Preparation and Application of a Novel Liquid Oxygen-Compatible Epoxy Resin of Fluorinated Glycidyl Amine with Low Viscosity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TFEPA Monomer
2.3. Synthesis of PDGEP
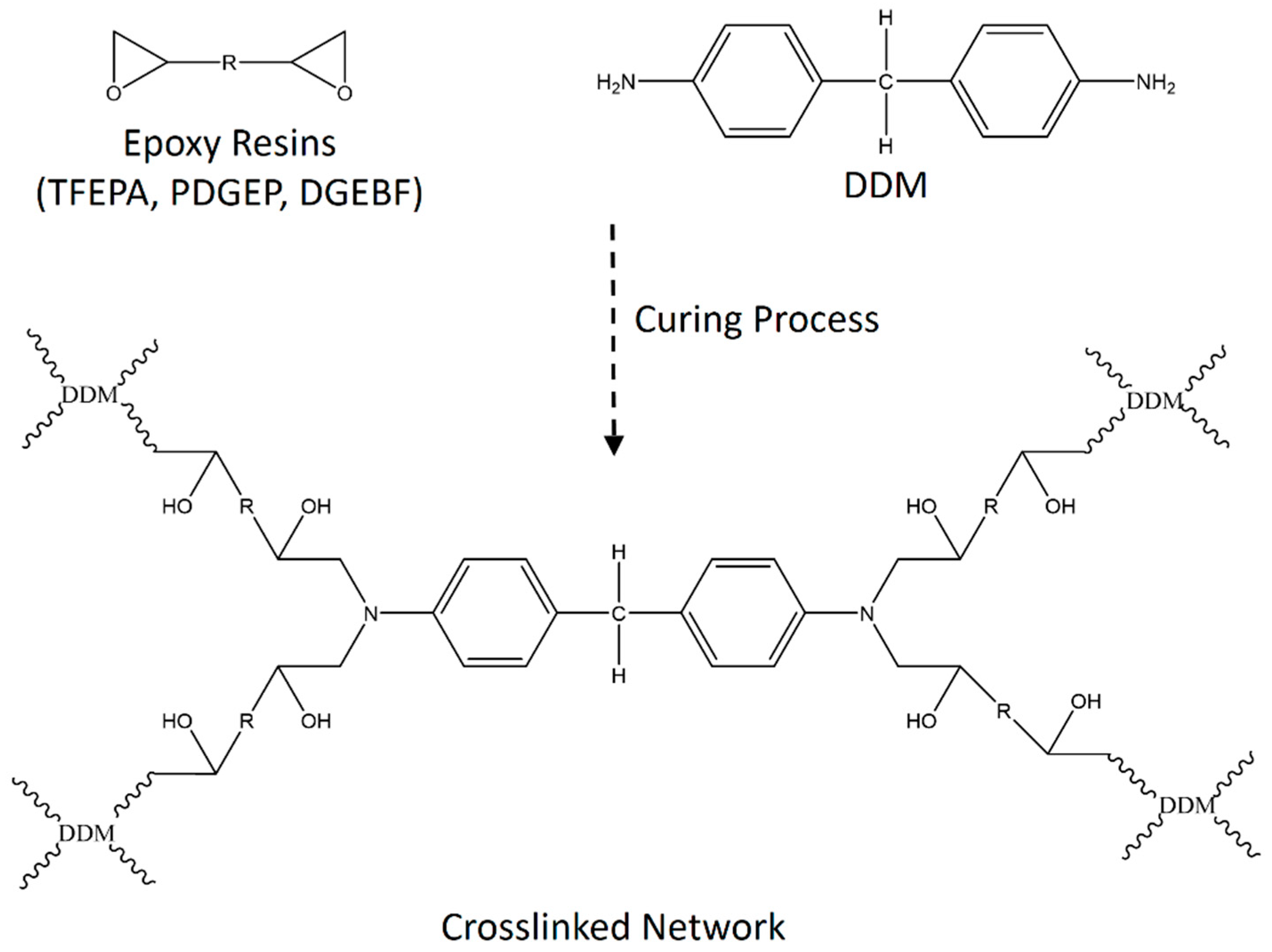
2.4. Preparation of Epoxy Matrix
2.5. Preparation of Carbon Fiber-Reinforced Epoxy Matrix Composites
2.6. Characterization
3. Results and Discussion
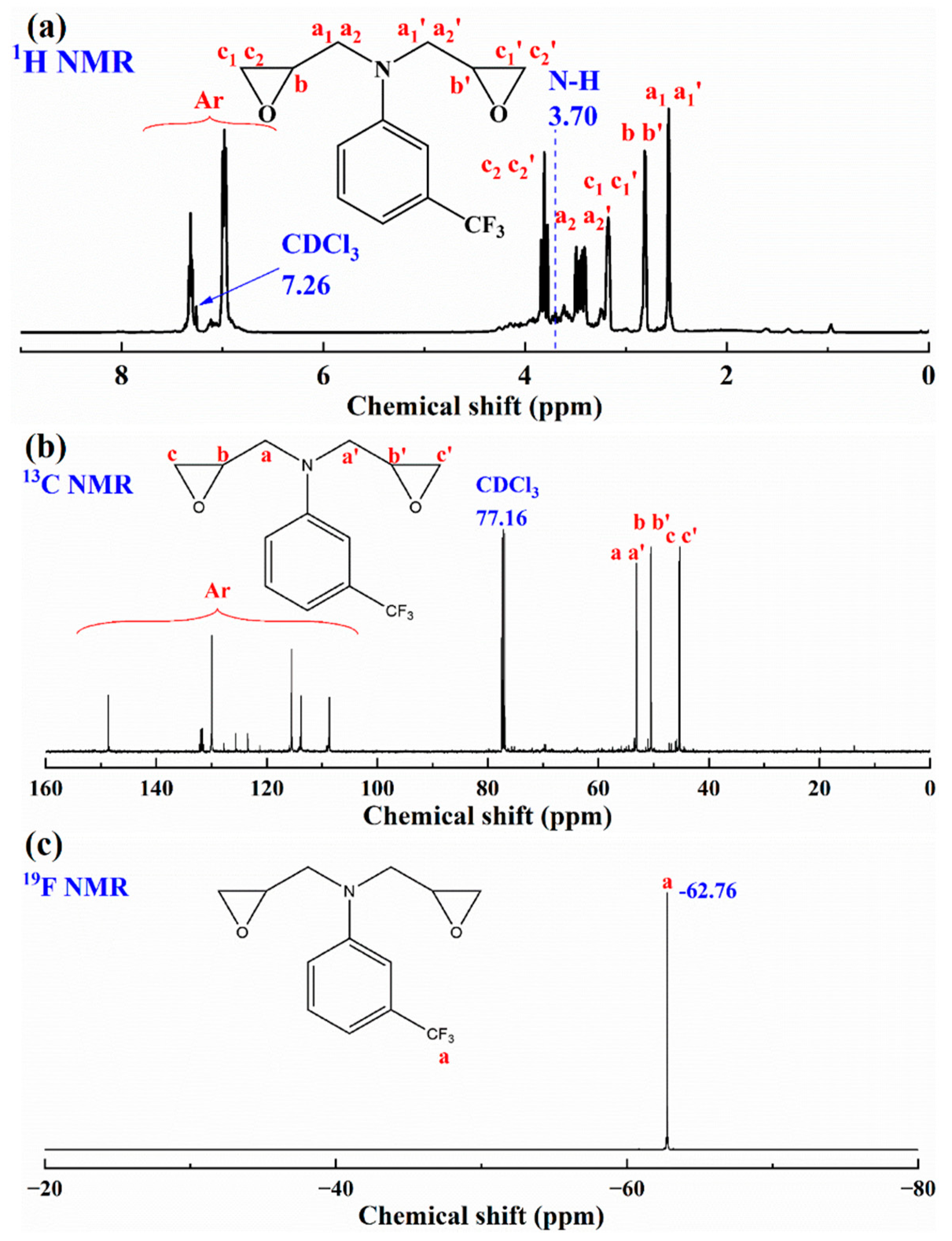
3.1. Characterization of TFEPA Monomer
3.2. Rheology Performance Analyses
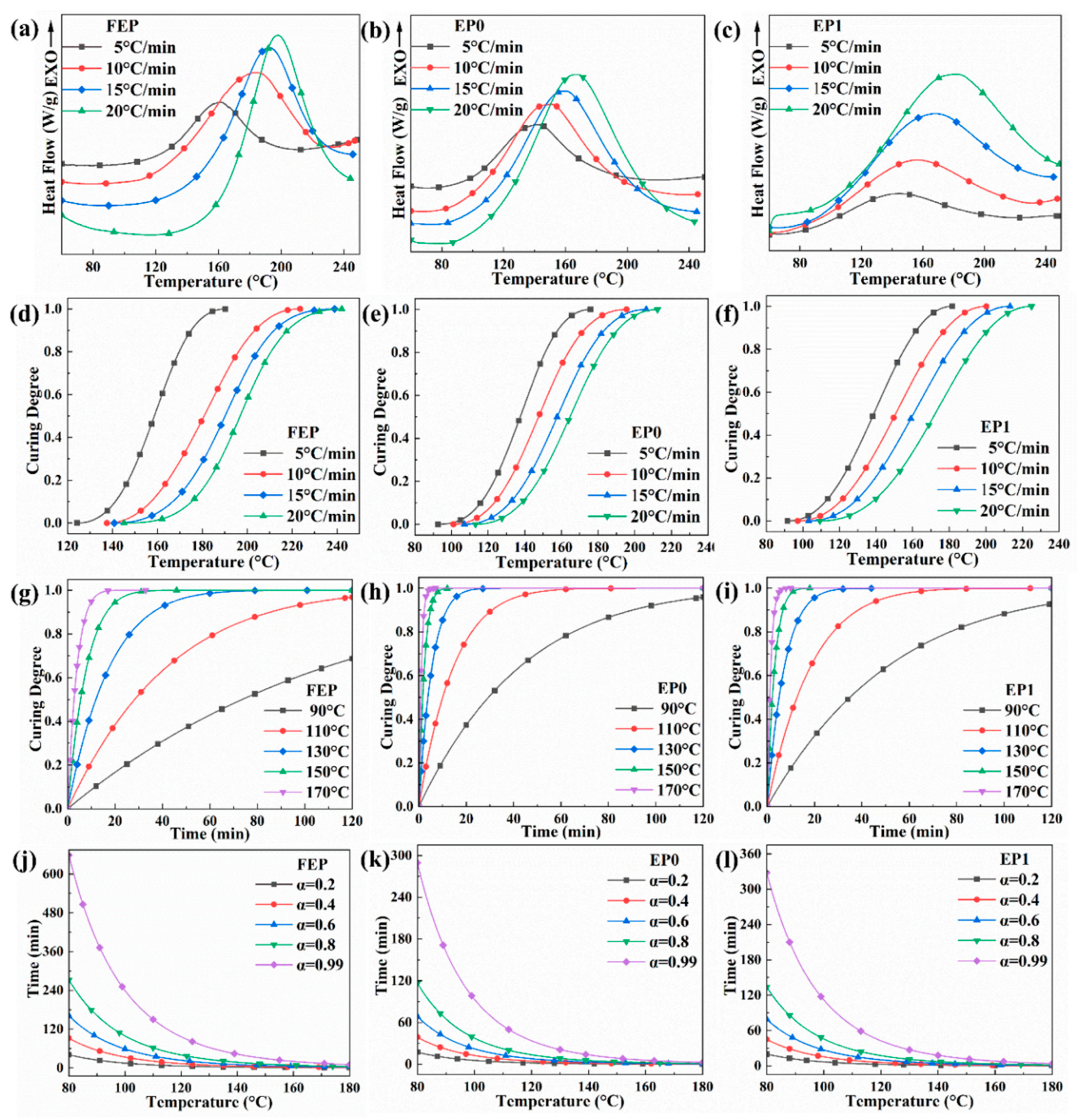
3.3. Curing Behavior
3.4. The Compatibility of Epoxy Matrix with Liquid Oxygen
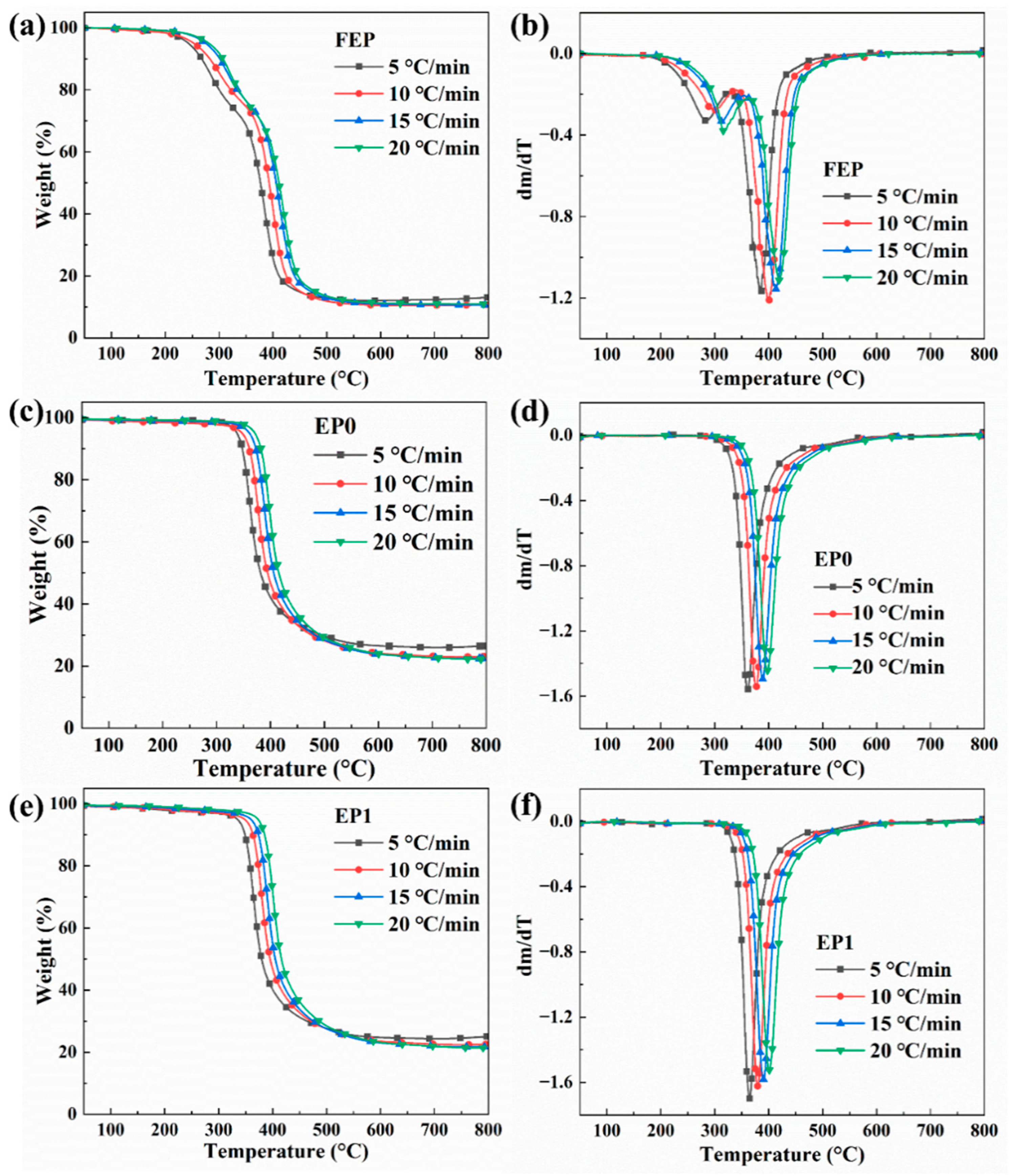
3.5. Thermal Analysis
3.6. Mechanical Properties of EP0 and EP1
3.7. Flexural Properties of the CF/EP Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, N.; Ma, B.; Liu, F.; Huang, W.; Xu, B.; Qu, L.; Yang, Y. Progress in research on composite cryogenic propellant tank for large aerospace vehicles. Compos. Part A-Appl. Sci. Manuf. 2021, 143, 106297. [Google Scholar] [CrossRef]
- Shi, X.-H.; Li, X.-L.; Liu, Q.-Y.; Wu, S.-J.; Xie, W.-M.; Zhao, N.; De La Vega, J.; Chen, M.-J.; Wang, D.-Y. Constructing Co-decorated layered double hydroxide via interfacial assembly and its application in flame-retardant epoxy resin. Compos. Commun. 2023, 43, 101712. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Li, J.; Cheng, S.; Bao, Q.; Wang, N.; Jian, X. Study on liquid oxygen compatibility of poly(phthalazinone ether) resins and their composites. Polym. Compos. 2024, 45, 7673–7688. [Google Scholar] [CrossRef]
