Mechanical and Biological Characterization of Ionic and Photo-Crosslinking Effects on Gelatin-Based Hydrogel for Cartilage Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
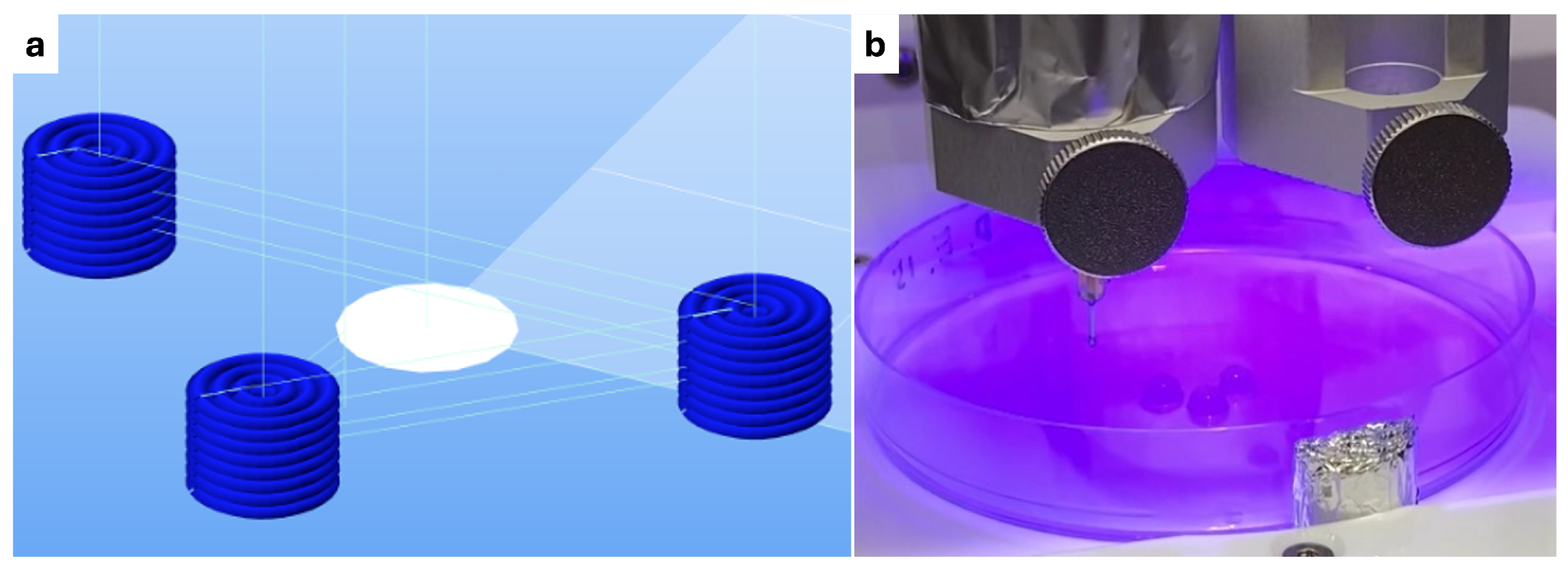
2.1. Crosslinking Experiment Set Up
- Ionic-crosslinking: After printing, the constructs were kept in a 50 mM calcium chloride solution for either 5 or 15 min, depending on the experimental condition. They were then washed with PBS and placed in incubator with media.
- Photo-crosslinking: During printing, the constructs were exposed to 405 nm blue light for 15 s, either after each layer or every two layers, depending on the experimental condition being tested. The light source was positioned at a distance of 5 cm from the constructs.
- Dual-crosslinking: Constructs underwent a combination of both methods, with the blue light exposure during printing followed by submersion in the Crosslinking Agent. A total of four conditions were tested.
