Grafting Starch with Acrylic Acid and Fenton’s Initiator: The Selectivity Challenge
Abstract
1. Introduction
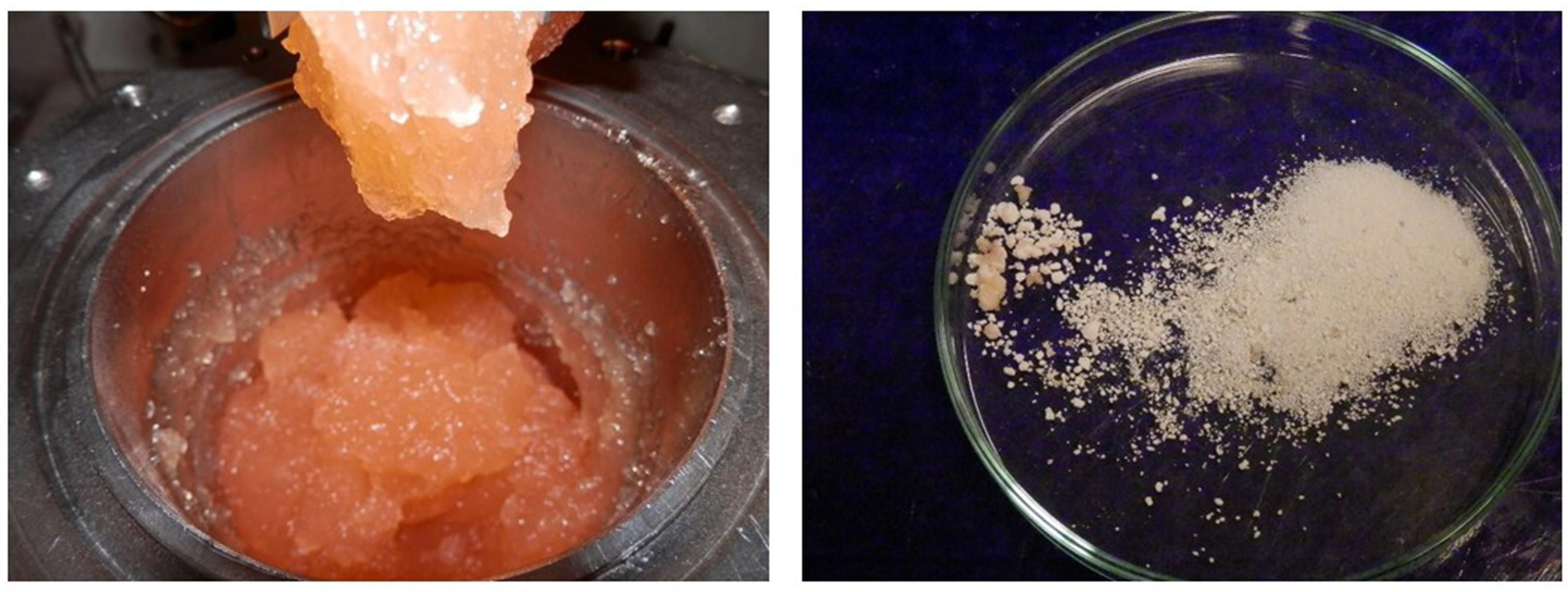
2. Experimental Procedures
3. Grafting Reactions: The Selectivity Challenge
3.1. Grafting and Homopolymerization Reactions
3.2. Initial and Final Graft Selectivity
4. Factors That Influence the Graft Selectivity
4.1. The Choice of the Initiator System
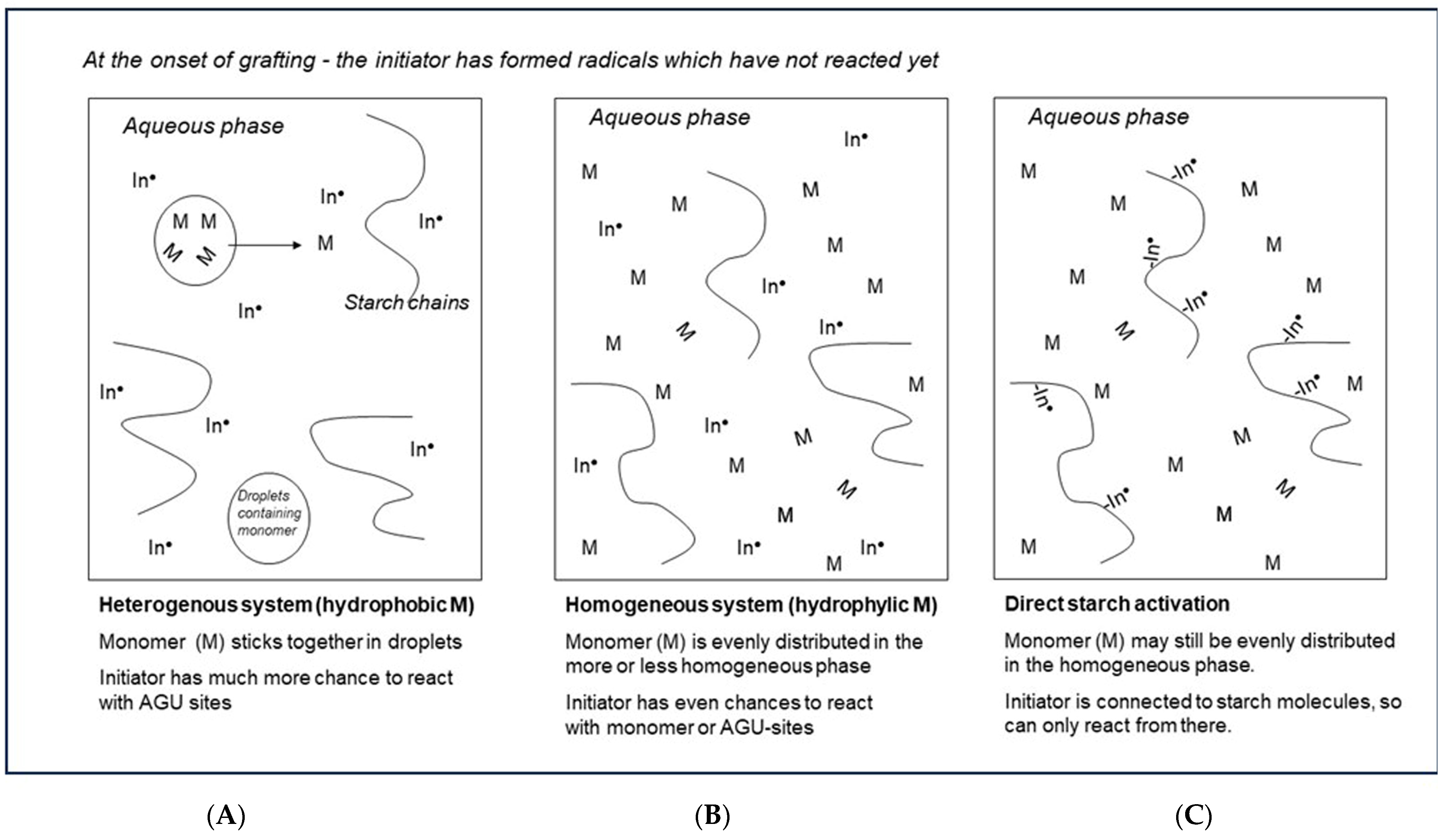
4.1.1. Class I: Activation by Radicals Created at Random in the Solution
4.1.2. Class II: Radicals via a Direct Reaction with Starch
4.1.3. Class III: Activation by High-Energy or Microwave Irradiation
4.1.4. Combinations of Initiators
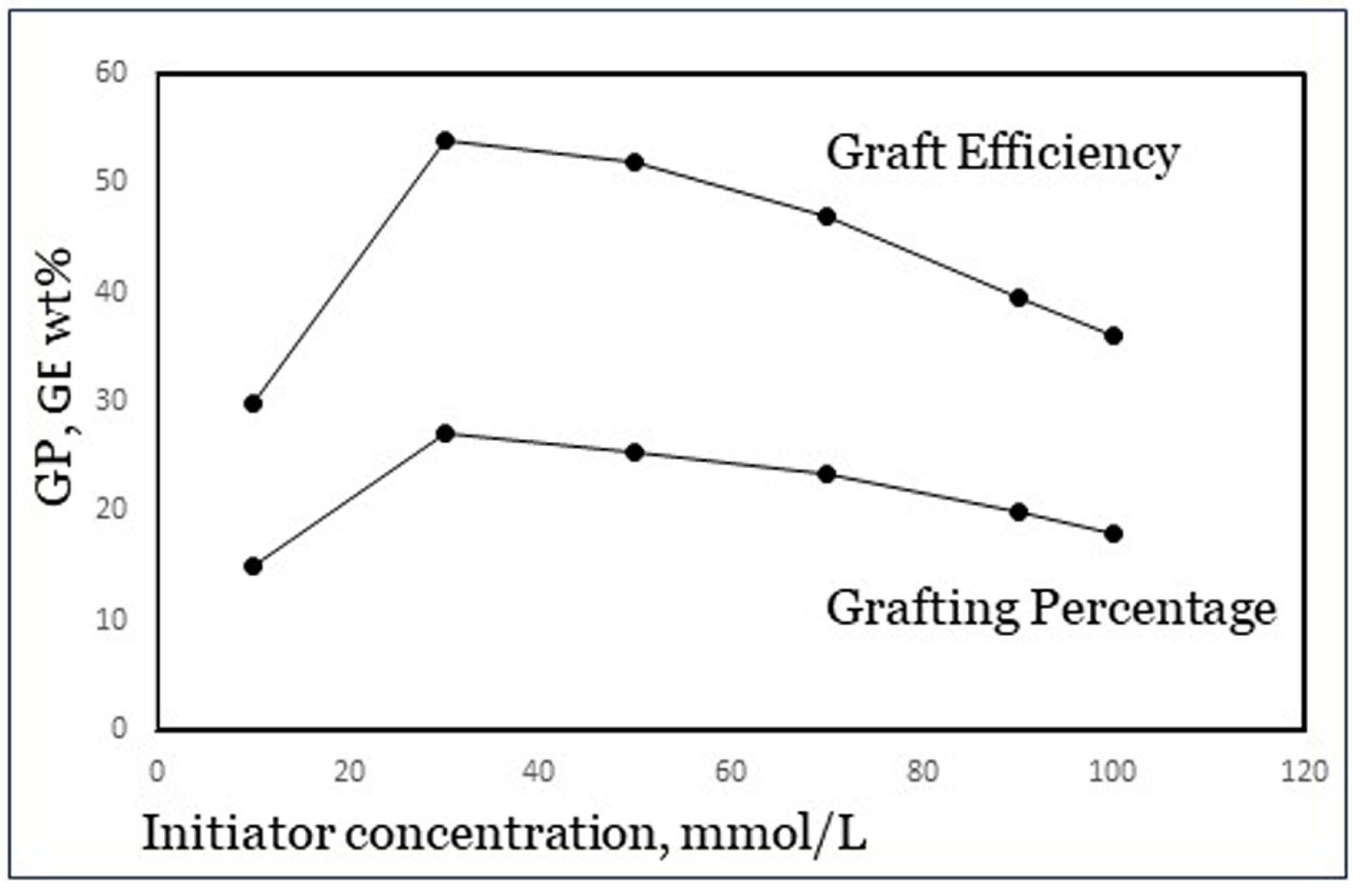
4.2. Reaction Variables Like Concentrations and Temperature
4.3. Water Solubility of the Monomer
5. A Chemical Engineering Approach to Different Grafting Systems
6. Methods to Improve the Graft Selectivity
6.1. General: Principal Approaches to the Selectivity Challenge
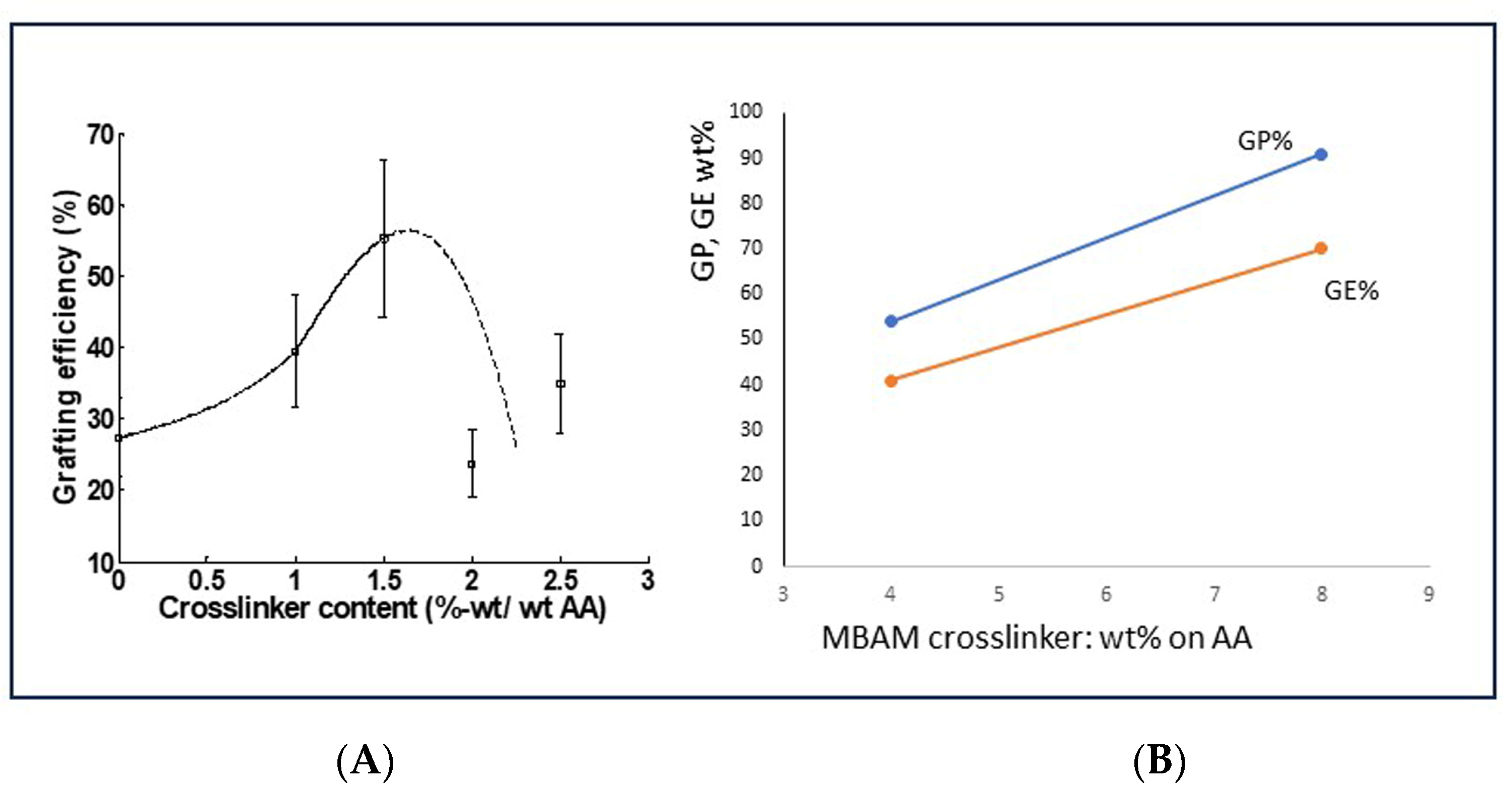
6.2. Approach A: Use of a Polymerization Crosslinker
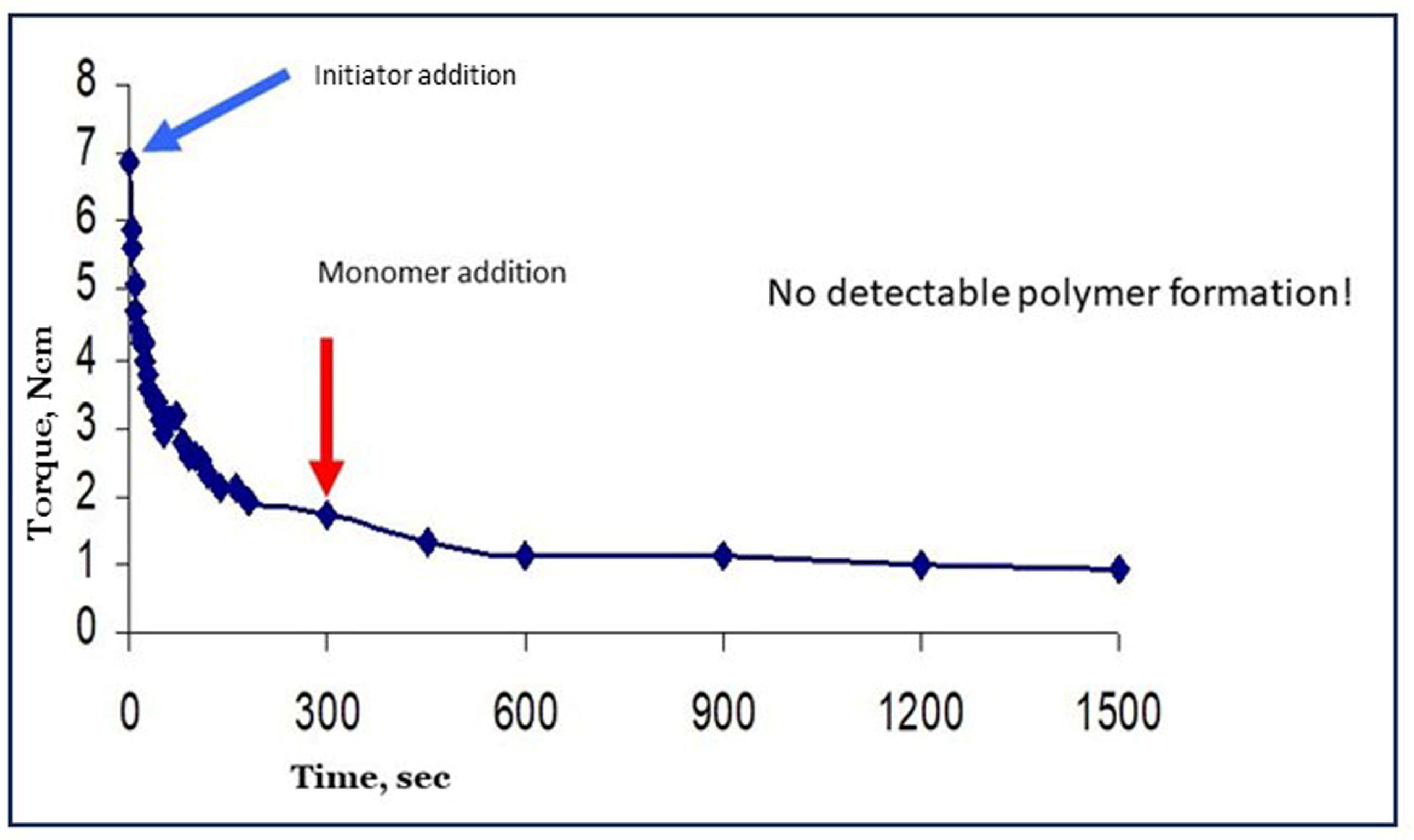
6.3. Approach B: Preceding Process Step(s) and Creation of Starch Macroradicals before Adding the Monomer
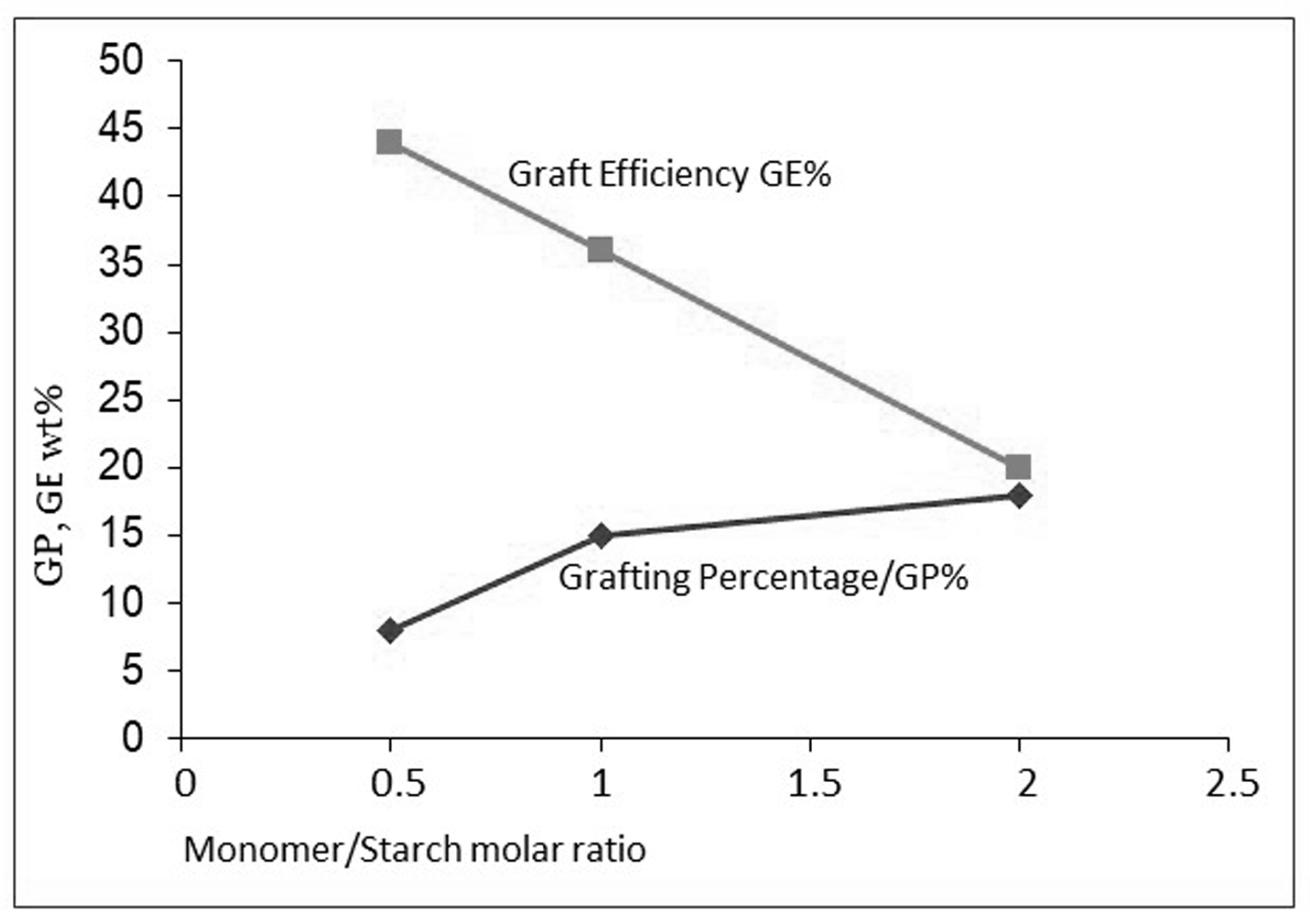
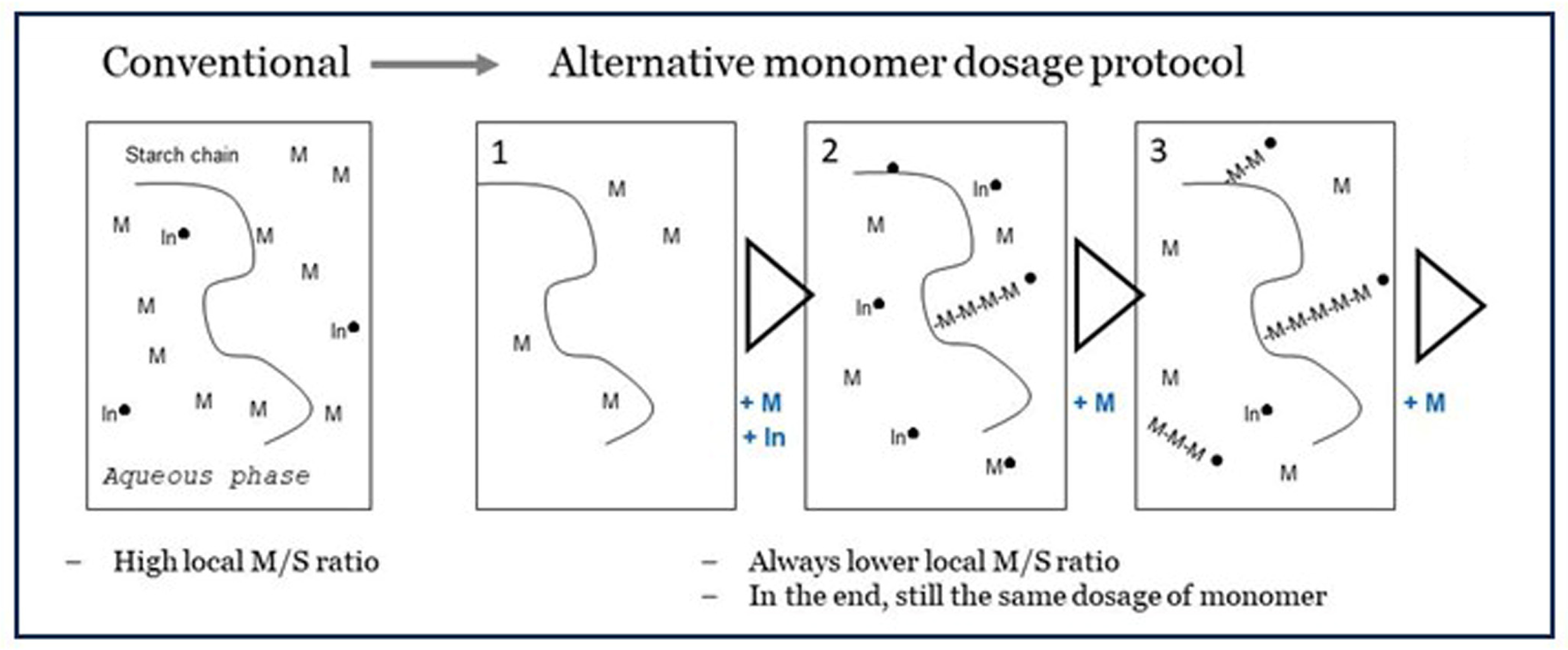
6.4. Approach C: Keeping the Monomer-to-Starch Ratio Low, by Dedicated Dosage of Monomer
6.5. Concluding Considerations about Methods to Improve the Graft Selectivity
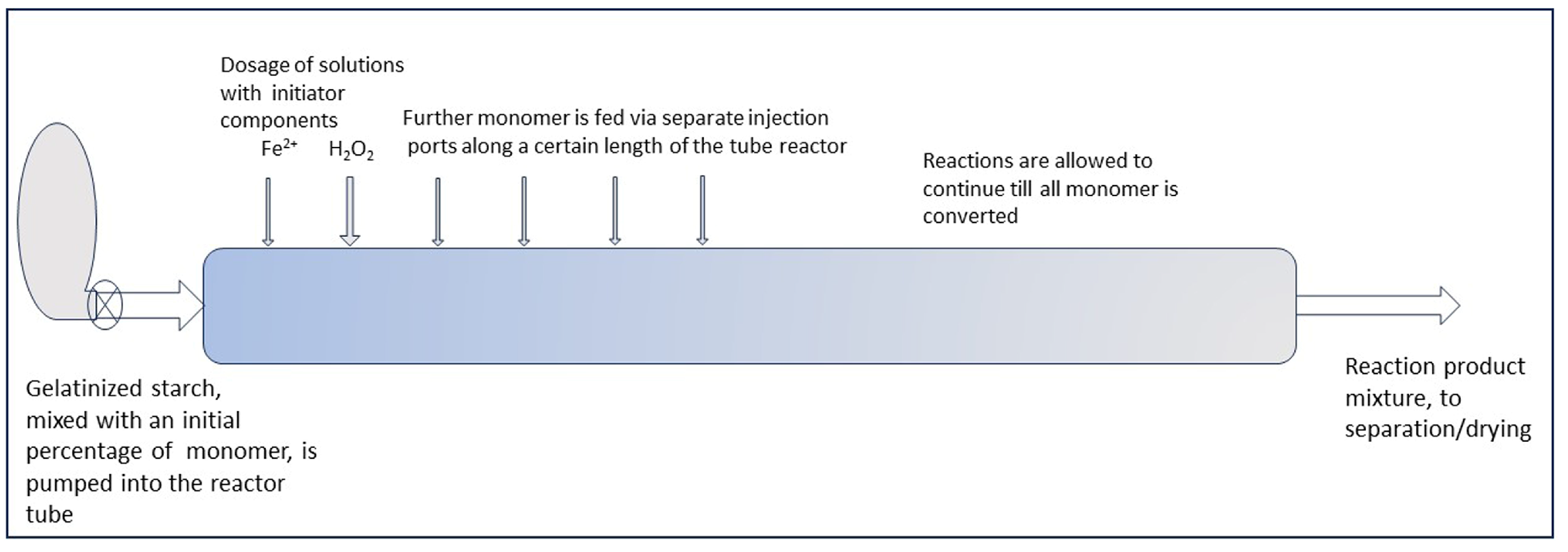
6.6. An Outlook towards Application of the Low M/S Principle in a Large-Scale Continuous Process
