Critical Cooling Rate of Fast-Crystallizing Polyesters: The Example of Poly(alkylene trans-1,4-cyclohexanedicarboxylate)
Abstract
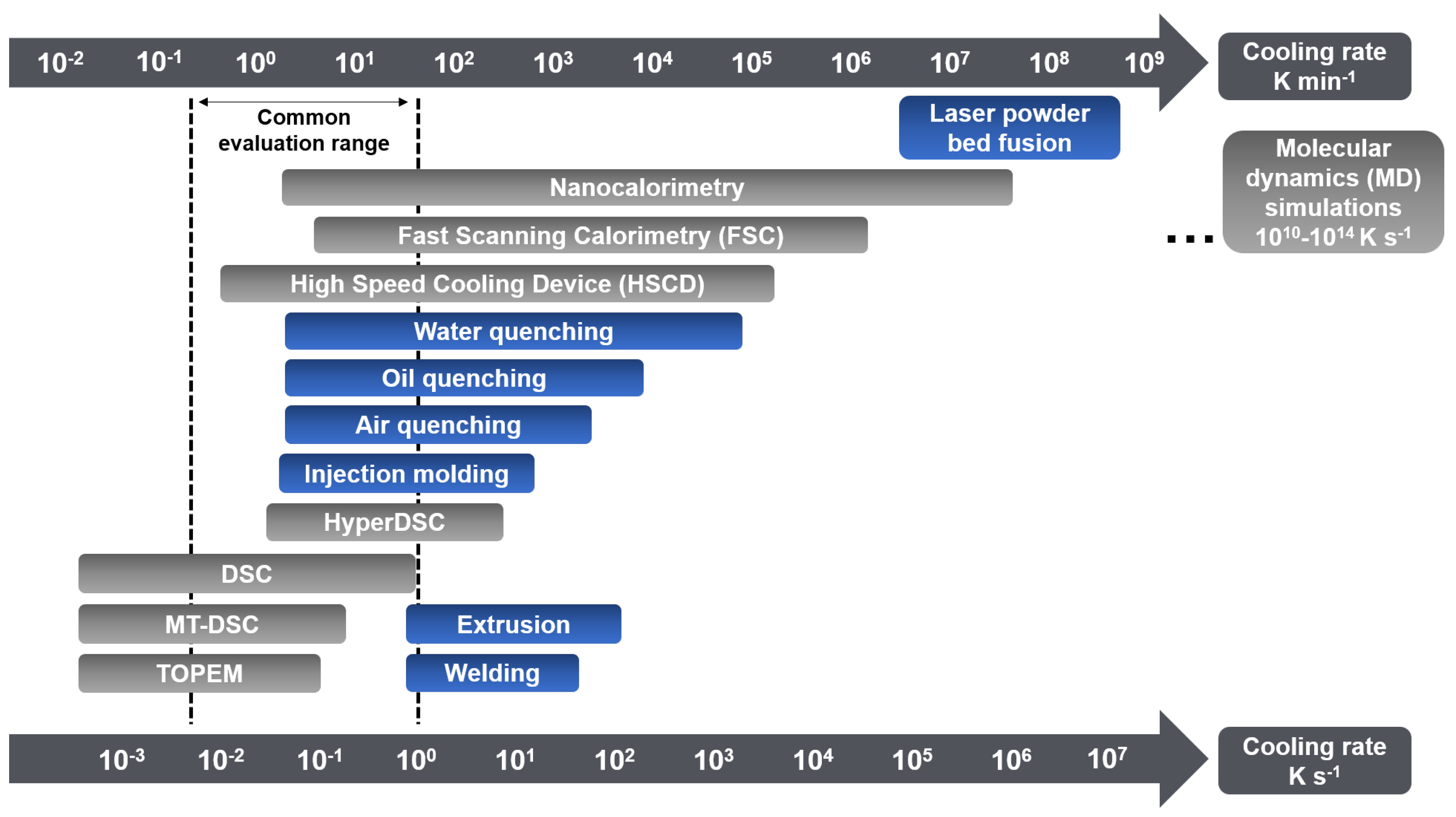
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Polymer Synthesis
2.3. Chemical Characterizations
2.4. Thermal Characterizations
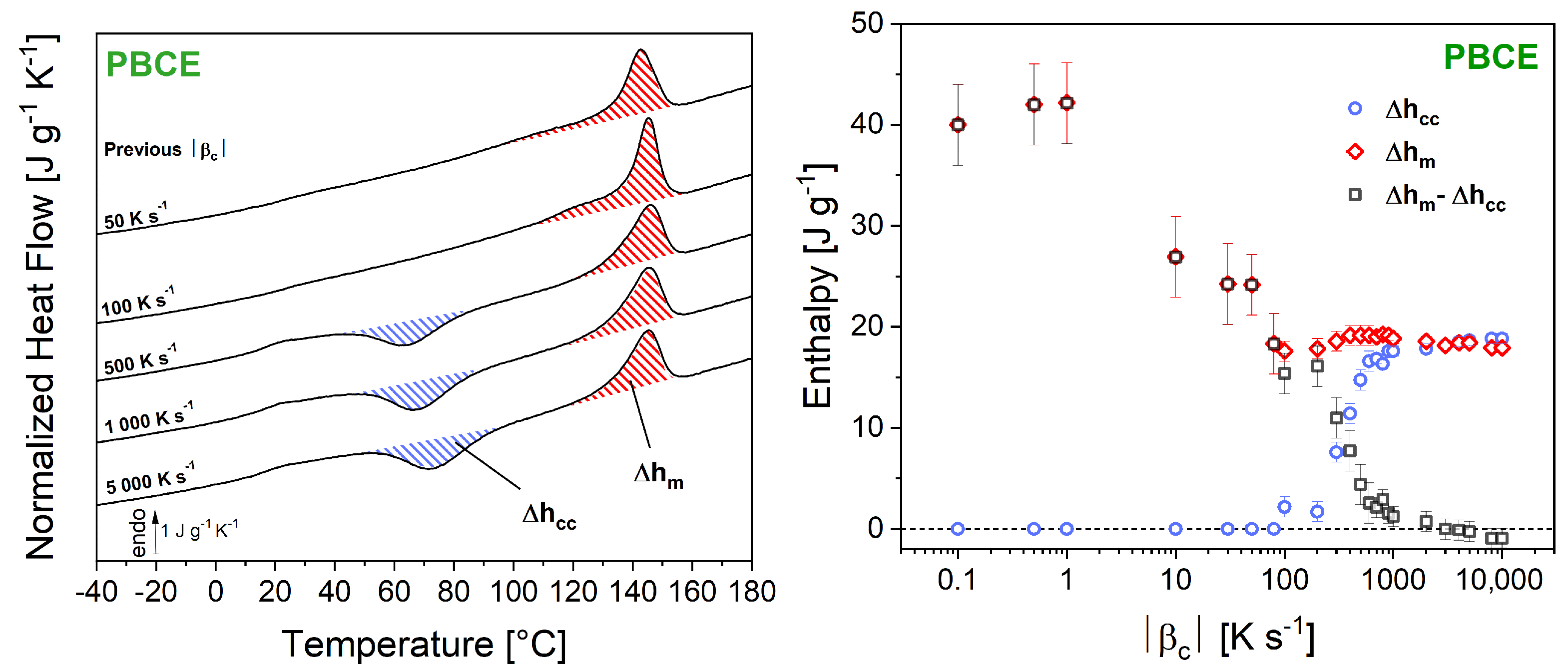
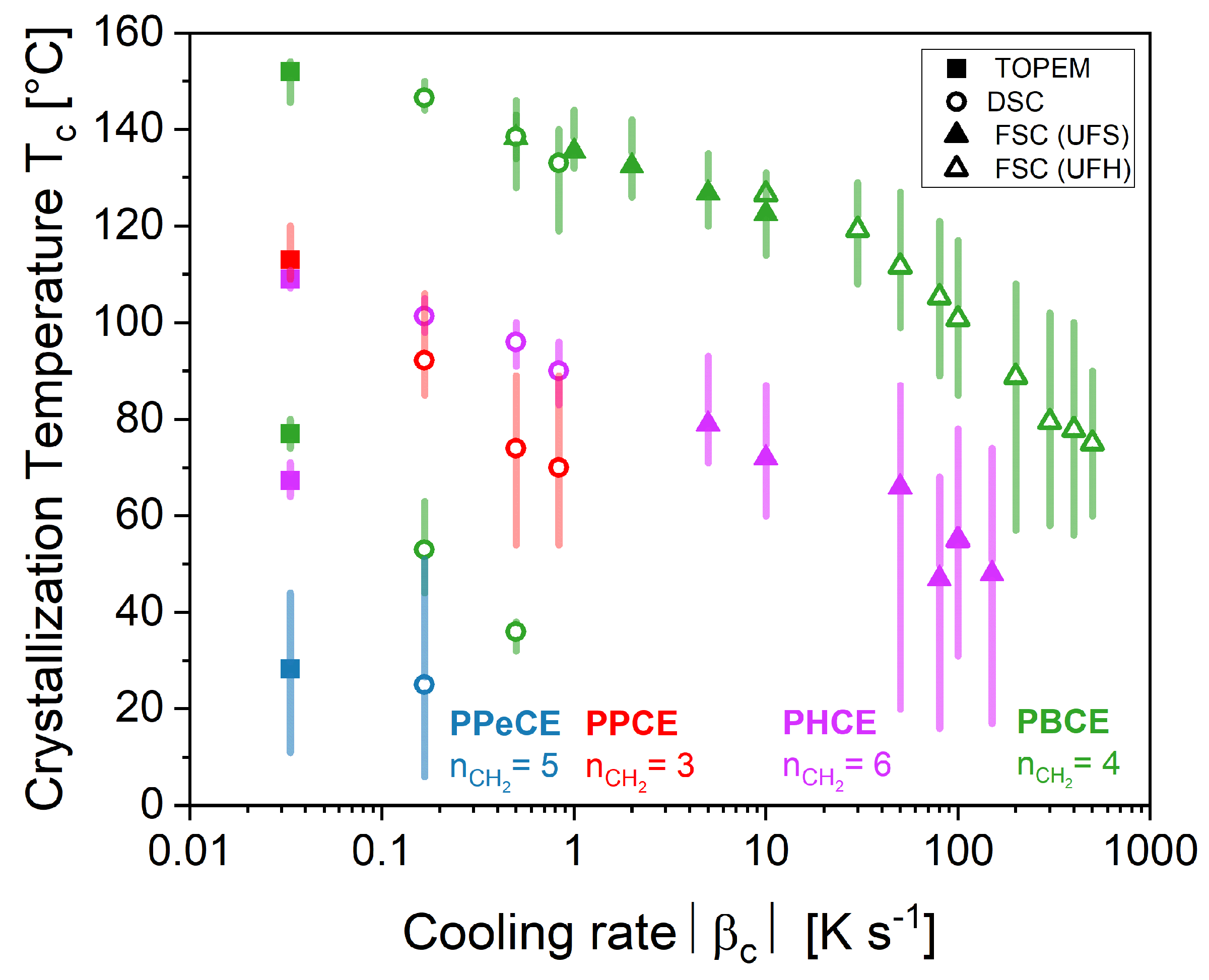
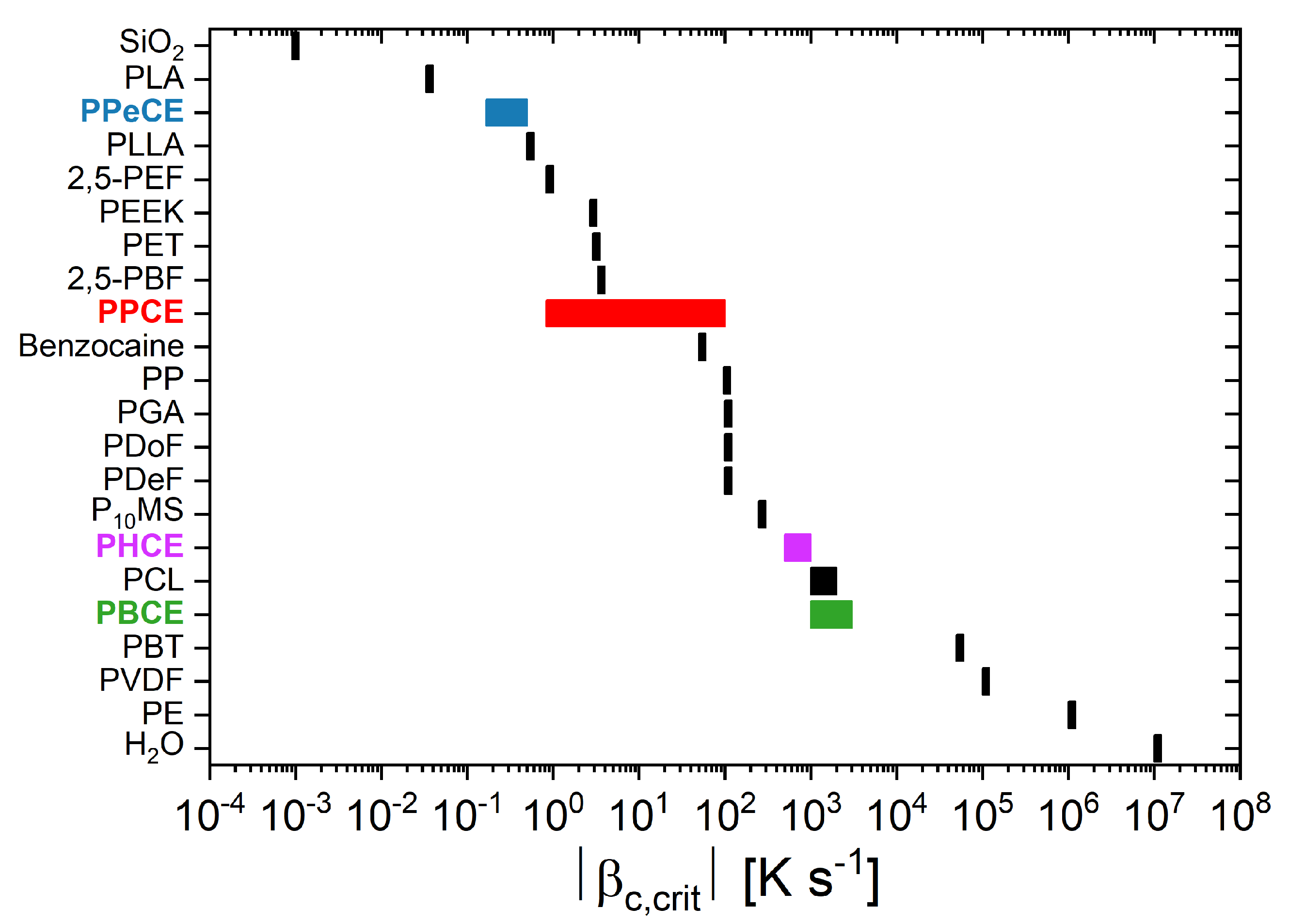
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Herrera, N.; Salaberria, A.M.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: Effects on mechanical, thermal and optical properties. Compos. Part A Appl. Sci. Manuf. 2016, 83, 89–97. [Google Scholar] [CrossRef]
- Adamovsky, S.; Minakov, A.; Schick, C. Scanning microcalorimetry at high cooling rate. Thermochim. Acta 2003, 403, 55–63. [Google Scholar] [CrossRef]
- Bardelcik, A.; Salisbury, C.P.; Winkler, S.; Wells, M.A.; Worswick, M.J. Effect of cooling rate on the high strain rate properties of boron steel. Int. J. Impact Eng. 2010, 37, 694–702. [Google Scholar] [CrossRef]
- Buczek, A.; Telejko, T. Investigation of heat transfer coefficient during quenching in various cooling agents. Int. J. Heat Fluid Flow 2013, 44, 358–364. [Google Scholar] [CrossRef]
- Carmeli, E.; Cavallo, D.; Tranchida, D. Instrument for mimicking fast cooling conditions of polymers: Design and case studies on polypropylene. Polym. Test. 2021, 97, 107164. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P.; Zhuravlev, E.; Schick, C. Kinetics of isothermal and non-isothermal crystallization of poly(vinylidene fluoride) by fast scanning calorimetry. Polymer 2016, 82, 40–48. [Google Scholar] [CrossRef]
- Han, T.; Chen, J.; Wei, Z.; Qu, N.; Liu, Y.; Yang, D.; Zhao, S.; Lai, Z.; Jiang, M.; Zhu, J. Effect of cooling rate on microstructure and mechanical properties of AlCrFe2Ni2 medium entropy alloy fabricated by laser powder bed fusion. J. Mater. Res. Technol. 2023, 25, 4063–4073. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Park, S.; Gottsfritz, G.; Benson, G.; Tolentino, B.; McWilliams, B.; Cho, K.; Sohn, Y. Understanding the Laser Powder Bed Fusion of AlSi10Mg Alloy. Metallogr. Microstruct. Anal. 2020, 9, 484–502. [Google Scholar] [CrossRef]
