Polyester Adhesives via One-Pot, One-Step Copolymerization of Cyclic Anhydride, Epoxide, and Lactide
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Instruments
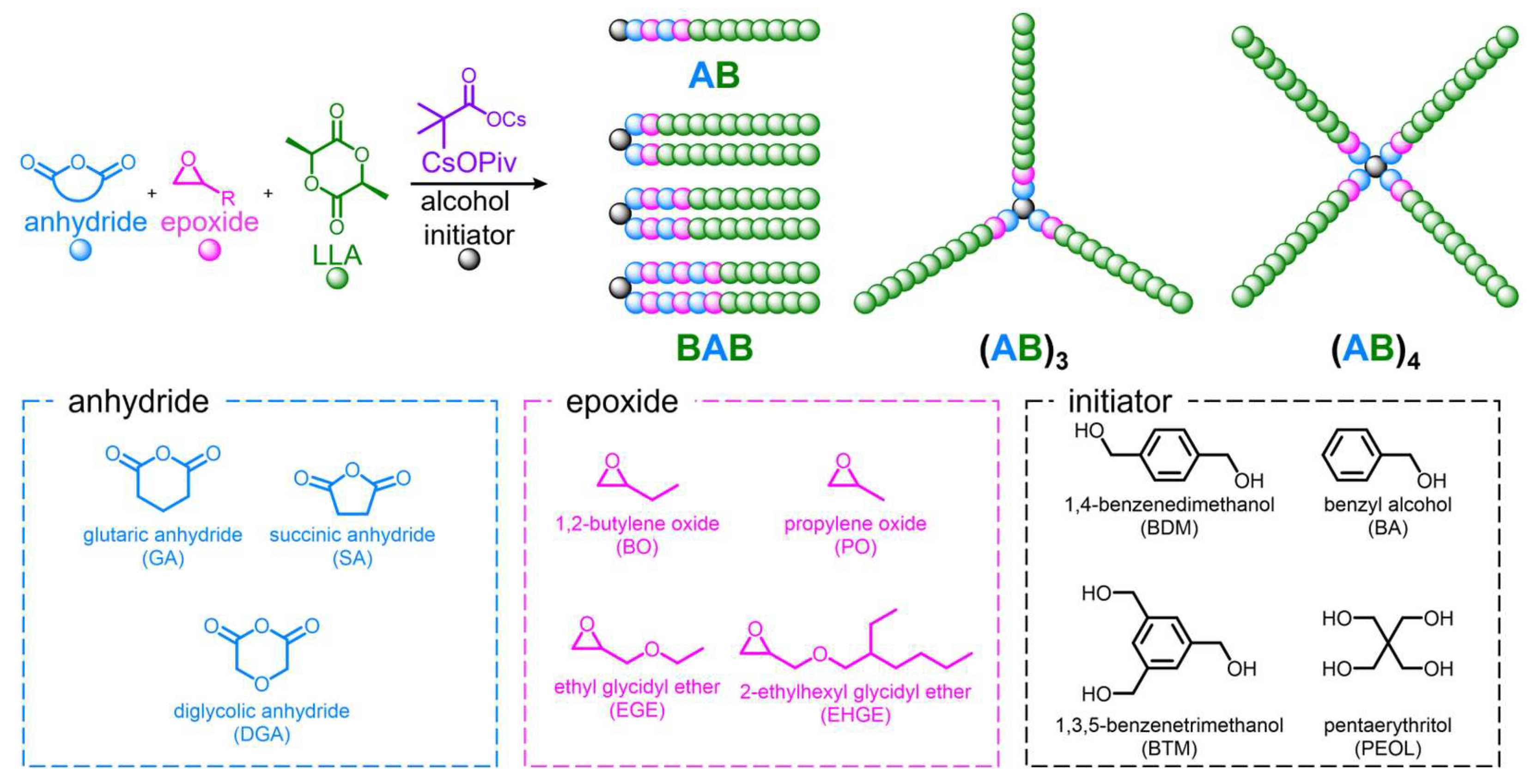
2.3. Typical Synthesis of the Block Copolymers via Self-Switchable Polymerization
2.3.1. Synthesis of PLLA-tb-poly(GA-alt-BO)-tb-PLLA (P1)
2.3.2. Synthesis of PLLA-b-poly(SA-alt-BO)-b-PLLA (P6)
2.3.3. Synthesis of PLLA-b-poly(DGA-alt-PO)-b-PLLA (P7)
2.3.4. Synthesis of PLLA-tb-poly(GA-alt-PO)-tb-PLLA (P8)
2.3.5. Synthesis of PLLA-tb-poly(GA-alt-EGE)-tb-PLLA (P9)
2.3.6. Synthesis of PLLA-tb-poly(GA-alt-EHGE)-tb-PLLA (P10)
2.3.7. Synthesis of AB-type poly(GA-alt-BO)-tb-PLLA (P11)
2.3.8. Synthesis of (poly(GA-alt-BO)-tb-PLLA)3 (P12)
2.3.9. Synthesis of PLLA-tb-poly(GA-alt-BO)-tb-PLLA (10k) (P13)
2.3.10. Synthesis of PLLA-tb-poly(GA-alt-BO)-tb-PLLA (40k) (P14)
2.3.11. Synthesis of (poly(GA-alt-BO)-tb-PLLA)4 (80k) (P15)
3. Results
3.1. Self-Switchable Polymerization Using GA, BO, and LLA
3.2. Thermal Properties of PLLA-tb-poly(GA-alt-BO)-tb-PLLA
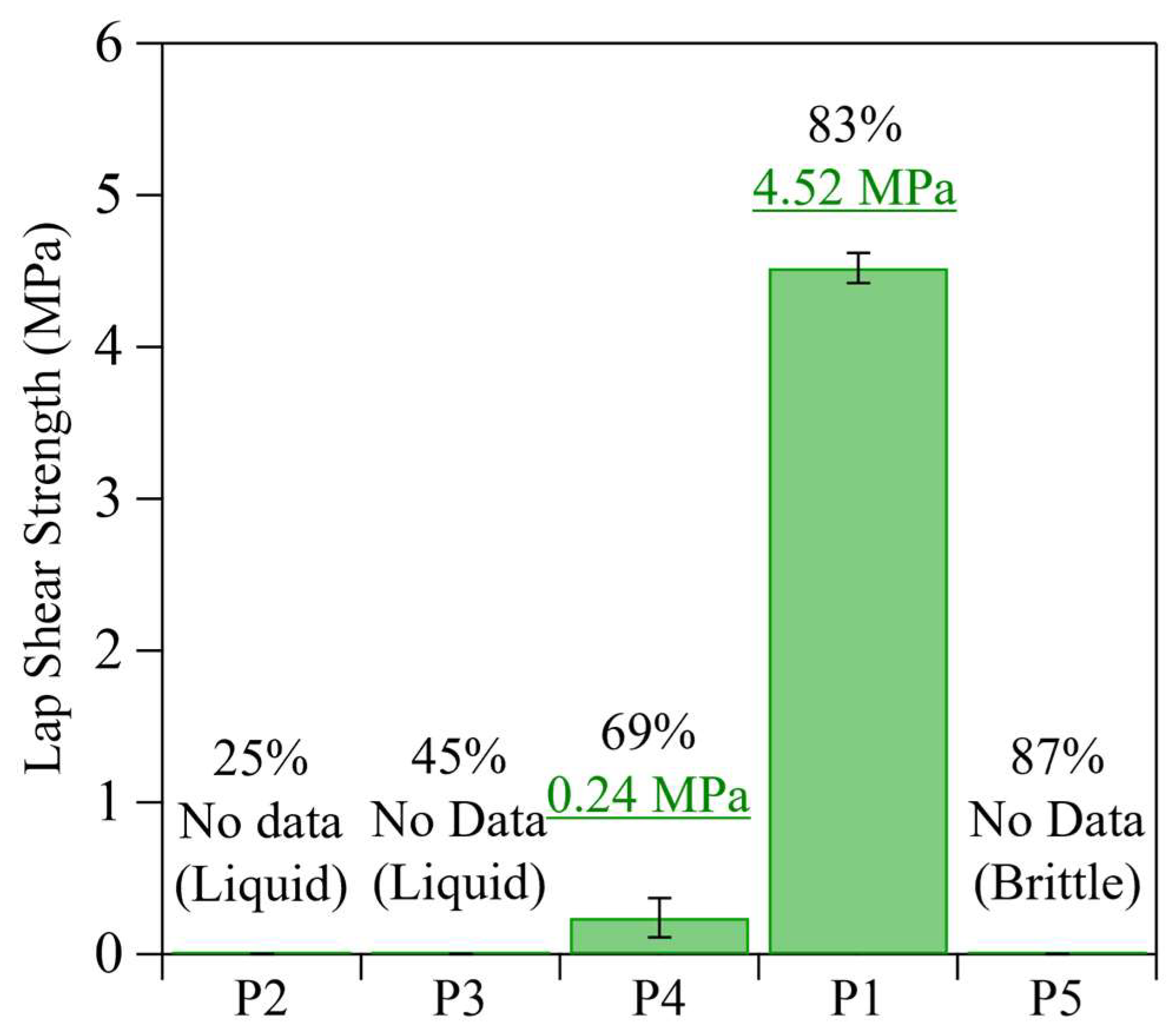
3.3. Evaluation of Adhesive Properties Using Lap Shear Test
3.4. Effect of Monomer Combinations
3.5. Effect of Branched Structure and Molecular Weight on Adhesive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cohn, D.; Salomon, A.H. Designing biodegradable multiblock PCL/PLA thermoplastic elastomers. Biomaterials 2005, 26, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Vilay, V.; Mariatti, M.; Ahmad, Z.; Pasomsouk, K.; Todo, M. Improvement of microstructures and properties of biodegradable PLLA and PCL blends compatibilized with a triblock copolymer. Mater. Sci. Eng. A 2010, 527, 6930–6937. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Sonseca, A.; Gimenez, E.; Marcos-Fernandez, A.; Kenny, J.M. Synthesis and characterization of PCL–PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013, 49, 893–903. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Marcos-Fernández, A.; Fernández-Torres, A.; Kenny, J.M.; Peponi, L. Synthesis of PLLA-b-PCL-b-PLLA linear tri-block copolymers and their corresponding poly(ester-urethane)s: Effect of the molecular weight on their crystallisation and mechanical properties. RSC Adv. 2014, 4, 8510–8524. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.; Bian, X.; Zhang, B.; Sun, B.; Liu, Y.; Li, G.; Chen, X. Toughening modification of PLLA with PCL in the presence of PCL-b-PLLA diblock copolymers as compatibilizer. Polym. Adv. Techs 2019, 30, 963–972. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, S.-H.; Kim, S.H.; Lee, Y.M.; Kim, Y.H. Synthesis and characterization of poly(L-lactide)−poly(ε-caprolactone) multiblock copolymers. Macromolecules 2003, 36, 5585–5592. [Google Scholar] [CrossRef]
- Shin, J.; Martello, M.T.; Shrestha, M.; Wissinger, J.E.; Tolman, W.B.; Hillmyer, M.A. Pressure-sensitive adhesives from renewable triblock copolymers. Macromolecules 2011, 44, 87–94. [Google Scholar] [CrossRef]
- Krajovic, D.M.; Haugstad, G.; Hillmyer, M.A. Crystallinity-independent toughness in renewable poly(L-lactide) triblock plastics. Macromolecules 2024, 57, 2818–2834. [Google Scholar] [CrossRef]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: Structure–property relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef] [PubMed]
- Pappuru, S.; Chakraborty, D. Progress in metal-free cooperative catalysis for the ring-opening copolymerization of cyclic anhydrides and epoxides. Eur. Polym. J. 2019, 121, 109276. [Google Scholar] [CrossRef]
- Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Martinez, J.; Alonso-Moreno, C.; Fernández-Baeza, J.; Tejeda, J.; Niza, E.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Alternating Copolymerization of Epoxides and Anhydrides Catalyzed by Aluminum Complexes. ACS Omega 2018, 3, 17581–17589. [Google Scholar] [CrossRef]
- Hirschmann, M.; Andriani, F.; Fuoco, T. Functional and degradable copolyesters by ring-opening copolymerization of epoxides and anhydrides. Eur. Polym. J. 2023, 183, 111766. [Google Scholar] [CrossRef]