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Julien Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch/Stärke 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Lele, V.V.; Kumari, S.; Niju, H. Syntheses, Characterization and Applications of Graft Copolymers of Sago Starch—A Review. Starch/Stärke 2018, 70, 1700133. [Google Scholar] [CrossRef]
- Athawale, V.D.; Rathi, S.C. Graft Polymerization: Starch as a Model Substrate. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1999, 39, 445–480. [Google Scholar] [CrossRef]
- Fanta, G.F.; Doane, W.M. Grafted Starches. In Modified Starches: Properties and Uses; Wurzburg, O.B., Ed.; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Witono, J.R. New Materials by Grafting of Acrylic Acid onto Cassava Starch. Ph.D. Thesis, Chemical Engineering Dept., University of Groningen, Groningen, The Netherlands, 2012. [Google Scholar]
- Noordergraaf, I.W.; Fourie, T.K.; Raffa, P. Free-Radical Graft Polymerization onto Starch as a Tool to Tune Properties in Relation to Potential Applications. A Review. Processes 2018, 6, 31. [Google Scholar] [CrossRef]
- Bucholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; Wiley VCH: New York, NY, USA, 1998. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Graft Copolymerization of Acrylic Acid to Cassava Starch—Evaluation of the Influences of Process Parameters by an Experimental Design Method. Carbohydr. Polym. 2012, 90, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasak, A. Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Torque measurement as a tool to monitor the breakdown of cassava starch gels, by the effect of Fenton’s initiator for graft copolymerization. Results Chem. 2022, 4, 100314. [Google Scholar] [CrossRef]
