Atmospheric Pressure Plasma Jet Exposure of Polylactic Acid Surfaces for Better Adhesion: Plasma Parameters towards Polymer Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Source and Electro-Optical Diagnosis
2.2. Surface and Volume Polymeric Sample Characterization Methods
2.2.1. Surface Morphology: Atomic Force Microscopy and Scanning Electron Microscopy
2.2.2. Static Contact Angle and Surface Energy
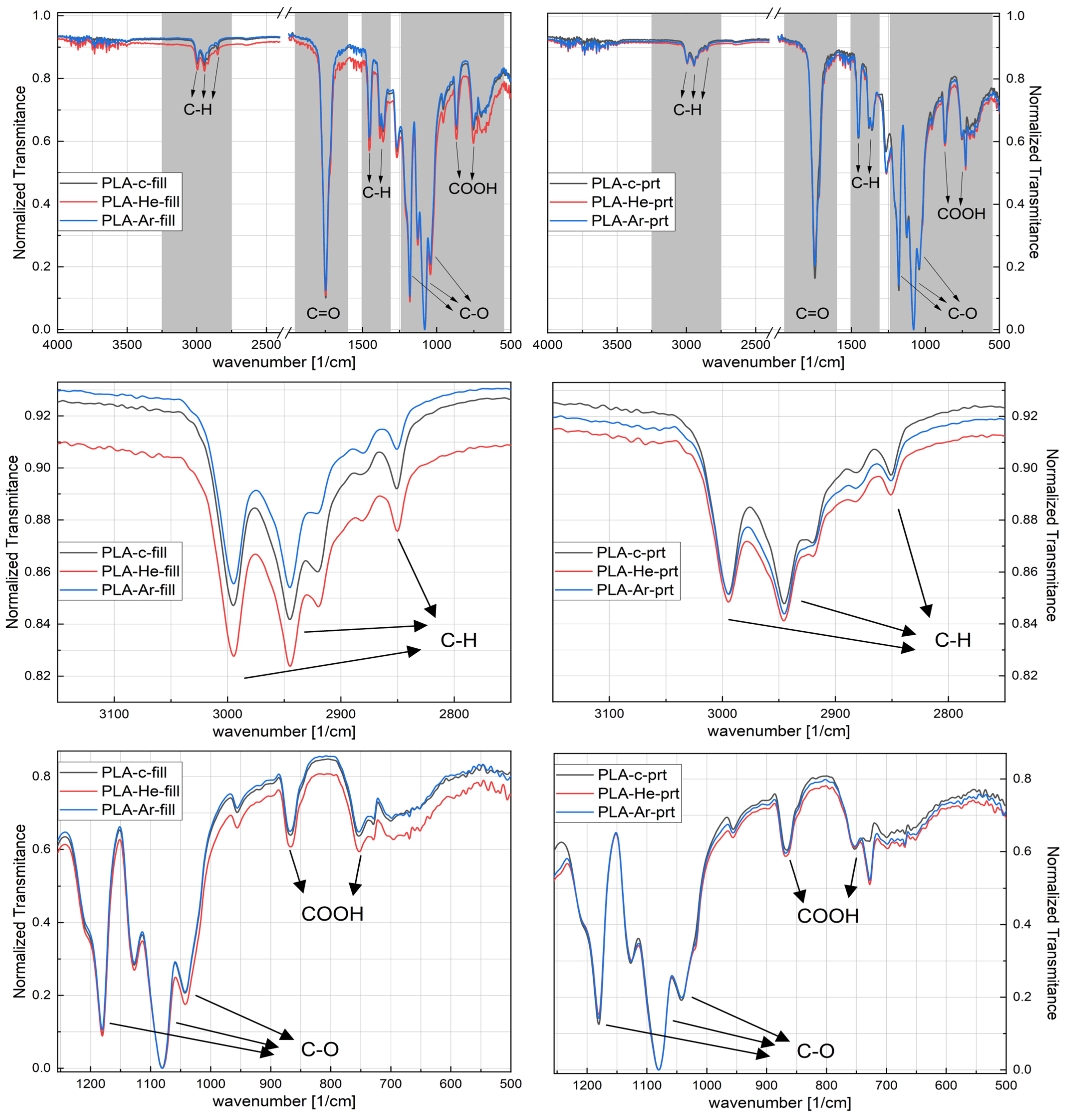
2.2.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
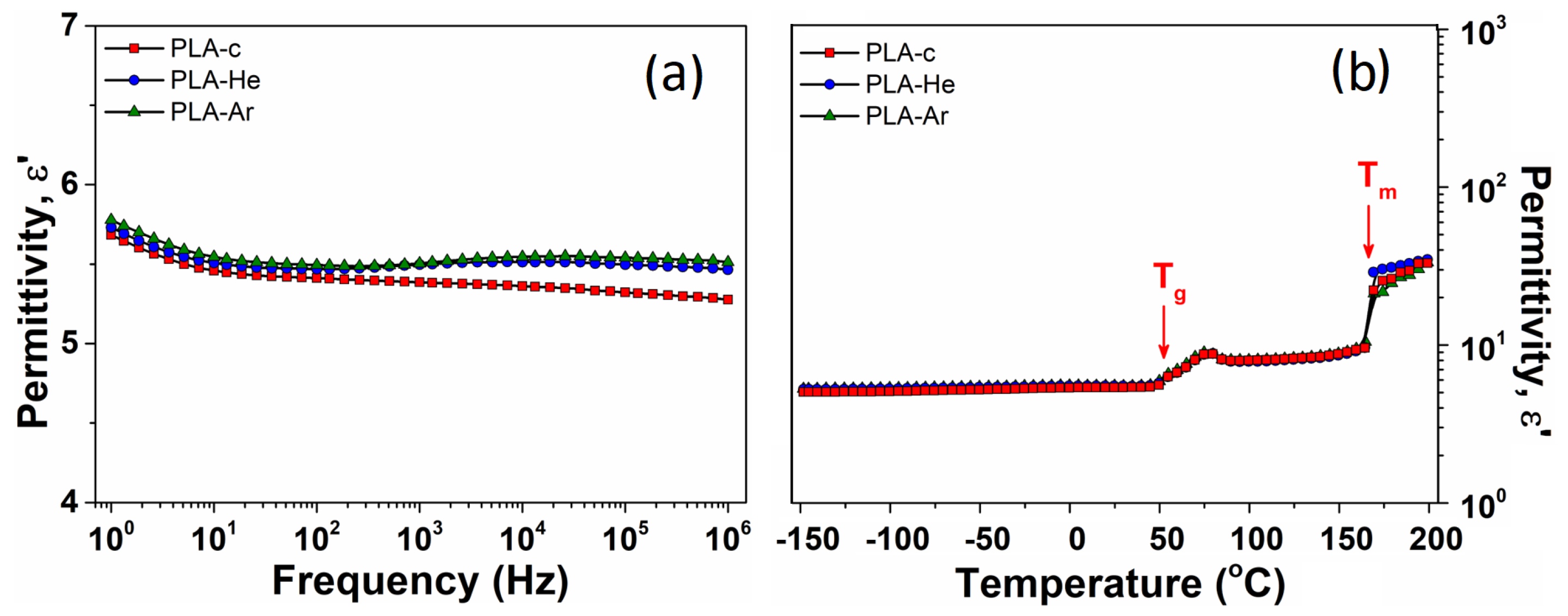
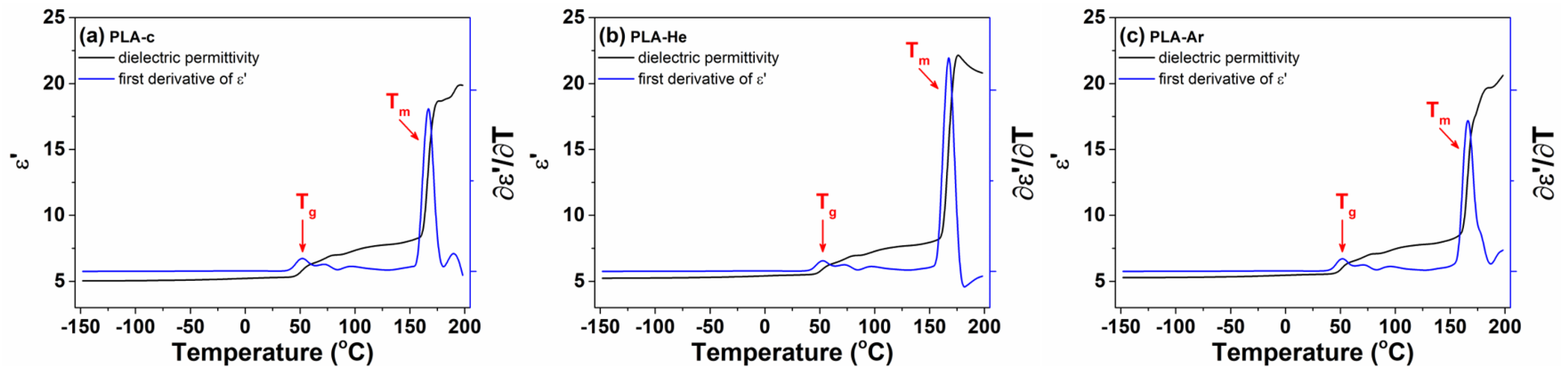
2.2.4. Broadband Dielectric Spectroscopy (BDS)
2.2.5. Differential Scanning Calorimetry (DSC) Measurements—Thermal Transitions
2.2.6. X-ray Diffraction
3. Results and Discussion
3.1. Plasma Source Electro-Optical Characterization
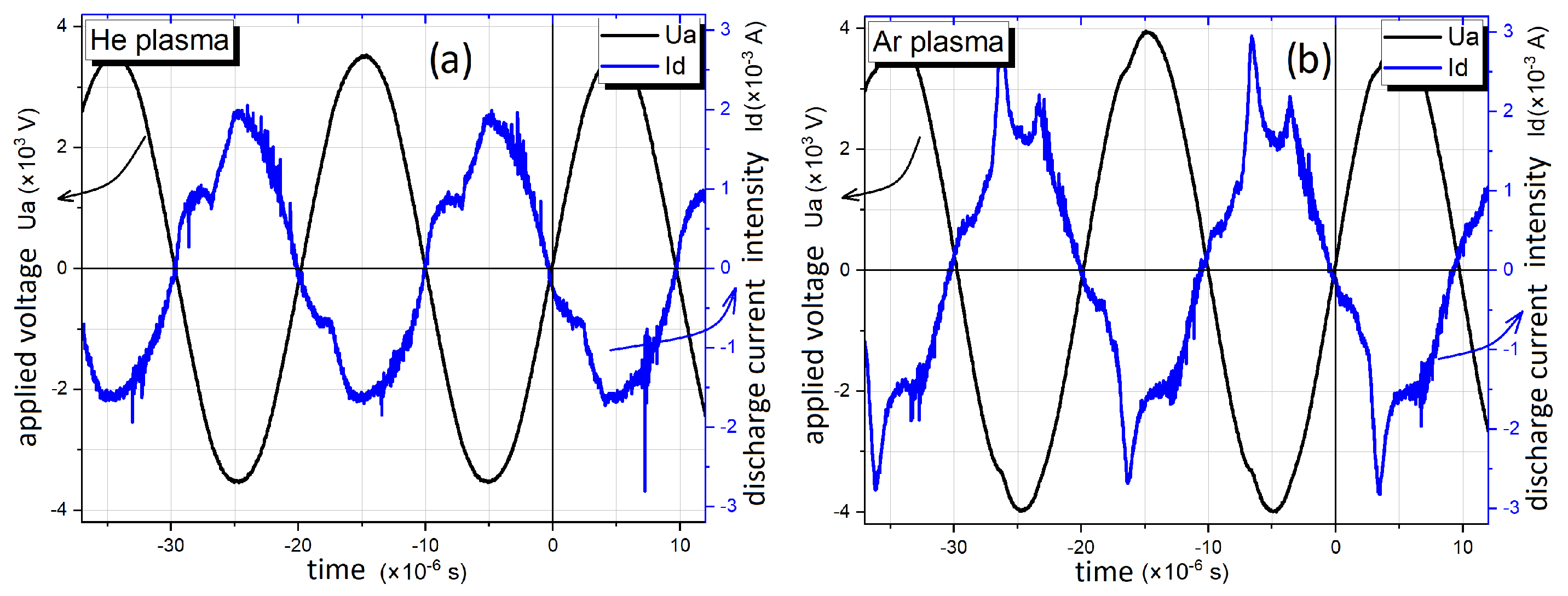
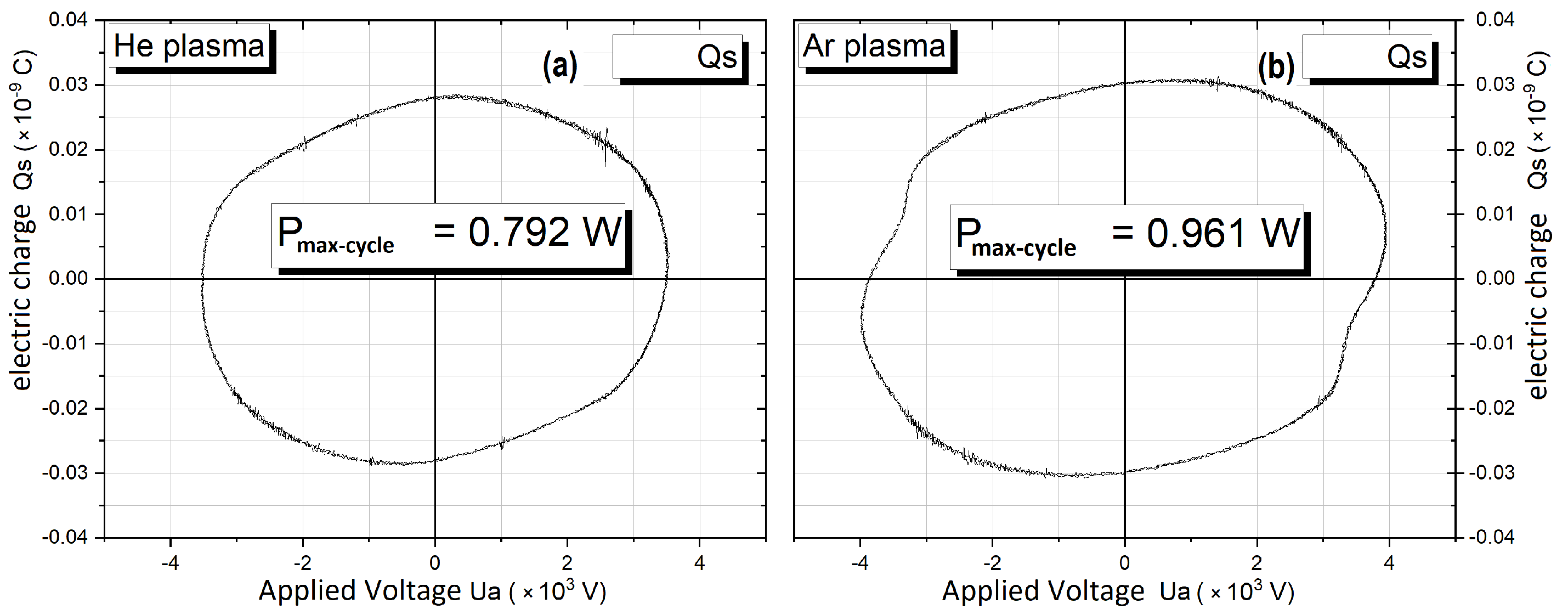
3.1.1. Plasma Jet Electrical Diagnosis
3.1.2. Plasma Jet Optical Diagnosis
3.2. Surface and Volume Polymeric Sample Characterization Methods
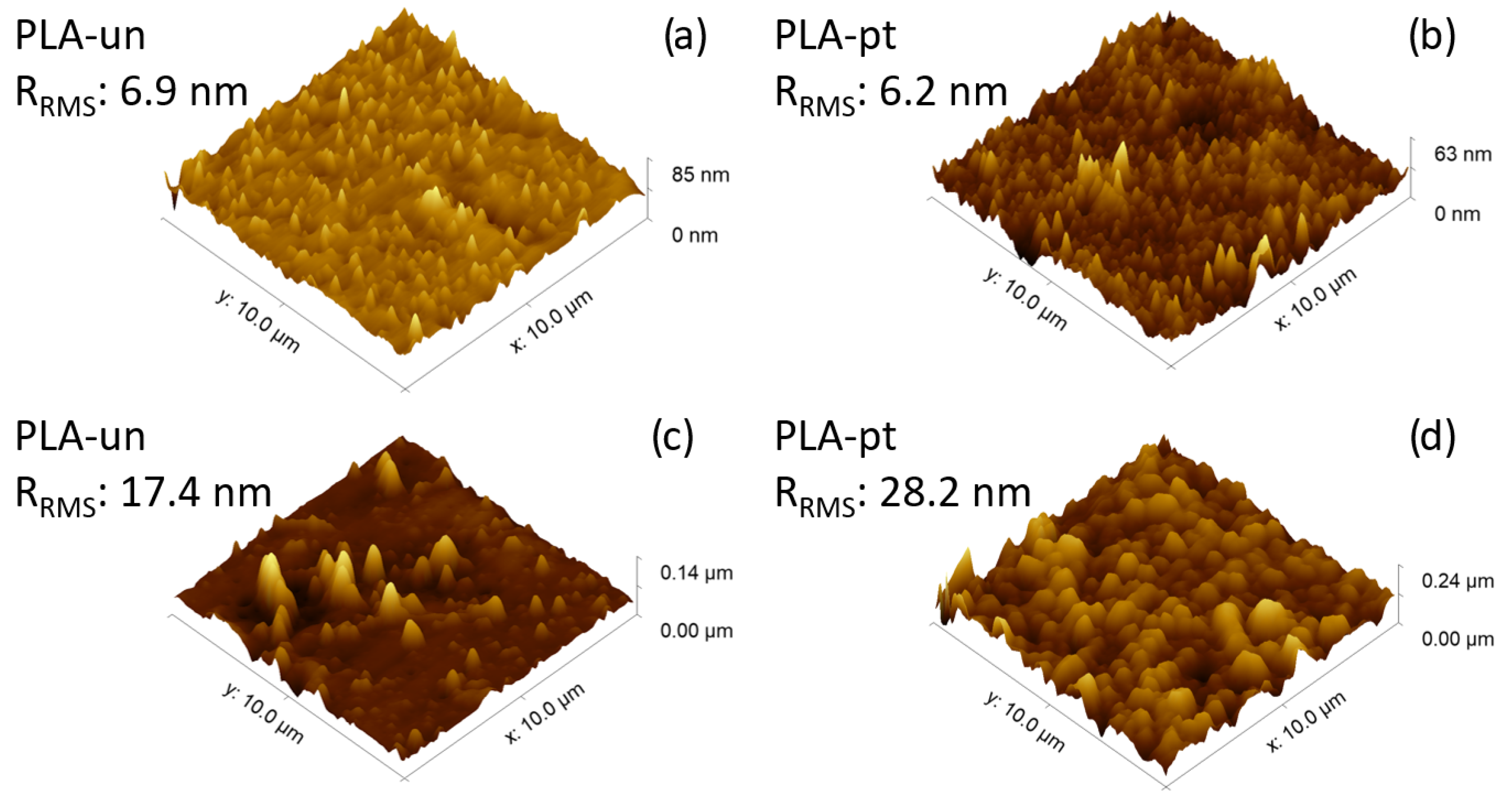
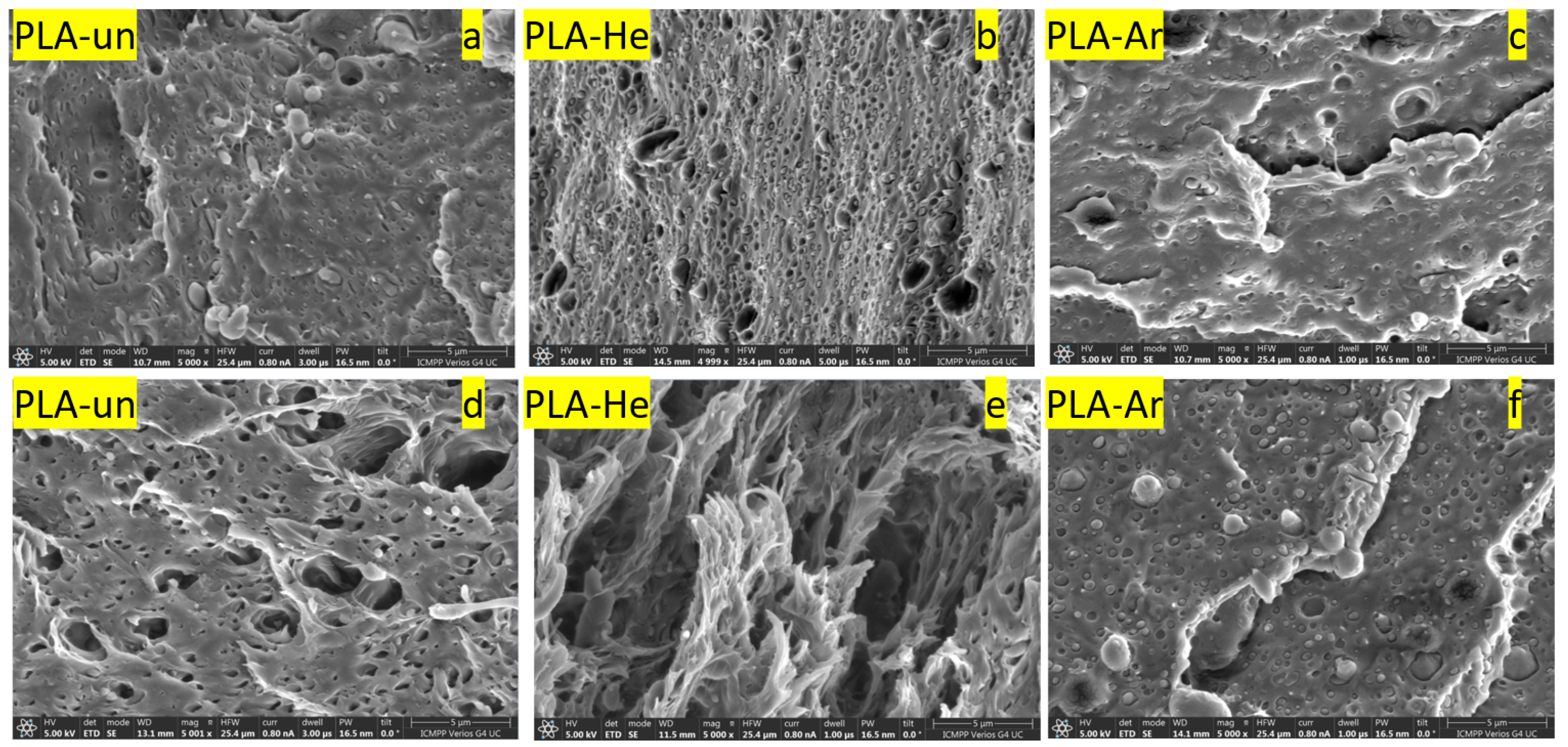
3.2.1. Surface Morphology: AFM and SEM
3.2.2. Static CA and Surface Energy
3.2.3. ATR-FTIR Spectroscopy
3.2.4. Dielectric Properties
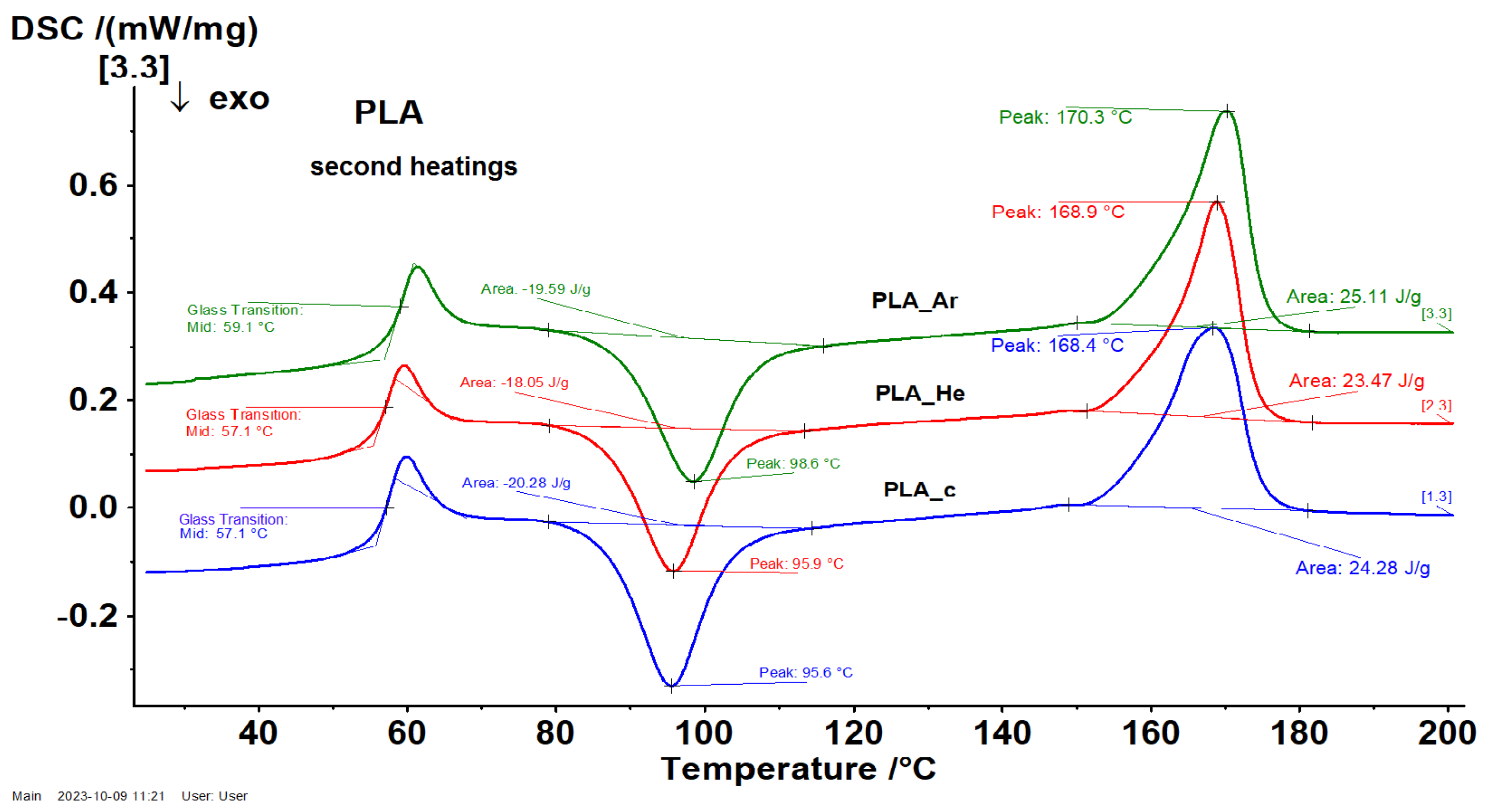
3.2.5. Differential Scanning Calorimetry (DSC)
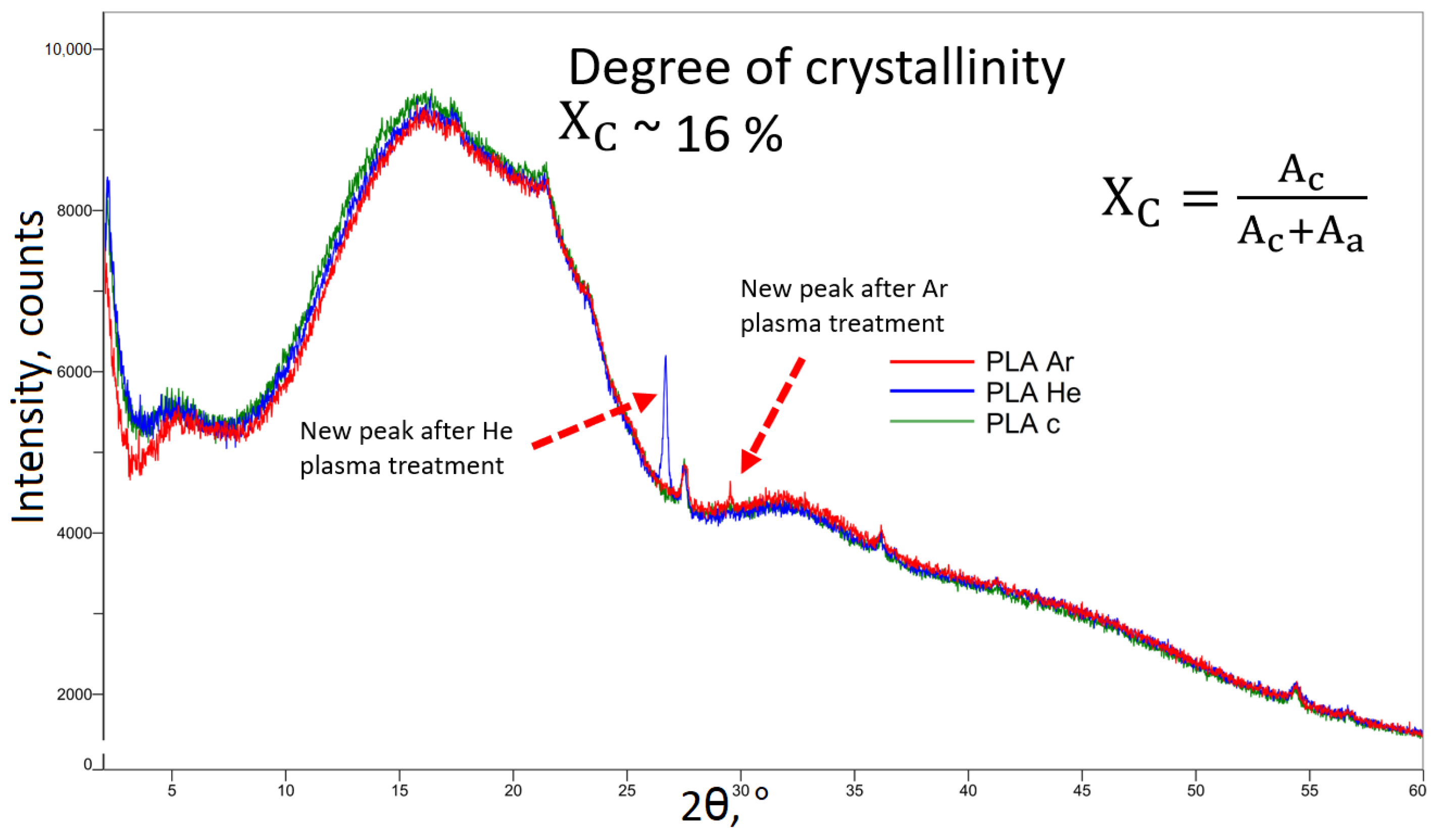
3.2.6. X-ray Diffraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cvelbar, U.; Walsh, J.L.; Černák, M.; de Vries, H.W.; Reuter, S.; Belmonte, T.; Corbella, C.; Miron, C.; Hojnik, N.; Jurov, A.; et al. White paper on the future of plasma science and technology in plastics and textiles. Plasma Process. Polym. 2019, 16, 1700228. [Google Scholar] [CrossRef]
