Enhanced Mechanical and Thermal Properties of Polyimide Films Using Hydrophobic Fumed Silica Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PI Oligomer Powder
2.3. Preparation of Pristine PI and PI/FS Composite Films
2.4. Characterization
3. Results and Discussion
3.1. Characterizations of FS Particles
3.2. Preparation of PI Films
3.3. Mechanical Properties of the Films
3.4. Optical Properties of the Films
3.5. Thermal Properties of Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Li, P.; Wu, Z.; Luo, D.; Yu, H.Y.; Lu, Z.H. Review and perspective of materials for flexible solar cells. Mater. Rep. Energy 2021, 1, 100001. [Google Scholar] [CrossRef]
- Koo, J.H.; Kim, D.C.; Shim, H.J.; Kim, T.H.; Kim, D.H. Flexible and Stretchable Smart Display: Materials, Fabrication, Device Design, and System Integration. Adv. Funct. Mater. 2018, 28, 1801834. [Google Scholar] [CrossRef]
- Han, S.T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, S.; Wang, H.; Cheng, W.; Li, Y.; Pan, L.; Shi, Y. Advanced electronic skin devices for healthcare applications. J. Mater. Chem. B 2019, 7, 173–197. [Google Scholar] [CrossRef]
- Li, L.; Han, L.; Hu, H.; Zhang, R. A review on polymers and their composites for flexible electronics. Mater. Adv. 2022, 4, 726–746. [Google Scholar] [CrossRef]
- Nam, K.H.; Jin, J.U.; Lee, D.H.; Han, H.; Goh, M.; Yu, J.; Ku, B.C.; You, N.H. Towards solution-processable, thermally robust, transparent polyimide-chain-end tethered organosilicate nanohybrids. Compos. Part B Eng. 2019, 163, 290–296. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, T.Y.; Hong, P.H.; Choi, S.; Lee, Y.J.; Kwon, H.; Jeon, H.; Ko, D.W.; Park, I.; Han, H.; et al. Highly Transparent, Colorless Optical Film with Outstanding Mechanical Strength and Folding Reliability Using Mismatched Charge-Transfer Complex Intensification. Adv. Funct. Mater. 2022, 32, 2111040. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wu, J.T.; Fu, S.Y.; Yang, S.Y.; Li, Y.; Fan, L.; Li, R.K.Y.; Li, L.F.; Yan, Q. Studies on characterization and cryogenic mechanical properties of polyimide-layered silicate nanocomposite films. Polymer 2004, 45, 7579–7587. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, J.H.; Chen, C.; Peng, W.F.; Liu, Z.Z.; Liu, Y.; Zeng, K.; Yang, G. Preparation and characterization of adenine-based polyimide/nano-silica hybrid films. Eur. Polym. J. 2018, 102, 209–218. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Xie, K.; Liu, J.G.; Zhou, H.W.; Zhang, S.Y.; He, M.H.; Yang, S.Y. Soluble fluoro-polyimides derived from 1, 3-bis (4-amino-2-trifluoromethyl-phenoxy) benzene and dianhydrides. Polymer 2001, 42, 7267–7274. [Google Scholar] [CrossRef]
- Choi, S.; Kim, Y.; Kim, I.; Ha, C.S. Effect of organosilica isomers on the interfacial interaction in polyimide/aromatic organosilica hybrids. J. Appl. Polym. Sci. 2007, 103, 2507–2513. [Google Scholar] [CrossRef]
- Sato, K.; Tominaga, Y.; Imai, Y. Optically transparent and thermally conductive composite films of α-alumina and highly refractive polyimide. Polym. Bull. 2023, 80, 9479–9488. [Google Scholar] [CrossRef]
- Jin, H.S.; Chang, J.H. Colorless polyimide nanocomposite films: Thermomechanical properties, morphology, and optical transparency. J. Appl. Polym. Sci. 2008, 107, 109–117. [Google Scholar] [CrossRef]
- Tsai, M.H.; Tseng, I.H.; Chiang, J.C.; Li, J.J. Flexible polyimide films hybrid with functionalized boron nitride and graphene oxide simultaneously to improve thermal conduction and dimensional stability. ACS Appl. Mater. Interfaces 2014, 6, 8639–8645. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.; Gu, J. Ordered Alignment of Liquid Crystalline Graphene Fluoride for Significantly Enhancing Thermal Conductivities of Liquid Crystalline Polyimide Composite Films. Macromolecules 2022, 55, 4134–4145. [Google Scholar] [CrossRef]
