In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of ZBG Powder
2.2. Electrospinning and Characterization of Electrospun ZBG/PCL Bioscaffolds
3. Results and Discussion
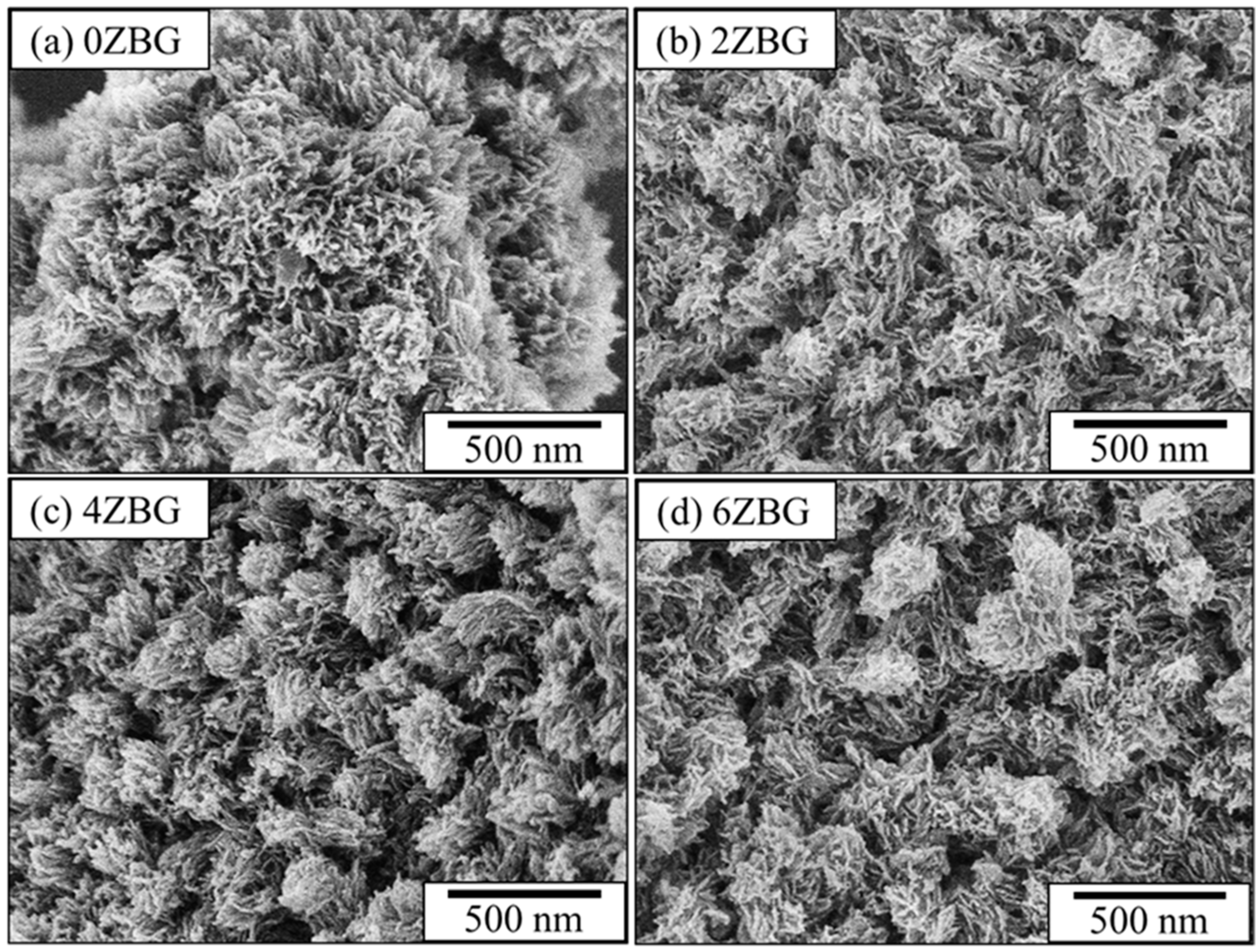
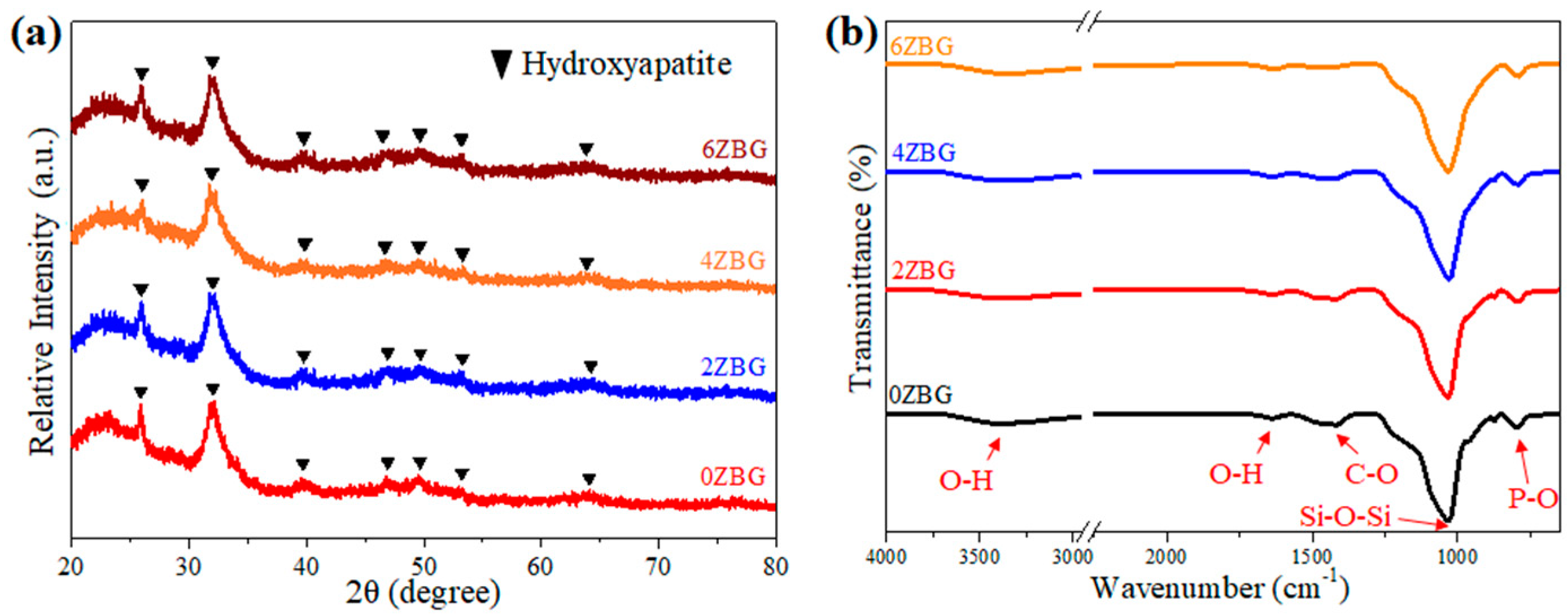
3.1. Characteristics of ZBG Powder
3.2. Evaluation of Electrospun ZBG/PCL Bioscaffold
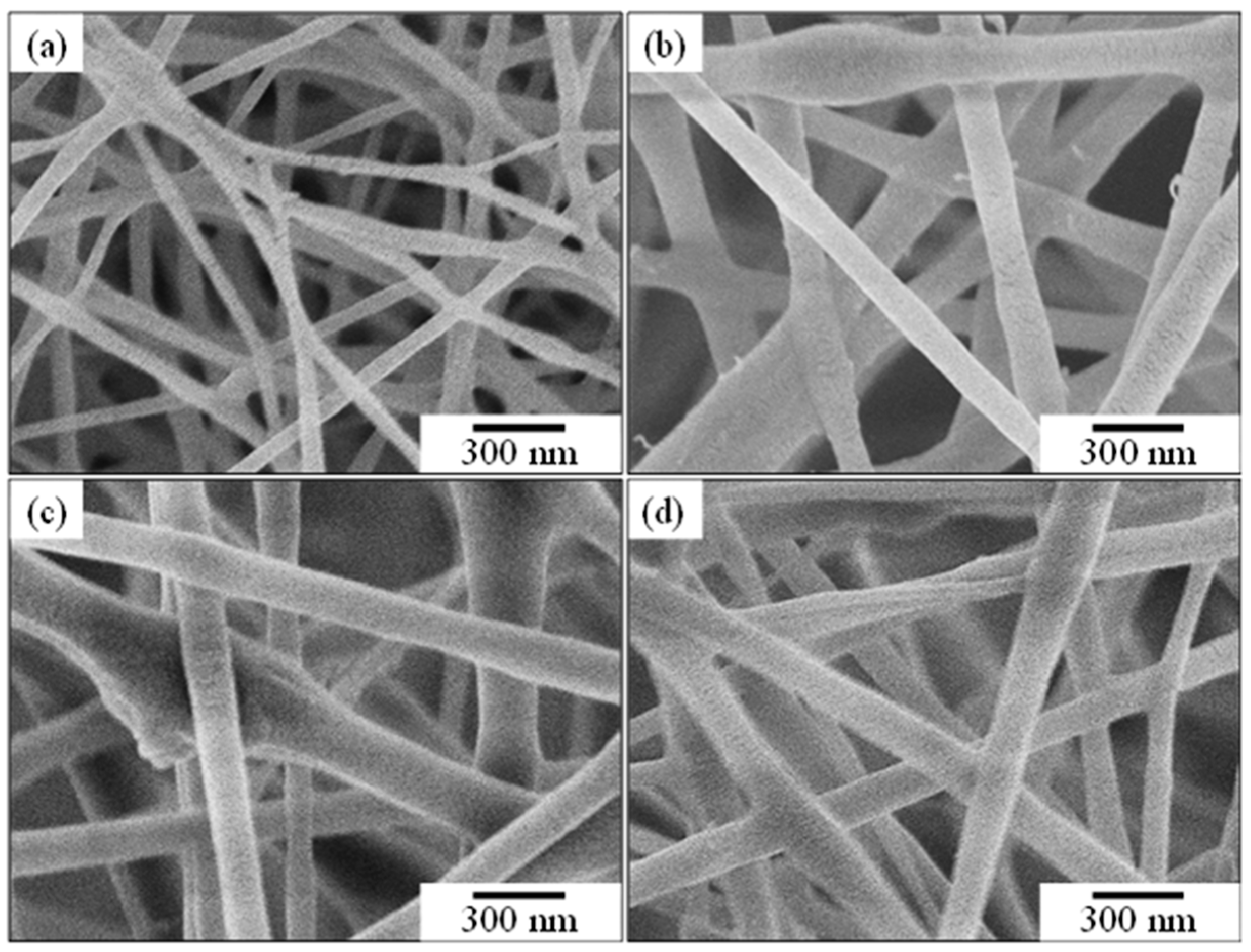
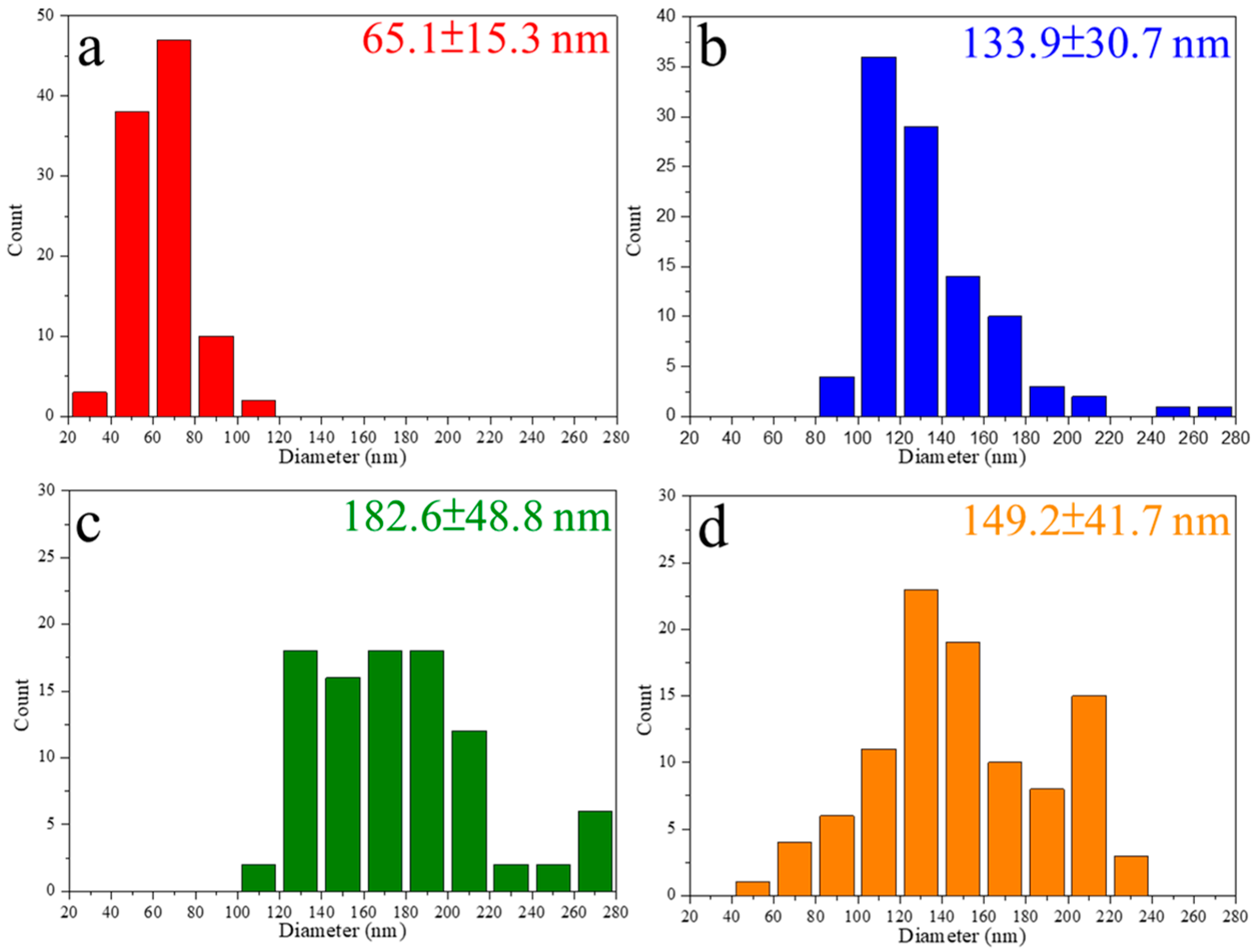
3.2.1. Effect of ZBG Powder Addition
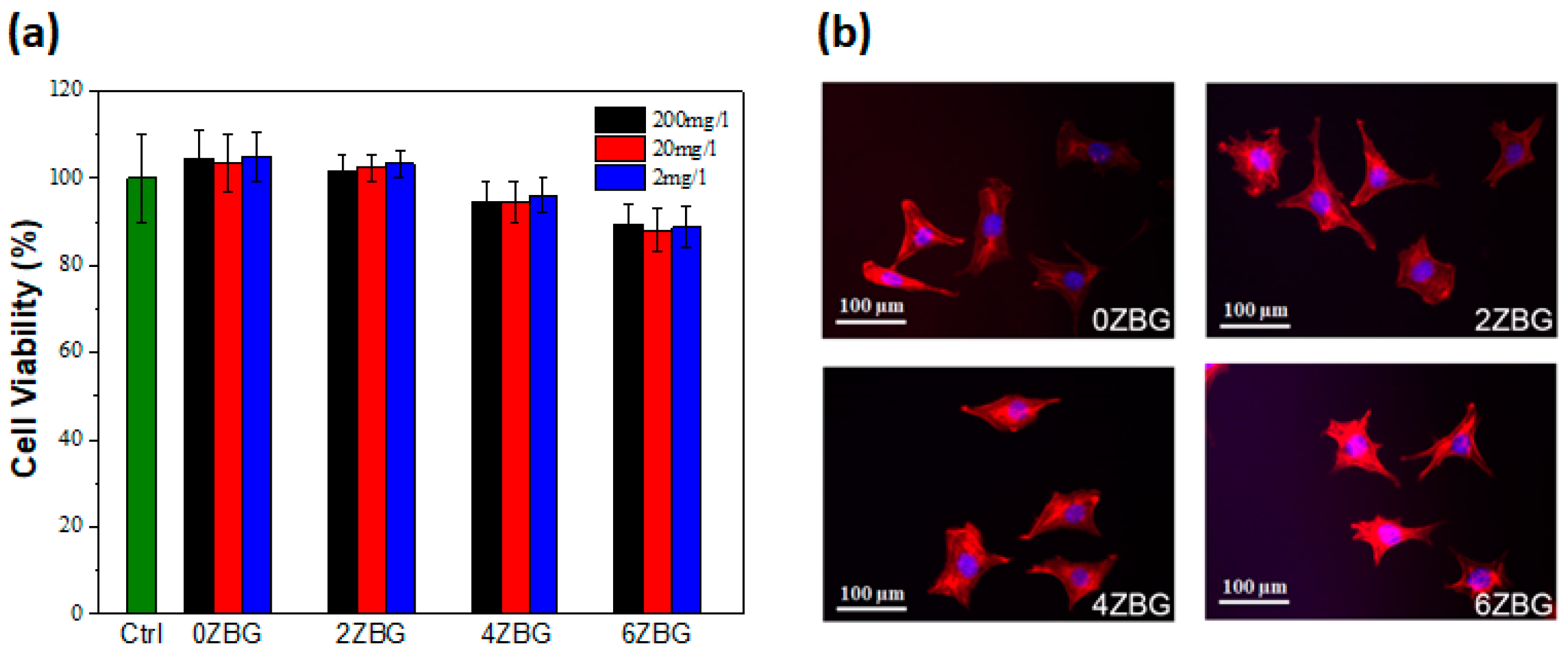
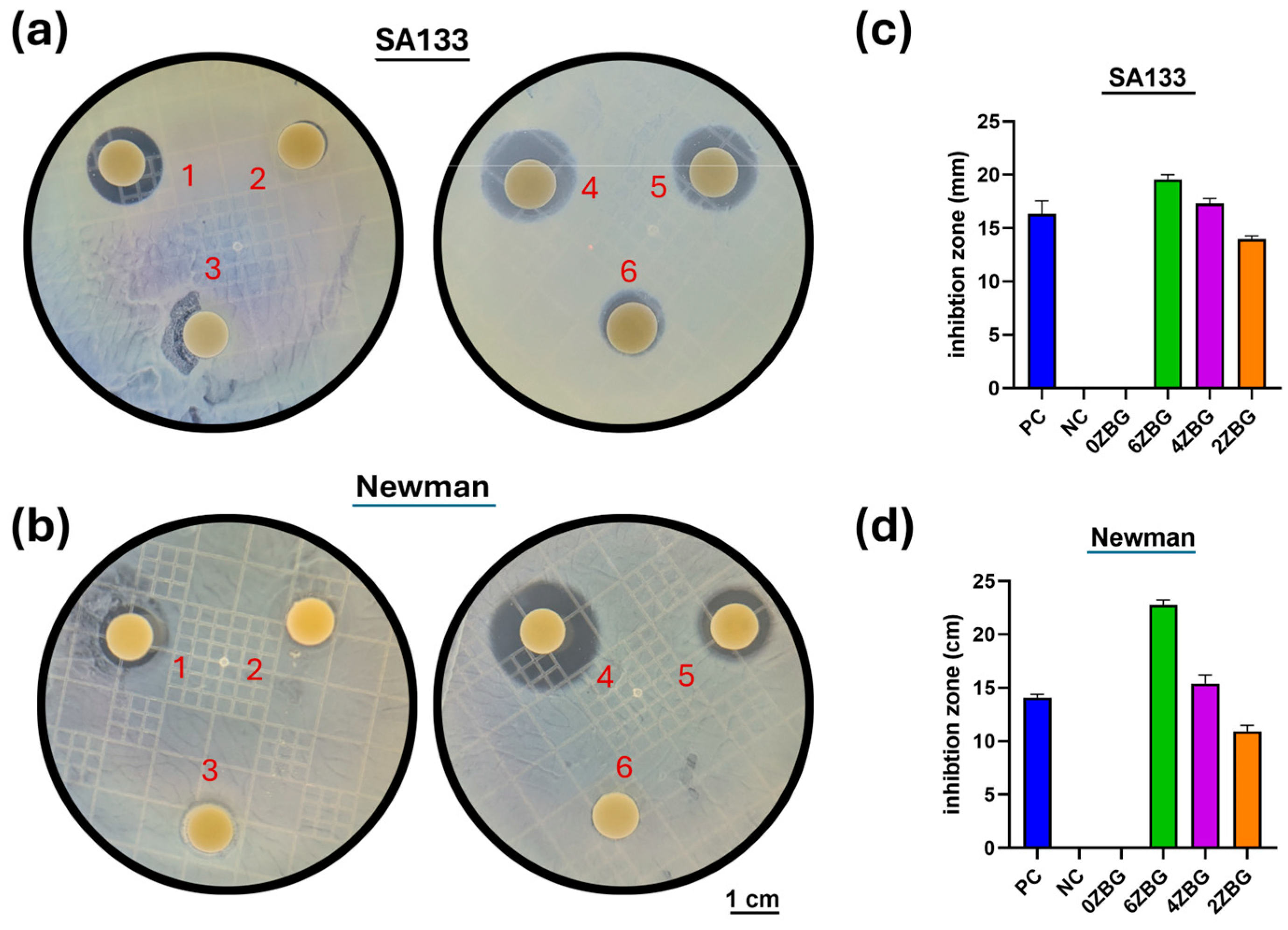
3.2.2. In Vitro Investigation of Electrospun PCL/ZBG Bioscaffold
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maji, K.; Pramanik, K. Electrospun scaffold for bone regeneration. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 842–857. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, X.; Gautrot, J.E.; Peijs, T. Nanoengineered electrospun fibers and their biomedical applications: A review. Nanocomposites 2021, 7, 1–34. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent advances in modification strategies of pre- and post-electrospinning of nanofiber scaffolds in tissue engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Coelho, S.; Kniep, J.; Barroca, N.; Almeida, J.; Fernandes, M. Nanofibrous hybrid scaffolds based on pcl-borosilicate system by a green sol-gel process. Mater. Today Chem. 2023, 29, 101396. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Cahill, P.; McGuinness, G. Solvent system effects on the physical and mechanical properties of electrospun poly (ε-caprolactone) scaffolds for in vitro lung models. J. Mech. Behav. Biomed. Mater. 2022, 136, 105493. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Schubert, D.W.; Boccaccini, A.R.; Roether, J.A.; Santos, H.A. An organic-inorganic hybrid scaffold with honeycomb-like structures enabled by one-step self-assembly-driven electrospinning. Mater. Sci. Eng. C 2021, 124, 112079. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of pcl/hap electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef]
- Saiding, Q.; Cui, W. Functional nanoparticles in electrospun fibers for biomedical applications. Nano Sel. 2022, 3, 999–1011. [Google Scholar] [CrossRef]
- Baran, A.; Barzowska, J.; Grinberg, M.; Mahlik, S.; Szczodrowski, K.; Zorenko, Y. Binding energies of eu2+ and eu3+ ions in β-Ca2SiO4 doped with europium. Opt. Mater. 2013, 35, 2107–2114. [Google Scholar] [CrossRef]
- Abel, S.B.; Liverani, L.; Boccaccini, A.R.; Abraham, G.A. Effect of benign solvents composition on poly (ε-caprolactone) electrospun fiber properties. Mater. Lett. 2019, 245, 86–89. [Google Scholar] [CrossRef]
- Pedrosa, M.C.G.; dos Anjos, S.A.; Mavropoulos, E.; Bernardo, P.L.; Granjeiro, J.M.; Rossi, A.M.; Dias, M.L. Structure and biological compatibility of polycaprolactone/zinc-hydroxyapatite electrospun nanofibers for tissue regeneration. J. Bioact. Compat. Polym. 2021, 36, 314–333. [Google Scholar] [CrossRef]
- Ismail, H.M.; Zamani, S.; Elrayess, M.A.; Kafienah, W.; Younes, H.M. New Three-Dimensional Poly(decanediol-co-tricarballylate) Elastomeric Fibrous Mesh Fabricated by Photoreactive Electrospinning for Cardiac Tissue Engineering Applications. Polymers 2018, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 2, 117–141. [Google Scholar] [CrossRef]
