Controlling the Architecture of Freeze-Dried Collagen Scaffolds with Ultrasound-Induced Nucleation
Abstract
1. Introduction
2. Materials and Methods
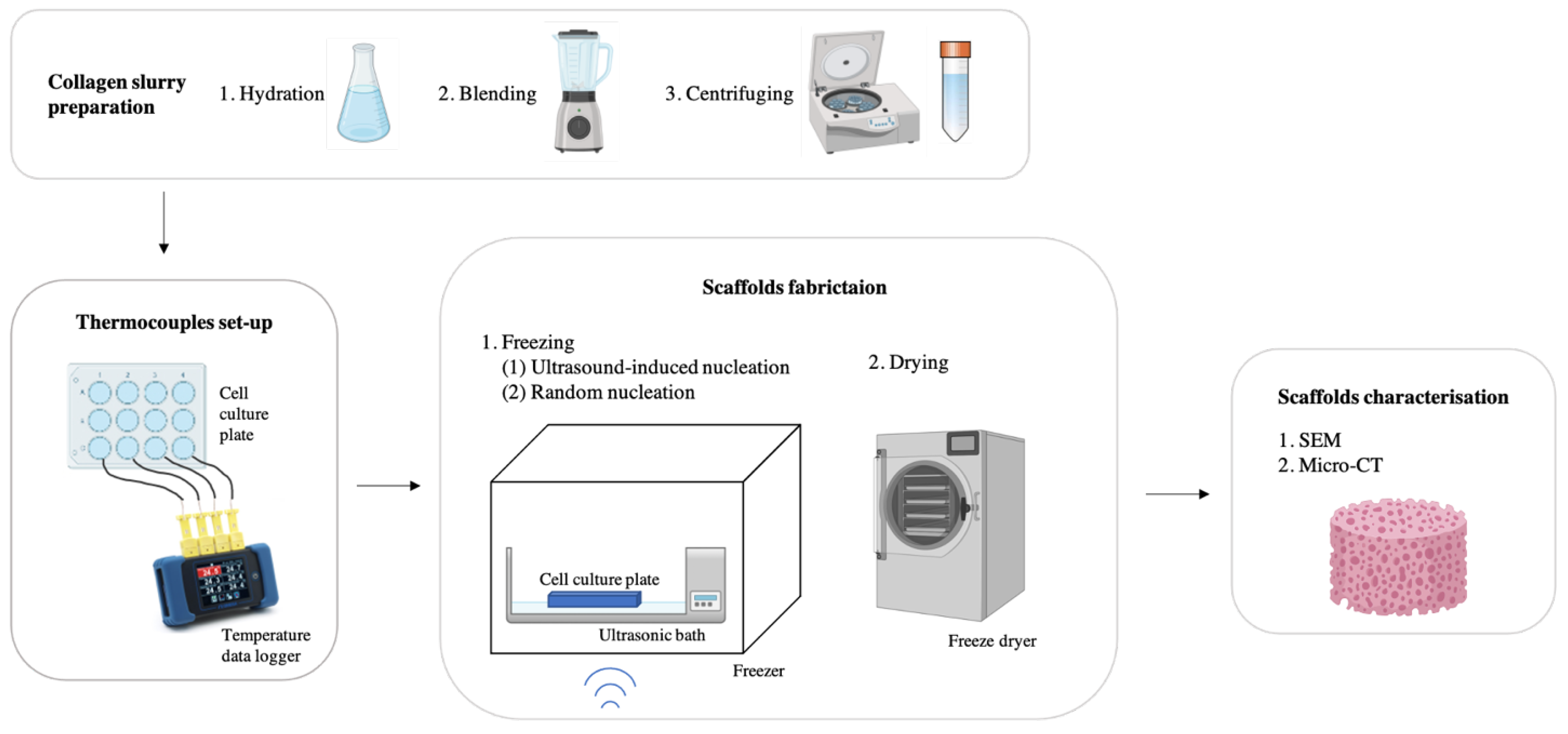
2.1. Collagen Slurry Preparation and Thermocouples Set-Up
2.2. Random Nucleation of Ice
2.3. Scaffold Fabrication
2.4. Scanning Electron Microscopy (SEM)
2.5. Micro-Computed Tomography (Micro-CT)
3. Results
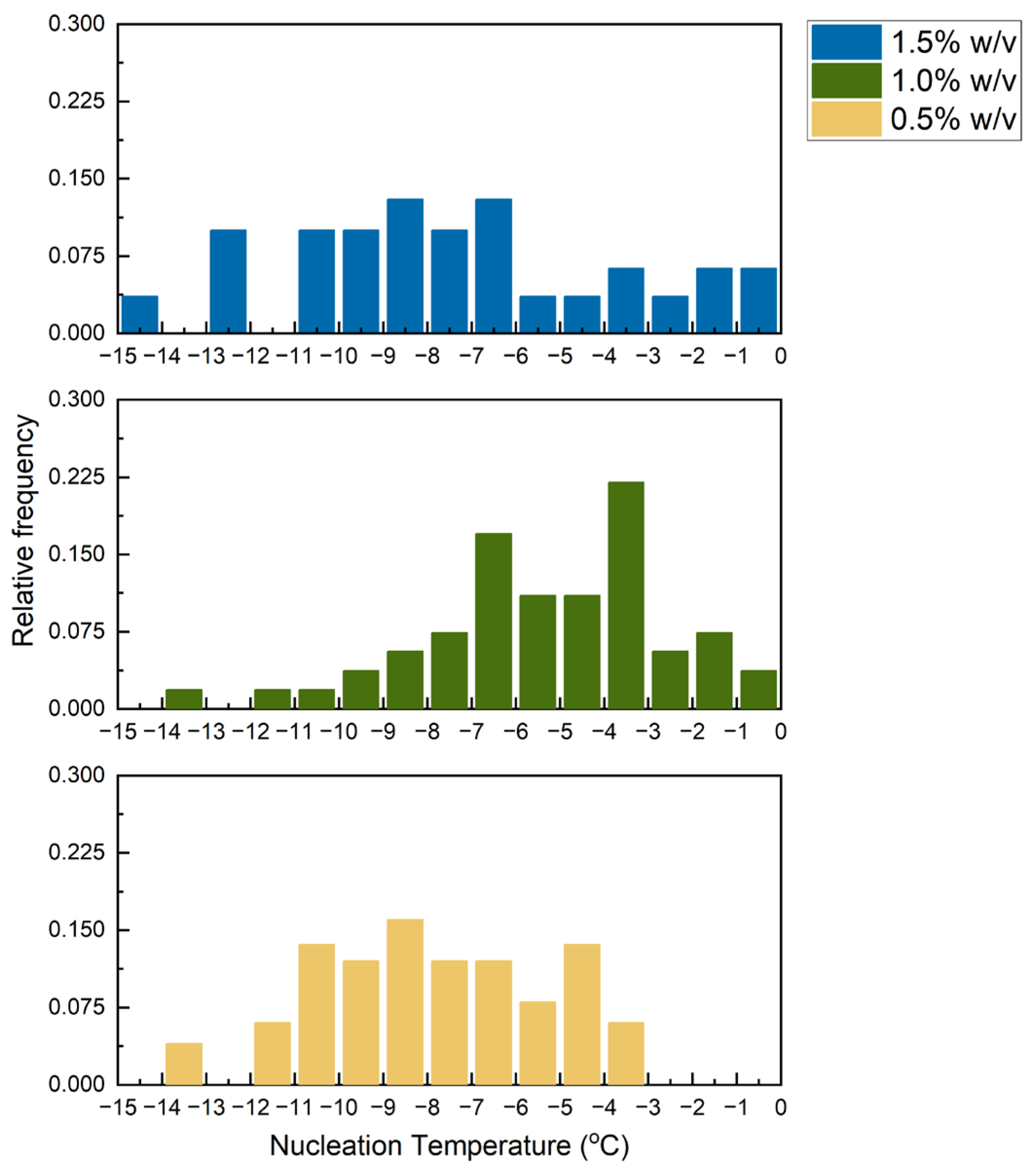
3.1. Nucleation Temperature Distribution in Collagen Slurry
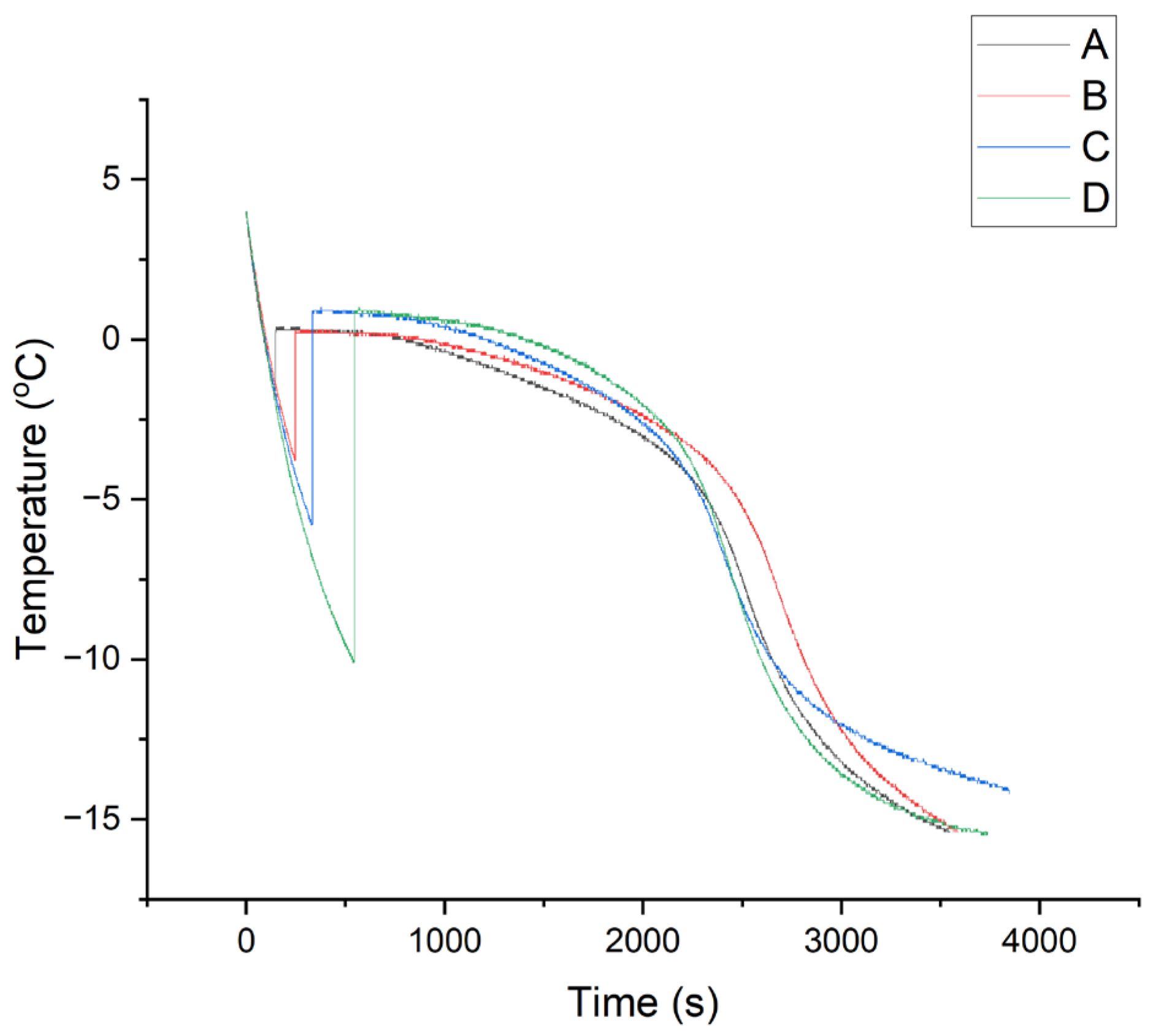
3.2. Thermal Profile of Freezing Collagen Slurry
3.3. Scaffold Architecture Visualisation
3.4. Relating Scaffold Architecture to Thermal Parameters
4. Discussion
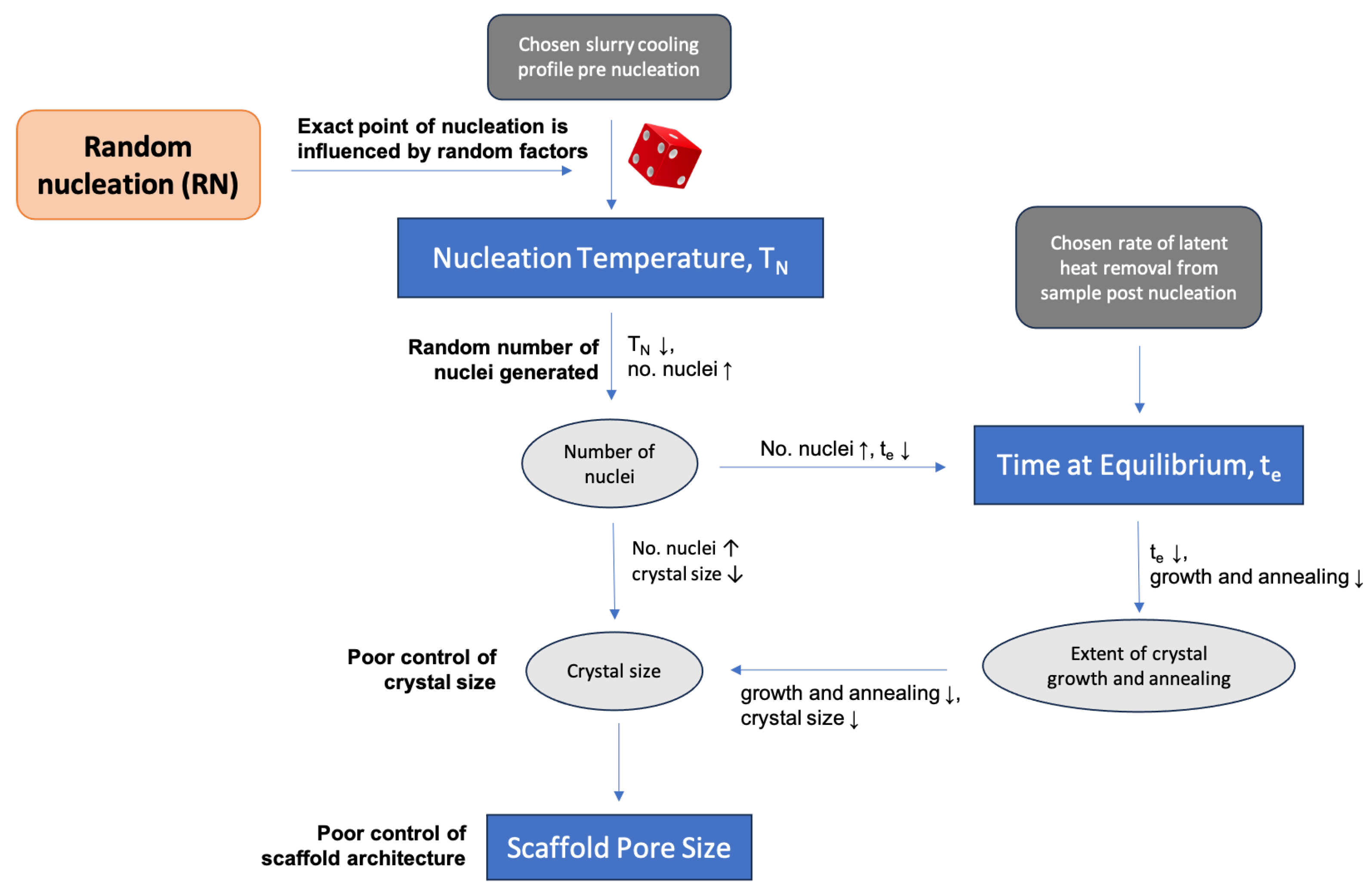
4.1. Stochastic Nature of Ice Nucleation
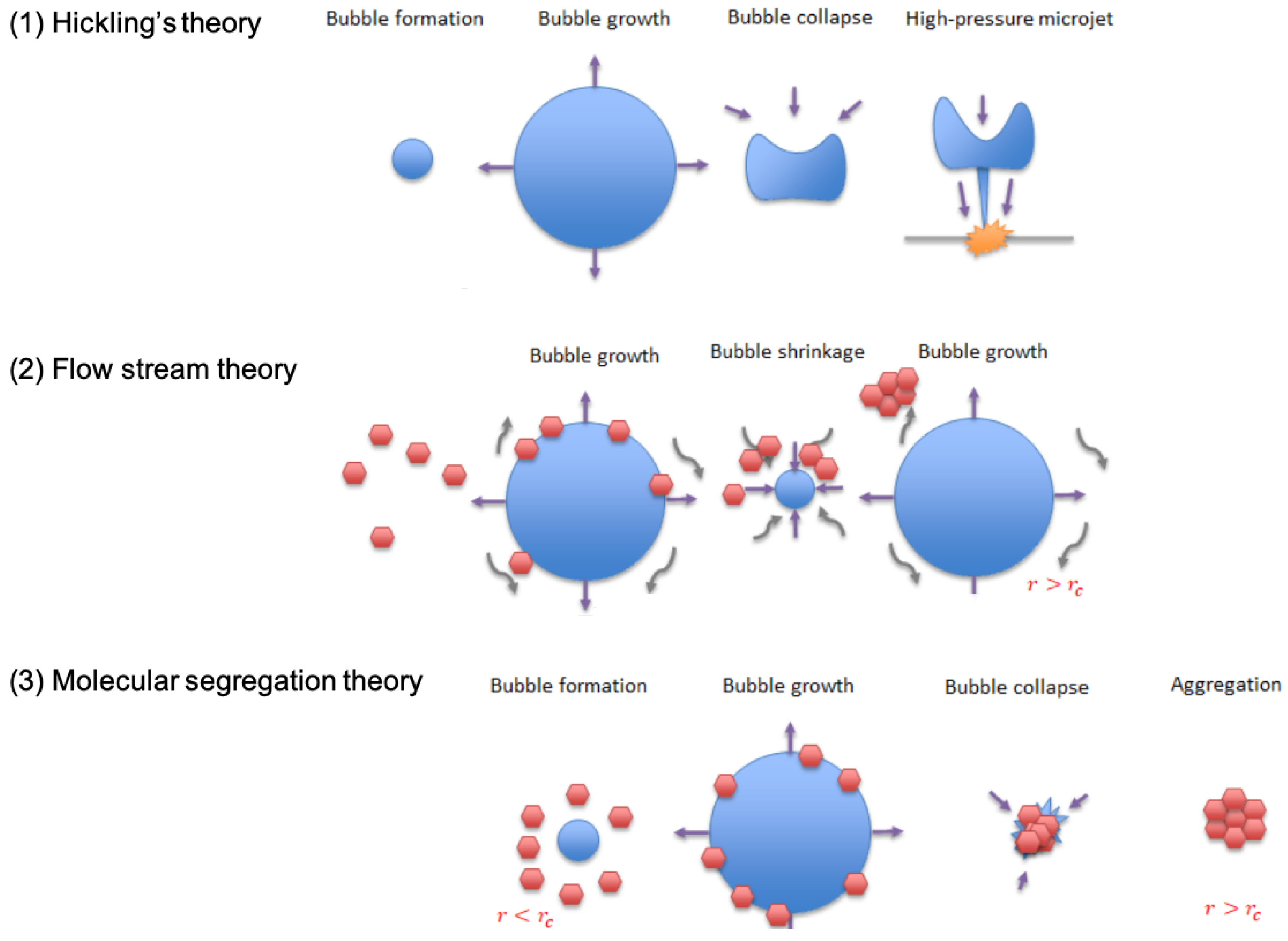
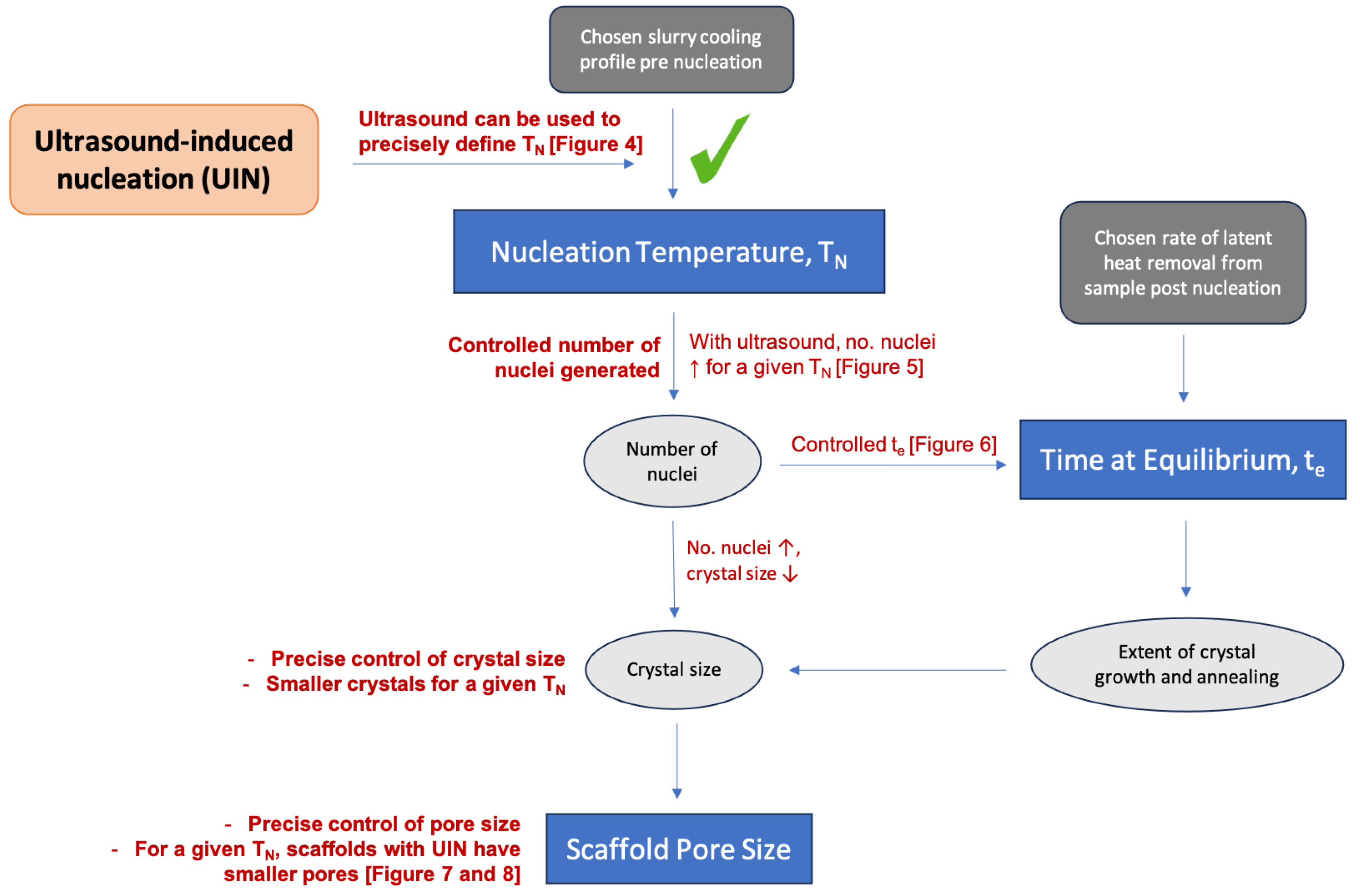
4.2. Ultrasound-Triggered Nucleation
4.3. Ultrasound-Induced Nucleation and Random Nucleation: Correlating Thermal Parameters to Scaffold Architecture
4.3.1. Pore Morphology
4.3.2. Time at Equilibrium against Nucleation Temperature
4.3.3. Pore Size against Nucleation Temperature and Time at Equilibrium
4.3.4. Pore Size Distribution
4.3.5. Degree of Anisotropy against Nucleation Temperature
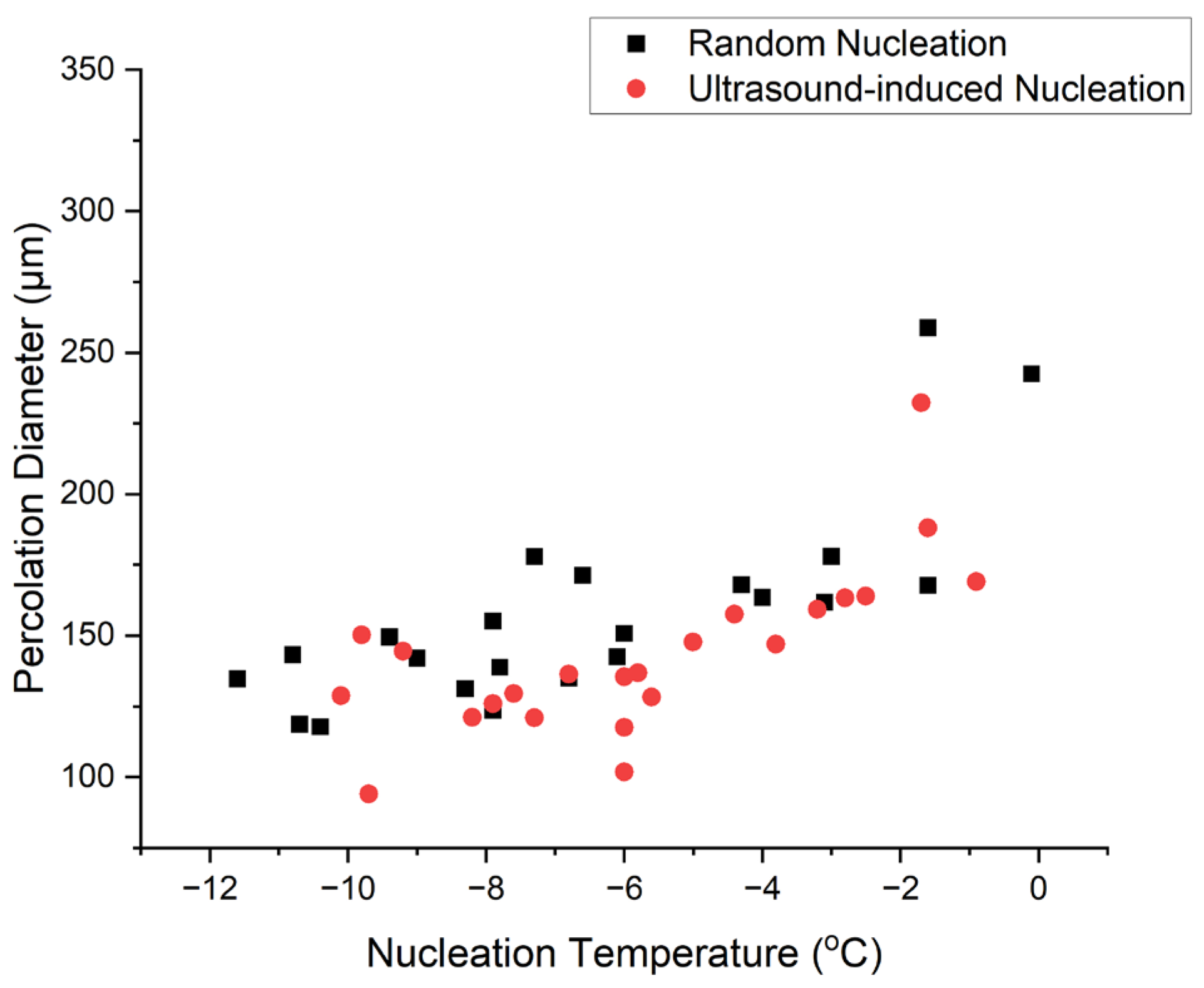
4.3.6. Percolation Diameter against Nucleation Temperature
