Formulation and Characterization of Deep Eutectic Solvents and Potential Application in Recycling Packaging Laminates
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents
2.2. Packaging Films and Blister Packaging
2.3. Delamination
2.3.1. Screening Tests
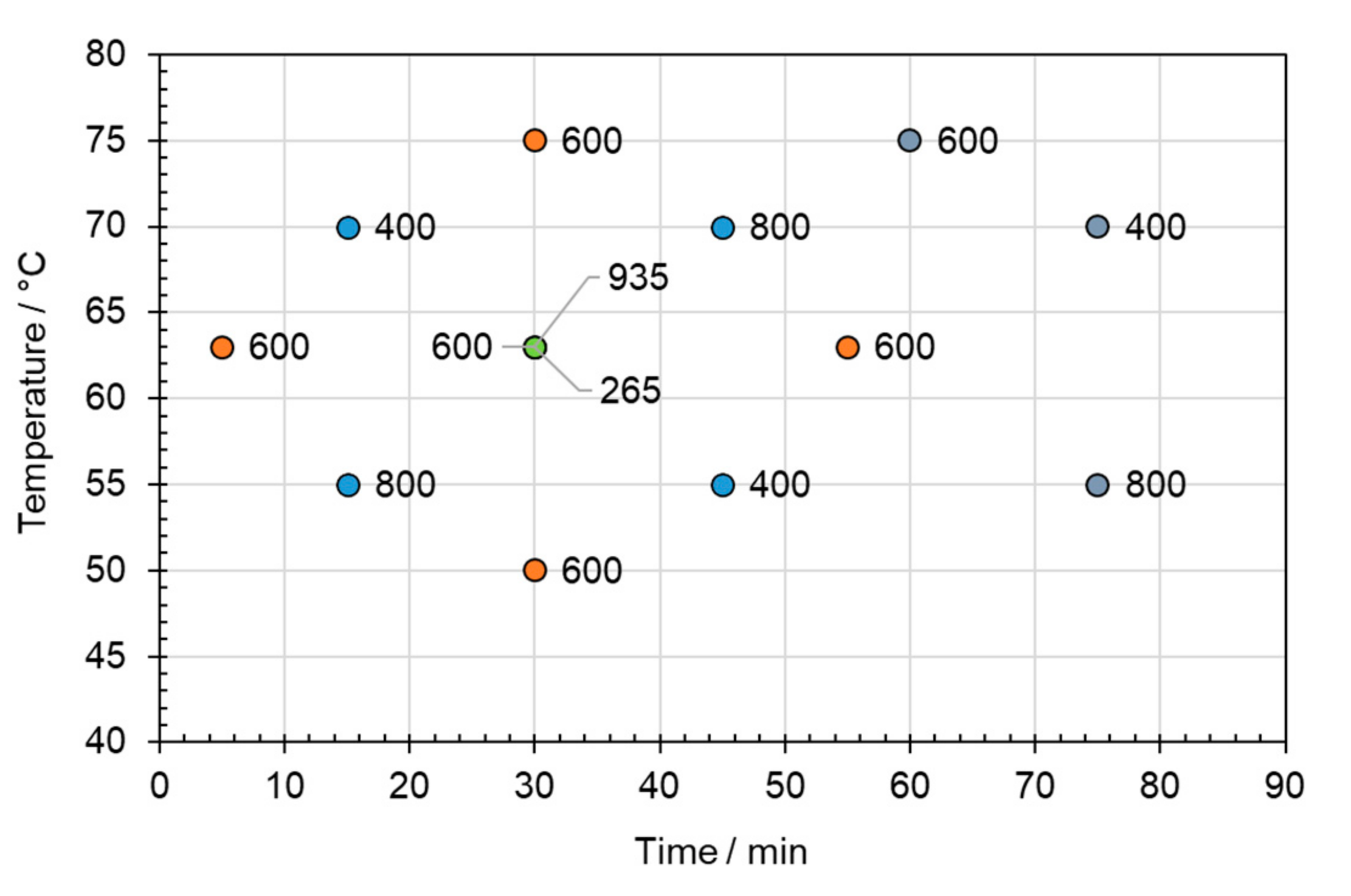
2.3.2. Design of Experiments
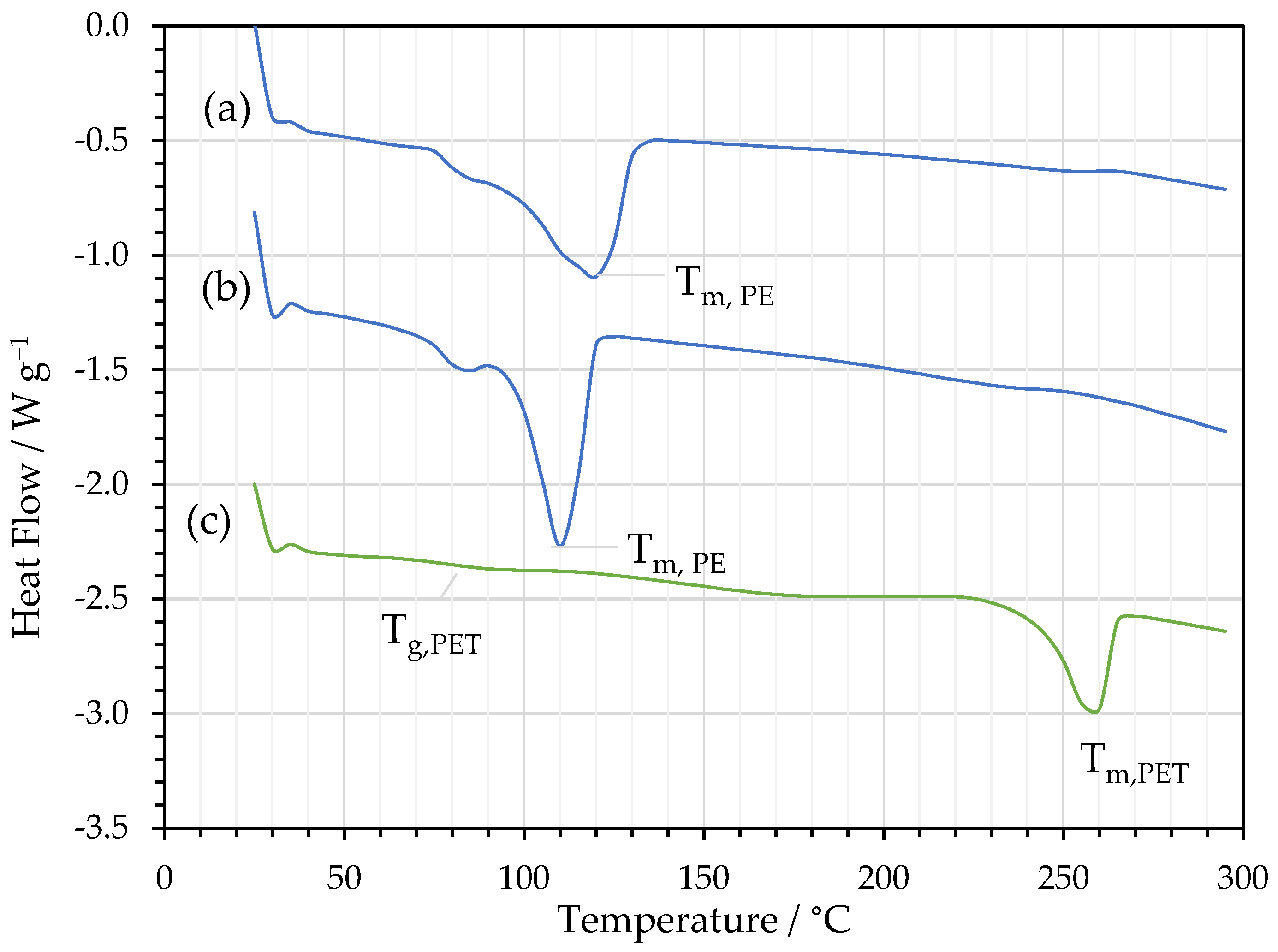
2.4. DSC
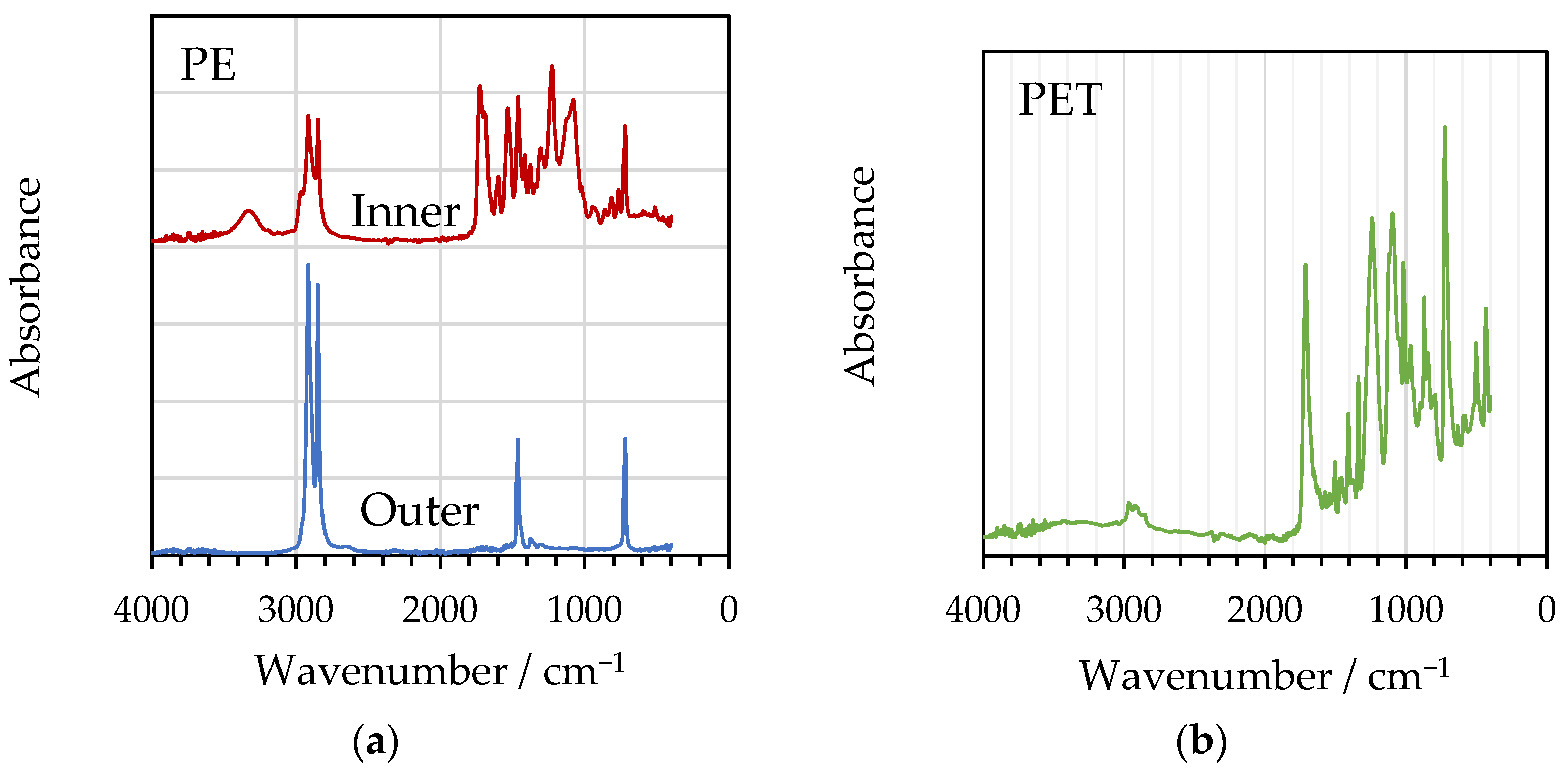
2.5. FTIR
3. Results and Discussion
3.1. Delamination
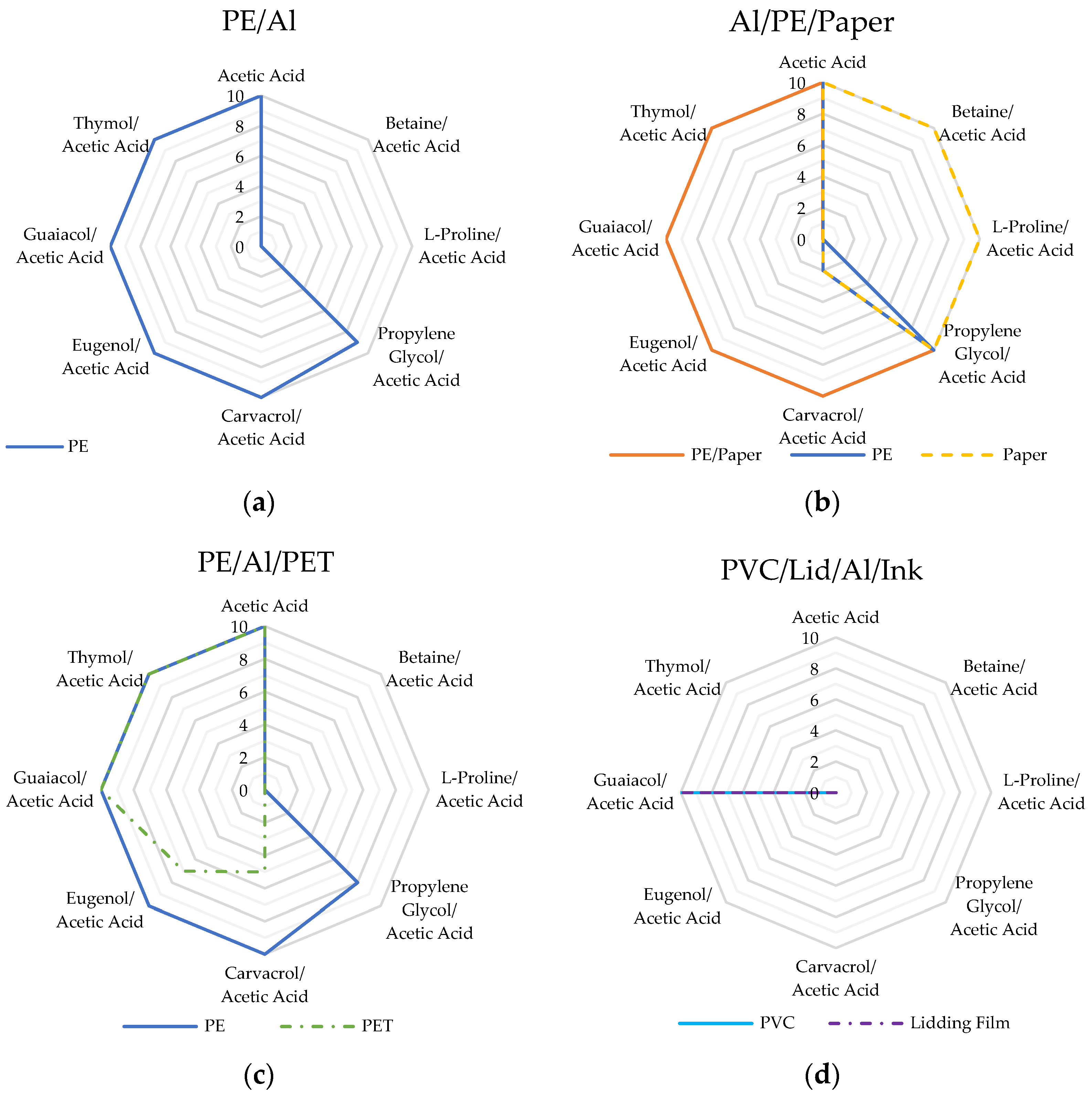
3.1.1. Screening Experiments
3.1.2. Design of Experiments
3.1.3. Thymol/Acetic Acid
3.1.4. Betaine/Acetic Acid
3.2. FTIR and DSC Analysis of Delaminated Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, J.; Grau, L.; Auer, M.; Maletz, R.; Woidasky, J. Multilayer Packaging in a Circular Economy. Polymers 2022, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Ügdüler, S.; De Somer, T.; Van Geem, K.M.; De Wilde, J.; Roosen, M.; Deprez, B.; De Meester, S. Analysis of the kinetics, energy balance and carbon footprint of the delamination of multilayer flexible packaging films via carboxylic acids. Resour. Conserv. Recycl. 2022, 181, 106256. [Google Scholar] [CrossRef]
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—A Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Giorgia Faraca, G.; Thomas Astrup, T. Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability. Waste Manag. 2019, 95, 388–398. [Google Scholar] [CrossRef]