- Peng, T.-Y.; Tsai, P.-Y.; Chen, M.-S.; Mine, Y.; Wu, S.-H.; Chen, C.-Y.; Lin, D.-J.; Lin, C.-K. Mesoporous Properties of Bioactive Glass Synthesized by Spray Pyrolysis with Various Polyethylene Glycol and Acid Additions. Polymers 2021, 13, 618. [Google Scholar] [CrossRef]
- Sergi, R.; Cannillo, V.; Boccaccini, A.R.; Liverani, L. Incorporation of Bioactive Glasses Containing Mg, Sr, and Zn in Electrospun PCL Fibers by Using Benign Solvents. Appl. Sci. 2020, 10, 5530. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents. Bioact. Mater. 2018, 3, 55–63. [Google Scholar] [CrossRef]
- Liverani, L.; Liguori, A.; Zezza, P.; Gualandi, C.; Toselli, M.; Boccaccini, A.R.; Focarete, M.L. Nanocomposite electrospun fibers of poly(epsilon-caprolactone)/bioactive glass with shape memory properties. Bioact Mater 2022, 11, 230–239. [Google Scholar]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, P.; Zhang, X.; Jingguo, X.; Yongjie, W.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (peek) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; An, Y.; Campoccia, D.; Donati, M.; Montanaro, L. Etiology of implant orthopedic infections: A survey on 1027 clinical isolates. Int. J. Artif. Organs 2005, 28, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Hudetz, S.U.; Harris, L.G.; Luginbühl, R.; Friederich, N.F.; Landmann, R. Weak effect of metal type and ica genes on staphylococcal infection of titanium and stainless steel implants. Clin. Microbiol. Infect. 2008, 14, 1135–1145. [Google Scholar] [CrossRef]
- Forson, A.M.; Rosman, C.W.K.; van Kooten, T.G.; van der Mei, H.C.; Sjollema, J. Micrococcal Nuclease stimulates Staphylococcus aureus Biofilm Formation in a Murine Implant Infection Model. Front. Cell. Infect. Microbiol. 2022, 11, 799845. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional coatings on implant materials—A systematic review of the current scenario. Coatings 2022, 13, 69. [Google Scholar] [CrossRef]
- Lin, H.-N.; Peng, T.-Y.; Kung, Y.-R.; Chiou, Y.-J.; Chang, W.-M.; Wu, S.-H.; Mine, Y.; Chen, C.-Y.; Lin, C.