4.4. Impact of Ultrasound-Trigged Nucleation on Ice Solidification and Final Scaffold Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Schecter, M.; Donius, A.E.; Hunger, P.M. Biomaterials by freeze casting. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2099–2121. [Google Scholar] [CrossRef] [PubMed]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. Ice-templated structures for biomedical tissue repair: From physics to final scaffolds. Appl. Phys. Rev. 2014, 1, 021301. [Google Scholar] [CrossRef]
- Elliott, R. Solidification of cast irons. In Cast Iron Technology; Elsevier: Amsterdam, The Netherlands, 1988; pp. 91–125. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, S.; Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature 2002, 416, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Komal, P.; Gautam, P.; Sharma, A.; Kumar, N.; Jung, J.P. Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications. Metals 2021, 11, 329. [Google Scholar] [CrossRef]
- Akyurt, M.; Zaki, G.; Habeebullah, B. Freezing phenomena in ice–water systems. Energy Convers. Manag. 2002, 43, 1773–1789. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Wilson, P.W.; Haymet, A.D.J. Heterogeneous nucleation of supercooled water, and the effect of an added catalyst. Proc. Natl. Acad. Sci. USA 2002, 99, 9631–9634. [Google Scholar] [CrossRef]
- Salas, G.; Costo, R.; del Puerto Morales, M. Synthesis of Inorganic Nanoparticles. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2012; pp. 35–79. [Google Scholar] [CrossRef]