- Bîrleanu, E.; Mihăilă, I.; Topală, I.; Borcia, C.; Borcia, G. Adhesion Properties and Stability of Non-Polar Polymers Treated by Air Atmospheric-Pressure Plasma. Polymers 2023, 15, 2443. [Google Scholar] [CrossRef]
- Chiper, A.S.; Borcia, G. Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers. Polymers 2023, 15, 4172. [Google Scholar] [CrossRef] [PubMed]
- Dufour, T. From basics to frontiers: A comprehensive review of plasma-modified and plasma-synthesized polymer films. Polymers 2023, 15, 3607. [Google Scholar] [CrossRef]
- Kusano, Y. Atmospheric pressure plasma processing for polymer adhesion: A review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lim, W.S.; Kim, D.E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Gogolides, E. Hydrophobic and superhydrophobic surfaces fabricated using atmospheric pressure cold plasma technology: A review. Adv. Colloid Interface Sci. 2018, 254, 1–21. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Peran, J.; Ercegović Ražić, S. Application of atmospheric pressure plasma technology for textile surface modification. Text. Res. J. 2020, 90, 1174–1197. [Google Scholar] [CrossRef]
- Levchenko, I.; Xu, S.; Baranov, O.; Bazaka, O.; Ivanova, E.P.; Bazaka, K. Plasma and polymers: Recent progress and trends. Molecules 2021, 26, 4091. [Google Scholar] [CrossRef]