- Robinson, M.; Stolzfus, J.; Owens, T. Composite material compatibility with liquid and gaseous oxygen. In Proceedings of the 19th AIAA Applied Aerodynamics Conference, Anaheim, CA, USA, 11–14 June 2001. [Google Scholar]
- Long, J.-F.; Zhou, Z.-L.; Wu, T.; Li, Y.-Q.; Fu, S.-Y. Enhancements in LOX compatibility and fracture toughness of carbon fiber/epoxy composite for liquid oxygen cryotank by simultaneously introducing Al(OH)3 and phosphorous-nitrogen flame retardants. Compos. Commun. 2023, 39, 101562. [Google Scholar] [CrossRef]
- Higuchi, K.; Takeuchi, S.; Sato, E.; Naruo, Y.; Inatani, Y.; Namiki, F.; Tanaka, K.; Watabe, Y. Development and flight test of metal-lined CFRP cryogenic tank for reusable rocket. Acta Astronaut. 2005, 57, 432–437. [Google Scholar] [CrossRef]
- McCarville, D.A.; Guzman, J.C.; Dillon, A.K.; Jackson, J.R.; Birkland, J.O. Design, Manufacture and Test of Cryotank Components. In Comprehensive Composite Materials II; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 153–179. [Google Scholar]
- Olsson, R.; Cameron, C.; Moreau, F.; Marklund, E.; Merzkirch, M.; Pettersson, J. Design, Manufacture, and Cryogenic Testing of a Linerless Composite Tank for Liquid Hydrogen. Appl. Compos. Mater. 2024, 31, 1131–1154. [Google Scholar] [CrossRef]