-K. Effects of the methyl methacrylate addition, polymerization temperature and time on the MBG@PMMA core-shell structure and its application as addition in electrospun composite fiber bioscaffold. Ceram. Int. 2023, 49, 7630–7639. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Synthesis and in vitro studies of sol-gel derived lithium substituted 58s bioactive glass. Ceram. Int. 2017, 43, 12835–12843. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Characterization, in vitro bioactivity and biological studies of sol-gel synthesized sro substituted 58s bioactive glass. Ceram. Int. 2017, 43, 14880–14890. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Xue, Q. Synthesis and Upconversion Emission of β-Ca2SiO4:(Er3+, Yb3+). Mater. Manuf. Process. 2016, 31, 194–197. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Chang, J. Functional mesoporous bioactive glass nanospheres: Synthesis, high loading efficiency, controllable delivery of doxorubicin and inhibitory effect on bone cancer cells. J. Mater. Chem. B 2013, 1, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol–gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Maisetta, G.; Batoni, G.; Cannillo, V. Zinc containing bioactive glasses with ultra-high crystallization temperature, good biological performance and antibacterial effects. Mater. Sci. Eng. C 2019, 104, 109910. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-A.; Kim, S.-H.; Won, J.-E.; Kim, J.-J.; Shin, U.S.; Kim, H.-W. Effects on growth and osteogenic differentiation of mesenchymal stem cells by the zinc-added sol-gel bioactive glass granules. J. Tissue Eng. 2010, 1, 475260. [Google Scholar] [CrossRef]
- Huang, M.; Hill, R.G.; Rawlinson, S.C. Zinc bioglasses regulate mineralization in human dental pulp stem cells. Dent. Mater. 2017, 33, 543–552. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Schroeder, K.; Nebe, J.B. Time-dependent metabolic activity and adhesion of human osteoblast-like cells on sensor chips with a plasma polymer nanolayer. Int. J. Artif. Organs 2010, 33, 738–748. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]



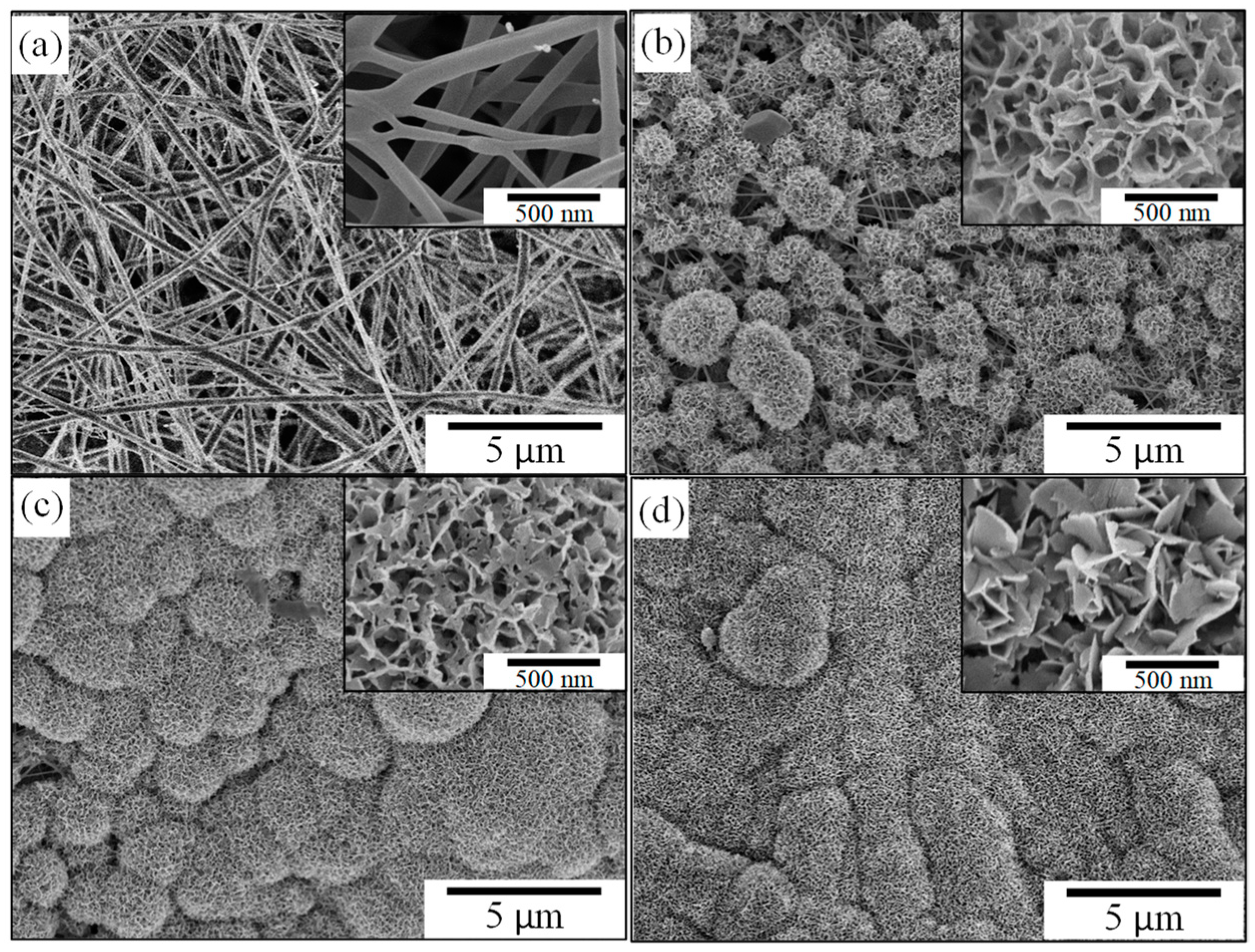



| Sample Code | SiO2 (wt.%) | CaO (wt.%) | P2O5 (wt.%) | ZnO (wt.%) |
|---|---|---|---|---|
| 0ZBG | 58 | 33 | 9 | 0 |