- Ma, C.; Wang, L.; Nikiforov, A.; Onyshchenko, Y.; Cools, P.; Ostrikov, K.K.; De Geyter, N.; Morent, R. Atmospheric-pressure plasma assisted engineering of polymer surfaces: From high hydrophobicity to superhydrophilicity. Appl. Surf. Sci. 2021, 535, 147032. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kolb, J.F.; Holub, M.; Uhrlandt, D.; Šimek, M.; Ostrikov, K.; Hamaguchi, S.; Cvelbar, U.; Černák, M.; Locke, B.; et al. The future for plasma science and technology. Plasma Process. Polym. 2019, 16, 1800118. [Google Scholar] [CrossRef]
- Booth, J.P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of plasma surface functionalization of polymers for industrial and biological applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Chen, Y.; Zhou, Y.; Wang, S.; Yuan, Z.; Jin, Z.; Liu, X. A review of low-temperature plasma-assisted machining: From mechanism to application. Front. Mech. Eng. 2023, 18, 18. [Google Scholar] [CrossRef]
- Merche, D.; Vandencasteele, N.; Reniers, F. Atmospheric plasmas for thin film deposition: A critical review. Thin Solid Films 2012, 520, 4219–4236. [Google Scholar] [CrossRef]
- Van Deynse, A.; Morent, R.; De Geyter, N. Surface modification of polymers using atmospheric pressure cold plasma technology. In Polymer Science: Research Advances, Pratical Applications and Educational Aspects; Méndez-Vilas, A., Solano, A., Eds.; Formatex Research Center: Badajoz, Spain, 2016; pp. 506–516. [Google Scholar]
- Berckmans, J.; Demaude, A.; Merche, D.; Baert, K.; Terryn, H.; Hauffman, T.; Reniers, F. Revisiting the surface characterization of plasma-modified polymers. Plasma Process. Polym. 2022, 19, 2100169. [Google Scholar] [CrossRef]