- Ügdüler, S.; De Somer, T.; Van Geem, K.M.; Roosen, M.; Kulawig, A.; Leineweber, R.; De Meester, S. Towards a Better Understanding of Delamination of Multilayer Flexible Packaging Films by Carboxylic Acids. ChemSusChem 2021, 14, 4198–4213. [Google Scholar] [CrossRef]
- Šleiniūtė, A.; Denafas, G.; Mumladze, T. Analysis of the Delamination Process with Nitric Acid in Multilayer Composite Food Packaging. Appl. Sci. 2023, 13, 5669. [Google Scholar] [CrossRef]
- Nieminen, J.; Anugwom, I.; Kallioinen, M.; Mänttäri, M. Green solvents in recovery of aluminium and plastic from waste pharmaceutical blister packaging. Waste Manag. 2020, 107, 20–27. [Google Scholar] [CrossRef]
- Fávaro, S.L.; Freitas, A.R.; Ganzerli, T.A.; Pereira, A.G.B.; Cardozo, A.L.; Baron, O.; Muniz, E.C.; Girotto, E.M.; Radovanovic, E. PET and aluminum recycling from multilayer food packaging using supercritical ethanol. J. Supercrit. Fluids 2013, 75, 138–143. [Google Scholar] [CrossRef]
- O’Rourke, G.; Houbrechts, M.; Nees, M.; Roosen, M.; De Meester, S.; De Vos, D. Delamination of polyamide/polyolefin multilayer films by selective glycolysis of polyurethane adhesive. Green Chem. 2022, 24, 6867–6878. [Google Scholar] [CrossRef]