- Xie, X.; Huo, Z.; Jang, E.; Tong, R. Recent advances in enantioselective ring-opening polymerization and copolymerization. Commun. Chem. 2023, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Liu, S.; Li, Z. Phosphazene/lewis acids as highly efficient cooperative catalyst for synthesis of high-molecular-weight polyesters by ring-opening alternating copolymerization of epoxide and anhydride. J. Polym. Sci. 2020, 58, 803–810. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Kim, Y.-W.; Shin, J. Preparation and characterization of a renewable pressure-sensitive adhesive system derived from ε-decalactone, L-Lactide, epoxidized soybean oil, and rosin ester. ACS Sustain. Chem. Eng. 2015, 3, 2309–2320. [Google Scholar] [CrossRef]
- Gregory, G.L.; Sulley, G.S.; Carrodeguas, L.P.; Chen, T.T.D.; Santamarti, A.; Terrill, N.J.; Lee, K.-Y.; Williams, C.K. Triblock polyester thermoplastic elastomers with semi-aromatic polymer end blocks by ring-opening copolymerization. Chem. Sci. 2020, 11, 6567–6581. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.L.; Williams, C.K. Exploiting sodium coordination in alternating monomer sequences to toughen degradable block polyester thermoplastic elastomers. Macromolecules 2022, 55, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Pang, X.; Chen, X. Self-switchable polymerization: A smart approach to sequence-controlled degradable copolymers. Macromolecules 2022, 55, 1879–1893. [Google Scholar] [CrossRef]
- Xia, X.; Suzuki, R.; Takojima, K.; Jiang, D.-H.; Isono, T.; Satoh, T. Smart access to sequentially and architecturally controlled block polymers via a simple catalytic polymerization system. ACS Catal. 2021, 11, 5999–6009. [Google Scholar] [CrossRef]
- Xia, X.; Suzuki, R.; Gao, T.; Isono, T.; Satoh, T. One-step synthesis of sequence-controlled multiblock polymers with up to 11 segments from monomer mixture. Nat. Commun. 2022, 13, 163. [Google Scholar] [CrossRef]