- Pawelec, K.; Husmann, A.; Best, S.; Cameron, R. Understanding anisotropy and architecture in ice-templated biopolymer scaffolds. Mater. Sci. Eng. C 2014, 37, 141–147. [Google Scholar] [CrossRef]
- Deville, S.; Maire, E.; Lasalle, A.; Bogner, A.; Gauthier, C.; Leloup, J.; Guizard, C. Influence of Particle Size on Ice Nucleation and Growth during the Ice-Templating Process: Rapid Communications of the American Ceramic Society. J. Am. Ceram. Soc. 2010, 93, 2507–2510. [Google Scholar] [CrossRef]
- Lasalle, A.; Guizard, C.; Leloup, J.; Deville, S.; Maire, E.; Bogner, A.; Gauthier, C.; Adrien, J.; Courtois, L. Ice-Templating of Alumina Suspensions: Effect of Supercooling and Crystal Growth During the Initial Freezing Regime. J. Am. Ceram. Soc. 2011, 95, 799–804. [Google Scholar] [CrossRef]
- Hindmarsh, J.; Russell, A.; Chen, X. Measuring dendritic growth in undercooled sucrose solution droplets. J. Cryst. Growth 2005, 285, 236–248. [Google Scholar] [CrossRef]
- Hallett, J. Experimental Studies of the Crystallization of Supercooled Water. J. Atmos. Sci. 1964, 21, 671–682. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. A design protocol for tailoring ice-templated scaffold structure. J. R. Soc. Interface 2014, 11, 20130958. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.; Zhang, Z.; Delgado, A.; Sun, D.-W. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res. Int. 2011, 44, 2915–2921. [Google Scholar] [CrossRef]