- Kamal, M.R.; Kalyon, D. Heat transfer and microstructure in extrusion blowmolding. Polym. Eng. Sci. 1983, 23, 503–509. [Google Scholar] [CrossRef]
- Li, X.; Song, W.; Yang, K.; Krishnan, N.A.; Wang, B.; Smedskjaer, M.M.; Mauro, J.C.; Sant, G.; Balonis, M.; Bauchy, M. Cooling rate effects in sodium silicate glasses: Bridging the gap between molecular dynamics simulations and experiments. J. Chem. Phys. 2017, 147, 074501. [Google Scholar] [CrossRef] [PubMed]
- Minakov, A.A.; Schick, C. Dynamics of the temperature distribution in ultra-fast thin-film calorimeter sensors. Thermochim. Acta 2015, 603, 205–217. [Google Scholar] [CrossRef]
- Pijpers, T.F.; Mathot, V.B.; Goderis, B.; Scherrenberg, R.L.; van der Vegte, E.W. High-Speed Calorimetry for the Study of the Kinetics of (De)vitrification, Crystallization, and Melting of Macromolecules. Macromolecules 2002, 35, 3601–3613. [Google Scholar] [CrossRef]
- Poorhaydari, K.; Patchett, B.M.; Ivey, D.G. Estimation of cooling rate in the welding of plates with intermediate thickness. Weld. J. 2005, 84, 149s–155s. [Google Scholar]
- Seppala, J.E.; Migler, K.D. Infrared thermography of welding zones produced by polymer extrusion additive manufacturing. Addit. Manuf. 2016, 12, 71–76. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Chen, C.; Tsai, H.L. 3D printing of large, complex metallic glass structures. Mater. Des. 2017, 117, 213–222. [Google Scholar] [CrossRef]
- Suplicz, A.; Szabo, F.; Kovacs, J. Injection molding of ceramic filled polypropylene: The effect of thermal conductivity and cooling rate on crystallinity. Thermochim. Acta 2013, 574, 145–150. [Google Scholar] [CrossRef]
- Vanden Poel, G.; Mathot, V.B. High performance differential scanning calorimetry (HPer DSC): A powerful analytical tool for the study of the metastability of polymers. Thermochim. Acta 2007, 461, 107–121. [Google Scholar] [CrossRef]
- Takata, N.; Liu, M.; Li, H.; Suzuki, A.; Kobashi, M. Fast scanning calorimetry study of Al alloy powder for understanding microstructural development in laser powder bed fusion. Mater. Des. 2022, 219, 110830. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schick, C. Fast scanning power compensated differential scanning nano-calorimeter: 1. The device. Thermochim. Acta 2010, 505, 1–13. [Google Scholar] [CrossRef]
