Synergistic Effects of Liquid Rubber and Thermoplastic Particles for Toughening Epoxy Resin
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Untoughened EP
2.3. Preparation of EP/CTBN Composites
2.4. Preparation of EP/PEK-C Composites
2.5. Preparation of EP/CTBN/PEK-C Composites
2.6. Characterization
3. Results and Discussion
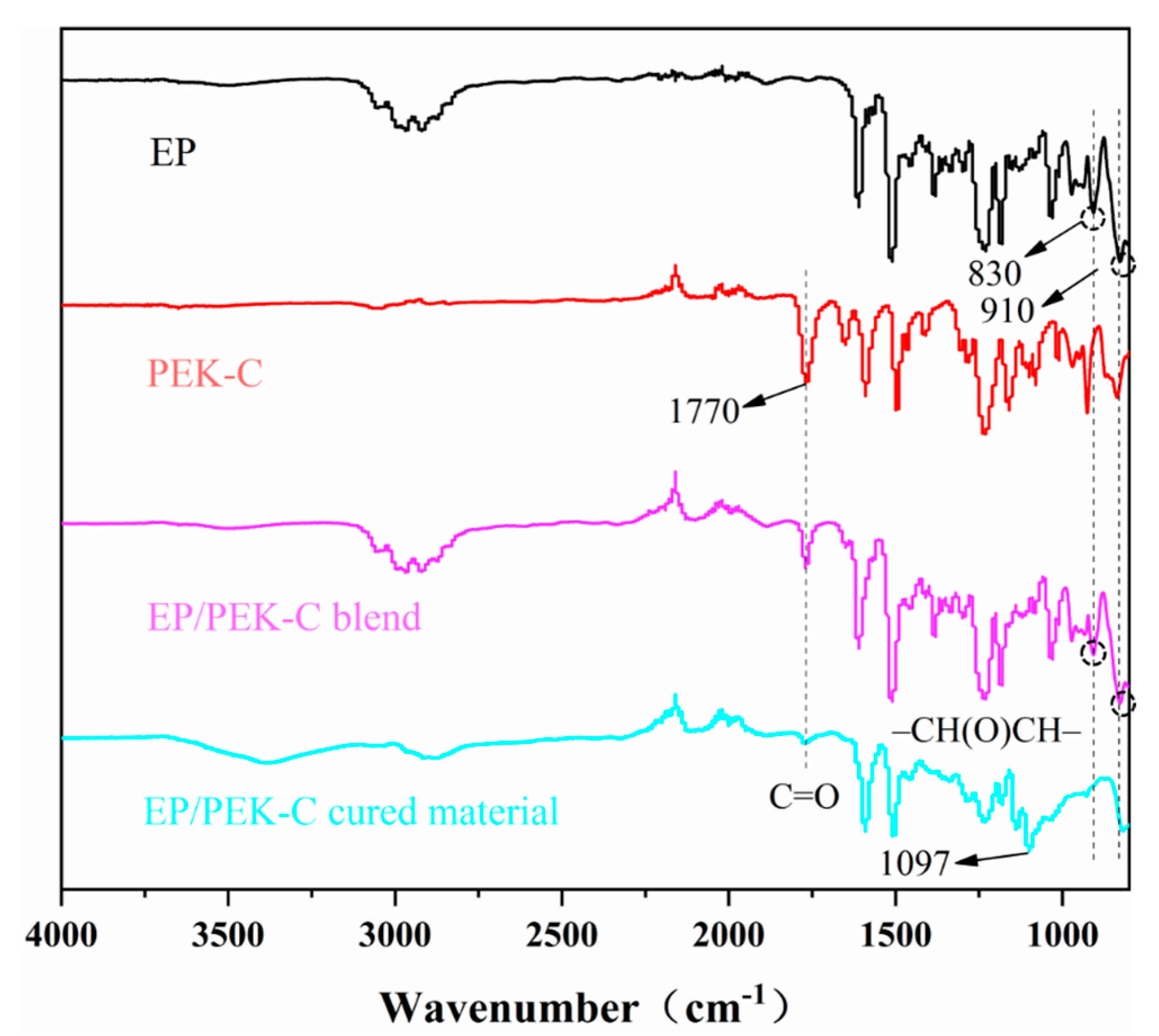
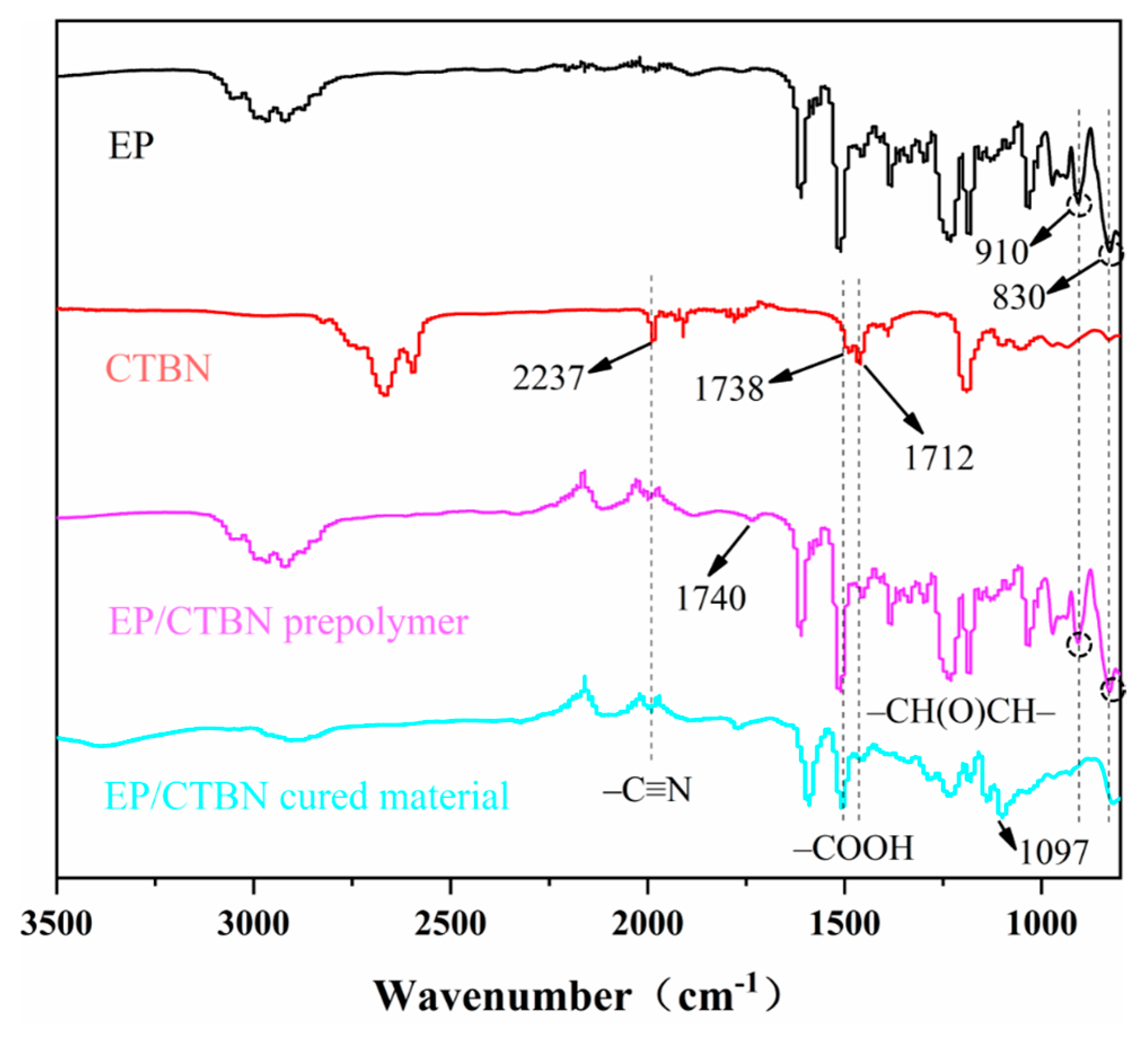
3.1. FT-IR Analysis of Different Toughened Systems
3.2. Analysis of Mechanical Properties
3.2.1. Flexural Properties
3.2.2. Impact Performance
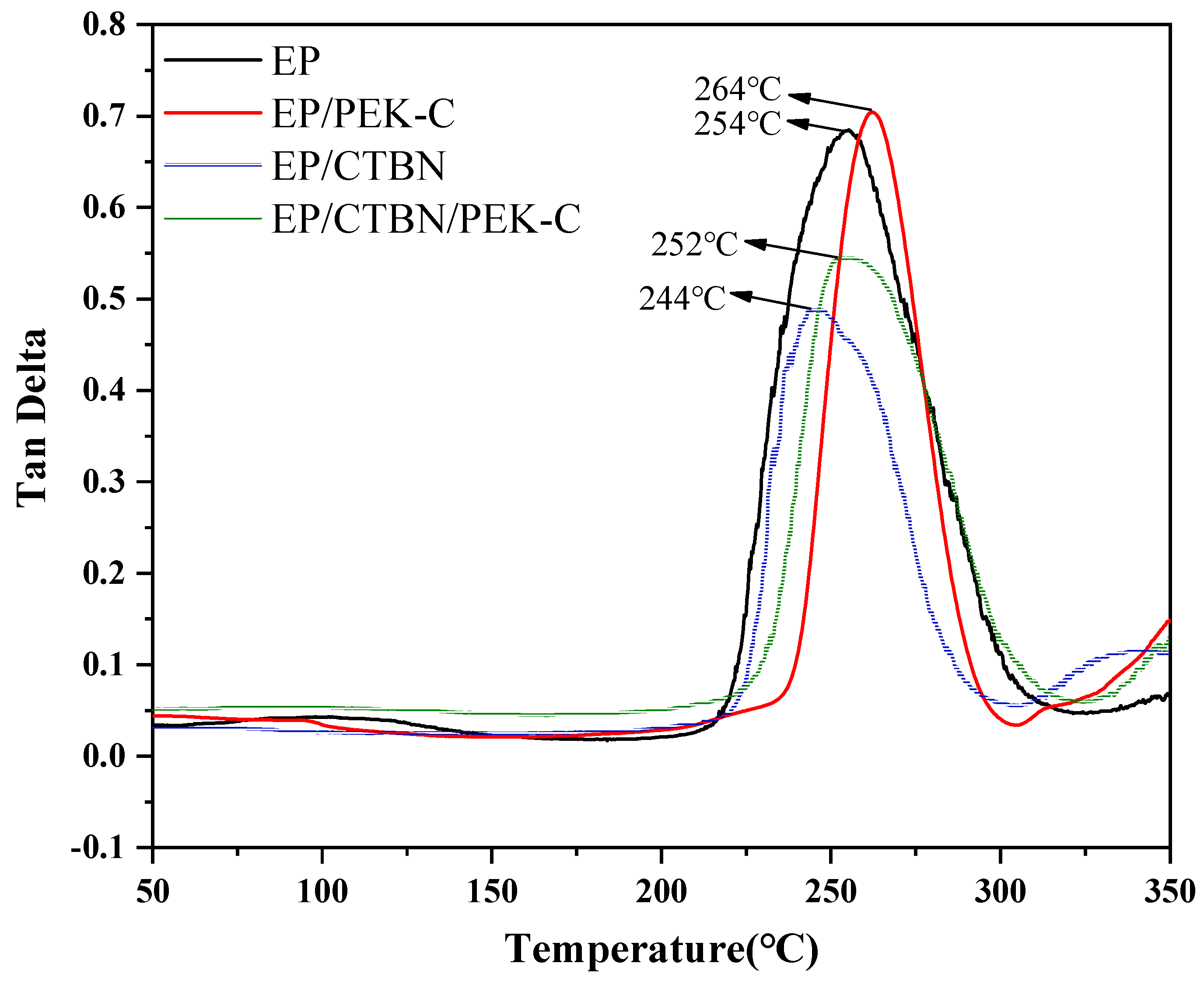
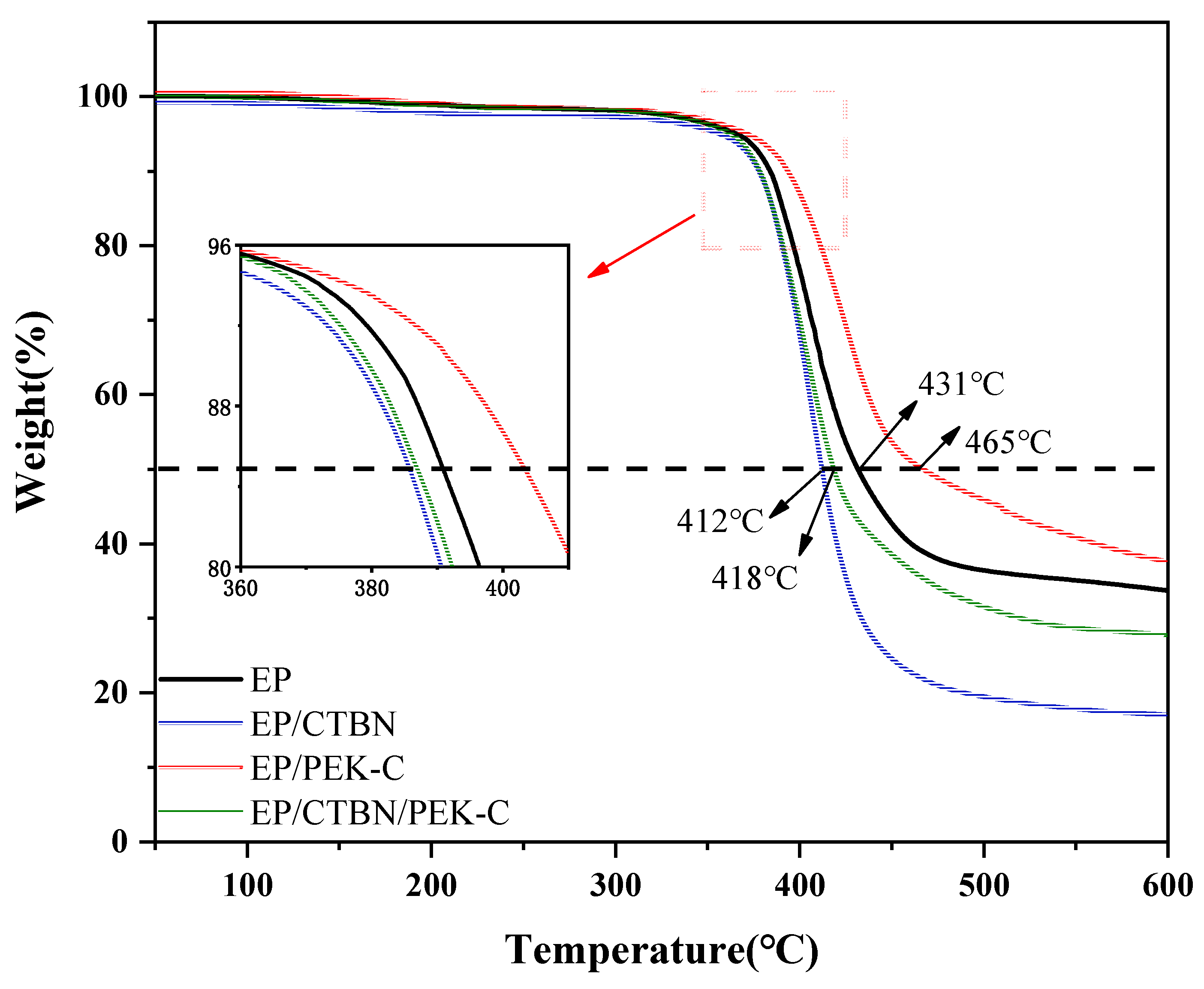
3.3. Analysis of Heat Resistance Performance
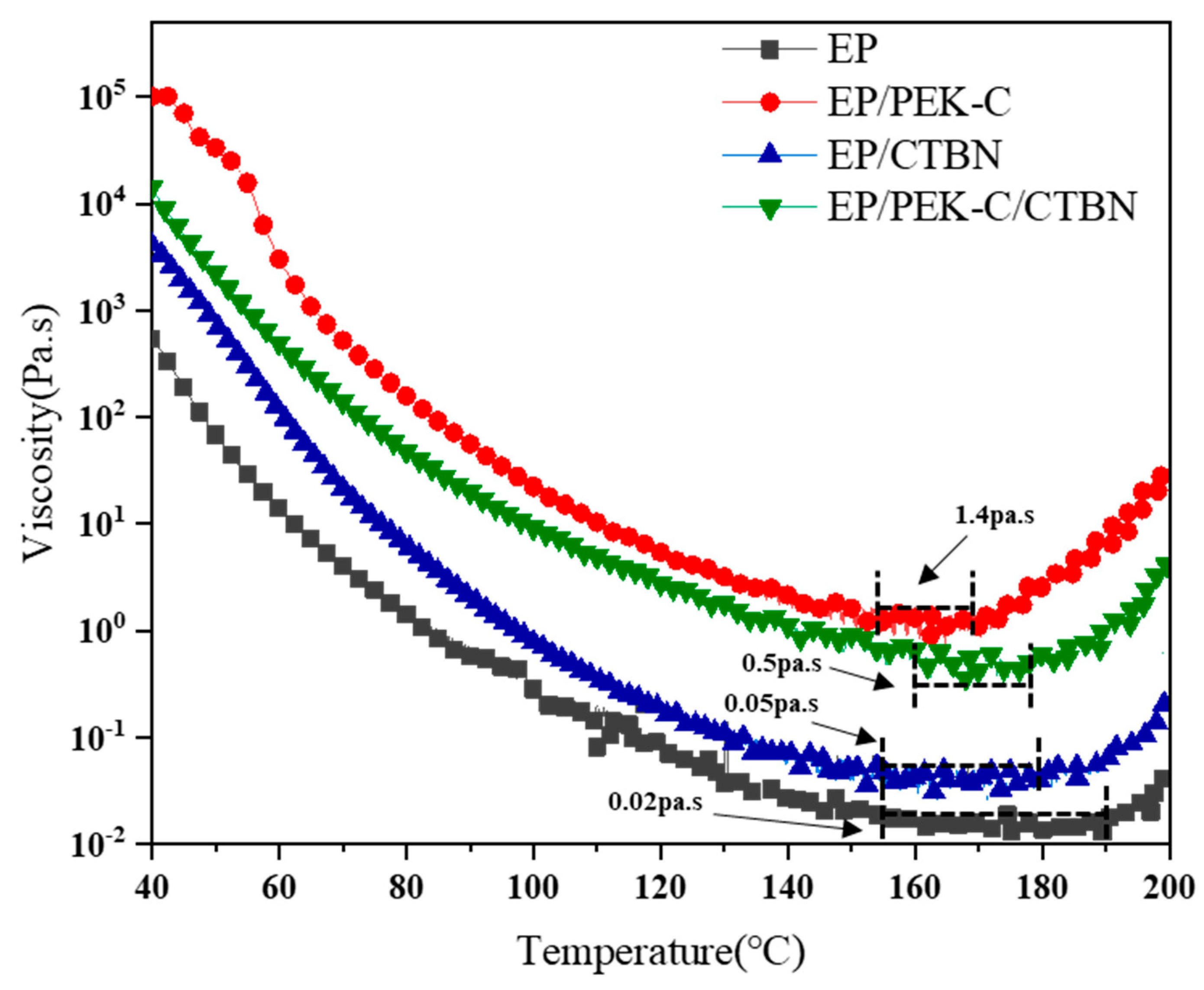
3.4. Rheological Properties of Modified Resin
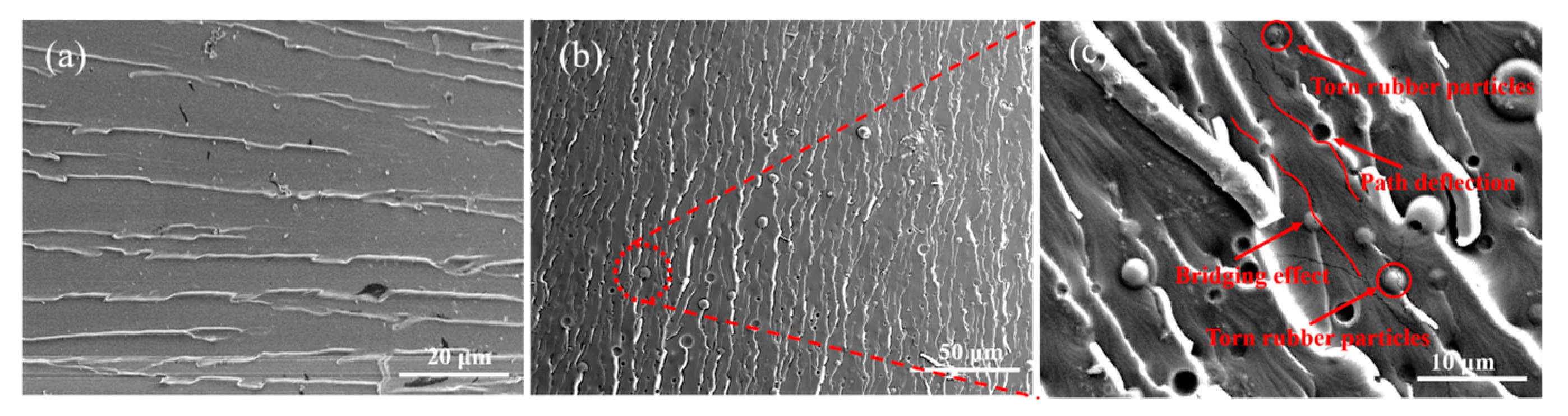
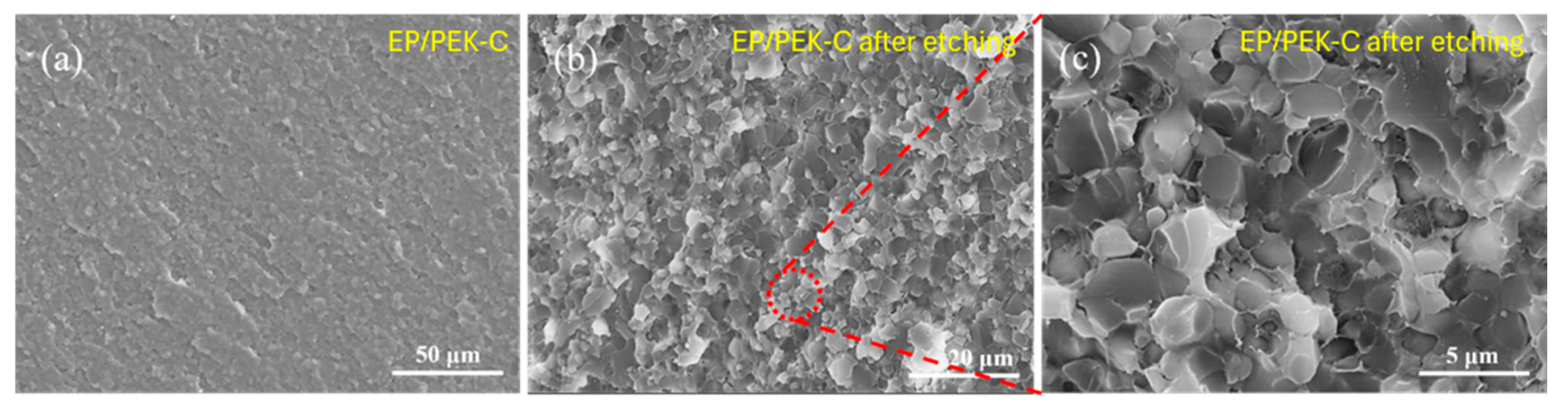
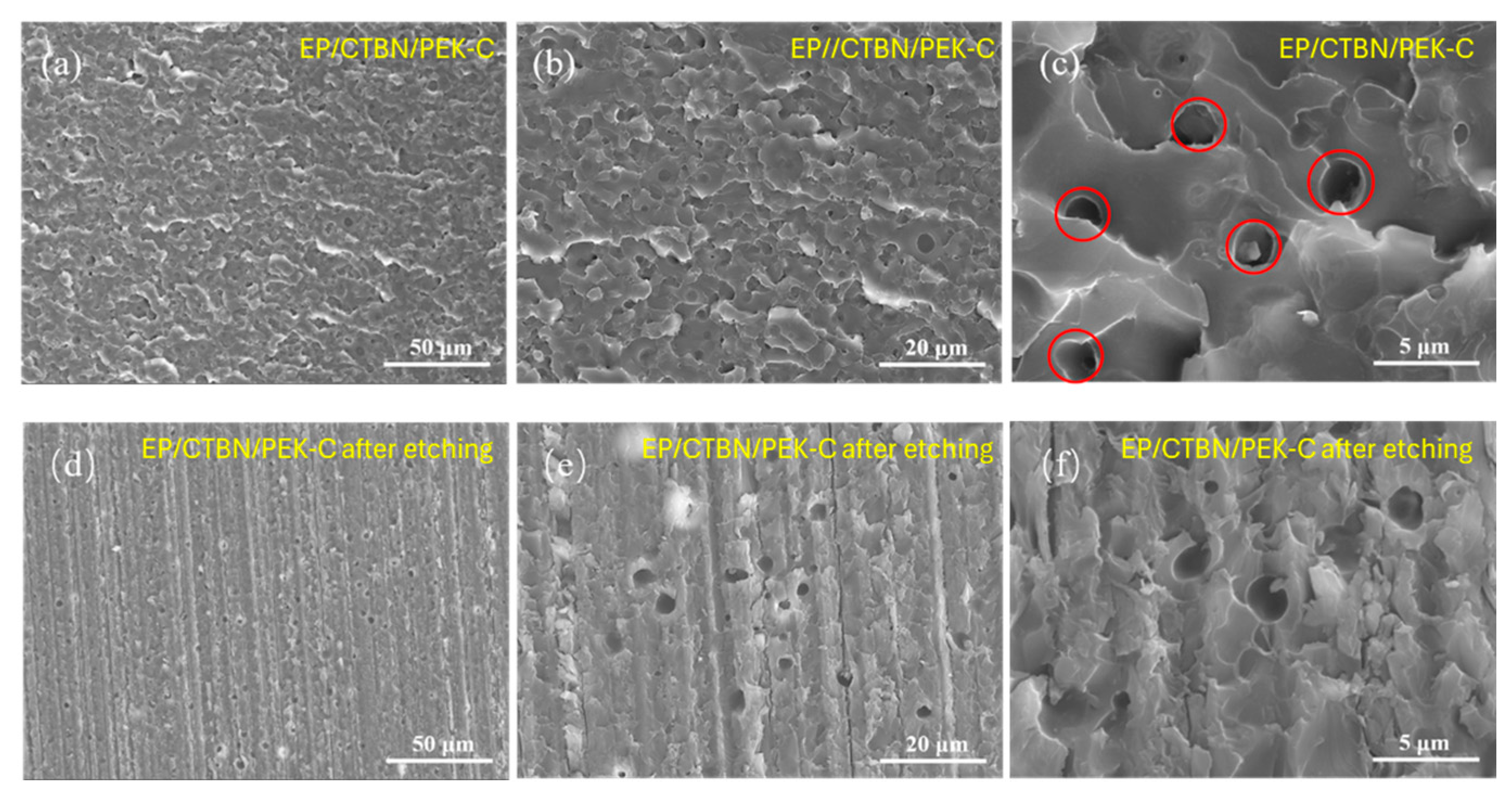
3.5. Microstructure of Modified Resin System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Białkowska, A.; Bakar, M.; Kucharczyk, W.; Zarzyka, I. Hybrid Epoxy Nanocomposites: Improvement in Mechanical Properties and Toughening Mechanisms—A Review. Polymers 2023, 15, 1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, Z.; Li, Y.; Li, G.; Wang, Y.; Zhong, W.-H.; Yang, X. Synergistically effects of copolymer and core-shell particles for toughening epoxy. Polymer 2018, 140, 39–46. [Google Scholar] [CrossRef]
- Mansour, G.; Tsongas, K.; Tzetzis, D. Investigation of the dynamic mechanical properties of epoxy resins modified with elastomers. Compos. Part B Eng. 2016, 94, 152–159. [Google Scholar] [CrossRef]
- Ricciardi, M.; Papa, I.; Langella, A.; Langella, T.; Lopresto, V.; Antonucci, V. Mechanical properties of glass fibre composites based on nitrile rubber toughened modified epoxy resin. Compos. Part B Eng. 2018, 139, 259–267. [Google Scholar] [CrossRef]