- Wilson, P.; Heneghan, A.; Haymet, A. Ice nucleation in nature: Supercooling point (SCP) measurements and the role of heterogeneous nucleation. Cryobiology 2003, 46, 88–98. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hottot, A.; Vessot, S.; Andrieu, J. Influence of controlled nucleation by ultrasounds on ice morphology of frozen formulations for pharmaceutical proteins freeze-drying. Chem. Eng. Process.-Process Intensif. 2006, 45, 783–791. [Google Scholar] [CrossRef]
- Candoni, N.; Hammadi, Z.; Grossier, R.; Ildefonso, M.; Zhang, S.; Morin, R.; Veesler, S. Addressing the Stochasticity of Nucleation: Practical Approaches. In Advances in Organic Crystal Chemistry; Tamura, R., Miyata, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 95–113. [Google Scholar] [CrossRef]
- Barber, E.R.; Ward, M.R.; Ward, A.D.; Alexander, A.J. Laser-induced nucleation promotes crystal growth of anhydrous sodium bromide. CrystEngComm 2021, 23, 8451–8461. [Google Scholar] [CrossRef]
- Allahyarov, E.; Sandomirski, K.; Egelhaaf, S.; Löwen, H. Crystallization seeds favour crystallization only during initial growth. Nat. Commun. 2015, 6, 7110. [Google Scholar] [CrossRef]
- Saclier, M.; Peczalski, R.; Andrieu, J. Effect of ultrasonically induced nucleation on ice crystals’ size and shape during freezing in vials. Chem. Eng. Sci. 2010, 65, 3064–3071. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Xanthakis, E.; Le-Bail, A. Review on the control of ice nucleation by ultrasound waves, electric and magnetic fields. J. Food Eng. 2017, 195, 222–234. [Google Scholar] [CrossRef]