- Witono, J.R.; Marsman, J.H.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Improved homopolymer separation to enable the application of 1H-NMR and HPLC for the determination of the reaction parameters in the graft copolymerization of acrylic acid onto starch. Carbohydr. Res. 2013, 370, 38–45. [Google Scholar] [CrossRef]
- Odian, G. Principle of Polymerization, 4th ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Vazquez, B.; Goni, I.; Gurruchaga, M.; Valero, M.; Guzman, G.M. A study of the graft copolymerization of methacrylic acid onto starch using the H2O2/Fe++ redox system. J. Polym. Sci. 1989, A27, 595–603. [Google Scholar] [CrossRef]
- Gao, J.; Yu, J.; Wang, W.; Chang, L.; Tian, R. Comparison of transition metals in the graft copolymerization of vinyl monomers to starch. J. Macromol. Sci. Pure Appl. Chem. 1998, A35, 483–494. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Yang, L.; Shi, Z.; Deng, K. Graft copolymerization of methyl methacrylate onto starch using potassium ditelluratocuprate(III). J. Macromol. Sci. Pure Appl. Chem. 2004, A41, 1025–1035. [Google Scholar] [CrossRef]
- Gurruchaga, M.B.; Goni, I.; Vazquez, B.; Valero, M.; Guzman, G.M. An Approach to the Knowledge of the Graft Polymerization of Acrylic Monomers onto Polysaccharides Using Ce (IV) as Initiator. J. Polym. Sci. Part C Polym. Lett. 1989, 27, 149–152. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Said, O.B. Studies on the graft copolymerization of methyl methacrylate onto starch. Acta Polym. 1989, 40, 708–710. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Egharevba, F.; Jideonwo, A. Graft copolymers of starch and (poly)ethyl acrylaat. Angew. Makromol. Chem. 1991, 184, 20–26. [Google Scholar] [CrossRef]
- Park, I.H.; Song, S.Y.; Song, B.K. Graft polymerization of acrylic acid onto corn starch in aqueous isopropanol solution. Angew. Makromol. Chem. 1999, 267, 20–26. [Google Scholar] [CrossRef]
- Reyes, Z.; Syz, M.G.; Huggins, M.L. Grafting acrylic acid to starch by preirradiation. J. Polym. Sci. 1963, C23, 401–408. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Pandey, S.; Singh, S.K. Microwave-accelerated synthesis and characterization of potato starchg-poly(acrylamide). Starch/Staerke 2006, 58, 536–543. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, F.; Duan, Q.; Bao, X.; Jiang, S.; Liu, H.; Chen, L.; Yu, L. Designing and application of reactive extrusion with twice initiations for graft copolymerization of acrylamide on starch. Eur. Polymer J. 2022, 165, 111008. [Google Scholar] [CrossRef]
- Khalil, M.I.; Mostafa, K.M.; Hebeish, A. Graft polymerization of acrylamide onto maize starch using potassium persulfate as initiator. Angew. Makromol. Chem. 1993, 213, 43–54. [Google Scholar] [CrossRef]
- Athawale, V.D.; Rathi, S.C. Effect of chain length of the alkyl group of alkyl methacrylates on graft polymerization onto starch using ceric ammonium nitrate as initiator. Eur. Polym. J. 1997, 33, 1067–1071. [Google Scholar] [CrossRef]
