Applied Investigation of Methyl, Ethyl, Propyl, and Butyl Mercaptan as Potential Poisons in the Gas Phase Polymerization Reaction of Propylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Polymerization
2.3. Melt Flow Index (MFI) and Average Molecular Weight (Mw)
2.4. Infrared (IR) Spectroscopy
2.5. Molecular Electrostatic Potentials
2.6. Fukui Function
3. Results
3.1. Analysis of the Impact of Different Mercaptans on the Reduction of Metric Tons of Polypropylene (PP) Produced Depending on the Concentration at Various Sampling Points
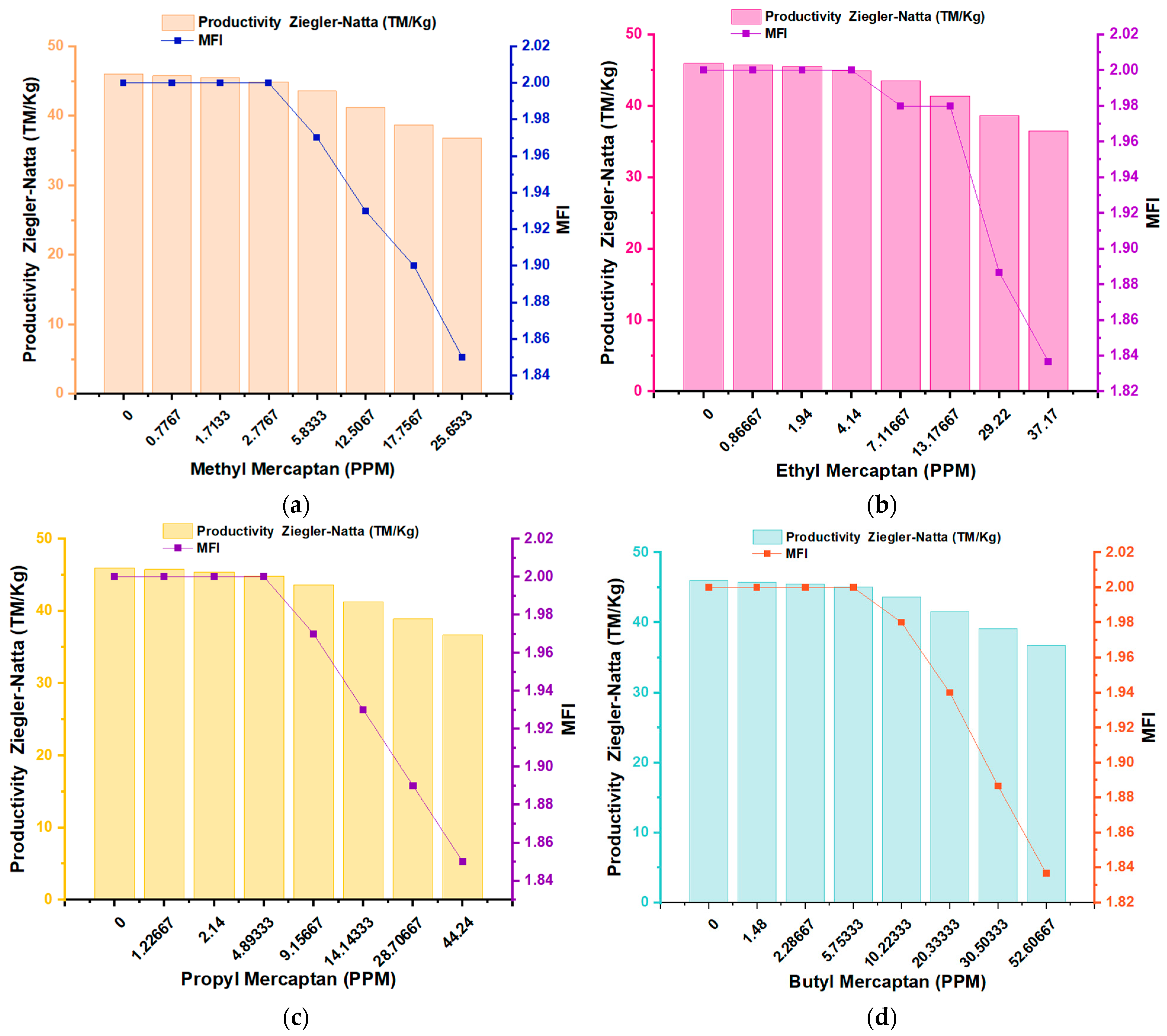
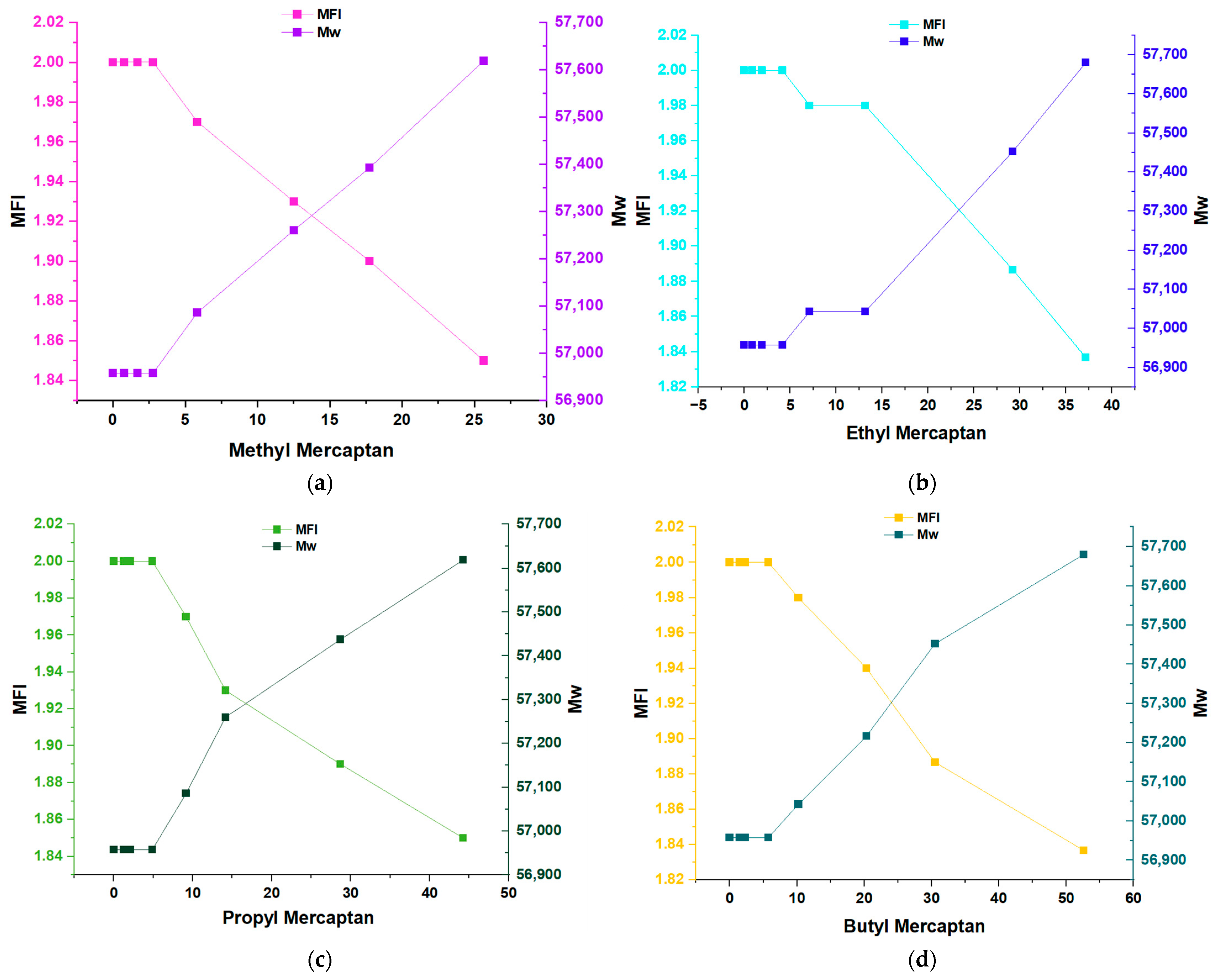
3.2. Impact and Effects on Flow Index (MFI) and Mw of PP
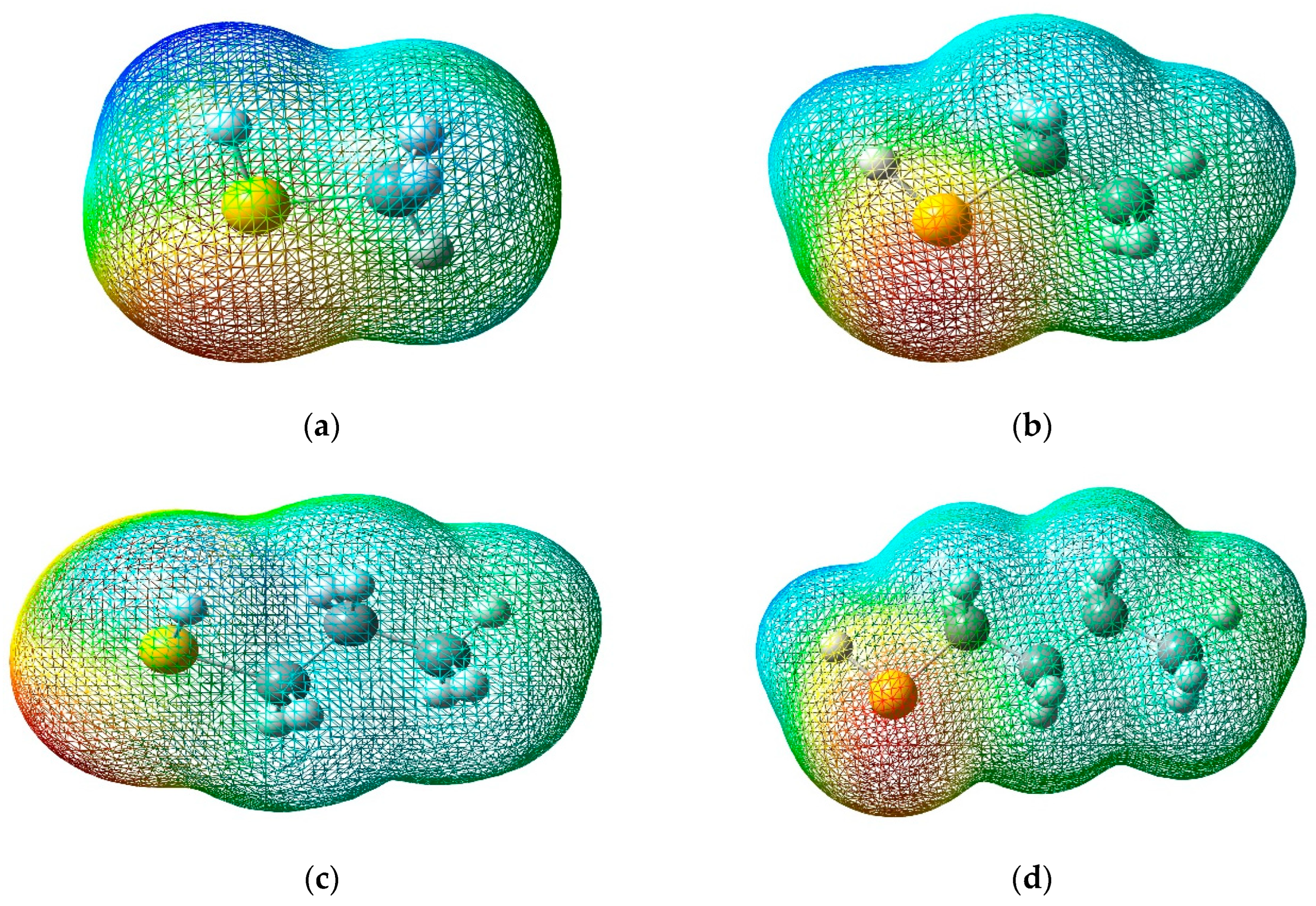
3.3. Molecular Electrostatic Potential
3.4. Analysis of Methyl Mercaptan, Ethyl Mercaptan, Propyl Mercaptan, and Butyl Mercaptan as Inhibitors of the ZN Catalyst
Fukui Features
3.5. Experimental Analysis by FTIR of the Reaction Product of ZN With Each Mercaptan of Interest
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Rempel, G.L. Ziegler-Natta catalysts for olefin polymerization: Mechanistic insights from metallocene systems. Prog. Polym. Sci. 1995, 20, 459–526. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Jiang, X.; He, A. Stereospecific polymerization of olefins with supported Ziegler–Natta catalysts. Polym. Int. 2014, 63, 179–183. [Google Scholar] [CrossRef]
- Kalita, A.; Boruah, M.; Das, D.; Dolui, S.K. Ethylene polymerization on polymer supported Ziegler-Natta catalyst. J. Polym. Res. 2012, 19, 9892. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The influence of Ziegler-Natta and metallocene catalysts on polyolefin structure, properties, and processing ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, R. Catalytic Polymerization and Post Polymerization Catalysis Fifty Years after the Discovery of Ziegler’s Catalysts. Macromol. Chem. Phys. 2003, 204, 289–327. [Google Scholar] [CrossRef]
- Bora, R.R.; Wang, R.; You, F. Waste polypropylene plastic recycling toward climate change mitigation and circular economy: Energy, environmental, and technoeconomic perspectives. ACS Sustain. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Pernusch, D.C.; Spiegel, G.; Paulik, C.; Hofer, W. Influence of Poisons Originating from Chemically Recycled Plastic Waste on the Performance of Ziegler–Natta Catalysts. Macromol. React. Eng. 2022, 16, 2100020. [Google Scholar] [CrossRef]