| 2ZBG | 58 | 31 | 9 | 2 |
| 4ZBG | 58 | 29 | 9 | 4 |
| 6ZBG | 58 | 27 | 9 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Chiou, Y.-J.; Chang, P.-J.; Chang, W.-M.; Yeh, Y.-C.; Chen, C.-Y.; Chang, Y.-K.; Lin, C.-K. In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition. Polymers 2024, 16, 2811. https://doi.org/10.3390/polym16192811
Chen Y-Y, Chiou Y-J, Chang P-J, Chang W-M, Yeh Y-C, Chen C-Y, Chang Y-K, Lin C-K. In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition. Polymers. 2024; 16(19):2811. https://doi.org/10.3390/polym16192811
Chicago/Turabian StyleChen, Ya-Yi, Yuh-Jing Chiou, Pei-Jung Chang, Wei-Min Chang, Yu-Cheng Yeh, Chin-Yi Chen, Yu-Kang Chang, and Chung-Kwei Lin. 2024. "In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition" Polymers 16, no. 19: 2811. https://doi.org/10.3390/polym16192811
APA StyleChen, Y.-Y., Chiou, Y.-J., Chang, P.-J., Chang, W.-M., Yeh, Y.-C., Chen, C.-Y., Chang, Y.-K., & Lin, C.-K. (2024). In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition. Polymers, 16(19), 2811. https://doi.org/10.3390/polym16192811