- Leong, T.; Ashokkumar, M.; Kentish, S. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- Hickling, R. Nucleation of Freezing by Cavity Collapse and its Relation to Cavitation Damage. Nature 1965, 206, 915–917. [Google Scholar] [CrossRef]
- Feng, H.; Barbosa-Canovas, G.; Weiss, J. (Eds.) Ultrasound Technologies for Food and Bioprocessing. In Food Engineering Series; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Morey, M.D.; Deshpande, N.S.; Barigou, M. Foam Destabilization by Mechanical and Ultrasonic Vibrations. J. Colloid Interface Sci. 1999, 219, 90–98. [Google Scholar] [CrossRef]
- Ahmed, S.E.; Martins, A.M.; Husseini, G.A. The use of ultrasound to release chemotherapeutic drugs from micelles and liposomes. J. Drug Target. 2014, 23, 16–42. [Google Scholar] [CrossRef]
- Yasui, K. Influence of ultrasonic frequency on multibubble sonoluminescence. J. Acoust. Soc. Am. 2002, 112, 1405–1413. [Google Scholar] [CrossRef]
- Inada, T.; Zhang, X.; Yabe, A.; Kozawa, Y. Active control of phase change from supercooled water to ice by ultrasonic vibration 1. Control of freezing temperature. Int. J. Heat Mass Transf. 2001, 44, 4523–4531. [Google Scholar] [CrossRef]
- Chow, R.; Blindt, R.; Chivers, R.; Povey, M. The sonocrystallisation of ice in sucrose solutions: Primary and secondary nucleation. Ultrasonics 2003, 41, 595–604. [Google Scholar] [CrossRef]
- Zhang, X.; Inada, T.; Tezuka, A. Ultrasonic-induced nucleation of ice in water containing air bubbles. Ultrason. Sonochem. 2002, 10, 71–76. [Google Scholar] [CrossRef]