- Zuidema, H.; Peters, G.W.M.; Meijer, H.E.H. Influence of cooling rate on pVT-data of semicrystalline polymers. J. Appl. Polym. Sci. 2001, 82, 1170–1186. [Google Scholar] [CrossRef]
- Monnier, X.; Maigret, J.E.; Lourdin, D.; Saiter, A. Glass transition of anhydrous starch by fast scanning calorimetry. Carbohydr. Polym. 2017, 173, 77–83. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Gazzano, M.; Finelli, L.; Munari, A. New eco-friendly random copolyesters based on poly(propylene cyclohexanedicarboxylate): Structure-properties relationships. EXPRESS Polym. Lett. 2015, 9, 972–983. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Finelli, L.; Munari, A.; Dalla Rosa, M. Fully Aliphatic Copolyesters Based on Poly(butylene 1,4-cyclohexanedicarboxylate) with Promising Mechanical and Barrier Properties for Food Packaging Applications. Ind. Eng. Chem. Res. 2013, 52, 12876–12886. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Munari, A.; Lotti, N. Novel Random Copolymers of Poly(butylene 1,4-cyclohexane dicarboxylate) with Outstanding Barrier Properties for Green and Sustainable Packaging: Content and Length of Aliphatic Side Chains as Efficient Tools to Tailor the Material’s Final Performance. Polymers 2018, 10, 866. [Google Scholar] [CrossRef]
- Soccio, M.; Lotti, N.; Finelli, L.; Gazzano, M.; Munari, A. Aliphatic poly(propylene dicarboxylate)s: Effect of chain length on thermal properties and crystallization kinetics. Polymer 2007, 48, 3125–3136. [Google Scholar] [CrossRef]
- Siracusa, V.; Genovese, L.; Ingrao, C.; Munari, A.; Lotti, N. Barrier Properties of Poly(Propylene Cyclohexanedicarboxylate) Random Eco-Friendly Copolyesters. Polymers 2018, 10, 502. [Google Scholar] [CrossRef]
- Kumar, N.; Chaudhary, S.; Singh, P.; Thapa, K.B.; Kumar, D. Electro-optical odd-even effect of APAPA liquid crystal molecules studied under the influence of an extraneous electric field (THz): A theoretical approach. J. Mol. Liq. 2020, 318, 114254. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, Z.; Yu, Y.; Shao, S.; Leng, X.; Wang, Y.; Li, Y. Biobased long-chain aliphatic polyesters of 1,12-dodecanedioic acid with a variety of diols: Odd-even effect and mechanical properties. Mater. Today Commun. 2019, 19, 450–458. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, X.; Ortmann, P.; Mecking, S.; Alamo, R.G. Crystallization of Long-Spaced Precision Polyacetals I: Melting and Recrystallization of Rapidly Formed Crystallites. Macromolecules 2019, 52, 4934–4948. [Google Scholar] [CrossRef]
- Pérez-Camargo, R.A.; Meabe, L.; Liu, G.; Sardon, H.; Zhao, Y.; Wang, D.; Müller, A.J. Even–Odd Effect in Aliphatic Polycarbonates with Different Chain Lengths: From Poly (Hexamethylene Carbonate) to Poly (Dodecamethylene Carbonate). Macromolecules 2021, 54, 259–271. [Google Scholar] [CrossRef]
- Flores, I.; Pérez-Camargo, R.A.; Gabirondo, E.; Caputo, M.R.; Liu, G.; Wang, D.; Sardon, H.; Müller, A.J. Unexpected Structural Properties in the Saturation Region of the Odd–Even Effects in Aliphatic Polyethers: Influence of Crystallization Conditions. Macromolecules 2022, 55, 584–594. [Google Scholar] [CrossRef]
- Masubuchi, T.; Sakai, M.; Kojio, K.; Furukawa, M.; Aoyagi, T. Structure and Properties of Aliphatic Poly(carbonate) glycols with Different Methylene Unit Length. e-J. Soft Mater. 2007, 3, 55–63. [Google Scholar] [CrossRef]
- Guidotti, G.; Fosse, C.; Soccio, M.; Gazzano, M.; Siracusa, V.; Delbreilh, L.; Esposito, A.; Lotti, N. Synthesis and properties of poly(alkylene trans-1,4-cyclohexanedicarboxylate)s with different glycolic subunits. Polym. Degrad. Stab. 2024. under review. [Google Scholar]
- Salmerón Sánchez, M.; Mathot, V.B.F.; Vanden Poel, G.; Gómez Ribelles, J.L. Effect of the Cooling Rate on the Nucleation Kinetics of Poly(l-Lactic Acid) and Its Influence on Morphology. Macromolecules 2007, 40, 7989–7997. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, Y.; Zheng, Y.; Ikeda, K.; Okabe, R.; Wu, R.; Ozaki, R.; Xu, J.; Xu, Q. Influence of Cooling Rate on Crystallization Behavior of Semi-Crystalline Polypropylene: Experiments and Mathematical Modeling. Polymers 2022, 14, 3646. [Google Scholar] [CrossRef]
- Boyer, S.; Haudin, J.M. Crystallization of polymers at constant and high cooling rates: A new hot-stage microscopy set-up. Polym. Test. 2010, 29, 445–452. [Google Scholar] [CrossRef]