- Blaakmeer, E.M.; Antinucci, G.; Correa, A.; Busico, V.; van Eck, E.R.; Kentgens, A.P. Structural characterization of electron donors in Ziegler–Natta catalysts. J. Phys. Chem. C 2018, 122, 5525–5536. [Google Scholar] [CrossRef]
- Tangjituabun, K.; Kim, S.Y.; Hiraoka, Y.; Taniike, T.; Terano, M.; Jongsomjit, B.; Praserthdam, P. Effects of various poisoning compounds on the activity and stereospecificity of heterogeneous Ziegler–Natta catalyst. Sci. Technol. Adv. Mater. 2008, 9, 024402. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Development and validation of a methodology for quantifying partsper-billion levels of arsine and phosphine in nitrogen, hydrogen and liquefied petroleum gas using a variable pressure sampler coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2021, 1637, 461833. [Google Scholar] [CrossRef] [PubMed]
- Chacon, H.; Cano, H.; Fernández, J.H.; Guerra, Y.; Puello-Polo, E.; Ríos-Rojas, J.F.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef]
- Soga, K.; Sano, T.; Yamamoto, K.; Shiono, T. The role of additives on the improvement of the isotacticity of polypropylene—A possible interpretation. Chem. Lett. 1982, 11, 425–428. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tritto, I.; Locatelli, P. The function of amines in conventional and supported Ziegler-Natta catalysts. Eur. Polym. J. 1988, 24, 137–140. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; López-Martínez, J. Experimental study of the auto-catalytic effect of triethylaluminum and TiCl4 residuals at the onset of non-additive polypropylene degradation and their impact on thermo-oxidative degradation and pyrolysis. J. Anal. Appl. Pyrolysis 2021, 155, 105052. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; Hernández-Fernández, J.; López-Martínez, J. Comparative characterization of gum rosins for their use as sustainable additives in polymeric matrices. J. Appl. Polym. Sci. 2021, 139, 51734. [Google Scholar] [CrossRef]
- Mier, J.; Artiaga, R.; Soto, L.G. Síntesis de Polímeros. Pesos Moleculares. Conformación y Configuración; Universidade da Coruña: La Coruña, Spain, 1997. [Google Scholar]