- Thomas, R.; Durix, S.; Sinturel, C.; Omonov, T.; Goossens, S.; Groeninckx, G.; Moldenaers, P.; Thomas, S. Cure kinetics, morphology and miscibility of modified DGEBA-based epoxy resin—Effects of a liquid rubber inclusion. Polymer 2007, 48, 1695–1710. [Google Scholar] [CrossRef]
- Gunwant, D.; Sah, P.L.; Zaidi, M. Morphology and micromechanics of liquid rubber toughened epoxies. e-Polymers 2018, 18, 511–527. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, X.; Wang, D.; Fan, X.; Li, H.; Liu, C.; Feng, H.; Wang, R.; Guo, K.; Tian, Y.; et al. Residual stress and thermal properties of rubber-modified epoxy systems for semiconductor package. J. Appl. Polym. Sci. 2022, 139, 51786. [Google Scholar] [CrossRef]
- Sprenger, S. Nanosilica-toughened epoxy resins. Polymers 2020, 12, 1777. [Google Scholar] [CrossRef]
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and mechanism of epoxy resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Thomas, R.; Yumei, D.; Yuelong, H.; Le, Y.; Moldenaers, P.; Weimin, Y.; Czigany, T.; Thomas, S. Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 2008, 49, 278–294. [Google Scholar] [CrossRef]
- Dong, L.; Zhou, W.; Sui, X.; Wang, Z.; Cai, H.; Wu, P.; Zuo, J.; Liu, X. A Carboxyl-Terminated Polybutadiene Liquid Rubber Modified Epoxy Resin with Enhanced Toughness and Excellent Electrical Properties. J. Electron. Mater. 2016, 45, 3776–3785. [Google Scholar] [CrossRef]
- Imanaka, M.; Narita, I.; Nakamura, Y.; Hisaka, S.; Yoshida, S.; Hara, K. Effect of matrix deformability on the fracture properties of epoxy resins modified with core-shell and cross-linked rubber particles. J. Appl. Polym. Sci. 2022, 139, 52316. [Google Scholar] [CrossRef]