- Fanta, G.F.; Burr, R.C.; Doane, W.M.; Russell, C.R. Influence of starch granule swelling on graft copolymer Composition. A comparison of monomers. J. Appl. Polym. Sci. 1971, 15, 2651–2660. [Google Scholar] [CrossRef]
- De Graaf, R.A. The Use of Twin Screw Extruders as Starch Modification Reactors. Ph.D. Thesis, Groningen University, Groningen, The Netherlands, 1995. [Google Scholar]
- Athawale, V.D.; Rathi, S.C. Role and relevance of polarity and solubility of vinyl monomers in graft polymerization onto starch. React. Funct. Polym. 1997, 34, 11–17. [Google Scholar] [CrossRef]
- Trimnell, D.; Fanta, G.F.; Salch, J.H. Graft polymerization of methyl acrylate onto granular starch: Comparison of the Fe2+/H2O2 and ceric initiation systems. J. Appl. Polym. Sci. 1996, 60, 285–292. [Google Scholar] [CrossRef]
- Gao, J.; Tian, R.; Zhang, L. Graft copolymerization of vinylic monomers onto stach initiated by transition metal-thiourea redox systems. Chin. J. Polym. Sci. 1996, 14, 163–171. [Google Scholar]
- Shogren, R.L.; Willet, J.L.; Biswas, A. HRP-mediated synthesis of starch-polyacrylamide grafts copolymers. Carbohydr. Polym. 2009, 75, 189–191. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Nkumah, J.E.; Egharevba, F. Studies on the grafting of acrylic acid to starch. Eur. Polym. J. 1989, 25, 423–426. [Google Scholar] [CrossRef]
- Kislenko, V.N. Emulsion graft polymerization: Mechanism of formation of dispersions. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 199–203. [Google Scholar] [CrossRef]