- Hernández-Fernández, J.; Puello-Polo, E.; Marquez, E. Study of the Chemical Activities of Carbon Monoxide, Carbon Dioxide, and Oxygen Traces as Critical Inhibitors of Polypropylene Synthesis. Polymers 2024, 16, 605. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction. Polymers 2023, 15, 3619. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Albeladi, A.; Moman, A.; McKenna, T.F. Impact of Process Poisons on the Performance of Post-Phthalate Supported Ziegler–Natta Catalysts in Gas Phase Propylene Polymerization. Macromol. React. Eng. 2023, 17, 2200049. [Google Scholar] [CrossRef]
- Bonachela, S.; López, J.C.; Granados, M.R.; Magán, J.J.; Hernández, J.; Baille, A. Effects of gravel mulch on surface energy balance and soil thermal regime in an unheated plastic greenhouse. Biosyst. Eng. 2020, 192, 1–13. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Arrieta, M.P. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef]
- Zülfikaroğlu, A.; Batı, H.; Dege, N. A theoretical and experimental study on isonitrosoacetophenone nicotinoyl hydrazone: Crystal structure, spectroscopic properties, NBO, NPA and NLMO analyses and the investigation of interaction with some transition metals. J. Mol. Struct. 2018, 1162, 125–139. [Google Scholar] [CrossRef]
- Shukla, B.K.; Yadava, U. DFT calculations on molecular structure, MEP and HOMO-LUMO study of 3-phenyl-1-(methyl-sulfonyl)-1H-pyrazolo [3,4-d] pyrimidine-4-amine. Mater. Today Proc. 2022, 49, 3056–3060. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.; Younis, S.A.; Kim, K.H. Adsorption of environmental contaminants on micro-and nano-scale plastic polymers and the influence of weathering processes on their adsorptive attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef]
- Joaquin, H.F.; Juan, L. Quantification of poisons for Ziegler Natta catalysts and effects on the production of polypropylene by gas chromatographic with simultaneous detection: Pulsed discharge helium ionization, mass spectrometry and flame ionization. J. Chromatogr. A 2020, 1614, 460736. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Hanifpour, A.; Mirmohammadi, S.A.; Poater, A.; Nekoomanesh-Haghighi, M.; Talarico, G.; Cavallo, L. Computational modeling of heterogeneous Ziegler-Natta catalysts for olefins polymerization. Prog. Polym. Sci. 2018, 84, 89–114. [Google Scholar] [CrossRef]
- Tangjituabun, K.; Kim, S.Y.; Hiraoka, Y.; Taniike, T.; Terano, M.; Jongsomjit, B.; Praserthdam, P. Poisoning of active sites on ziegler-natta catalyst for propylene polymerization. Chin. J. Polym. Sci. 2008, 26, 547–552. [Google Scholar] [CrossRef]