- Huzum, R.; Nastuta, A.V. Helium atmospheric pressure plasma jet source treatment of white grapes juice for winemaking. Appl. Sci. 2021, 11, 8498. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Gerling, T. Cold atmospheric pressure plasma jet operated in Ar and He: From basic plasma properties to vacuum ultraviolet, electric field and safety thresholds measurements in plasma medicine. Appl. Sci. 2022, 12, 644. [Google Scholar] [CrossRef]
- Burducea, I.; Burducea, C.; Mereuta, P.E.; Sirbu, S.R.; Iancu, D.A.; Istrati, M.B.; Straticiuc, M.; Lungoci, C.; Stoleru, V.; Teliban, G.C.; et al. Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L. Foods 2023, 12, 208. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Pohoata, V.; Topala, I. Atmospheric pressure plasma jet—Living tissue interface: Electrical, optical, and spectral characterization. J. Appl. Phys. 2013, 113, 183302. [Google Scholar] [CrossRef]
- Nastuta, A.; Topala, I.; Pohoata, V.; Mihaila, I.; Agheorghiesei, C.; Dumitrascu, N. Atmospheric pressure plasma jets in inert gases: Electrical, optical and mass spectrometry diagnosis. Rom. Rep. Phys. 2017, 69, 407. [Google Scholar]
- Nastuta, A.; Popa, G. Surface oxidation and enhanced hydrophilization of polyamide fiber surface after He/Ar atmospheric pressure plasma exposure. Rom. Rep. Phys. 2019, 71, 413. [Google Scholar]
- Grace, J.M.; Gerenser, L.J. Plasma treatment of polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Slepička, P.; Trostová, S.; Slepičková Kasálková, N.; Kolská, Z.; Sajdl, P.; Švorčík, V. Surface modification of biopolymers by argon plasma and thermal treatment. Plasma Process. Polym. 2012, 9, 197–206. [Google Scholar] [CrossRef]