- Clark, A.F.; Hust, J.G. A review of the compatibility of structural materials with oxygen. AIAA J. 1974, 12, 441–454. [Google Scholar] [CrossRef]
- Wu, Z.; Li, S.; Liu, M.; Wang, Z.; Liu, X. Liquid oxygen compatible epoxy resin: Modification and characterization. RSC Adv. 2015, 5, 11325–11333. [Google Scholar] [CrossRef]
- Wu, Z.; Li, J.; Chen, Y.; Wang, Z.; Li, S. Effect of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide on liquid oxygen compatibility of bisphenol a epoxy resin. J. Appl. Polym. Sci. 2014, 131, 40848. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Wu, Z.; Wang, Z. The effect of 10-(2,5-dihydroxyphenyl)-9, 10-dihydro-9-oxa-10-phosphaphenanthrene- 10-oxide on liquid oxygen compatibility and cryogenic mechanical properties of epoxy resins. High Perform. Polym. 2016, 28, 820–830. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Z.; Xiao, Y.; Chen, Y.; Meng, X.; Ge, L.; Wang, Z. Development and Properties of Temperature-resistant Epoxy System forWetWinding. Eng. Plast. Appl. 2022, 50, 44–48. [Google Scholar] [CrossRef]
- Yan, C.; Wei, J.; Zhu, Y.; Xu, H.; Liu, D.; Chen, G.; Liu, X.; Dai, J.; Lv, D. Preparation and properties of carbon fiber reinforced bio-based degradable acetal-linkage-containing epoxy resin composites by RTM process. Polym. Compos. 2023, 44, 4081–4094. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, J.; Xing, S.; Yang, J.; Yang, F. Chemorheological characteristics of high-temperature resistant glycidylamine epoxy. Acta Mater. Compos. Sin. 2016, 33, 741–748. [Google Scholar]
- Chen, D.; Li, J.; Yuan, Y.; Gao, C.; Cui, Y.; Li, S.; Wang, H.; Peng, C.; Liu, X.; Wu, Z.; et al. A new strategy to improve the toughness of epoxy thermosets by introducing the thermoplastic epoxy. Polymer 2022, 240, 124518. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, J.; Li, J.; Chen, D.; Wang, Z.; Yin, W.; Wu, Z. Cryogenic Mechanical Properties and Stability of Polymer Films for Liquid Oxygen Hoses. Polymers 2023, 15, 3423. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Chen, D.; Cui, Y.; Wei, J.; Wang, Z.; Huang, H.; Wu, Z. A low-cost and liquid oxygen-compatible epoxy matrix of composites by introducing aryl phosphinate diglycidyl ether. Compos. Part A-Appl. Sci. Manuf. 2024, 177, 107944. [Google Scholar] [CrossRef]
- GB/T 1677-2008; Determinating the Epoxy Value of Plasticizers. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2008.
- ASTM G86-17; Test Method for Determining Ignition Sensitivity of Materials to Mechanical Impact in Ambient Liquid Oxygen and Pressurized Liquid and Gaseous Oxygen Environments. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D638; Standard Test Method for Tensile Properties of Plastics (Metric). ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D7264; Standard Test Method for Flexural Properties of Polymer Matrix Composite Materials. ASTM: West Conshohocken, PA, USA, 2021.