- Xia, X.; Gao, T.; Li, F.; Suzuki, R.; Isono, T.; Satoh, T. Multidimensional control of repeating unit/sequence/topology for one-step synthesis of block polymers from monomer mixtures. J. Am. Chem. Soc. 2022, 144, 17905–17915. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Gao, T.; Li, F.; Suzuki, R.; Isono, T.; Satoh, T. Sequential polymerization from complex monomer mixtures: Access to multiblock copolymers with adjustable sequence, topology, and gradient strength. Macromolecules 2023, 56, 92–103. [Google Scholar] [CrossRef]
- Ota, I.; Suzuki, R.; Mizukami, Y.; Xia, X.; Tajima, K.; Yamamoto, T.; Li, F.; Isono, T.; Satoh, T. Organobase-catalyzed ring-opening copolymerization of cyclic anhydrides and Oxetanes: Establishment and application in block copolymer synthesis. Macromolecules 2024, 57, 3741–3750. [Google Scholar] [CrossRef]
- Yuntawattana, N.; Gregory, G.L.; Carrodeguas, L.P.; Williams, C.K. Switchable polymerization catalysis using a tin(II) catalyst and commercial monomers to toughen poly(l-lactide). ACS Macro Lett. 2021, 10, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Gao, X.; Zhang, F.; Zhou, W.; Qi, G.; Song, K.; Cheng, S.; Ding, Y.; Winter, H.H. Triblock Elastomeric Vitrimers: Preparation, Morphology, Rheology, and Applications. Macromolecules 2022, 55, 10900–10911. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Antonov, S.V.; Ilyin, S.O. Asphaltenes as a tackifier for hot-melt adhesives based on the styrene-isoprene-styrene block copolymer. Polym. Eng. Sci. 2020, 60, 2224–2234. [Google Scholar] [CrossRef]
- Maw, M.R.; Tanas, A.K.; Dashtimoghadam, E.; Nilitina, E.A.; Ivanov, D.A.; Dobrynin, A.V.; V.-Varnosfaderani, M.; Sheiko, S.S. Bottlebrush Thermoplastic Elastomers as Hot-Melt Pressure-Sensitive Adhesives. ACS Appl. Mater. Interfaces 2023, 15, 41870–41879. [Google Scholar] [CrossRef] [PubMed]
- Arbenz, A.; Avérous, L. Synthesis and characterization of fully biobased aromatic polyols–oxybutylation of condensed tannins towards new macromolecular architectures. RSC Adv. 2014, 4, 61564–61572. [Google Scholar] [CrossRef]