- Packham, D.E. Surface energy, surface topography and adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Faix, O. Fourier Transform Infrared Spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 83–109. [Google Scholar] [CrossRef]
- Naumann, D. FT-infrared and FT-Raman spectroscopy in biomedical research. Appl. Spectrosc. Rev. 2001, 36, 239–298. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C. Surface characterization of plasma-modified polyethylene by contact angle experiments and ATR-FTIR spectroscopy. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Films 2008, 40, 608–611. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS-and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Films 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Blum, M.M.; John, H. Historical perspective and modern applications of attenuated total reflectance—Fourier transform infrared spectroscopy (ATR-FTIR). Drug Test. Anal. 2012, 4, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy, 1st ed.; XXI; Springer: Berlin/Heidelberg, Germany, 2002; 729p. [Google Scholar] [CrossRef]
- Serghei, A.; Lutkenhaus, J.L.; Miranda, D.F.; McEnnis, K.; Kremer, F.; Russell, T.P. Density fluctuations and phase transitions of ferroelectric polymer nanowires. Small 2010, 6, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial interactions, crystallization and molecular mobility in nanocomposites of Poly (lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential Scanning Calorimetry (DSC). In Thermal Analysis of Polymers; Menczel, J., Prime, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Chapter 2; pp. 7–239. [Google Scholar] [CrossRef]