- Magni, E.; Somorjai, G.A. Preparation and surface science characterization of model Ziegler−Natta catalysts. Role of undercoordinated surface magnesium atoms in the chemisorption of TiCl4 on MgCl2 thin films. J. Phys. Chem. B 1998, 102, 8788–8795. [Google Scholar] [CrossRef]
- Udhayakalaa, P.; Rajendiranb, T.V.; Gunasekaran, S. Theoretical evaluation of corrosion inhibition performance of some triazole derivatives. J. Adv. Sci. Res. 2012, 3, 71–77. [Google Scholar]
- Jumabaev, A.; Holikulov, U.; Hushvaktov, H.; Issaoui, N.; Absanov, A. Intermolecular interactions in ethanol solution of OABA: Raman, FTIR, DFT, M062X, MEP, NBO, FMO, AIM, NCI, RDG analysis. J. Mol. Liq. 2023, 377, 121552. [Google Scholar] [CrossRef]
- Pandey, A.K.; Baboo, V.; Mishra, V.N.; Singh, V.K.; Dwivedi, A. Comparative Study of Molecular Docking, Structural, Electronic, Vibrational Spectra and Fukui Function Studies of Thiadiazole Containing Schiff Base—A Complete Density Functional Study. Polycycl. Aromat. Compd. 2021, 42, 13–39. [Google Scholar] [CrossRef]
- Bharathy, G.; Prasana, J.C.; Muthu, S.; Irfan, A.; Asif, F.B.; Saral, A.; Aayisha, S. Evaluation of electronic and biological interactions between N-[4-(Ethylsulfamoyl) phenyl] acetamide and some polar liquids (IEFPCM solvation model) with Fukui function and molecular docking analysis. J. Mol. Liq. 2021, 340, 117271. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Evaluation of the Reactivity of Methanol and Hydrogen Sulfide Residues with the Ziegler–Natta Catalyst during Polypropylene Synthesis and Its Effects on Polymer Properties. Polymers 2023, 15, 4061. [Google Scholar] [CrossRef] [PubMed]
- Lowe, P.A. Sulphur Analogues of the Alcohols and their Derivatives. In Supplements to the 2nd Edition of Rodd’s Chemistry of Carbon Compounds; Elsevier: Amsterdam, The Netherlands, 1975; pp. 109–123. [Google Scholar]
- Hernández-Fernández, J.; Ortega-Toro, R.; Castro-Suares, J. Multiple Traces of Families of Epoxy Derivatives as New Inhibitors of the Industrial Polymerization Reaction of Propylene. Polymers 2024, 16, 2028. [Google Scholar] [CrossRef]
- Beaman, C.W.; Lees, R.M.; Xu, L.-H.; Billinghurst, B.E. FTIR synchrotron spectroscopy of lower modes of methyl-D3 mercaptan (CD3SH). J. Mol. Spectrosc. 2023, 392, 111739. [Google Scholar] [CrossRef]