- Dodds, J.; Espitalier, F.; Louisnard, O.; Grossier, R.; David, R.; Hassoun, M.; Baillon, F.; Gatumel, C.; Lyczko, N. The Effect of Ultrasound on Crystallisation-Precipitation Processes: Some Examples and a New Segregation Model. Part. Part. Syst. Charact. 2007, 24, 18–28. [Google Scholar] [CrossRef]
- Dalecki, D.; Hocking, D.C. Ultrasound Technologies for Biomaterials Fabrication and Imaging. Ann. Biomed. Eng. 2014, 43, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.; Johal, R.; Glover, Z.; Reinwald, Y.; White, L.; Ghaemmaghami, A.; Morgan, S.; Rose, F.; Povey, M.; Parker, N. Post-processing of polymer foam tissue scaffolds with high power ultrasound: A route to increased pore interconnectivity, pore size and fluid transport. Mater. Sci. Eng. C 2013, 33, 4825–4832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sirsi, S.R.; Borden, M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lv, J.-B.; Tan, W.; He, X.; Chen, M.-H.; Zeng, K.; Hu, J.-H.; Yang, G. Ultrasound-assisted freeze-drying process for polyimide aerogels. Chem. Eng. J. 2022, 450, 138344. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.; Sun, D.-W. Effect of ultrasound irradiation on ice crystal size distribution in frozen agar gel samples. Innov. Food Sci. Emerg. Technol. 2013, 18, 126–131. [Google Scholar] [CrossRef]