- Xie, Y.; Liu, W.; Liang, L.; Liu, C.; He, S.; Zhang, F.; Shi, H.; Yang, M. Enhancement of anticorrosion property and hydrophobicity of modified epoxy coatings with fluorinated polyacrylate. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 579, 123659. [Google Scholar] [CrossRef]
- Xiao, C.; Li, D.; Lang, F.; Xiang, Y.; Lin, Y.; Ou, B. Preparation and properties of MDA-BAPP-BTDA copolyimide/18-crown ether-6 supramolecular films with inclusion structure and ultralow dielectric constants. Mater. Res. Express 2021, 8, 075310. [Google Scholar] [CrossRef]
- Cheng, H.; Guo, J.; Zhao, T.; Ye, Y.; Yang, B.; Cui, J.; Mu, B.; Bao, X.; Tian, L.; Zhang, X.; et al. Molecular design and properties of a P-N synergistic flame retardant epoxy resin curing agent. J. Vinyl Addit. Technol. 2023, 30, 383–397. [Google Scholar] [CrossRef]
- Sons, J.W.; SpectraBase, I. SpectraBase Compound ID=GjKQx5apH7B SpectraBase Spectrum ID=1ZdPQq2iHbQ. Available online: https://spectrabase.com/spectrum/1ZdPQq2iHbQ (accessed on 1 September 2023).
- Sons, J.W.; SpectraBase, I. SpectraBase Compound ID=GjKQx5apH7B SpectraBase Spectrum ID=6y1RfPXVncV. Available online: https://spectrabase.com/spectrum/6y1RfPXVncV (accessed on 1 September 2023).