- Hamilton, J.A. Transport of fatty acids across membranes by the diffusion mechanism. Prostaglandins Leukot. Essent. Fat. Acids 1999, 60, 291–297. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Wong, W.F. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Row, K.H. Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction. TrAC Trends Anal. Chem. 2019, 120, 115651. [Google Scholar] [CrossRef]
- Cardellini, F.; Germani, R.; Cardinali, G.; Corte, L.; Roscini, L.; Spreti, N.; Tiecco, M. Room temperature deep eutectic solvents of (1S)-(+)-10-camphorsulfonic acid and sulfobetaines: Hydrogen bond-based mixtures with low ionicity and structure-dependent toxicity. RSC Adv. 2015, 5, 31772–31786. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.H.; Ali Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering-Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Yang, R.; Cao, Q.; Liang, Y.; Hong, S.; Xia, C.; Wu, Y.; Li, J.; Cai, L.; Sonne, C.; Le, Q.V.; et al. High capacity oil absorbent wood prepared through eco-friendly deep eutectic solvent delignification. Chem. Eng. J. 2020, 401, 126150. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]



| Solvent | Molar Ratio | Water Miscibility | Density/g cm−3 | Viscosity/mPa s | Crystallization Temperature/°C |
|---|---|---|---|---|---|
| Acetic Acid | - | miscible | 1.049 | 2.4 ± 0.6 | −16.7 |
| Betaine/Acetic acid | 1:4 | miscible | 1.118 | 41.7 ± 0.7 | Not detected |
| L-Proline/Acetic Acid | 1:3 | miscible | 1.166 | 117 ± 2 | Not detected |