- Ali-Asgari Dehaghi, H.; Mazinani, S.; Zaarei, D.; Kalaee, M.; Jabari, H.; Sedaghat, N. Thermal and morphological characteristics of solution blended epoxy/NBR compound. J. Therm. Anal. Calorim. 2013, 114, 185–194. [Google Scholar] [CrossRef]
- Ma, H.; Aravand, M.A.; Falzon, B.G. Phase morphology and mechanical properties of polyetherimide modified epoxy resins: A comparative study. Polymer 2019, 179, 121640. [Google Scholar] [CrossRef]
- Szymańska, J.; Bakar, M.; Białkowska, A.; Kostrzewa, M. Study on the adhesive properties of reactive liquid rubber toughened epoxy-clay hybrid nanocomposites. J. Polym. Eng. 2018, 38, 231–238. [Google Scholar] [CrossRef]
- Tripathi, G.; Srivastava, D. Effect of carboxyl-terminated poly(butadiene-co-acrylonitrile) (CTBN) concentration on thermal and mechanical properties of binary blends of diglycidyl ether of bisphenol-A (DGEBA) epoxy resin. Mat. Sci. Eng. A Struct. 2007, 443, 262–269. [Google Scholar] [CrossRef]
- Chikhi, N.; Fellahi, S.; Bakar, M. Modification of epoxy resin using reactive liquid (ATBN) rubber. Eur. Polym. J. 2002, 38, 251–264. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Zhang, H.; Wang, H.; Liu, L.; Xu, Z.; Liu, P.; Peng, Z. Influence of addition of hydroxyl-terminated liquid nitrile rubber on dielectric properties and relaxation behavior of epoxy resin. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2258–2269. [Google Scholar] [CrossRef]
- Lee, S.-E.; Jeong, E.; Lee, M.Y.; Lee, M.-K.; Lee, Y.-S. Improvement of the mechanical and thermal properties of polyethersulfone-modified epoxy composites. J. Ind. Eng. Chem. 2016, 33, 73–79. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, B.; Yang, J.-P.; Xu, G.-S.; Fu, S.-Y. Preparation and cryogenic mechanical properties of epoxy resins modified by poly(ethersulfone). J. Polym. Sci. Part A Polym. Chem. 2008, 46, 612–624. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Yuan, Y.; Gao, C.; Cui, Y.; Li, S.; Wang, H.; Peng, C.; Liu, X.; Wu, Z. A new strategy to improve the toughness of epoxy thermosets by introducing the thermoplastic epoxy. Polymer 2022, 240, 124518. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Wang, P.; Li, Y.; Sui, J. Mechanical and thermal properties of epoxy resins modified by a novel thermoplastic-polyimide. Fiber Polym. 2021, 22, 205–212. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Robert, T.M.; Mathew, D.; Suma, D.D.; Thomas, D. Novel epoxy resin adhesives toughened by functionalized poly (ether ether ketone) s. Int. J. Adhes. Adhes. 2021, 106, 102816. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Su, K.; Feng, H.; Wang, D.; Qu, C. A comparative study on the rheological, thermal, and mechanical performance of epoxy resin modified with thermoplastics. J. Adhes. Sci. Technol. 2021, 35, 1393–1403. [Google Scholar] [CrossRef]
- Mathis, E.; Michon, M.-L.; Billaud, C.; Vergelati, C.; Clarke, N.; Jestin, J.; Long, D.R. Controlling the morphology in epoxy/thermoplastic systems. ACS Appl. Polym. Mater. 2022, 4, 2091–2104. [Google Scholar] [CrossRef]
- Jung, H.-S.; Park, Y.; Nah, C.-W.; Lee, J.-C.; Kim, K.-Y.; Lee, C.S. Evaluation of the mechanical properties of polyether sulfone-toughened epoxy resin for carbon fiber composites. Fiber Polym. 2021, 22, 184–195. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Z.; Tusiime, R.; Cheng, C.; Sun, Z.; Xu, L.; Liu, Y.; Jiang, M.; Zhou, J.; Zhang, H. Highly improving the mechanical and thermal properties of epoxy resin via blending with polyetherketone cardo. Compos. Commun. 2019, 13, 80–84. [Google Scholar] [CrossRef]
- GB/T 2567-2008; Test Method for Impact Resistance of Resin Casting Body. Standards Press of China: Beijing, China, 2008.