- Huang, T.; Lu, R.; Su, C.; Wang, H.; Guo, Z.; Liu, P.; Huang, Z.; Chen, H.; Li, T. Chemically modified graphene/polyimide composite films based on utilization of covalent bonding and oriented distribution. ACS Appl. Mater. Interfaces 2012, 4, 2699–2708. [Google Scholar] [CrossRef]
- Chen, Y.; Iroh, J.O. Synthesis and characterization of polyimide/silica hybrid composites. Chem. Mater. 1999, 11, 1218–1222. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Ding, H.; Li, J. Microstructure and properties of organosoluble polyimide/silica hybrid films. Eur. Polym. J. 2006, 42, 2921–2930. [Google Scholar] [CrossRef]
- Shang, Z.; Lü, C.; Lü, X.; Gao, L. Synthesis and properties of silica-polyimide hybrid films derived from colloidal silica particles and polyamic acid. J. Appl. Polym. Sci. 2008, 109, 3477–3483. [Google Scholar] [CrossRef]
- Yen, J.H.; Wang, Y.J.; Hsieh, C.A.; Chen, Y.C.; Chen, L.Y. Enhanced light extraction from organic light-emitting devices through non-covalent or covalent polyimide-silica light scattering hybrid films. J. Mater. Chem. C 2020, 8, 4102–4111. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Dai, H.Y.; Chen, J.; Xue, R.Z.; Liu, D.W.; Wang, M.M. Preparation and characterization of low-κ polyhedral oligomeric silsesquioxane/polyimide hybrid films. Mater. Chem. Phys. 2022, 278, 125716. [Google Scholar] [CrossRef]
- Huang, C.; Li, J.; Xie, G.; Han, F.; Huang, D.; Zhang, F.; Zhang, B.; Zhang, G.; Sun, R.; Wong, C.P. Low-Dielectric Constant and Low-Temperature Curable Polyimide/POSS Nanocomposites. Macromol. Mater. Eng. 2019, 304, 1900505. [Google Scholar] [CrossRef]
- Lee, B.Y.; Park, S.; Chung, D.W.; Jang, K.S. Incorporation of alkyl-functionalized silica nanoparticles into hydrophilic epoxy and hydrophobic polystyrene matrices. J. Appl. Polym. Sci. 2022, 139, 51828. [Google Scholar] [CrossRef]