- Khaddazh, M.; Gritskova, I.A.; Litvinenko, G.I. An Advanced Approach on the Study of Emulsion Polymerization: Effect of the Initial Dispersion State of the System on the Reaction Mechanism, Polymerization Rate, and Size Distribution of Polymer-Monomer Particles. In Polymerization; De Souza Gomes, A., Ed.; Intech Open: Rijeka, Croatia, 2019. [Google Scholar]
- Pradan, S.; Mohanty, S. (Eds.) Bio-based Superabsorbents. Recent Trends, Types, Applications and Recycling; Springer Nature Singapore Pte Ltd.: Singapore, 2023. [Google Scholar]
- Athawale, V.D.; Lele, V. Recent trends in hydrogels based on starch-graft-acrylic acid: A review. Starch/Stärke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Water Absorption, Retention and the Swelling Characteristics of Cassava Starch Grafted with Polyacrylic Acid. Carbohydr. Polym. 2014, 103, 325–332. [Google Scholar] [CrossRef]
- Hebeish, A.; Zahran, M.K.; El-Rafie, M.H.; El-Tahlawy, K.F. Preparation and characterisation of poly(acrylic acid) starch polyblends. Polym. Polym. Compos. 1996, 4, 129–141. [Google Scholar] [CrossRef]
- Van Warners, A. Modification of Starch by Reaction with Ethylene Oxide. Ph.D. Thesis, Groningen University, Groningen, The Netherlands, 1992. [Google Scholar]
- Hebeish, A.; Guthrie, J.T. The Chemistry and Technology of Cellulosic Copolymers; Springer: New York, NY, USA, 1981. [Google Scholar]
- Bemiller, J.; Whistler, R.L. Starch: Chemistry and Technology, 3rd ed.; Academic: London, UK; Elsevier Academic Press: Burlington, MA, USA, 2009. [Google Scholar]