- Hu, B.; Yao, Z.-P. Electrospray ionization mass spectrometry with wooden tips: A review. Anal. Chim. Acta 2022, 1209, 339136. [Google Scholar] [CrossRef] [PubMed]
- Brostow, W.; Chonkaew, W.; Menard, K.P.; Scharf, T.W. Modification of an epoxy resin with a fluoroepoxy oligomer for improved mechanical and tribological properties. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2009, 507, 241–251. [Google Scholar] [CrossRef]
- Hong, X.; Xie, K.; Diao, Y.; Xiao, J. Progress of low viscosity epoxy resin used in vacuum assisted resin infusion. New Chem. Mater. 2006, 34, 11–13. [Google Scholar]
- Anna, R.; Mariaenrica, F. Effect of Diluents on Mechanical Characteristics of Epoxy Compounds. Polymers 2022, 14, 2277. [Google Scholar] [CrossRef] [PubMed]
- Suyeon, O.; Vishal, G.; Lee, W.-K. Synthesis and characteristics of cardanol-based acrylates as reactive diluents in UV-curing coatings. Mol. Cryst. Liquid Cryst. Acta 2023, 760, 68–75. [Google Scholar] [CrossRef]
- Yue, C.; Guan, L.; Zhang, X.; Wang, Y.; Weng, L. Thermally conductive epoxy/boron nitride composites with high glass transition temperatures for thermal interface materials. Mater. Des. 2021, 212, 110190. [Google Scholar] [CrossRef]
- Uicich, J.F.; Jouyandeh, M.; Fasce, D.; Montemartini, P.E.; Penoff, M.E.; Vahabi, H.; Saeb, M.R. Fluorinated-polyhedral oligomeric silsesquioxane (F-POSS) functionalized halloysite nanotubes (HNTs) as an antifouling additive for epoxy resin. J. Vinyl Addit. Technol. 2023, 30, 727–747. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ma, L.; Ge, H.; Gao, J.; Zhu, Z.; Weng, Y. Facile synthesis of phosphorus-containing benzotriazole flame retardant for enhancement of mechanical and fire properties of epoxy resins. Eur. Polym. J. 2024, 202, 112610. [Google Scholar] [CrossRef]
- Chen, M.-F.; Zhou, Q.; Ni, L.-Z.; Wang, G.-C. Synthesis, cure kinetics, and thermal properties of a novel boron–silicon hybrid polymer. J. Therm. Anal. Calorim. 2013, 114, 1317–1324. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Crane, L.W.; Dynes, P.J.; Kaelble, D.H. Analysis of curing kinetics in polymer composites. J. Polym. Sci. 2003, 11, 533–540. [Google Scholar] [CrossRef]
- Borchardt, H.; Daniels, F. The application of differential thermal analysis to the study of reaction kinetics. J. Am. Chem. Soc. 1956, 79, 41–46. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Cui, Y.; Ye, J.; Chen, D.; Yuan, Y.; Liu, X.; Liu, M.; Peng, C.; Wu, Z. Liquid oxygen compatibility of epoxy matrix and carbon fiber reinforced epoxy composite. Compos. Part A-Appl. Sci. Manuf. 2022, 154, 106771. [Google Scholar] [CrossRef]
- Wu, Z.; Li, J.; Wang, Z. Liquid oxygen compatibility and thermal stability of bisphenol A and bisphenol F epoxy resins modified by DOPO. Polym. Adv. Technol. 2015, 26, 153–159. [Google Scholar] [CrossRef]