- Son, M.; Han, S.; Han, D.; Kim, Y.; Lim, J.; Kim, I.; Ha, C.S. Organic/inorganic hybrid composite films from polyimide and organosilica: Effect of the type of organosilica precursors. Polym. Bull. 2008, 60, 713–723. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, P.; Li, G.; Lu, D.; Sun, R.; Wong, C.P. Effects of surface-modified alkyl chain length of silica fillers on the rheological and thermal mechanical properties of underfill. IEEE Trans. Compon. Packag. Manuf. Technol. 2016, 6, 1796–1803. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, J.H.; Cheng, L.P. Preparation of solvent-dispersible nano-silica powder by sol-gel method. J. Appl. Sci. Eng. 2016, 19, 401–408. [Google Scholar] [CrossRef]
- Al-Kandary, S.; Ali, A.A.M.; Ahmad, Z. Morphology and thermo-mechanical properties of compatibilized polyimide-silica nanocomposites. J. Appl. Polym. Sci. 2005, 98, 2521–2531. [Google Scholar] [CrossRef]
- Aydoğan, C.; Erdoğan, İ.Y.; El-Rassi, Z. Hydrophobic AEROSIL®R972 Fumed Silica Nanoparticles Incorporated Monolithic Nano-Columns for Small Molecule and Protein Separation by Nano-Liquid Chromatography. Molecules 2022, 27, 2306. [Google Scholar] [CrossRef]
- Barthel, H.; Rösch, L.; Weis, J. Fumed Silica—Production, Properties, and Applications. In Organosilicon Chemistry Set: From Molecules to Materials; Auner, N., Weis, J., Eds.; Wiley: Weinheim, Germany, 2005; pp. 761–778. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Mironyuk, I.F.; Zarko, V.I.; Voronin, E.F.; Turov, V.V.; Pakhlov, E.M.; Goncharuk, E.V.; Nychiporuk, Y.M.; Vlasova, N.N.; Gorbik, P.P.; et al. Morphology and surface properties of fumed silicas. J. Colloid Interface Sci. 2005, 289, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.K.; Moo, J.G.S.; Sing, S.L.; Yeong, W.Y. Use of Fumed Silica Nanostructured Additives in Selective Laser Melting and Fabrication of Steel Matrix Nanocomposites. Materials 2022, 15, 1869. [Google Scholar] [CrossRef]
- Sarath, P.S.; Prasad, V.; Pahovnik, D.; Thomas, S.; Haponiuk, J.T.; George, S.C. Study the effect of fumed silica on the mechanical, thermal and tribological properties of silicone rubber nanocomposites. J. Polym. Res. 2022, 29, 53. [Google Scholar] [CrossRef]