| Propylene Glycol/Acetic Acid | 1:2 | miscible | 1.043 | 16.0 ± 0.5 | Not detected |
| Carvacrol/Acetic Acid | 1:1 | immiscible | 0.993 | 6.9 ± 0.5 | Not detected |
| Eugenol/Acetic Acid | 1:1 | immiscible | 1.061 | 5.0 ± 0.4 | Not detected |
| Guaiacol/Acetic Acid | 1:1 | immiscible | 1.107 | 4.0 ± 0.8 | Not detected |
| Thymol/Acetic Acid | 1:1 | immiscible | 0.991 | 7.0 ± 0.5 | Not detected |
| Material | Film Thickness/μm | ||||
|---|---|---|---|---|---|
| Al | PVC | PE | PET | Paper | |
| PE/Al | 16 ± 1 | - | 83 ± 1 | - | - |
| Al/PE/Paper | 10 ± 2 | - | 18 ± 2 | - | 58 ± 3 |
| PE/Al/PET | 10 ± 1 | - | 87 ± 1 | 21 ± 1 | - |
| Blister Pack | 26 ± 1 | 211 ± 1 | - | - | - |
| Factor | Unit | Low | High | Centre | −α | +α | |
|---|---|---|---|---|---|---|---|
| A | Temperature | °C | 55 | 70 | 63 | 50 | 75 |
| B | Time | min | 15 | 45 | 30 | 5 | 55 |
| C | Stirrer Speed | rpm | 400 | 800 | 600 | 265 | 935 |
| Solvent | Molar Ratio | PE/Al | Al/PE/Paper | PE/Al/PET | Blister Pack | ||||
|---|---|---|---|---|---|---|---|---|---|
| PE | PE/Paper | PE | Paper | PE | PET | PVC | Lidding Film | ||
| Acetic Acid | - | 10 | 10a | 10 | 10 | 10 | 10 | 0 | 0 |
| Betaine/Acetic Acid | 1:4 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| L-Proline/Acetic Acid | 1:3 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| Propylene Glycol/Acetic Acid | 1:2 | 9 | 10a | 10 | 10 | 8 | 0 | 0 | 0 |
| Carvacrol/Acetic Acid | 1:1 | 10 | 10a | 2 | 2 | 10 | 5 | 0 | 0 |