- Offeddu, G.; Ashworth, J.; Cameron, R.; Oyen, M. Structural determinants of hydration, mechanics and fluid flow in freeze-dried collagen scaffolds. Acta Biomater. 2016, 41, 193–203. [Google Scholar] [CrossRef]
- Meek, M.C.; Best, S.; Cameron, R. The effects of despeckling filters on pore size measurements in collagen scaffold micro-CT data. J. Microsc. 2021, 284, 142–156. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; White, D.L.; Genant, H.K. Micro CT and Micro MR imaging of 3D architecture of animal skeleton. J. Musculoskelet. Neuronal Interact. 2000, 1, 45–51. [Google Scholar]
- Ashworth, J.C.; Mehr, M.; Buxton, P.G.; Best, S.M.; Cameron, R.E. Cell Invasion in Collagen Scaffold Architectures Characterized by Percolation Theory. Adv. Healthc. Mater. 2015, 4, 1317–1321. [Google Scholar] [CrossRef]
- Nair, M.; Shepherd, J.H.; Best, S.M.; Cameron, R.E. MicroCT analysis of connectivity in porous structures: Optimizing data acquisition and analytical methods in the context of tissue engineering. J. R. Soc. Interface 2020, 17, 20190833. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, M.; Toramaru, A. Inclusion of Viscosity Into Classical Homogeneous Nucleation Theory for Water Bubbles in Silicate Melts: Reexamination of Bubble Number Density in Ascending Magmas. J. Geophys. Res. Solid Earth 2019, 124, 8250–8266. [Google Scholar] [CrossRef]
- Grossier, R.; Louisnard, O.; Vargas, Y. Mixture segregation by an inertial cavitation bubble. Ultrason. Sonochem. 2007, 14, 431–437. [Google Scholar] [CrossRef][Green Version]