- Mejres, M.; Hallavant, K.; Guidotti, G.; Soccio, M.; Lotti, N.; Esposito, A.; Saiter-Fourcin, A. Physical aging of a biodegradable alicyclic polymer: Poly (pentamethylene trans-1,4-cyclohexanedicarboxylate). J. Non-Cryst. Solids 2024, 629, 122874. [Google Scholar] [CrossRef]
- Hallavant, K.; Mejres, M.; Schawe, J.E.K.; Esposito, A.; Saiter-Fourcin, A. Influence of Chemical Composition and Structure on the Cooperative Fluctuation in Supercooled Glass-Forming Liquids. J. Phys. Chem. Lett. 2024, 15, 4508–4514. [Google Scholar] [CrossRef] [PubMed]
- Monnier, X.; Saiter, A.; Dargent, E. Vitrification of PLA by fast scanning calorimetry: Towards unique glass above critical cooling rate? Thermochim. Acta 2017, 658, 47–54. [Google Scholar] [CrossRef]
- Schawe, J.E.; Löffler, J. Existence of multiple critical cooling rates which generate different types of monolithic metallic glass. Nat. Commun. 2019, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, P. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G. Polymorphism in polymers. In Macromolecules: Synthesis, Order And Advanced Properties; Springer: Berlin/Heidelberg, Germany, 1992; pp. 183–217. [Google Scholar]
- Di Lorenzo, M.L.; Cocca, M.; Malinconico, M. Crystal polymorphism of poly(l-lactic acid) and its influence on thermal properties. Thermochim. Acta 2011, 522, 110–117. [Google Scholar] [CrossRef]
- Cocca, M.; Lorenzo, M.L.D.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Esposito, A.; Delpouve, N.; Causin, V.; Dhotel, A.; Delbreilh, L.; Dargent, E. From a Three-Phase Model to a Continuous Description of Molecular Mobility in Semicrystalline Poly(hydroxybutyrate-co-hydroxyvalerate). Macromolecules 2016, 49, 4850–4861. [Google Scholar] [CrossRef]
- Fosse, C.; Esposito, A.; Lemechko, P.; Salim, Y.S.; Causin, V.; Gaucher, V.; Bruzaud, S.; Sudesh, K.; Delbreilh, L.; Dargent, E. Effect of Chemical Composition on Molecular Mobility and Phase Coupling in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with Different Comonomer Contents. J. Polym. Environ. 2023, 31, 4430–4447. [Google Scholar] [CrossRef]