- Lees, R.M.; Xu, L.-H.; Billinghurst, B.E. High-resolution Fourier transform synchrotron spectroscopy of the C–S stretching band of methyl mercaptan, CH332SH. J. Mol. Spectrosc. 2016, 319, 30–38. [Google Scholar] [CrossRef]
- Hernández-Fernández, J. Quantification of oxygenates, sulphides, thiols and permanent gases in propylene. A multiple linear regression model to predict the loss of efficiency in polypropylene production on an industrial scale. J Chromatogr. A 2020, 1628, 461478. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Puello-Polo, E.; Marquez, E. Effects of Different Concentrations of Arsine on the Synthesis and Final Properties of Polypropylene. Polymers 2022, 14, 3123. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Juan, L.M. Autocatalytic influence of different levels of arsine on the thermal stability and pyrolysis of polypropylene. J. Anal. Appl. Pyrolysis. 2022, 161, 105385. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Vivas-Reyes, R.; Toloza, C.A.T. Experimental Study of the Impact of Trace Amounts of Acetylene and Methylacetylene on the Synthesis, Mechanical and Thermal Properties of Polypropylene. Int. J. Mol. Sci. 2022, 23, 12148. [Google Scholar] [CrossRef]
- Hernandez-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Environ. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial Polypropylene Waste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Ortega-Toro, R.; Castro-Suarez, J.R. Theoretical–Experimental Study of the Action of Trace Amounts of Formaldehyde, Propionaldehyde, and Butyraldehyde as Inhibitors of the Ziegler–Natta Catalyst and the Synthesis of an Ethylene–Propylene Copolymer. Polymers 2023, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Puello-Polo, E.; Márquez, E. Furan as Impurity in Green Ethylene and Its Effects on the Productivity of Random Ethylene–Propylene Copolymer Synthesis and Its Thermal and Mechanical Properties. Polymers 2023, 15, 2264. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Marquez, E. Experimental–Density Functional Theory (DFT) Study of the Inhibitory Effect of Furan Residues in the Ziegler–Natta Catalyst during Polypropylene Synthesis. Int. J. Mol. Sci. 2023, 24, 14368. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Dimethylformamide Impurities as Propylene Polymerization Inhibitor. Polymers 2023, 15, 3806. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Cano, H.; Aldas, M. Impact of Traces of Hydrogen Sulfide on the Efficiency of Ziegler–Natta Catalyst on the Final Properties of Polypropylene. Polymers 2022, 14, 3910. [Google Scholar] [CrossRef]