- Goertzen, W.K.; Kessler, M.R. Dynamic mechanical analysis of fumed silica/cyanate ester nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 761–768. [Google Scholar] [CrossRef]
- Padinjakkara, A.; Salim, N.; Thomas, S. Effect of Hexamethyldisilazane-Modified Nano Fumed Silica on the Properties of Epoxy/Carboxyl-Terminated Poly(butadiene-co-acrylonitrile) Blend: A New Hybrid Approach. Ind. Eng. Chem. Res. 2020, 59, 2883–2891. [Google Scholar] [CrossRef]
- ASTM D1003; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D1925; Standard Test Method for Yellowness Index of Plastics. ASTM International: West Conshohocken, PA, USA, 1988.
- ASTM D-638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Alam, M.; Singh, H.; Brunner, S.; Naziris, C. Experimental characterisation and evaluation of the thermo-physical properties of expanded perlite—Fumed silica composite for effective vacuum insulation panel (VIP) core. Energy Build. 2014, 69, 442–450. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Hwang, H.S.; Lee, J.; Cha, D.A.; Park, I. n-Octadecane/fumed silica phase change composites as building envelope for high energy efficiency. Nanomaterilas 2021, 11, 566. [Google Scholar] [CrossRef]
- McElwee, J.; Helmy, R.; Fadeev, A.Y. Thermal stability of organic monolayers chemically grafted to minerals. J. Colloid Interface Sci. 2005, 285, 551–556. [Google Scholar] [CrossRef]
- Loan, S.; Cosutchi, A.I.; Hulubei, C.; Macocinschi, D.; Ioanid, G. Surface and interfacial properties of poly(amic acid)s and polyimides. Polym. Eng. Sci. 2007, 47, 381–389. [Google Scholar] [CrossRef]
- Wu, J.; Lee, N.Y. One-step surface modification for irreversible bonding of various plastics with a poly(dimethylsiloxane) elastomer at room temperature. Lab Chip 2014, 14, 1564–1571. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lo, T.N.H.; Lee, J.; Hwang, H.S.; Park, I. Nanoscale Coatings Derived from Fluoroalkyl and PDMS Alkoxysilanes on Rough Aluminum Surfaces for Improved Durability and Anti-Icing Properties. ACS Appl. Nano Mater. 2021, 4, 7493–7501. [Google Scholar] [CrossRef]