- Bourdet, A.; Fosse, C.; Garda, M.R.; Thiyagarajan, S.; Delbreilh, L.; Esposito, A.; Dargent, E. Microstructural consequences of isothermal crystallization in homo- and co-polyesters based on 2,5- and 2,4-furandicarboxylic acid. Polymer 2023, 272, 125835. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P.; Minakov, A.; Adamovsky, S.; Schick, C.; Hashimoto, T.; Saijo, K. Crystallization of polypropylene at various cooling rates. Mater. Sci. Eng. A 2005, 413–414, 442–446. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Mesophases in polyethylene, polypropylene, and poly(1-butene). Polymer 2010, 51, 4639–4662. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Li, T.; Peng, X.; Jiang, S.; Turng, L.S. Quantification of the Young’s modulus for polypropylene: Influence of initial crystallinity and service temperature. J. Appl. Polym. Sci. 2020, 137, 48581. [Google Scholar] [CrossRef]
- Wright, D.; Dunk, R.; Bouvart, D.; Autran, M. The effect of crystallinity on the properties of injection moulded polypropylene and polyacetal. Polymer 1988, 29, 793–796. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. (Eds.) Fast Scanning Calorimetry; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Schawe, J.; Hütter, T.; Heitz, C.; Alig, I.; Lellinger, D. Stochastic temperature modulation: A new technique in temperature-modulated DSC. Thermochim. Acta 2006, 446, 147–155. [Google Scholar] [CrossRef]
- Klein, J.P.; Gdowski, Z.M.; Register, R.A. Controlling thermomechanical behavior of semicrystalline hydrogenated polynorbornene through the cis- to trans-cyclopentylene ratio. J. Polym. Sci. 2022, 60, 266–275. [Google Scholar] [CrossRef]
- Brunelle, D.J.; Jang, T. Optimization of poly(1,4-cyclohexylidene cyclohexane-1,4-dicarboxylate) (PCCD) preparation for increased crystallinity. Polymer 2006, 47, 4094–4104. [Google Scholar] [CrossRef]
- Puente, J.A.S.; Esposito, A.; Chivrac, F.; Dargent, E. Effects of Size and Specific Surface Area of Boron Nitride Particles on the Crystallization of Bacterial Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Macromol. Symp. 2013, 328, 8–19. [Google Scholar] [CrossRef]