- Guo, Q. (Ed.) Polymer Morphology: Principles, Characterization, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Widjonarko, N.E. Introduction to advanced X-ray diffraction techniques for polymeric thin films. Coatings 2016, 6, 54. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Topala, I.; Grigoras, C.; Pohoata, V.; Popa, G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J. Phys. D Appl. Phys. 2011, 44, 105204. [Google Scholar] [CrossRef]
- Tan, D.K.; Münzenrieder, N.; Maniruzzaman, M.; Nokhodchi, A. A low-cost method to prepare biocompatible filaments with enhanced physico-mechanical properties for FDM 3D printing. Curr. Drug Deliv. 2021, 18, 700–711. [Google Scholar] [CrossRef]
- Zarei, M.; Shabani Dargah, M.; Hasanzadeh Azar, M.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Kaviani, A.; Asadollahi, M.; Azami, M.; Beheshtizadeh, N. Enhanced bone tissue regeneration using a 3D-printed poly (lactic acid)/Ti6Al4V composite scaffold with plasma treatment modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Sayedain, S.S.; Askarinya, A.; Sabbaghi, M.; Alizadeh, R. Improving physio-mechanical and biological properties of 3D-printed PLA scaffolds via in-situ argon cold plasma treatment. Sci. Rep. 2023, 13, 14120. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, N.; Ly, O. Effect of microstructure on hydrolytic degradation studies of poly (l-lactic acid) by FTIR spectroscopy and differential scanning calorimetry. Polym. Degrad. Stab. 2009, 94, 1364–1372. [Google Scholar] [CrossRef]