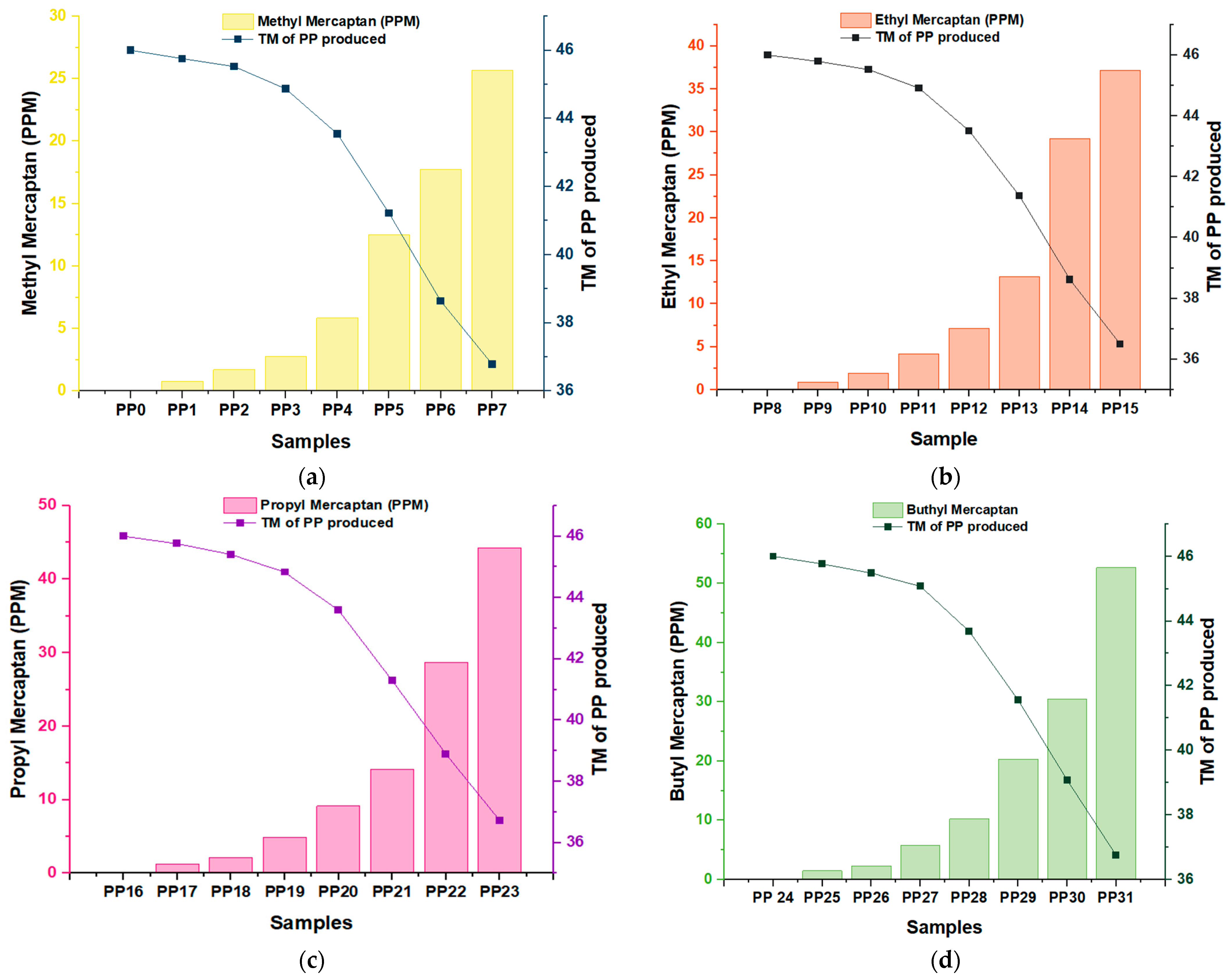


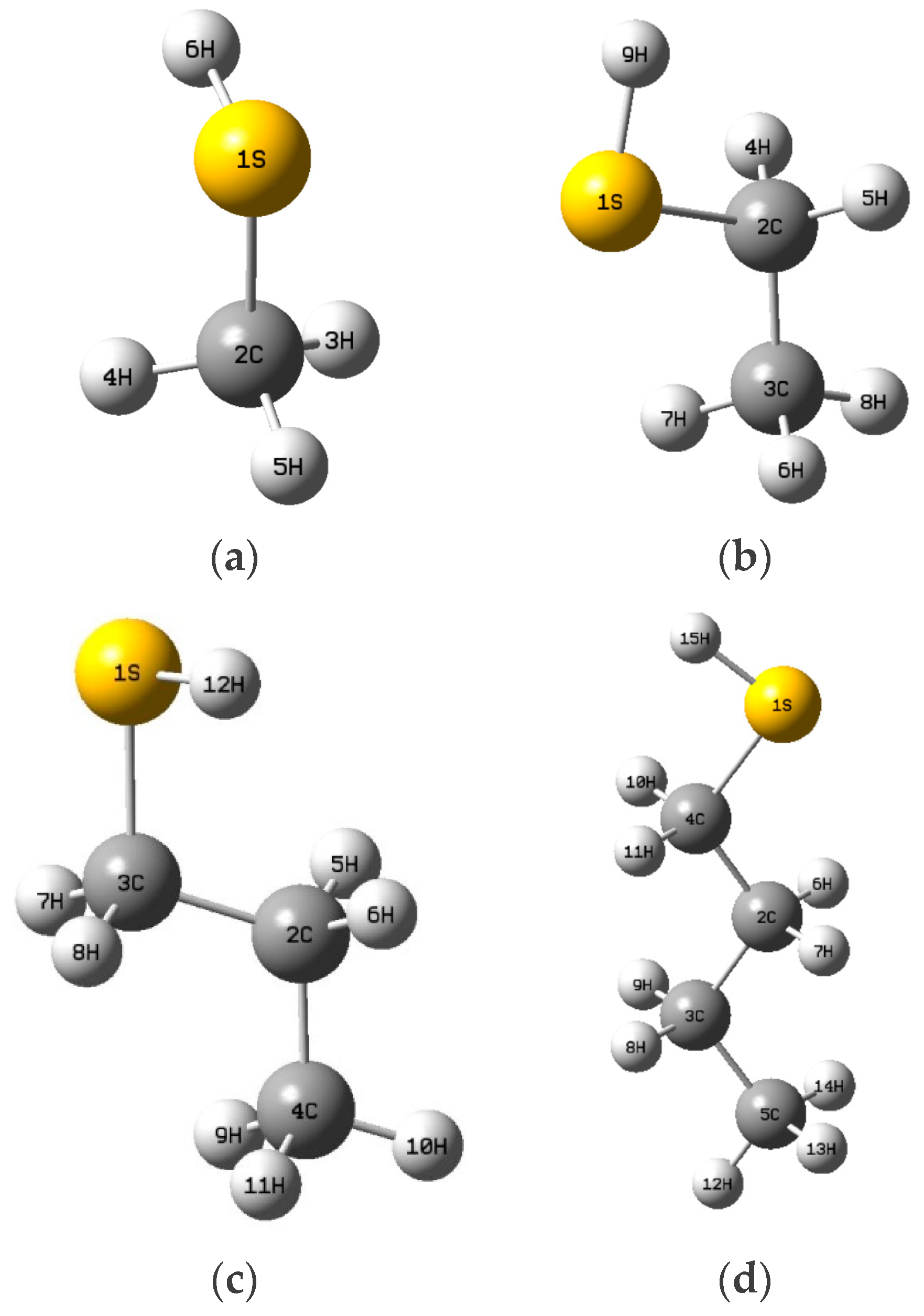

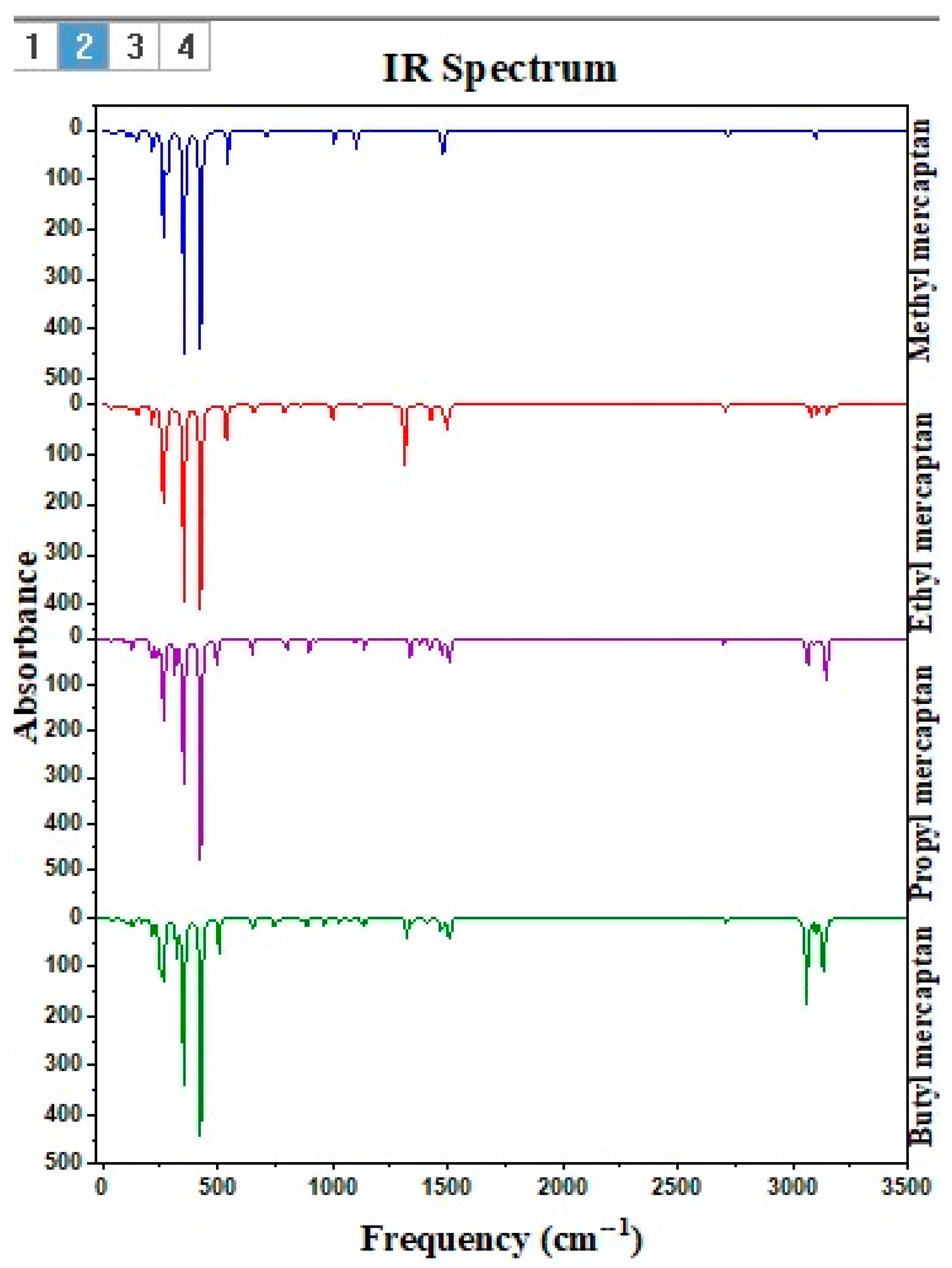
| Methyl Mercaptan (PPM) | 0.00 | 0.78 | 1.71 | 2.78 | 5.83 | 12.51 | 17.76 | 25.65 |
| TM of PP Produced | 46.00 | 45.75 | 45.52 | 44.87 | 43.54 | 41.22 | 38.64 | 36.78 |
| Productivity Ziegler–Natta (TM/Kg) | 46.00 | 45.75 | 45.52 | 44.87 | 43.54 | 41.22 | 38.64 | 36.78 |
| % Productivity Loss | 0.00 | 0.54 | 1.04 | 2.46 | 5.34 | 10.40 | 16.00 | 20.04 |
| MFI | 2.00 | 2.00 | 2.00 | 2.00 | 1.97 | 1.93 | 1.90 | 1.85 |
| % MFI loss | 0 | 0 | 0 | 0 | 1.50 | 3.50 | 5.0 | 7.50 |
| Ethyl Mercaptan (PPM) | 0.00 | 0.87 | 1.94 | 4.14 | 7.12 | 13.18 | 29.22 | 37.17 |
| TM of PP Produced | 46.00 | 45.79 | 45.52 | 44.91 | 43.51 | 41.36 | 38.62 | 36.50 |
| Productivity Ziegler–Natta (TM/Kg) | 46.00 | 45.79 | 45.52 | 44.91 | 43.51 | 41.36 | 38.62 | 36.50 |
| % Productivity Loss | 0.00 | 0.46 | 1.04 | 2.36 | 5.41 | 10.08 | 16.05 | 20.66 |
| MFI | 2.00 | 2.00 | 2.00 | 2.00 | 1.98 | 1.98 | 1.89 | 1.84 |
| % MFI loss | 0 | 0 | 0 | 0 | 1 | 1 | 5.65 | 8.15 |
| Propyl Mercaptan (PPM) | 0.00 | 1.23 | 2.14 | 4.89 | 9.16 | 14.14 | 28.71 | 44.24 |
| TM of PP Produced | 46.00 | 45.75 | 45.40 | 44.83 | 43.59 | 41.29 | 38.89 | 36.73 |
| Productivity Ziegler–Natta (TM/Kg) | 46.00 | 45.75 | 45.40 | 44.83 | 43.59 | 41.29 | 38.89 | 36.73 |
| % Productivity Loss | 0.00 | 0.54 | 1.30 | 2.55 | 5.23 | 10.24 | 15.46 | 20.16 |
| MFI | 2.00 | 2.00 | 2.00 | 2.00 | 1.97 | 1.93 | 1.89 | 1.85 |
| % MFI loss | 0 | 0 | 0 | 0 | 1.50 | 3.50 | 5.50 | 7.50 |
| Butyl Mercaptan (PPM) | 0.00 | 1.48 | 2.29 | 5.75 | 10.22 | 20.33 | 30.50 | 52.61 |
| TM of PP Produced | 46.00 | 45.76 | 45.48 | 45.08 | 43.68 | 41.55 | 39.08 | 36.75 |
| Productivity Ziegler–Natta (TM/Kg) | 46.00 | 45.76 | 45.48 | 45.08 | 43.68 | 41.55 | 39.08 | 36.75 |
| % Productivity Loss | 0.00 | 0.51 | 1.13 | 2.00 | 5.04 | 9.67 | 15.05 | 20.12 |
| MFI | 2.00 | 2.00 | 2.00 | 2.00 | 1.98 | 1.94 | 1.89 | 1.84 |
| % MFI loss | 0 | 0 | 0 | 1 | 1 | 3 | 6.67 | 8.16 |
| # | f− | f+ | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.9095 | 0.0435 | 0.4765 | −0.866 |
| 2 | 0.0272 | 0.6773 | 0.3523 | 0.6501 |
| 3 | 0.0314 | 0.0676 | 0.0495 | 0.0362 |
| 4 | 0.0314 | 0.0675 | 0.0495 | 0.0361 |