- Ahmad, Z.; Sarwar, M.I.; Wang, S.; Markt, J.E. Preparation and properties of hybrid organic-inorganic composites prepared from poly(phenylene terephthalamide) and titania. Polymer 1997, 38, 4523–4529. [Google Scholar] [CrossRef]
- Mominul Alam, S.M.; Agag, T.; Kawauchi, T.; Takeichi, T. Organic-inorganic hybrids containing polyimide, organically modified clay and in situ formed polydimethylsiloxane. React. Funct. Polym. 2007, 67, 1218–1224. [Google Scholar] [CrossRef]
- Takeichi, T.; Shirai, Y.; Shen, Z.; Alam, S.M.M.; Kawauchi, T. Effect of in situ-formed polydimethylsiloxane on the properties of polyimide hybrids. React. Funct. Polym. 2010, 70, 755–760. [Google Scholar] [CrossRef]
- Park, I.; Peng, H.G.; Gidley, D.W.; Xue, S.; Pinnavaia, T.J. Epoxy-silica mesocomposites with enhanced tensile properties and oxygen permeability. Chem. Mater. 2006, 18, 650–656. [Google Scholar] [CrossRef]
- Ma, X.; Lee, N.H.; Oh, H.J.; Hwang, J.S.; Kim, S.J. Preparation and characterization of silica/polyamide-imide nanocomposite thin films. Nanoscale Res. Lett. 2010, 5, 1846–1851. [Google Scholar] [CrossRef]
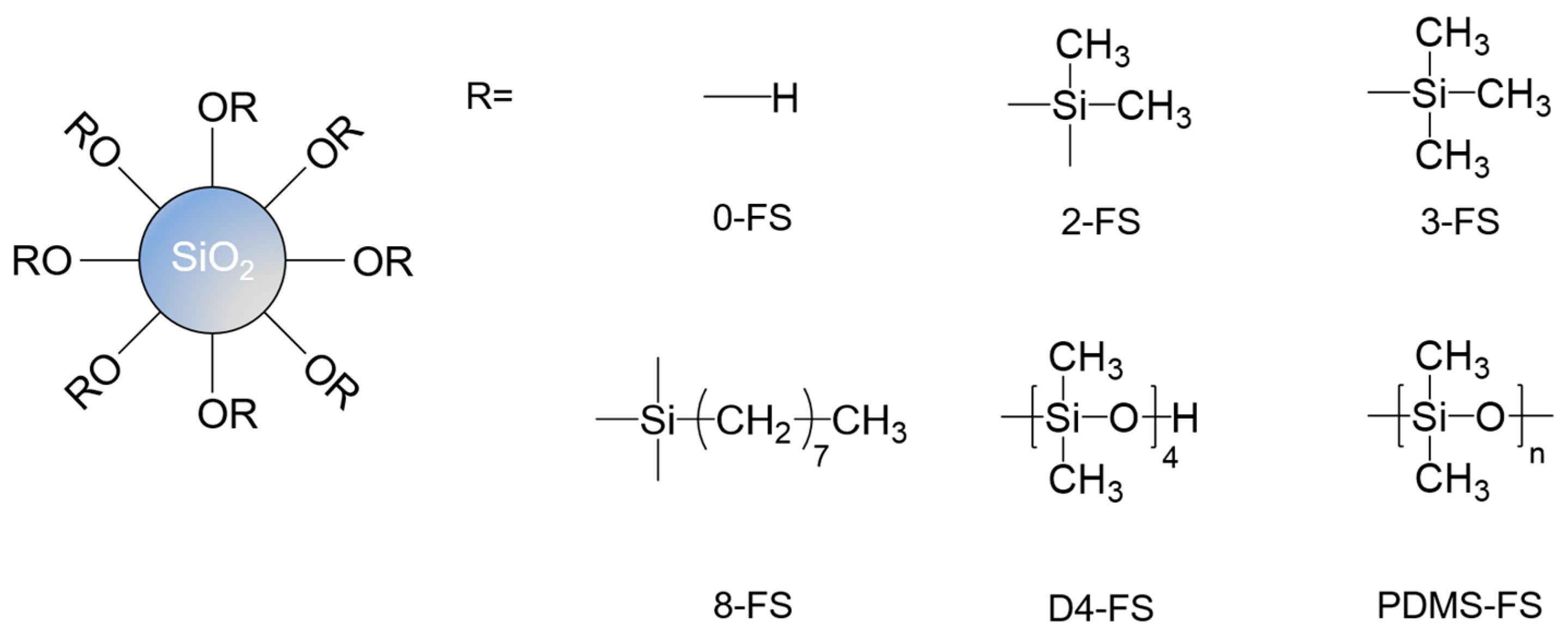
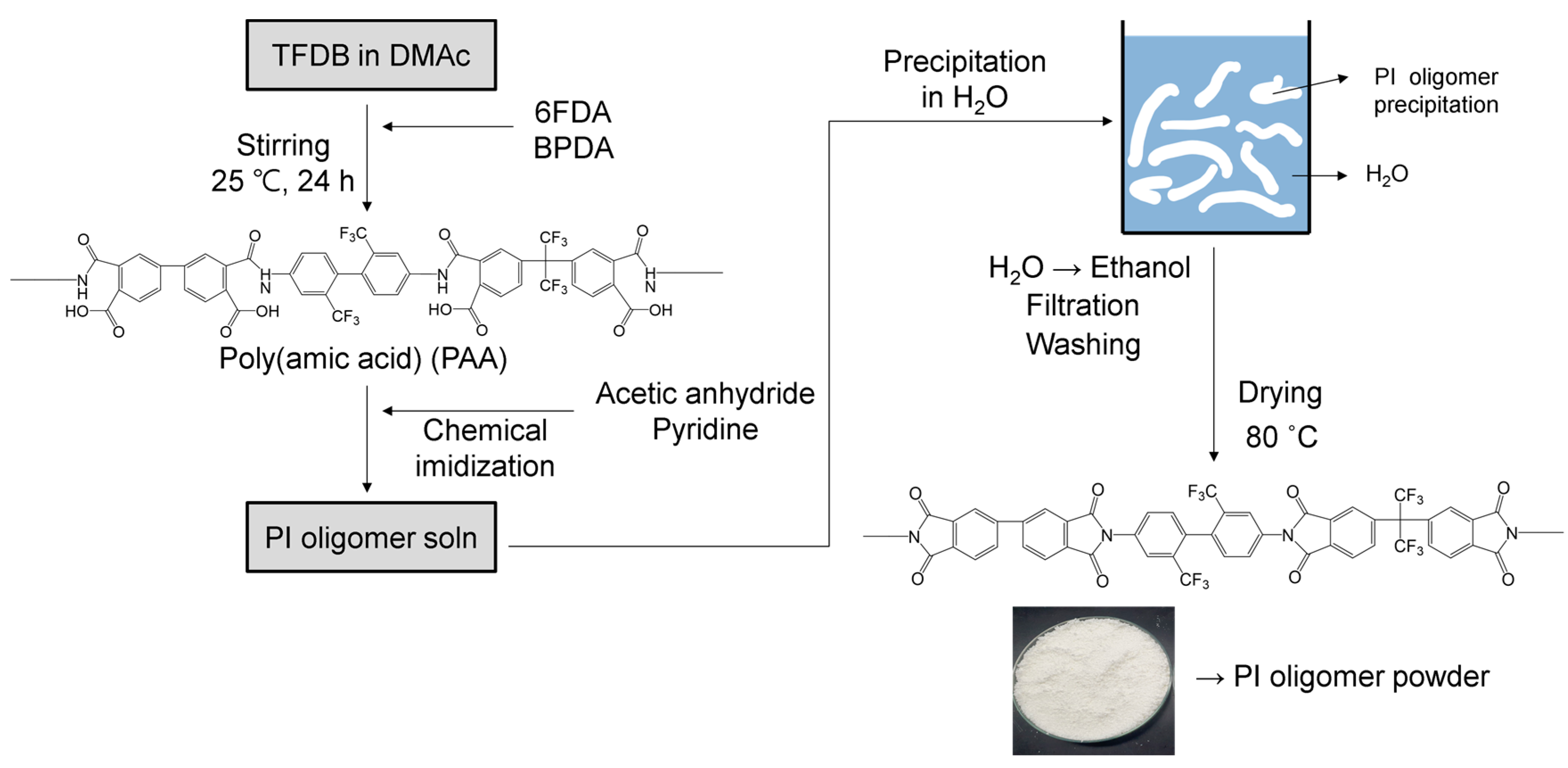
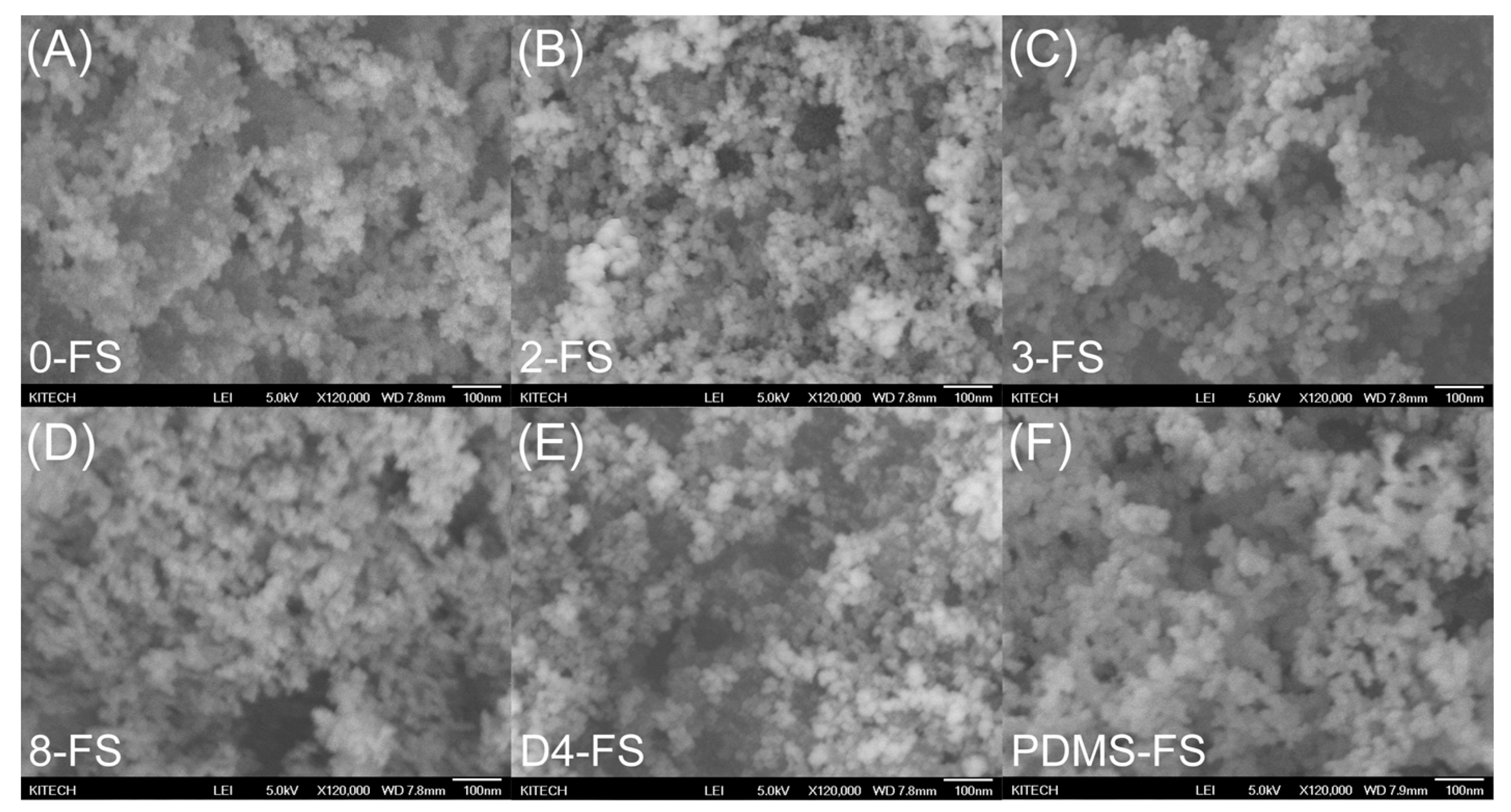
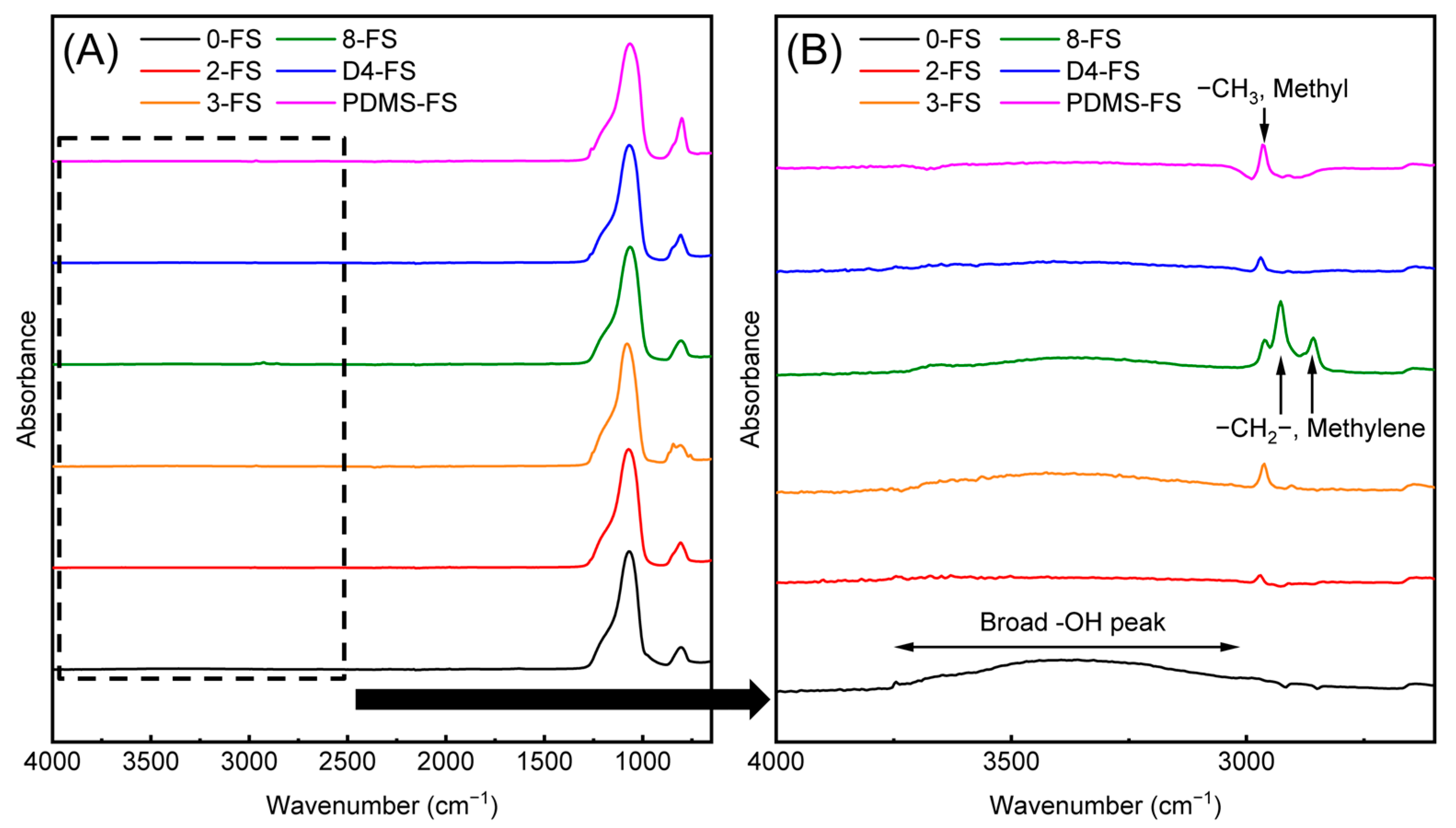

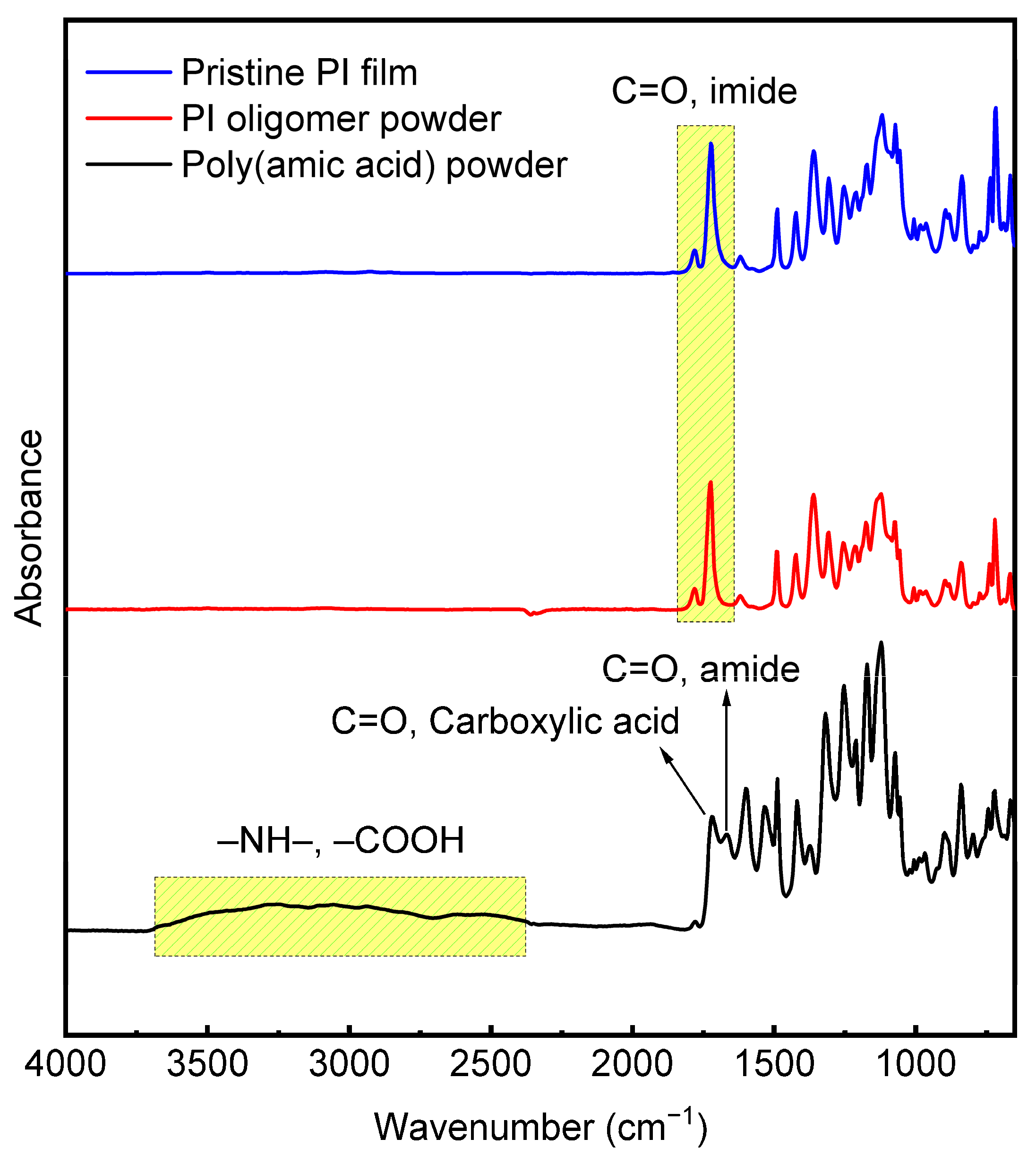

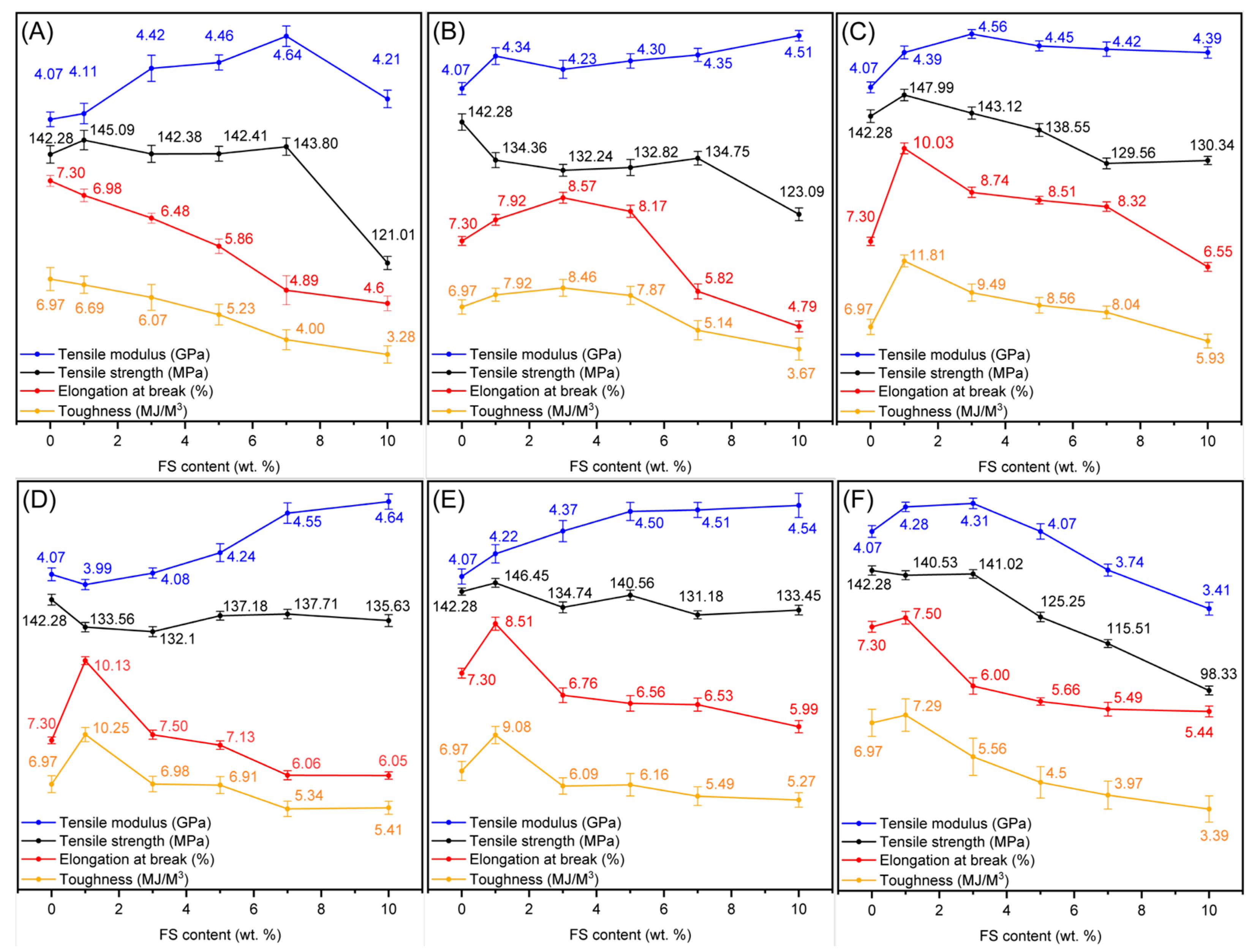
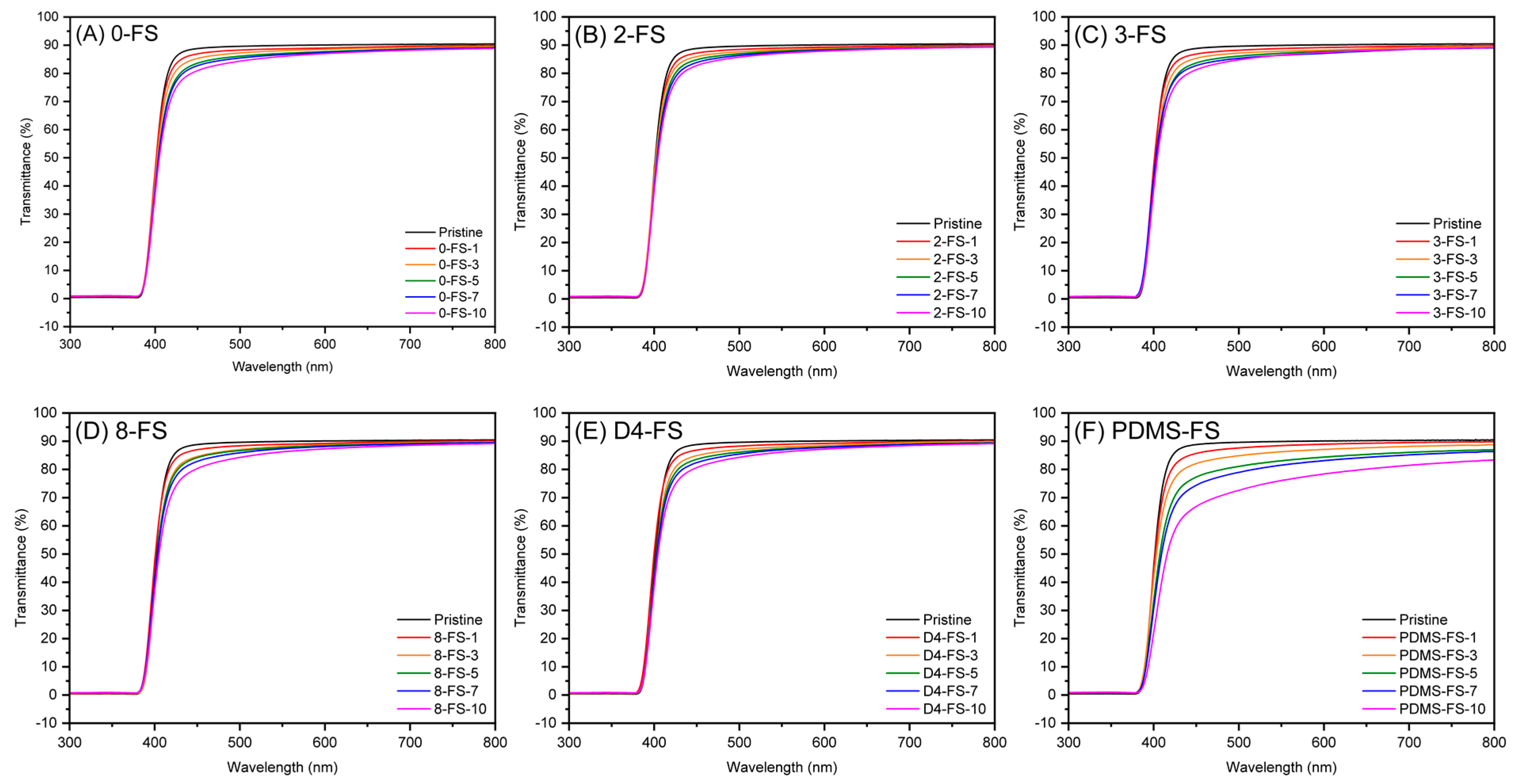
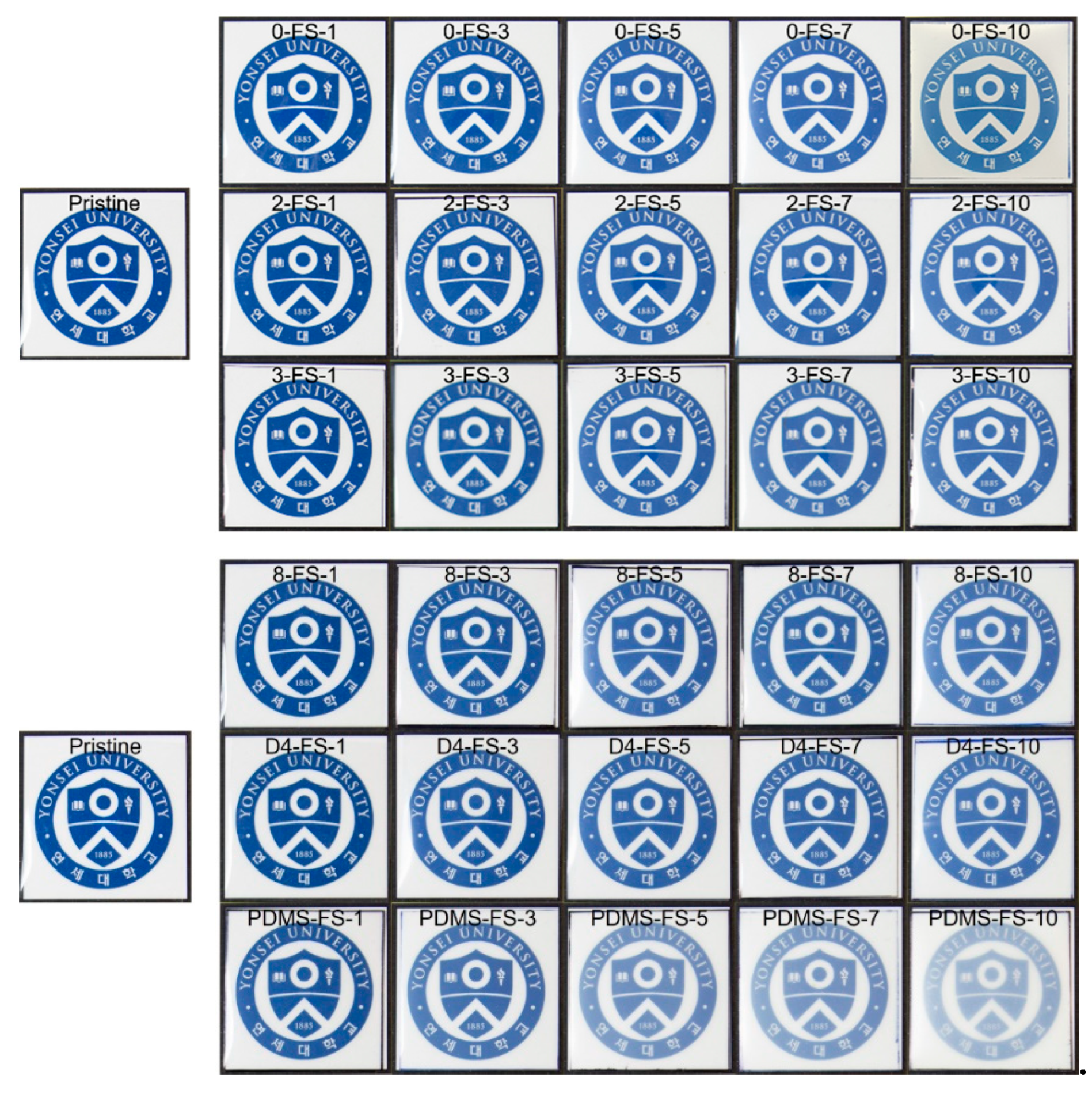
| Sample | Surface Grafting Ratio (wt. %) | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| 0-FS | 0 | 200 | 14.0 | 0.83 |
| 2-FS | 0.4 | 135 | 19.4 | 0.84 |
| 3-FS | 3.0 | 221 | 20.6 | 1.45 |
| 8-FS | 6.5 | 106 | 14.4 | 0.60 |
| D4-FS | 0.75 | 243 | 19.5 | 1.48 |
| PDMS-FS | 7.9 | 105 | 15.8 | 0.62 |
| Sample | Water CA (°) |
|---|---|
| Pristine | 65.2 |
| 0-FS-7 | 74.9 |
| 2-FS-7 | 74.6 |
| 3-FS-7 | 75.0 |