- Bronnikov, S.; Kostromin, S.; Musteaţa, V.; Cozan, V. Polyazomethine with m-tolylazo side groups: Thermal, dielectric and conductive behaviour. Liq. Cryst. 2015, 42, 1102–1110. [Google Scholar] [CrossRef]
- Wei, X.F.; Bao, R.Y.; Cao, Z.Q.; Yang, W.; Xie, B.H.; Yang, M.B. Stereocomplex crystallite network in asymmetric PLLA/PDLA blends: Formation, structure, and confining effect on the crystallization rate of homocrystallites. Macromolecules 2014, 47, 1439–1448. [Google Scholar] [CrossRef]


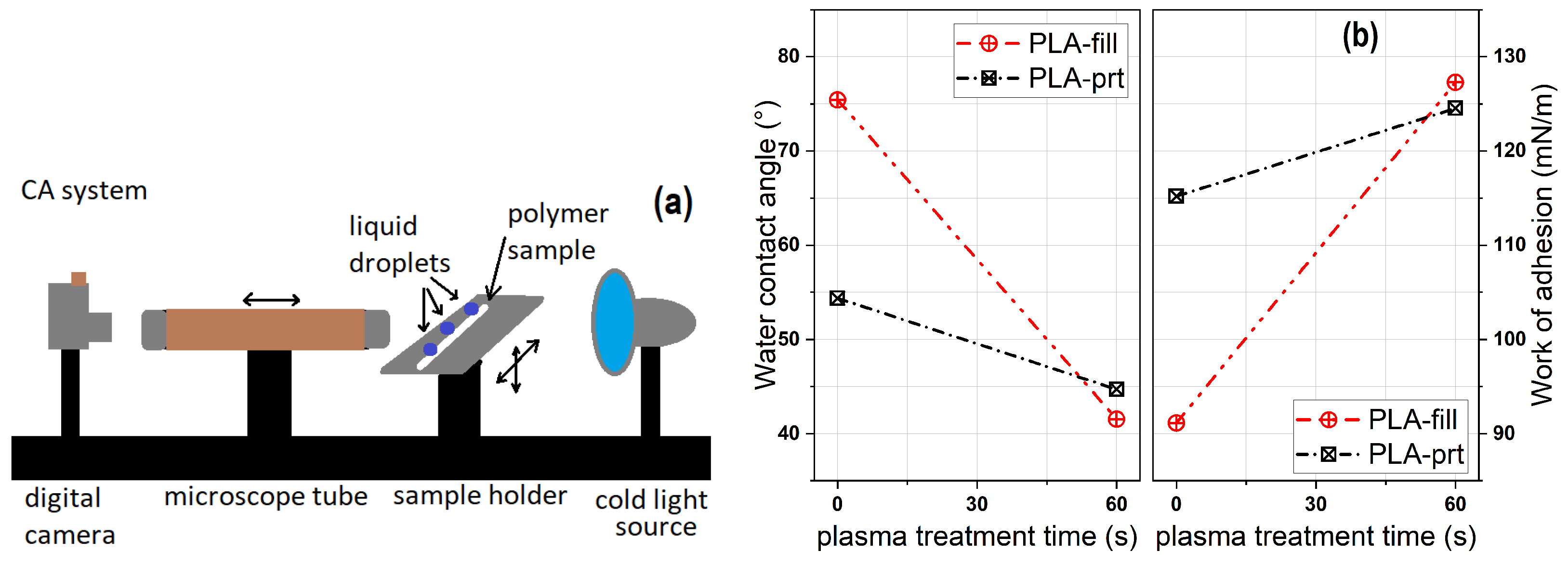

| Contact Angle | Work of Adhesion | Surface Free Energy | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (°) | () | () | (%) | (%) | ||||||
| Sample | Plasma ON (s) | Distilled Water | Glycerol | -Water | -Glycerol | / | / | |||
| PLA-c-fill 3 | 0 | 75 | 77 | 91.13 | 77.34 | 30.80 | 2.73 | 28.06 | 0.088 | 0.911 |
| PLA-He-fill | 60 | 41 | 55 | 127.29 | 100.66 | 72.21 | 0.45 | 71.75 | 0.006 | 0.993 |
| PLA-Ar-fill | 60 | 46 | 64 | 107.78 | 91.62 | 36.03 | 3.19 | 32.83 | 0.087 | 0.911 |
| PLA-c-prt 4 | 0 | 54 | 58 | 115.20 | 97.13 | 38.93 | 3.45 | 35.47 | 0.088 | 0.911 |
| PLA-He-prt | 60 | 44 | 48 | 124.53 | 106.32 | 70.64 | 0.44 | 70.19 | 0.006 | 0.993 |
| PLA-Ar-prt | 60 | 50 | 53 | 118.90 | 102.53 | 40.18 | 3.56 | 36.60 | 0.088 | 0.910 |
| Sample | BDS Measurements | ||
|---|---|---|---|
| (r.t. and f = 1 kHz) | (°C) | (°C) | |