- GB/T 2571-1995; Test Method for Impact Resistance of Resin Casting Body. Standards Press of China: Beijing, China, 1995.
- Yin, X.; Xie, Z.; Liu, Q.; Yuan, X.; Hou, X.; Zhao, J. Synergistic toughening of epoxy resin by CTBN and CM-β-CD. J. Appl. Polym. Sci. 2021, 138, 51248. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Z.; Patil, D.; Bajaj, D.; Verghese, N.; Jiang, Z.; Sue, H.-J. Mechanical properties of reactive polyetherimide-modified tetrafunctional epoxy systems. Polymer 2023, 270, 125763. [Google Scholar] [CrossRef]
- Ramos, V.D.; da Costa, H.M.; Soares, V.L.; Nascimento, R.S. Hybrid composites of epoxy resin modified with carboxyl terminated butadiene acrilonitrile copolymer and fly ash microspheres. Polym. Test. 2005, 24, 219–226. [Google Scholar] [CrossRef]
- Tripathi, G.; Srivastava, D. Studies on the physico-mechanical and thermal characteristics of blends of DGEBA epoxy, 3, 4 epoxy cyclohexylmethyl, 3′, 4′-epoxycylohexane carboxylate and carboxyl terminated butadiene co-acrylonitrile (CTBN). Mat. Sci. Eng. A Struct. 2008, 496, 483–493. [Google Scholar] [CrossRef]
- Akbari, R.; Beheshty, M.H.; Shervin, M. Toughening of dicyandiamide-cured DGEBA-based epoxy resins by CTBN liquid rubber. Iran. Polym. J. 2013, 22, 313–324. [Google Scholar] [CrossRef]
- Nigam, V.; Setua, D.K.; Mathur, G.N. Characterization of liquid carboxy terminated copolymer of butadiene acrylonitrile modified epoxy resin. Polym. Eng. Sci. 1999, 39, 1425–1432. [Google Scholar] [CrossRef]
- Januszewski, R.; Dutkiewicz, M.; Nowicki, M.; Szołyga, M.; Kownacki, I. Synthesis and properties of epoxy resin modified with novel reactive liquid rubber-based systems. Ind. Eng. Chem. Res. 2021, 60, 2178–2186. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, R.; Liu, H. Investigation on viscoelastic phase separation of phenolphthalein poly (ether ether ketone)/epoxy blends system. Polym. Polym. Compos. 2020, 28, 199–208. [Google Scholar] [CrossRef]