| 8-FS-7 | 80.5 |
| D4-FS-7 | 81.3 |
| PDMS-FS-7 | 81.7 |
| Sample | T550 * | YI | Haze |
|---|---|---|---|
| Pristine | 89.9 | 4.0 | 0.26 |
| 0-FS-1 | 88.7 | 4.6 | 3.57 |
| 0-FS-3 | 88.0 | 5.3 | 6.61 |
| 0-FS-5 | 87.1 | 6.3 | 11.67 |
| 0-FS-7 | 86.7 | 7.3 | 13.62 |
| 0-FS-10 | 86.0 | 6.3 | 29.04 |
| 2-FS-1 | 88.9 | 4.5 | 1.69 |
| 2-FS-3 | 88.3 | 5.6 | 5.60 |
| 2-FS-5 | 87.8 | 6.8 | 9.19 |
| 2-FS-7 | 87.5 | 7.2 | 11.41 |
| 2-FS-10 | 87.1 | 9.0 | 15.60 |
| 3-FS-1 | 88.8 | 4.8 | 2.85 |
| 3-FS-3 | 87.8 | 5.4 | 7.95 |
| 3-FS-5 | 87.1 | 6.6 | 13.35 |
| 3-FS-7 | 86.4 | 7.0 | 17.20 |
| 3-FS-10 | 86.5 | 8.4 | 17.95 |
| 8-FS-1 | 88.9 | 4.6 | 4.04 |
| 8-FS-3 | 88.1 | 6.6 | 10.77 |
| 8-FS-5 | 87.8 | 6.8 | 14.76 |
| 8-FS-7 | 87.3 | 7.9 | 15.20 |
| 8-FS-10 | 86.3 | 9.5 | 18.30 |
| D4-FS-1 | 88.9 | 4.5 | 3.27 |
| D4-FS-3 | 87.9 | 6.1 | 8.12 |
| D4-FS-5 | 87.3 | 6.8 | 10.03 |
| D4-FS-7 | 86.9 | 7.7 | 13.10 |
| D4-FS-10 | 86.2 | 9.0 | 14.43 |
| PDMS-FS-1 | 88.4 | 5.7 | 11.65 |
| PDMS-FS-3 | 86.3 | 6.7 | 24.95 |
| PDMS-FS-5 | 83.0 | 8.4 | 46.50 |
| PDMS-FS-7 | 81.5 | 10.0 | 50.39 |
| PDMS-FS-10 | 76.1 | 13.2 | 67.71 |
| TGA | DMA | TMA | ||||
|---|---|---|---|---|---|---|
| Td5% (°C) | Residue Amount (wt. %) | tan δ | Tg (°C) | CTE (ppm/°C) | ||
| Experimental | Calculated | |||||
| Pristine | 528.3 | 42.9 | N/A | 0.65 | 360.8 | 47.3 |
| 0-FS-1 | 534.5 | 44.6 | 43.5 | 0.72 | 358.0 | 39.7 |
| 0-FS-3 | 534.1 | 46.0 | 44.6 | 0.68 | 360.2 | 39.6 |
| 0-FS-5 | 536.1 | 46.1 | 45.8 | 0.63 | 370.3 | 38.2 |
| 0-FS-7 | 535.2 | 47.8 | 46.9 | 0.64 | 367.3 | 37.2 |
| 0-FS-10 | 537.7 | 50.0 | 48.5 | 0.56 | 366.2 | 28.9 |
| 2-FS-1 | 534.7 | 44.9 | 43.5 | 0.71 | 367.1 | 39.9 |
| 2-FS-3 | 534.8 | 45.5 | 44.6 | 0.71 | 366.6 | 37.0 |
| 2-FS-5 | 534.6 | 46.4 | 45.7 | 0.70 | 364.0 | 40.1 |
| 2-FS-7 | 531.6 | 48.6 | 46.9 | 0.63 | 357.7 | 35.9 |
| 2-FS-10 | 534.4 | 50.4 | 48.6 | 0.62 | 357.8 | 30.4 |
| 3-FS-1 | 531.3 | 44.8 | 43.4 | 0.68 | 367.9 | 37.4 |
| 3-FS-3 | 532.5 | 45.7 | 44.5 | 0.69 | 359.0 | 34.0 |