- Rohles, C.M.; Gläser, L.; Kohlstedt, M.; Gießelmann, G.; Pearson, S.; del Campo, A.; Becker, J.; Wittmann, C. A bio-based route to the carbon-5 chemical glutaric acid and to bionylon-6,5 using metabolically engineered Corynebacterium glutamicum. Green Chem. 2018, 20, 4662–4674. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V.L. Poly-l-lactic acid (PLLA)-based biomaterials for regenerative medicine: A review on processing and applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, T.; Norström, E.; Khabbaz, F.; Brücher, J.; Malmström, E.; Fogelström, L. A fully bio-based wood adhesive valorising hemicellulose-rich sidestreams from the pulp industry. Green Chem. 2021, 23, 3322–3333. [Google Scholar] [CrossRef]






| Sample | Monomer Combination | [CsOPiv]/[BDM]0/ [anhydride]0/ [epoxide]0/[LLA]0 | fLLA b | Time (h) | Mn,theo. c | Mn,NMR d | Mn,sec [Ð] e | FLLA f | Tg g | Td,5% h |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | GA/BO/ LLA | 1/1/20/80/110 | 0.81 | 5.5 | 19,700 | 17,800 | 3990 [1.72] | 0.83 | 19.0 | 218 |
| P2 | GA/BO/ LLA | 1/1/65/260/55 | 0.40 | 13.5 | 20,200 | 14,800 | 9550 [1.20] | 0.25 | −15.6 | 244 |
| P3 | GA/BO/ LLA | 1/1/45/180/85 | 0.59 | 7.0 | 20,800 | 18,300 | 8090 [1.30] | 0.45 | −6.1 | 189 |
| P4 | GA/BO/ LLA | 1/1/36/144/110 | 0.70 | 6.0 | 22,700 | 28,200 | 5200 [1.57] | 0.69 | 13.7 | 196 |
| P5 | GA/BO/ LLA | 1/1/11/44/130 | 0.90 | 4.5 | 20,900 | 23,500 | 6420 [1.36] | 0.87 | 32.5 | 184 |
| P6 | SA/BO/ LLA | 1/1/23/92/110 | 0.80 | 6.0 | 20,000 | 20,000 | 8610 [1.46] | 0.81 | 31.4 | 207 |
| P7 | DGA/BO/ LLA | 1/1/20/80/110 | 0.81 | 3.5 | 19,800 | 23,700 | 11,200 [1.18] | 0.80 | 34.9 | 216 |
| P8 | GA/PO/ LLA | 1/1/25/100/110 | 0.79 | 5.0 | 20,300 | 22,800 | 5380 [1.51] | 0.74 | 15.7 | 194 |
| P9 | GA/EGE/ LLA | 1/1/20/80/110 | 0.79 | 4.0 | 20,300 | 24,700 | 4160 [1.84] | 0.77 | 1.4 | 188 |
| P10 | GA/EHGE/ LLA | 1/1/15/60/120 | 0.79 | 6.5 | 21,900 | 23,000 | 8260 [1.39] | 0.77 | 1.3 | 210 |
