8] |
| Chitosan | Biopolymer | Low risk of immunogenicity, antimicrobial properties reduce risk of infection, supports cell adhesion and proliferation | Low spinnability, lacks mechanical strength, low solubility, batch-to-batch variability, degrades rapidly | [39,40,41] |
| Collagen | Biopolymer | Provides structural support, naturally degrades, promotes cell adhesion and proliferation | Low spinnability, high-cost, batch-to-batch variability, lacks mechanical strength | [42,43,44] |
| Silk fibroin | Biopolymer | Promotes cell attachment and proliferation, naturally degrades, mechanically strong | Low spinnability, batch-to-batch variability, high cost | [25,45,46] |
| Alginate | Biopolymer | Can form hydrogels, naturally degrades, low risk of immunogenicity, low cost | Batch-to-batch variability, limited cell adhesion, may contain impurities | [36,47] |
| PLA | Synthetic polymer | Low risk of immunogenicity, provides mechanical strength, renewable and biodegradable resource | Temperature sensitive, can be brittle | [26] |
| PVA | Synthetic polymer | Capacity for high water absorption, easily chemically modified through hydroxyl groups | Limited cell adhesion, hydrophilicity and swelling impact mechanical stability | [21,48] |
| PEG | Synthetic polymer | Generally biocompatible, water-soluble | Risk of immunogenicity in some individuals, lacks mechanical strength, not biodegradable | [49,50] |
| PU | Synthetic polymer | Good mechanical strength and flexibility, generally biocompatible | Risk of immunogenicity, byproducts may be inflammatory or toxic | [51] |
| PVP | Synthetic polymer | Low risk of immunogenicity, water-soluble | Readily absorbs moisture, not biodegradable, lacks mechanical strength | [52,53,54] |
| Technique | Impact On PCL-Based Scaffold Properties | Reference |
|---|---|---|
| Scaffold multi-layering | Emulate properties of organized multi-level tissue layers, enhance bioactivity or mechanical strength | [45,79] |
| Acetone treatment | Increase scaffold porosity, improve cell adhesion and infiltration | [80] |
| Surfactant treatment | Promotes water absorption, cell infiltration into scaffold | [81] |
| Cross-linked hydrogels | Improved mechanical strength, reduce pore size and improve scaffold hydrophilicity | [36] |
| Aminolysis treatment | Reduce water contact angle, reduce tensile strength, increased strain, improve bioacticity | [82] |
| Hydrolysis treatment | Increase fiber diameter, reduce water contact angle, reduce tensile strength and strain, improve bioactivity | [82] |
| Plasma polymerization | Surface functionalization of biomolecules, reduce water contact angle, and improve bioactivity | [75,83] |
| Polydopamine polymerization | Increase inter and intramolecular interactions to support bioactivity | [84] |
| Dip-coating | Modify surface wettability and decorate with biomolecules, reduce water contact angle, modify drug-release profile | [84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, K.N.; Zahra, F.t.; Mu, R.; Giorgio, T. Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering. Polymers 2024, 16, 2853. https://doi.org/10.3390/polym16202853
Robles KN, Zahra Ft, Mu R, Giorgio T. Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering. Polymers. 2024; 16(20):2853. https://doi.org/10.3390/polym16202853
Chicago/Turabian StyleRobles, Karla N., Fatima tuz Zahra, Richard Mu, and Todd Giorgio. 2024. "Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering" Polymers 16, no. 20: 2853. https://doi.org/10.3390/polym16202853
APA StyleRobles, K. N., Zahra, F. t., Mu, R., & Giorgio, T. (2024). Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering. Polymers, 16(20), 2853. https://doi.org/10.3390/polym16202853