- Shu, Q.; Li, Q.; Medeiros, S.L.S.; Klug, J.L. Development of Non-reactive F-Free Mold Fluxes for High Aluminum Steels: Non-isothermal Crystallization Kinetics for Devitrification. Met. Mater. Trans. B 2020, 51, 1169–1180. [Google Scholar] [CrossRef]
- Fang, L.; Gao, Z.; Wu, S.; Jia, S.; Wang, J.; Rohani, S.; Gong, J. Ultrasound-assisted solution crystallization of fotagliptin benzoate: Process intensification and crystal product optimization. Ultrason. Sonochem. 2021, 76, 105634. [Google Scholar] [CrossRef]
- De Castro, M.L.D.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Philpott, M.A.; Best, S.M.; Cameron, R.E. Controlling the Architecture of Freeze-Dried Collagen Scaffolds with Ultrasound-Induced Nucleation. Polymers 2024, 16, 213. https://doi.org/10.3390/polym16020213
Song X, Philpott MA, Best SM, Cameron RE. Controlling the Architecture of Freeze-Dried Collagen Scaffolds with Ultrasound-Induced Nucleation. Polymers. 2024; 16(2):213. https://doi.org/10.3390/polym16020213
Chicago/Turabian StyleSong, Xinyuan, Matthew A. Philpott, Serena M. Best, and Ruth E. Cameron. 2024. "Controlling the Architecture of Freeze-Dried Collagen Scaffolds with Ultrasound-Induced Nucleation" Polymers 16, no. 2: 213. https://doi.org/10.3390/polym16020213
APA StyleSong, X., Philpott, M. A., Best, S. M., & Cameron, R. E. (2024). Controlling the Architecture of Freeze-Dried Collagen Scaffolds with Ultrasound-Induced Nucleation. Polymers, 16(2), 213. https://doi.org/10.3390/polym16020213