- Wu, Z.; Li, S.; Liu, M.; Wang, Z.; Li, J. Synthesis and characterization of a liquid oxygen-compatible epoxy resin. High Perform. Polym. 2014, 27, 74–84. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Fu, X.-L.; Chu, F.-K.; Hu, Y. Synthesis of zirconium aminotrimethylene phosphonate nanorods and their application in toughened and flame retarded epoxy composites. Compos. Part A-Appl. Sci. Manuf. 2024, 179, 108059. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Chen, L.; Qin, Y.; Xiao, M.; Wang, Y. Methyl-substitution affects dielectric, thermal, mechanical properties, and shrinkage of fluorinated epoxy. Polymer 2022, 253, 125012. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Wu, M.; Liu, S.; Zhao, J. Design and synthesis of anhydride-terminated imide oligomer containing phosphorus and fluorine for high-performance flame-retarded epoxy resins. Chem. Eng. J. 2023, 461, 142063. [Google Scholar] [CrossRef]
- Kratz, J.; Paris, C.; Gaska, K.; Maes, V.; Partridge, I.; Olivier, P. Effects of accelerated curing in thermoplastic particle interleaf epoxy laminates. Compos. Part A-Appl. Sci. Manuf. 2024, 177, 107922. [Google Scholar] [CrossRef]
- Wei, M.; Wang, B.; Zhang, X.; Wei, W.; Li, X. Cycloaliphatic epoxy-functionalized polydimethylsiloxanes for comprehensive modifications of epoxy thermosets. Eur. Polym. J. 2024, 202, 112656. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Bu, M.; Lei, C. Toughening and strengthening epoxy resins with a new bi-DOPO biphenyl reactive flame retardant. Eur. Polym. J. 2022, 178, 111488. [Google Scholar] [CrossRef]
- Tian, P.-X.; Li, Y.-D.; Weng, Y.; Hu, Z.; Zeng, J.-B. Reprocessable, chemically recyclable, and flame-retardant biobased epoxy vitrimers. Eur. Polym. J. 2023, 193, 112078. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, X.; Bu, M.; Lei, C. Tuning the properties of bio-based epoxy resins by varying the structural unit rigidity in oligomers and curing procedures. Eur. Polym. J. 2023, 197, 112326. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Yan, J.; Wu, Z. Room-to-low temperature thermo-mechanical behavior and corresponding constitutive model of liquid oxygen compatible epoxy composites. Compos. Sci. Technol. 2024, 245, 110357. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Zhou, P.; Yan, Z.; Wang, D. Design and synthesis of mechanochromic poly(ether-ester-urethane) elastomer with high toughness and resilience mediated by crystalline domains. Polym. Chem. 2022, 13, 2155–2164. [Google Scholar] [CrossRef]
- Choudhury, D.N.; Pareta, A.S.; Rajesh, A.K.; Panda, S.K. Enhanced mechanical and interfacial performances of carbon fiber reinforced composites with low percentage of amine functionalized graphene. Polym. Compos. 2024, 1–19. [Google Scholar] [CrossRef]




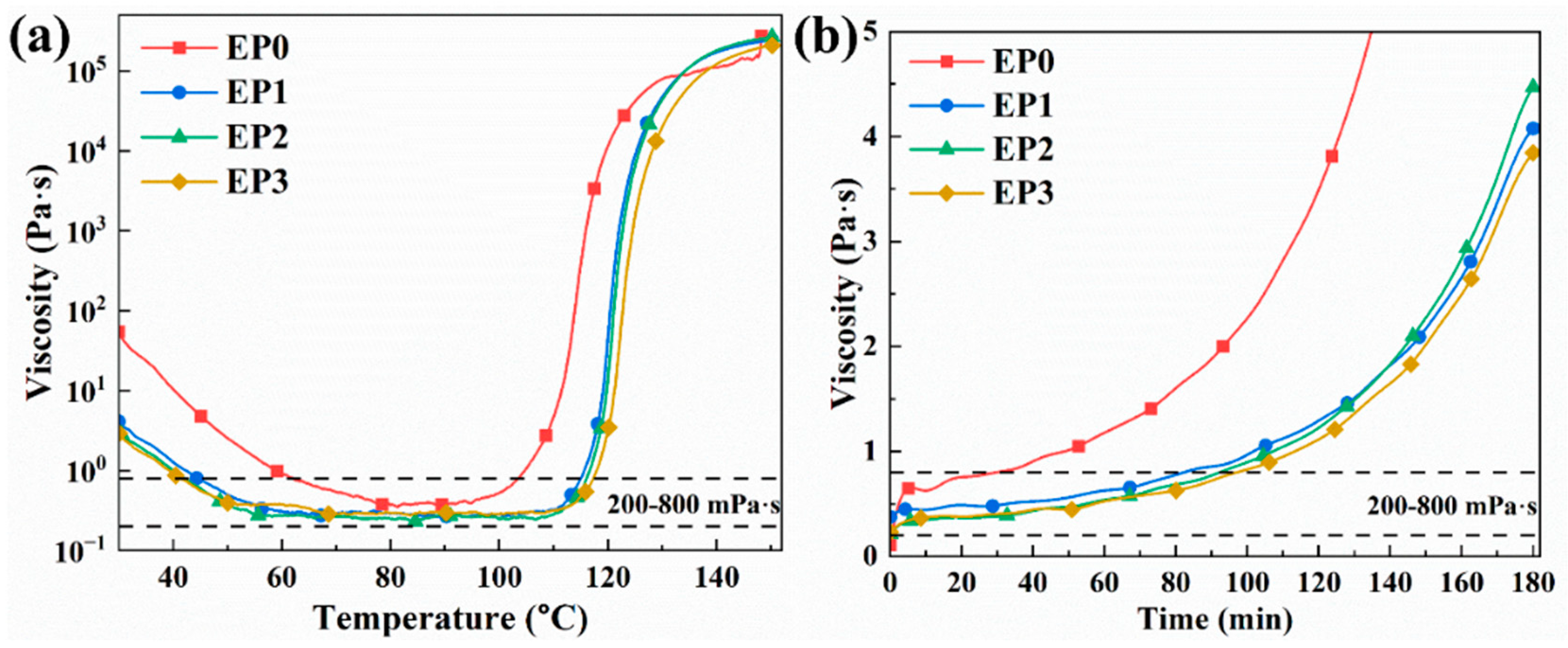





| Sample | Processing Onset Temperature (°C) | Processing Termination Temperature (°C) | Processing Temperature Window (°C) | Processing Time at 50 °C (min) |
|---|---|---|---|---|
| EP0 | 62 | 103 | 41 | 29 |
| EP1 | 44 | 115 | 71 | 83 |
| EP2 | 42 | 116 | 74 | 91 |
| EP3 | 41 | 117 | 76 | 96 |
| Sample | Heating Rate (°C/min) | To (°C) | Tp (°C) | Te (°C) |