| 5.4 | 52.2 | 166.8 | |
| 5.5 | 52.8 | 167.5 | |
| 5.5 | 52.0 | 166.3 | |
| Sample | (°C) | (°C) | (°C) | |
|---|---|---|---|---|
| 1 | 2 | |||
| 51.6 | 103.7 | 148.7 | 164.9 | |
| 53.6 | 110.1 | 151.3 | 166.6 | |
| 54.7 | 112.3 | 152.3 | 167.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastuta, A.V.; Asandulesa, M.; Doroftei, F.; Dascalu, I.-A.; Varganici, C.-D.; Tiron, V.; Topala, I. Atmospheric Pressure Plasma Jet Exposure of Polylactic Acid Surfaces for Better Adhesion: Plasma Parameters towards Polymer Properties. Polymers 2024, 16, 240. https://doi.org/10.3390/polym16020240
Nastuta AV, Asandulesa M, Doroftei F, Dascalu I-A, Varganici C-D, Tiron V, Topala I. Atmospheric Pressure Plasma Jet Exposure of Polylactic Acid Surfaces for Better Adhesion: Plasma Parameters towards Polymer Properties. Polymers. 2024; 16(2):240. https://doi.org/10.3390/polym16020240
Chicago/Turabian StyleNastuta, Andrei Vasile, Mihai Asandulesa, Florica Doroftei, Ioan-Andrei Dascalu, Cristian-Dragos Varganici, Vasile Tiron, and Ionut Topala. 2024. "Atmospheric Pressure Plasma Jet Exposure of Polylactic Acid Surfaces for Better Adhesion: Plasma Parameters towards Polymer Properties" Polymers 16, no. 2: 240. https://doi.org/10.3390/polym16020240
APA StyleNastuta, A. V., Asandulesa, M., Doroftei, F., Dascalu, I.-A., Varganici, C.-D., Tiron, V., & Topala, I. (2024). Atmospheric Pressure Plasma Jet Exposure of Polylactic Acid Surfaces for Better Adhesion: Plasma Parameters towards Polymer Properties. Polymers, 16(2), 240. https://doi.org/10.3390/polym16020240