- Mimura, K.; Ito, H.; Fujioka, H. Improvement of thermal and mechanical properties by control of morphologies in PES-modified epoxy resins. Polymer 2000, 41, 4451–4459. [Google Scholar] [CrossRef]
- Son, B.T.; Trung, N.N.; Lim, D.-G.; Shin, S.; Bae, J.-Y. Improvements in thermal, mechanical, and dielectric properties of epoxy resin by chemical modification with a novel amino-terminated liquid-crystalline copoly (ester amide). React. Funct. Polym. 2012, 72, 542–548. [Google Scholar] [CrossRef]
- Liu, X.-F.; Luo, X.; Liu, B.-W.; Zhong, H.-Y.; Guo, D.-M.; Yang, R.; Chen, L.; Wang, Y.-Z. Toughening epoxy resin using a liquid crystalline elastomer for versatile application. ACS Appl. Polym. Mater. 2019, 1, 2291–2301. [Google Scholar] [CrossRef]
- Tiwari, S.N.; Agnihotri, P.K. Effect of crumb rubber addition on the deformation and fracture behavior of ductile epoxy matrix. J. Appl. Polym. Sci. 2023, 140, e53255. [Google Scholar] [CrossRef]
- Ozturk, A.; Kaynak, C.; Tincer, T. Effects of liquid rubber modification on the behaviour of epoxy resin. Eur. Polym. J. 2001, 37, 2353–2363. [Google Scholar] [CrossRef]
- Ahmad, H.; Shah, A.; Afaq, S.K.; Azad, M.; Arif, S.; Siddiqi, M.; Xie, L. Development and characterization of kevlar and glass fibers reinforced epoxy/vinyl ester hybrid resin composites. Polym. Compos. 2024, 45, 8133–8146. [Google Scholar] [CrossRef]
- Dispenza, C.; Spadaro, G.; McGrail, P.T. The influence of the rubber carboxyl functionality on the processability and properties of solid rubber modified epoxy mixtures. Macromol. Chem. Phys. 2005, 206, 393–403. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Y.; Xu, Y.; Li, S. Synthesis and characterization of a liquid crystalline epoxy containing azomethine mesogen for modification of epoxy resin. J. Appl. Polym. Sci. 2007, 105, 1861–1868. [Google Scholar] [CrossRef]