| 5 | 0 | 0.0739 | 0.037 | 0.0739 |
| 6 | 0.0005 | 0.0701 | 0.0353 | 0.0696 |
| # | f− | f+ | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.8866 | 0.0466 | 0.4666 | −0.84 |
| 2 | 0.0201 | 0.2927 | 0.1564 | 0.2726 |
| 3 | 0.0045 | 0.3019 | 0.1532 | 0.2974 |
| 4 | 0.0352 | 0.074 | 0.0546 | 0.0388 |
| 5 | 0.0352 | 0.074 | 0.0546 | 0.0388 |
| 6 | 0.0092 | 0.0303 | 0.0198 | 0.0211 |
| 7 | 0.0092 | 0.0303 | 0.0198 | 0.0211 |
| 8 | 0 | 0.0506 | 0.0253 | 0.0506 |
| 9 | 0 | 0.0995 | 0.0497 | 0.0995 |
| # | f− | f+ | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.8271 | 0.0277 | 0.4274 | −0.7994 |
| 2 | 0.0397 | 0.0337 | 0.0367 | −0.006 |
| 3 | 0.0654 | 0.3692 | 0.2173 | 0.3038 |
| 4 | 0.0288 | 0.3444 | 0.1866 | 0.3156 |
| 5 | 0.0046 | 0.0145 | 0.0095 | 0.0099 |
| 6 | 0.001 | 0.0385 | 0.0197 | 0.0375 |
| 7 | 0.0012 | 0.037 | 0.0191 | 0.0358 |
| 8 | 0.0278 | 0.0334 | 0.0306 | 0.0056 |
| 9 | 0.0011 | 0.0132 | 0.0071 | 0.0121 |
| 10 | 0.0024 | 0.0296 | 0.016 | 0.0272 |
| 11 | 0.0006 | 0.0242 | 0.0124 | 0.0236 |
| 12 | 0.0004 | 0.0346 | 0.0175 | 0.0342 |
| # | f− | f+ | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.7416 | 0.0483 | 0.395 | −0.6933 |
| 2 | 0.0491 | 0.0423 | 0.0457 | −0.0068 |
| 3 | 0.0361 | 0.1348 | 0.0855 | 0.0987 |
| 4 | 0.0143 | 0.3328 | 0.1735 | 0.3185 |
| 5 | 0.0018 | 0.0999 | 0.0508 | 0.0981 |
| 6 | 0.0302 | 0.0171 | 0.0237 | −0.0131 |
| 7 | 0.0313 | 0.014 | 0.0226 | −0.0173 |
| 8 | 0.0154 | 0.0209 | 0.0182 | 0.0055 |
| 9 | 0.0111 | 0.0238 | 0.0174 | 0.0127 |
| 10 | 0.0344 | 0.0665 | 0.0504 | 0.0321 |
| 11 | 0.0348 | 0.0669 | 0.0509 | 0.0321 |
| 12 | 0 | 0.0117 | 0.0058 | 0.0117 |
| 13 | 0.0001 | 0.0121 | 0.0061 | 0.012 |
| 14 | 0 | 0.0109 | 0.0055 | 0.0109 |
| 15 | −0.0001 | 0.0982 | 0.049 | 0.0983 |
| # | f− | f+ | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.0001 | 0.7968 | 0.3986 | 0.7968 |
| 2 | 0.3741 | 0.0506 | 0.2125 | −0.3234 |
| 3 | 0.163 | 0.0503 | 0.1073 | −0.1137 |
| 4 | 0.373 | 0.0513 | 0.2125 | −0.3229 |
| 5 | 0.0878 | 0.0509 | 0.0693 | −0.0368 |
| # | f− | f+ | f0 | Δf |
|---|---|---|---|---|
| 1 | 0.0313 | 0.0304 | 0.0309 | −0.0009 |
| 2 | 0.0013 | 0.4433 | 0.2223 | 0.442 |
| 3 | 0.5554 | 0.0032 | 0.2793 | −0.5522 |
| 4 | 0.0109 | 0.0029 | 0.0069 | −0.008 |
| 5 | 0.0011 | 0.0351 | 0.0181 | 0.034 |
| 6 | 0.0004 | 0.0198 | 0.0101 | 0.0194 |
| 7 | 0.0003 | 0.0036 | 0.0019 | 0.0033 |
| 8 | 0.0007 | 0.0287 | 0.0147 | 0.028 |