- Puente, J.A.S.; Esposito, A.; Chivrac, F.; Dargent, E. Effect of boron nitride as a nucleating agent on the crystallization of bacterial poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2013, 128, 2586–2594. [Google Scholar] [CrossRef]
- Duer, M.J.; Roper, C. A solid-state NMR investigation of the odd–even effect in a series of liquid-crystal dimers. Phys. Chem. Chem. Phys. 2003, 5, 3034–3041. [Google Scholar] [CrossRef]
- Hu, W.; Ma, T.; Zhou, Y.; Soccio, M.; Lotti, N.; Cavallo, D.; Wang, D.; Liu, G. Crystal Structure and Polymorphism of Poly(butylene-trans-1,4-cyclohexane dicarboxylate). Macromolecules 2024, 57, 4374–4384. [Google Scholar] [CrossRef]
- Martins, J.C.; Novack, K.M.; Gomes, A.S. Non-isothermal crystallization kinetics of thermotropic polyesters with flexible spacers in the main chain. Polymer 1998, 39, 6941–6944. [Google Scholar] [CrossRef]
- Barandiarán, J.; Colmenero, J. Continuous cooling approximation for the formation of a glass. J. Non-Cryst. Solids 1981, 46, 277–287. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, K.; He, Y.; Hu, W. Fast-Scanning Chip-Calorimetry Measurement of Crystallization Kinetics of Poly(Glycolic Acid). Polymers 2021, 13, 891. [Google Scholar] [CrossRef]
- Furushima, Y.; Kumazawa, S.; Umetsu, H.; Toda, A.; Zhuravlev, E.; Schick, C. Melting and recrystallization kinetics of poly(butylene terephthalate). Polymer 2017, 109, 307–314. [Google Scholar] [CrossRef]
- Kagawa, F.; Oike, H. Quenching of Charge and Spin Degrees of Freedom in Condensed Matter. Adv. Mater. 2017, 29, 1601979. [Google Scholar] [CrossRef]
- Mukhametzyanov, T.A.; Andrianov, R.A.; Bolmatenkov, D.N.; Yagofarov, M.I.; Solomonov, B.N.; Schick, C. Nucleation and crystallization of deeply supercooled benzocaine, a rapidly crystallizing organic compound: A Fast scanning calorimetry investigation. Thermochim. Acta 2023, 730, 179613. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furan dicarboxylate). Comparison of thermal behavior and solid state structure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast Crystallization and Melting Behavior of a Long-Spaced Aliphatic Furandicarboxylate Biobased Polyester, Poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Stoclet, G.; Gobius du Sart, G.; Yeniad, B.; de Vos, S.; Lefebvre, J. Isothermal crystallization and structural characterization of poly(ethylene-2,5-furanoate). Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]