- Wilham, C.A.; McGuire, T.A.; Rudolphi, A.S.; Mehltretter, C.L. Polymerization studies with allyl starch. J. Appl. Polym. Sci. 1963, 7, 1403. [Google Scholar] [CrossRef]
- Reyes, Z.; Clark, C.F.; Comas, M.; Russell, C.R.; Rist, C.E. Continuous Production of Graft Copolymers of Starch with Acrylamide and Acrylic Acid by Electron Preirradiation. Nucl. Appl. 1969, 6, 509–517. [Google Scholar] [CrossRef]
- Saade, H.; Torres, S.; Barrera, C.; Sánchez, J.; Garza, Y. Effect of pH and Monomer Dosing Rate in the Anionic Polymerization of Ethyl Cyanoacrylate in Semicontinuous Operation. In Prime Archives in Polymer Technology; Vide Leaf: Hyderabad, India, 2019. [Google Scholar]
- Trimnell, D.; Stout, E.I. Grafting acrylic acid onto starch and poly(vinyl alcohol) by photolysis. J. Appl. Polym. Sci. 1980, 25, 2431–2434. [Google Scholar] [CrossRef]
- Brockway, C.E. Efficiency and frequency of grafting of methylmethacrylate to granular corn starch. J. Polym. Sci. 1964, A2, 3721–3731. [Google Scholar]
- Swinkels, J.J.M. Composition and Properties of Commercial Native Starches. Starch/Staerke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Rheological behavior of reaction mixtures during the graft copolymerization of cassava starch with acrylic acid. Polym. Eng. Sci. 2017, 57, 1285–1292. [Google Scholar] [CrossRef]
- Lammers, G.; Beenackers, A.A.C.M. Heat transfer and the continous production of hydroxypropyl starch in a static mixer reactor. Chem. Eng. Sci. 1994, 49, 5107. [Google Scholar] [CrossRef]
- EP0778850A1; Method of Modifying Starch. European Patent Office: Rijswijk, The Netherlands, 1994.