| Sample | Arm | Ini. | [CsOPiv]/ [Initiator]0/[GA]0/ [BO]0/[LLA]0 | fLLA b | Time (h) | Mn,theo. c | Mn,NMR d | Mn,sec [Ð] e | FLLA f | Tg g | Td,5% h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P11 | 1 | BA | 1/1/12/48/60 | 0.80 | 5.5 | 11,000 | 10,800 | 5060 [1.59] | 0.80 | 23.2 | 254 |
| P12 | 3 | BTM | 1/1/35/140/170 | 0.79 | 8.0 | 31,200 | 32,600 | 8110 [1.22] | 0.76 | 23.2 | 226 |
| P13 | 2 | BDM | 1/1/10/40/55 | 0.81 | 4.0 | 9930 | 11,800 | 2180 [2.12] | 0.78 | 21.0 | 190 |
| P14 | 2 | BDM | 1/1/42/168/220 | 0.80 | 9.0 | 39,700 | 38,100 | 6510 [1.69] | 0.80 | 25.0 | 224 |
| P15 | 4 | PEOL | 1/1/84/336/440 | 0.80 | 23.0 | 79,200 | N.D. | 9170 [1.62] | 0.79 | 21.2 | 235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, R.; Miwa, T.; Nunokawa, R.; Sumi, A.; Ando, M.; Takahashi, K.; Takagi, A.; Yamamoto, T.; Tajima, K.; Li, F.; et al. Polyester Adhesives via One-Pot, One-Step Copolymerization of Cyclic Anhydride, Epoxide, and Lactide. Polymers 2024, 16, 2767. https://doi.org/10.3390/polym16192767
Suzuki R, Miwa T, Nunokawa R, Sumi A, Ando M, Takahashi K, Takagi A, Yamamoto T, Tajima K, Li F, et al. Polyester Adhesives via One-Pot, One-Step Copolymerization of Cyclic Anhydride, Epoxide, and Lactide. Polymers. 2024; 16(19):2767. https://doi.org/10.3390/polym16192767
Chicago/Turabian StyleSuzuki, Ryota, Toshiki Miwa, Ryosuke Nunokawa, Ayaka Sumi, Masaru Ando, Katsuaki Takahashi, Akira Takagi, Takuya Yamamoto, Kenji Tajima, Feng Li, and et al. 2024. "Polyester Adhesives via One-Pot, One-Step Copolymerization of Cyclic Anhydride, Epoxide, and Lactide" Polymers 16, no. 19: 2767. https://doi.org/10.3390/polym16192767
APA StyleSuzuki, R., Miwa, T., Nunokawa, R., Sumi, A., Ando, M., Takahashi, K., Takagi, A., Yamamoto, T., Tajima, K., Li, F., Isono, T., & Satoh, T. (2024). Polyester Adhesives via One-Pot, One-Step Copolymerization of Cyclic Anhydride, Epoxide, and Lactide. Polymers, 16(19), 2767. https://doi.org/10.3390/polym16192767