| Eugenol/Acetic Acid | 1:1 | 10 | 10 | 0 | 0 | 10 | 7 | 0 | 0 |
| Guaiacol/Acetic Acid | 1:1 | 10 | 10 | 0 | 0 | 10 | 10 | 10 | 10 |
| Thymol/Acetic Acid | 1:1 | 10 | 10 | 0 | 0 | 10 | 10 | 0 | 0 |
| # | Conditions | PE/Al | Al/PE/Paper | PE/Al/PET | |||||
|---|---|---|---|---|---|---|---|---|---|
| A: Temperature /°C | B: Time /min | C: Speed /rpm | PE | PE/Paper | PE | Paper | PE | PET | |
| 1 | 55 | 45 | 400 | 10 | 0 | 0 | 0 | 10 | 0 |
| 2 | 70 | 15 | 400 | 10 | 0 | 0 | 0 | 10 | 0 |
| 3 | 55 | 15 | 800 | 0 | 0 | 0 | 0 | 5 | 0 |
| 4 | 70 | 45 | 800 | 10 | 10 | 0 | 0 | 10 | 10 |
| 5 | 63 | 30 | 600 | 10 | 10 | 0 | 0 | 10 | 0 |
| 6 | 63 | 55 | 600 | 10 | 10a | 2 | 2 | 10 | 7 |
| 7 | 75 | 30 | 600 | 10 | 10a | 0 | 0 | 10 | 8 |
| 8 | 50 | 30 | 600 | 0 | 0 | 0 | 0 | 3 | 0 |
| 9 | 63 | 5 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 63 | 30 | 600 | 10 | 10 | 0 | 0 | 10 | 0 |
| 11 | 63 | 30 | 265 | 10 | 0 | 0 | 0 | 10 | 0 |
| 12 | 63 | 30 | 935 | 10 | 10 | 0 | 0 | 10 | 0 |
| 13 | 75 | 60 | 600 | 10 | 10 | 0 | 0 | 10 | 10 |
| 14 | 70 | 75 | 400 | 10 | 10 | 0 | 0 | 10 | 10 |
| 15 | 55 | 75 | 800 | 10 | 10a | 1 | 1 | 10 | 0 |
| Factor p-Values | |||||
|---|---|---|---|---|---|
| Material | Film | Model | A: Temperature (°C) | B: Time (min) | C: Stirrer Speed (rpm) |
| PE/Al | PE | <0.0001 | <0.0001 | <0.0001 | n/a |