- Morancho, J.; Salla, J. Relaxation in partially cured samples of an epoxy resin and of the same resin modified with a carboxyl-terminated rubber. Polymer 1999, 40, 2821–2828. [Google Scholar] [CrossRef]
- Dadfar, M.; Ghadami, F. Effect of rubber modification on fracture toughness properties of glass reinforced hot cured epoxy composites. Mater. Des. 2013, 47, 16–20. [Google Scholar] [CrossRef]
- Vijayan, P.P.; Pionteck, J.; Huczko, A.; Puglia, D.; Kenny, J.M.; Thomas, S. Liquid rubber and silicon carbide nanofiber modified epoxy nanocomposites: Volume shrinkage, cure kinetics and properties. Compos. Sci. Technol. 2014, 102, 65–73. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Fox, B. Structural and material properties of a rapidly cured thermoplastic-toughened epoxy system. J. Appl. Polym. Sci. 2009, 113, 485–491. [Google Scholar] [CrossRef]
- Xi, J.; Yu, Z. Toughening mechanism of rubber reinforced epoxy composites by thermal and microwave curing. J. Appl. Polym. Sci. 2018, 135, 45767. [Google Scholar] [CrossRef]
- Bian, X.; Tuo, R.; Yang, W.; Zhang, Y.; Xie, Q.; Zha, J.; Lin, J.; He, S. Mechanical, thermal, and electrical properties of BN–epoxy composites modified with carboxyl-terminated butadiene nitrile liquid rubber. Polymers 2019, 11, 1548. [Google Scholar] [CrossRef]
- Ocando, C.; Tercjak, A.; Mondragon, I. Nanostructured systems based on SBS epoxidized triblock copolymers and well-dispersed alumina/epoxy matrix composites. Compos. Sci. Technol. 2010, 70, 1106–1112. [Google Scholar] [CrossRef]