| Sample | Repeating Unit | (g mol−1) | (g mol−1) | (g mol−1) | Đ | DPn | cis-Isomers (%) |
|---|---|---|---|---|---|---|---|
| PPCE | 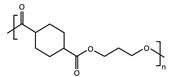 | 212 | 62,462 | 96,657 | 1.5 | 295 | 6.6 |
| PBCE | 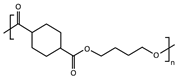 | 226 | 68,703 | 93,382 | 1.4 | 304 | 5.5 |
| PPeCE | 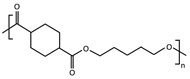 | 240 | 57,855 | 83,592 | 1.4 | 241 | 5.4 |
| PHCE |  | 254 | 38,666 | 58,734 | 1.5 | 152 | 9.4 |
| SAMPLE | ||||||
|---|---|---|---|---|---|---|
| (°C) | (°C) | (J g−1) | (°C) | (°C) | (J g−1) | |
| PPCE | 92.2 ± 0.5 | 85–106 | 33 ± 1 | 8 ± 2 | 136–160 | 32 ± 2 |
| PBCE | 146.5 ± 0.5 | 144–150 | 39 ± 2 | 7 ± 2 | 92–171 | 55 ± 5 |
| PPeCE | 25 ± 5 | 6–51 | 0.5 ± 0.1 | −15 ± 1 | 60–76 | 0.6 ± 0.1 |
| PHCE | 101.3 ± 0.5 | 98–105 | 43 ± 3 | −21 ± 2 | 50–129 | 57 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallavant, K.; Soccio, M.; Guidotti, G.; Lotti, N.; Esposito, A.; Saiter-Fourcin, A. Critical Cooling Rate of Fast-Crystallizing Polyesters: The Example of Poly(alkylene trans-1,4-cyclohexanedicarboxylate). Polymers 2024, 16, 2792. https://doi.org/10.3390/polym16192792
Hallavant K, Soccio M, Guidotti G, Lotti N, Esposito A, Saiter-Fourcin A. Critical Cooling Rate of Fast-Crystallizing Polyesters: The Example of Poly(alkylene trans-1,4-cyclohexanedicarboxylate). Polymers. 2024; 16(19):2792. https://doi.org/10.3390/polym16192792
Chicago/Turabian StyleHallavant, Kylian, Michelina Soccio, Giulia Guidotti, Nadia Lotti, Antonella Esposito, and Allisson Saiter-Fourcin. 2024. "Critical Cooling Rate of Fast-Crystallizing Polyesters: The Example of Poly(alkylene trans-1,4-cyclohexanedicarboxylate)" Polymers 16, no. 19: 2792. https://doi.org/10.3390/polym16192792
APA StyleHallavant, K., Soccio, M., Guidotti, G., Lotti, N., Esposito, A., & Saiter-Fourcin, A. (2024). Critical Cooling Rate of Fast-Crystallizing Polyesters: The Example of Poly(alkylene trans-1,4-cyclohexanedicarboxylate). Polymers, 16(19), 2792. https://doi.org/10.3390/polym16192792