| Al/PE/Paper | PE/Paper | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| PE/Al/PET | PE | <0.0001 | <0.0001 | <0.0001 | 0.0006 |
| PET | <0.0001 | <0.0001 | <0.0001 | 0.0192 | |
| # | Conditions | PE/Al | Al/PE/Paper | PE/Al/PET | |||||
|---|---|---|---|---|---|---|---|---|---|
| A: Temperature /°C | B: Time /min | C: Speed /rpm | PE | PE/Paper | PE | Paper | PE | PET | |
| 1 | 70 | 45 | 800 | 0 | 0 | 0 | 10 | 0 | 0 |
| 2 | 55 | 45 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 63 | 30 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 70 | 15 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 75 | 30 | 600 | 0 | 0 | 0 | 1 | 0 | 0 |
| 6 | 63 | 55 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 55 | 75 | 800 | 0 | 0 | 0 | 10 | 0 | 0 |
| 8 | 75 | 60 | 600 | 0 | 0 | 0 | 3 | 0 | 0 |
| 9 | 70 | 75 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 55 | 15 | 800 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | 70 | 15 | 800 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 70 | 45 | 800 | 0 | 0 | 0 | 10 | 0 | 0 |
| 13 | 63 | 30 | 935 | 0 | 0 | 0 | 3 | 0 | 0 |
| 14 | 63 | 30 | 265 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 50 | 30 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| Factor p-Values | |||||
|---|---|---|---|---|---|
| Material | Film | Model | A: Temperature (°C) | B: Time (min) | C: Stirrer Speed (rpm) |
| Al/PE/Paper | Paper | <0.0001 | 0.0018 | <0.0001 | <0.0001 |