- Ocando, C.; Tercjak, A.; Serrano, E.; Ramos, J.A.; Corona-Galván, S.; Parellada, M.D.; Fernández-Berridi, M.J.; Mondragon, I. Micro- and macrophase separation of thermosetting systems modified with epoxidized styrene-block-butadiene-block-styrene linear triblock copolymers and their influence on final mechanical properties. Polym. Int. 2008, 57, 1333–1342. [Google Scholar] [CrossRef]
- Pearson, R.A.; Yee, A.F. Influence of particle size and particle size distribution on toughening mechanisms in rubber-modified epoxies. J. Mater. Sci. 1991, 26, 3828–3844. [Google Scholar] [CrossRef]
- Klingler, A.; Bajpai, A.; Wetzel, B. The effect of block copolymer and core-shell rubber hybrid toughening on morphology and fracture of epoxy-based fibre reinforced composites. Eng. Fract. Mech. 2018, 203, 81–101. [Google Scholar] [CrossRef]
- Gilbert, A.H.; Bucknall, C.B. Epoxy resin toughened with thermoplastic. Makromol. Chemie. Macromol. Symp. 1991, 45, 289–298. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lai, Y.; Xu, P.; Ma, J.; Xu, Y.; Yang, X. Synergistic Effects of Liquid Rubber and Thermoplastic Particles for Toughening Epoxy Resin. Polymers 2024, 16, 2775. https://doi.org/10.3390/polym16192775
Wang Z, Lai Y, Xu P, Ma J, Xu Y, Yang X. Synergistic Effects of Liquid Rubber and Thermoplastic Particles for Toughening Epoxy Resin. Polymers. 2024; 16(19):2775. https://doi.org/10.3390/polym16192775
Chicago/Turabian StyleWang, Zhaodi, Yuanchang Lai, Peiwen Xu, Junchi Ma, Yahong Xu, and Xin Yang. 2024. "Synergistic Effects of Liquid Rubber and Thermoplastic Particles for Toughening Epoxy Resin" Polymers 16, no. 19: 2775. https://doi.org/10.3390/polym16192775
APA StyleWang, Z., Lai, Y., Xu, P., Ma, J., Xu, Y., & Yang, X. (2024). Synergistic Effects of Liquid Rubber and Thermoplastic Particles for Toughening Epoxy Resin. Polymers, 16(19), 2775. https://doi.org/10.3390/polym16192775