| 9 | 0.0007 | 0.3607 | 0.1807 | 0.36 |
| 10 | 0.0005 | 0.0236 | 0.0120 | 0.0231 |
| 11 | 0.0003 | 0.0123 | 0.0063 | 0.012 |
| 12 | 0.0000 | 0.0037 | 0.0019 | 0.0037 |
| 13 | 0.0068 | 0.0017 | 0.0043 | −0.0051 |
| 14 | 0.3523 | 0.0027 | 0.1775 | −0.3496 |
| 15 | 0.0194 | 0.0248 | 0.0221 | 0.0054 |
| 16 | 0.0064 | 0.0014 | 0.0039 | −0.005 |
| 17 | 0.0060 | 0.0006 | 0.0033 | −0.0054 |
| 18 | 0.0060 | 0.0014 | 0.0037 | −0.0046 |
| 19 | 0.0002 | 0.0001 | 0.0002 | −0.0001 |
| Bonds | ZN-Propyl | ZN-Methyl Mercaptan | ZN-Ethyl Mercaptan | ZN-Propyl Mercaptan | ZN-Butyl Mercaptan |
|---|---|---|---|---|---|
| Ti-S | -------- | 430 | 445 | 475 | 477 |
| Ti-Cl | 618–555 | 493 | 725 | 591 | 599 |
| Cl-Mg | 1510 | 1456 | 1510 | 1625 | 1634 |
| Cl-Ti-S-CH2 | -------- | -------- | 644–720 | 647–725 | 642–728 |
| Cl-Ti -S-CH3 | -------- | 644–722 | -------- | -------- | -------- |
| -CH3 | 2995–2969 | 3058 | 3054 | 2997 | 3115–2986 |
| -CH2 | 1517–1480 | -------- | 1518–1444 | 1511–1449 | 1513–1467 |
| Reagents | Products | |
|---|---|---|
| TiCl4 + CH3S | → | CH3STiCl4 |
| TiCl4 + CH3CH2S | → | CH3CH2S TiCl4 |
| TiCl4 + CH3CH2CH2S | → | CH3CH2CH2STiCl4 |
| TiCl4 + CH3CH2CH2CH2S | → | CH3CH2CH2CH2STiCl4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Fernandez, J.; Herrera Zabala, J.E.; Marquez, E. Applied Investigation of Methyl, Ethyl, Propyl, and Butyl Mercaptan as Potential Poisons in the Gas Phase Polymerization Reaction of Propylene. Polymers 2024, 16, 2851. https://doi.org/10.3390/polym16202851
Hernandez-Fernandez J, Herrera Zabala JE, Marquez E. Applied Investigation of Methyl, Ethyl, Propyl, and Butyl Mercaptan as Potential Poisons in the Gas Phase Polymerization Reaction of Propylene. Polymers. 2024; 16(20):2851. https://doi.org/10.3390/polym16202851
Chicago/Turabian StyleHernandez-Fernandez, Joaquin, Juan Esteban Herrera Zabala, and Edgar Marquez. 2024. "Applied Investigation of Methyl, Ethyl, Propyl, and Butyl Mercaptan as Potential Poisons in the Gas Phase Polymerization Reaction of Propylene" Polymers 16, no. 20: 2851. https://doi.org/10.3390/polym16202851
APA StyleHernandez-Fernandez, J., Herrera Zabala, J. E., & Marquez, E. (2024). Applied Investigation of Methyl, Ethyl, Propyl, and Butyl Mercaptan as Potential Poisons in the Gas Phase Polymerization Reaction of Propylene. Polymers, 16(20), 2851. https://doi.org/10.3390/polym16202851