| Material | Film | DSC Parameters | |||
|---|---|---|---|---|---|
| Tg /°C | Tm /°C | ΔHf /J g−1 | χc /% | ||
| PE/Al | PE | - | 118 | 103 | 35 |
| Al/PE/Paper | PE | - | 111 | 97.4 | 33 |
| PE/Al/PET | PET | 78 | 257 | 38.2 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukodimou, A.; Lovell, C.; Li, T.; Theodosopoulos, G.; Maniam, K.K.; Paul, S. Formulation and Characterization of Deep Eutectic Solvents and Potential Application in Recycling Packaging Laminates. Polymers 2024, 16, 2781. https://doi.org/10.3390/polym16192781
Loukodimou A, Lovell C, Li T, Theodosopoulos G, Maniam KK, Paul S. Formulation and Characterization of Deep Eutectic Solvents and Potential Application in Recycling Packaging Laminates. Polymers. 2024; 16(19):2781. https://doi.org/10.3390/polym16192781
Chicago/Turabian StyleLoukodimou, Adamantini, Christopher Lovell, Tianmiao Li, George Theodosopoulos, Kranthi Kumar Maniam, and Shiladitya Paul. 2024. "Formulation and Characterization of Deep Eutectic Solvents and Potential Application in Recycling Packaging Laminates" Polymers 16, no. 19: 2781. https://doi.org/10.3390/polym16192781
APA StyleLoukodimou, A., Lovell, C., Li, T., Theodosopoulos, G., Maniam, K. K., & Paul, S. (2024). Formulation and Characterization of Deep Eutectic Solvents and Potential Application in Recycling Packaging Laminates. Polymers, 16(19), 2781. https://doi.org/10.3390/polym16192781