|---|---|---|---|---|
| FEP | 5 | 125 | 161.93 | 191.3 |
| 10 | 138.32 | 176.12 | 204.15 | |
| 15 | 151.97 | 187.85 | 217.51 | |
| 20 | 164.96 | 199.76 | 228.17 | |
| EP0 | 5 | 92.6 | 135.23 | 175.83 |
| 10 | 101.04 | 150.67 | 195.58 | |
| 15 | 107.56 | 158.13 | 206.31 | |
| 20 | 113.07 | 165.44 | 212.48 | |
| EP1 | 5 | 91.56 | 142.4 | 186.62 |
| 10 | 97.2 | 153.56 | 200.3 | |
| 15 | 103.26 | 164.51 | 213.23 | |
| 20 | 109.32 | 174.64 | 224.97 |
| Sample | Total Number of Test Samples | Burning | Explosion | Flash | Charring | IRS (%) |
|---|---|---|---|---|---|---|
| FEP | 60 | 0 | 0 | 1 | 0 | 1 |
| EP0 | 20 | 0 | 0 | 0 | 0 | 0 |
| EP1 | 20 | 0 | 0 | 0 | 0 | 0 |
| E59 a | 20 | 2 | 0 | 1 | 2 | 17 |
| Sample | Heating Rate (°C/min) | Td5% (°C) | Td10% (°C) | Tdmax (°C) | Maximum Degradation Rate (%/°C) | Carbon Residue Rate at 800 °C (%) |
|---|---|---|---|---|---|---|
| FEP | 5 | 243 | 268 | 385 | −1.16 | 13.04 |
| 10 | 253 | 283 | 401 | −1.21 | 10.72 | |
| 15 | 275 | 301 | 414 | −1.16 | 10.68 | |
| 20 | 281 | 307 | 421 | −1.11 | 10.97 | |
| EP0 | 5 | 338 | 348 | 361 | −1.56 | 26.46 |
| 10 | 347 | 361 | 377 | −1.54 | 23.00 | |
| 15 | 361 | 373 | 389 | −1.50 | 22.43 | |
| 20 | 371 | 382 | 398 | −1.44 | 22.21 | |
| EP1 | 5 | 335 | 349 | 366 | −1.70 | 25.03 |
| 10 | 348 | 364 | 380 | −1.62 | 22.55 | |
| 15 | 360 | 374 | 390 | −1.58 | 21.71 | |
| 20 | 373 | 385 | 401 | −1.52 | 21.39 |
| Sample | Tg(DSC) a (°C) | Tg(DMA) b (°C) | E′ (MPa) | (MPa) | ρ (mol/m3) |
|---|---|---|---|---|---|
| FEP | 108 | 128 | 2680.86 | 7.21 | 671 |
| EP0 | 148 | 159 | 2247.01 | 23.21 | 2014 |
| EP1 | 144 | 154 | 2186.11 | 22.86 | 2006 |
| Temperature | Sample | Tensile Strain Energy (MJ/m3) | Relative Percentage a (%) | Flexural Strain Energy (MJ/m3) | Relative Percentage (%) |
|---|---|---|---|---|---|
| RT | EP0 | 6.343 | - | 8.696 | - |
| EP1 | 7.151 | +12.74 | 12.011 | +38.12 | |
| −196 °C | EP0 | 1.857 | - | 4.868 | - |
| EP1 | 1.877 | +1.08 | 5.926 | +21.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Yan, J.; Li, S.; Li, J.; Wu, Z. Preparation and Application of a Novel Liquid Oxygen-Compatible Epoxy Resin of Fluorinated Glycidyl Amine with Low Viscosity. Polymers 2024, 16, 2759. https://doi.org/10.3390/polym16192759
Wei J, Yan J, Li S, Li J, Wu Z. Preparation and Application of a Novel Liquid Oxygen-Compatible Epoxy Resin of Fluorinated Glycidyl Amine with Low Viscosity. Polymers. 2024; 16(19):2759. https://doi.org/10.3390/polym16192759
Chicago/Turabian StyleWei, Jianing, Jia Yan, Shichao Li, Juanzi Li, and Zhanjun Wu. 2024. "Preparation and Application of a Novel Liquid Oxygen-Compatible Epoxy Resin of Fluorinated Glycidyl Amine with Low Viscosity" Polymers 16, no. 19: 2759. https://doi.org/10.3390/polym16192759
APA StyleWei, J., Yan, J., Li, S., Li, J., & Wu, Z. (2024). Preparation and Application of a Novel Liquid Oxygen-Compatible Epoxy Resin of Fluorinated Glycidyl Amine with Low Viscosity. Polymers, 16(19), 2759. https://doi.org/10.3390/polym16192759