| 3-FS-5 | 530.4 | 47.4 | 45.6 | 0.69 | 368.5 | 38.4 |
| 3-FS-7 | 533.0 | 48.3 | 46.7 | 0.60 | 367.1 | 34.5 |
| 3-FS-10 | 531.3 | 49.8 | 48.3 | 0.61 | 370.1 | 36.9 |
| 8-FS-1 | 529.1 | 43.8 | 43.4 | 0.61 | 361.2 | 37.1 |
| 8-FS-3 | 532.4 | 45.6 | 44.4 | 0.65 | 355.7 | 37.5 |
| 8-FS-5 | 536.9 | 46.4 | 45.4 | 0.62 | 369.9 | 29.3 |
| 8-FS-7 | 532.6 | 47.4 | 46.4 | 0.63 | 357.4 | 39.8 |
| 8-FS-10 | 531.2 | 49.8 | 48.0 | 0.63 | 357.1 | 42.0 |
| D4-FS-1 | 534.5 | 44.6 | 43.5 | 0.70 | 363.4 | 45.9 |
| D4-FS-3 | 528.7 | 45.7 | 44.6 | 0.66 | 361.4 | 40.6 |
| D4-FS-5 | 530.3 | 47.2 | 45.7 | 0.61 | 356.1 | 38.2 |
| D4-FS-7 | 533.3 | 48.7 | 46.9 | 0.61 | 365.0 | 38.7 |
| D4-FS-10 | 532.1 | 48.3 | 48.5 | 0.58 | 357.2 | 36.7 |
| PDMS-FS-1 | 532.8 | 44.9 | 43.4 | 0.68 | 361.3 | 43.1 |
| PDMS-FS-3 | 533.8 | 47.2 | 44.4 | 0.70 | 361.6 | 41.4 |
| PDMS-FS-5 | 531.5 | 48.8 | 45.4 | 0.60 | 365.6 | 36.9 |
| PDMS-FS-7 | 536.9 | 50.4 | 46.3 | 0.66 | 359.4 | 36.5 |
| PDMS-FS-10 | 537.6 | 51.1 | 47.8 | 0.60 | 354.7 | 42.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeob, J.; Hong, S.W.; Koh, W.-G.; Park, I. Enhanced Mechanical and Thermal Properties of Polyimide Films Using Hydrophobic Fumed Silica Fillers. Polymers 2024, 16, 297. https://doi.org/10.3390/polym16020297
Yeob J, Hong SW, Koh W-G, Park I. Enhanced Mechanical and Thermal Properties of Polyimide Films Using Hydrophobic Fumed Silica Fillers. Polymers. 2024; 16(2):297. https://doi.org/10.3390/polym16020297
Chicago/Turabian StyleYeob, Jongin, Sung Woo Hong, Won-Gun Koh, and In Park. 2024. "Enhanced Mechanical and Thermal Properties of Polyimide Films Using Hydrophobic Fumed Silica Fillers" Polymers 16, no. 2: 297. https://doi.org/10.3390/polym16020297
APA StyleYeob, J., Hong, S. W., Koh, W.-G., & Park, I. (2024). Enhanced Mechanical and Thermal Properties of Polyimide Films Using Hydrophobic Fumed Silica Fillers. Polymers, 16(2), 297. https://doi.org/10.3390/polym16020297