- Willet, J.L.; Finkenstadt, V.L. Comparison of Cationic and Unmodified Starches in Reactive Extrusion of Starch–Polyacrylamide Graft Copolymers. J. Polym. Environ. 2009, 17, 248–253. [Google Scholar] [CrossRef]
- Kugler, S.; Spychaj, T.; Wilpiszewska, K.; Gor, K. Starch-Graft Copolymers of N-Vinylformamide and Acrylamide Modified with Montmorillonite Manufactured by Reactive Extrusion. J. Appl. Polym. Sci. 2013, 127, 2847–2854. [Google Scholar] [CrossRef]
- Evans, A.G.; Tyrrall, E. Heats of polymerization of acrylic acid and derivatives. J. Polym. Sci. 1947, 2, 387–396. [Google Scholar] [CrossRef]



 represents high energy radiation, ● = radical state.
represents high energy radiation, ● = radical state.
 represents high energy radiation, ● = radical state.
represents high energy radiation, ● = radical state.
| Monomer | Solubility in Water | Initiator (Class) | GE% Range | Reference |
|---|---|---|---|---|
| Styrene (Sty) | 0.3 g/L | KPS (I) | 82–88% | Fanta et al. [25] |
| KPS (I) | 20–45% | De Graaf [26] | ||
| CAN (II) | No grafting | Fanta et al. [25] | ||
| CAN (II) | 0% | Athawale et al. [27] | ||
| Methyl methacrylate (MAAt) | 12 g/L | Fenton’s (I) | 69–85% | Trimnell et al. [28] |
| Fenton’s (I) | 82–93% | Fanta et al. [25] | ||
| MnTU (I) | 83–85% | Gao et al. [29] | ||
| CAN (II) | 84–94% | Trimnell et al. [28] | ||
| CAN(II) | 65–80% | Fanta et al. [25] | ||
| CAN (II) | 56–94% | Okieimen et al. [17] | ||
| Acrylonitrile (AN) | 73 g/L | Fenton’s (I) | 39–91% | Fanta et al. [25] |
| KPS (I) | 34% | Khalil et al. [23] | ||
| MnTU (I) | 76–82% | Gao et al. [29] | ||
| CAN (II) | 89–97% | Fanta et al. [25] | ||
| CAN (II) | 62% | Athawale et al. [27] | ||
| Acrylamide (AAm) | 2000 g/L | Fenton’s (I) | 34–54% | Fanta et al. [25] |
| KPS (I) | 30–75% | Khalil et al. [23] | ||
| HRP (I) | 33–66% | Shogren et al. [30] | ||
| MnTU (I) | 69–71% | Gao et al. [29] | ||
| CAN (II) | 12–33% | Fanta et al. [25] | ||
| Acrylic acid (AA) | Fully soluble | Fenton’s (I) | 19–36% | Fanta et al. [25] |
| Fenton’s (I) | 20–44% | Witono et al. [8] | ||
| KPS (I) | 10% | Khalil et al. [23] | ||
| MnTU (I) | 25–29% | Gao et al. [29] | ||
| CAN (II) | 10–48% | Fanta et al. [25] | ||
| CAN (II) | 29–87% | Okieimen et al. [31] |
| Method | Run nr | Total M/S Molar Ratio | Results: Grafting Percentage/Efficiency | |
|---|---|---|---|---|
| Conventional dosage (A) = all monomer before adding the initiator | A-1 A-2 A-3 Average (StDev) | 2.0 2.0 2.0 | GP wt% 18% 15% 17% 17 (±1.3)% | GE wt% 20% 17% 20% 19 (±1.4)% |
| Monomer dosage in 20% portions, at 5 min intervals (B) | B-1 B-2 B-3 Average (StDev) | 2.0 2.0 2.0 | 28% 26% 29% 28 (±1.3)% | 32% 29% 33% 31 (±1.7)% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noordergraaf, I.-W.; Witono, J.R.; Heeres, H.J. Grafting Starch with Acrylic Acid and Fenton’s Initiator: The Selectivity Challenge. Polymers 2024, 16, 255. https://doi.org/10.3390/polym16020255
Noordergraaf I-W, Witono JR, Heeres HJ. Grafting Starch with Acrylic Acid and Fenton’s Initiator: The Selectivity Challenge. Polymers. 2024; 16(2):255. https://doi.org/10.3390/polym16020255
Chicago/Turabian StyleNoordergraaf, Inge-Willem, Judy R. Witono, and Hero J. Heeres. 2024. "Grafting Starch with Acrylic Acid and Fenton’s Initiator: The Selectivity Challenge" Polymers 16, no. 2: 255. https://doi.org/10.3390/polym16020255
APA StyleNoordergraaf, I.-W., Witono, J. R., & Heeres, H. J. (2024). Grafting Starch with Acrylic Acid and Fenton’s Initiator: The Selectivity Challenge. Polymers, 16(2), 255. https://doi.org/10.3390/polym16020255