2.2. Bioprinting Process Parameters
2.2.1. G-Code Formulation
2.2.2. Printing Protocol
2.3. Human Mesenchymal Stem Cell Culture
2.4. Mechanical Characterization
Compression Protocol and Mechanical Data Analysis
2.5. Biological Characterization
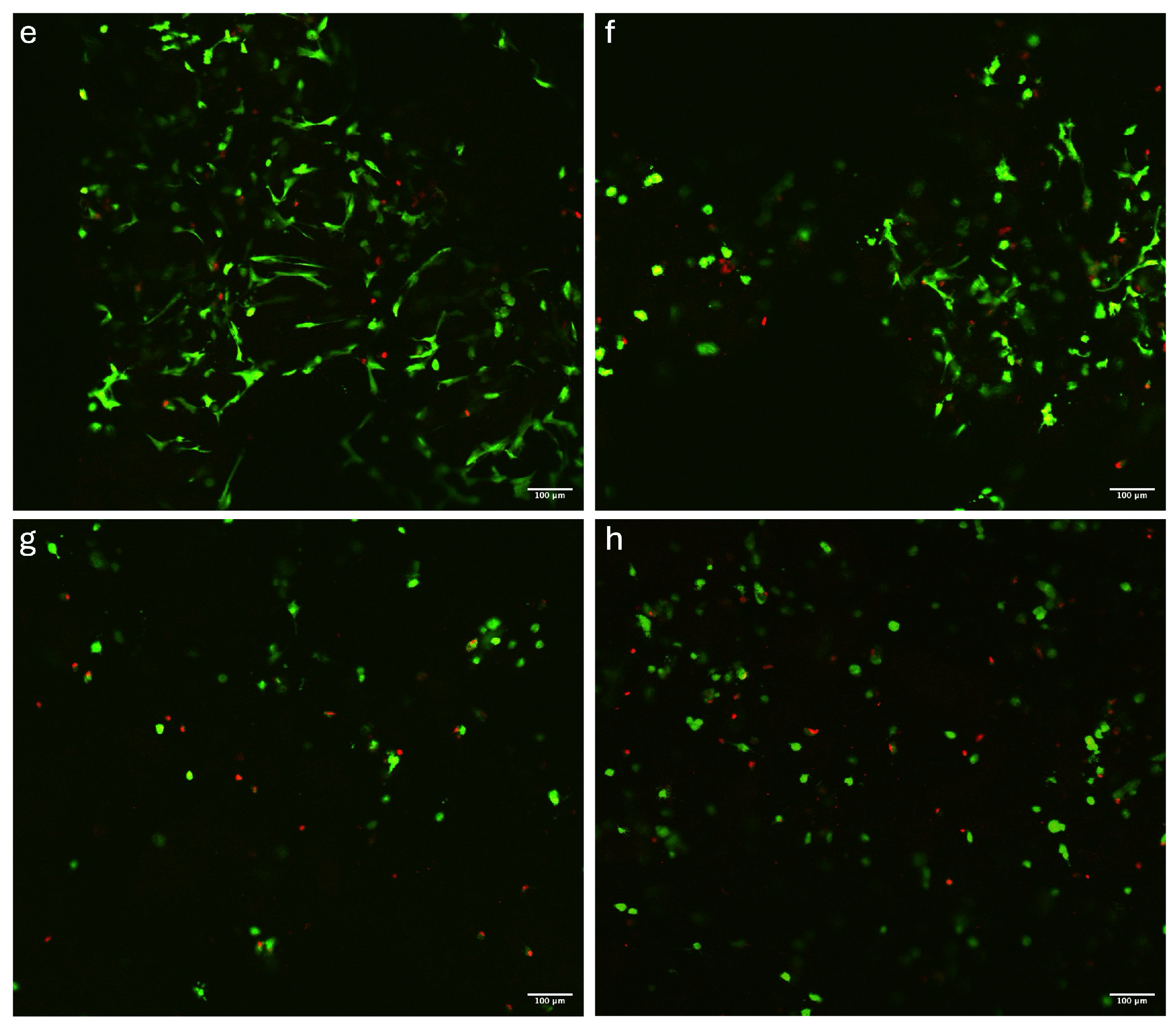
2.5.1. Live/Dead Assay and Images Analysis
3. Results and Discussion
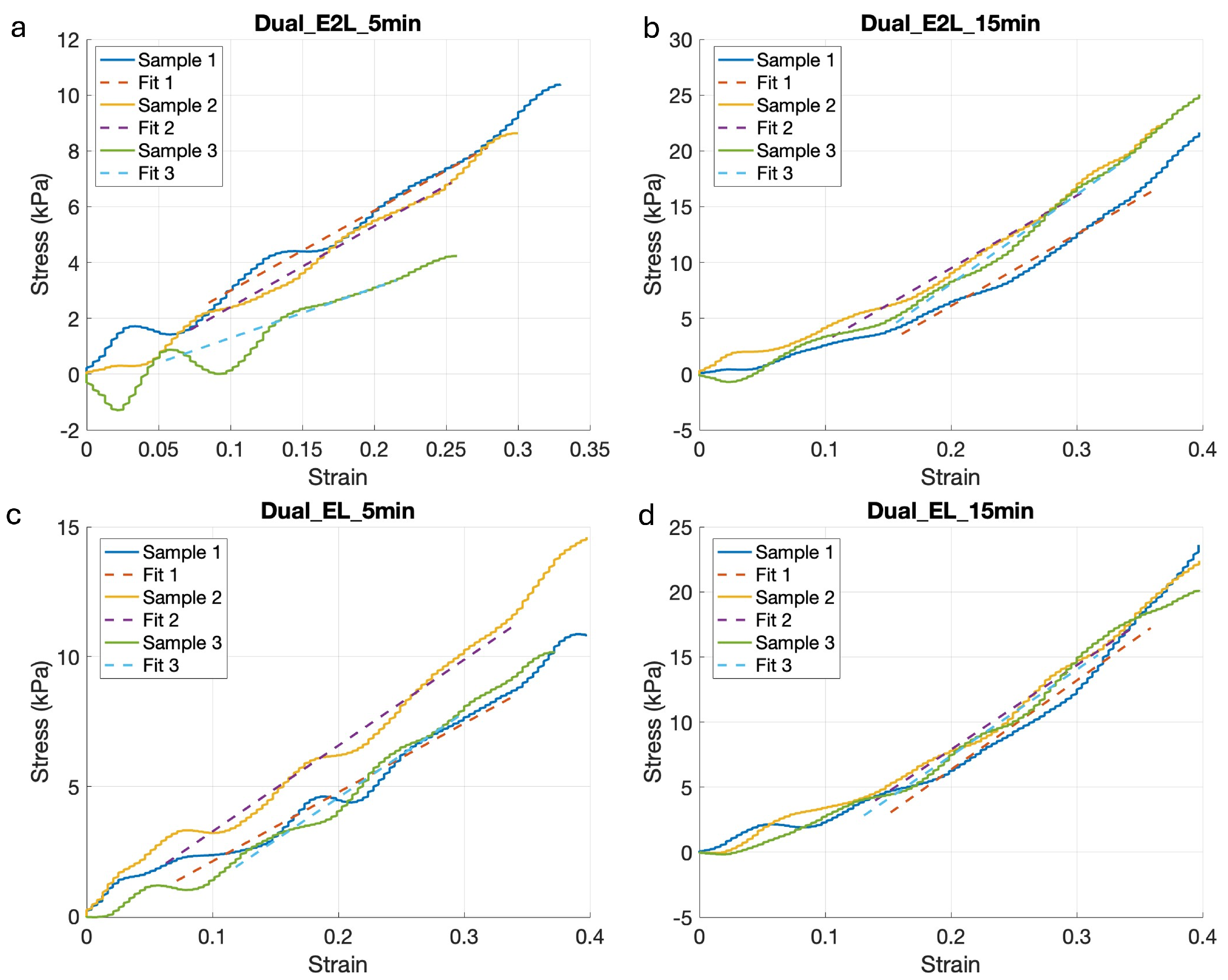
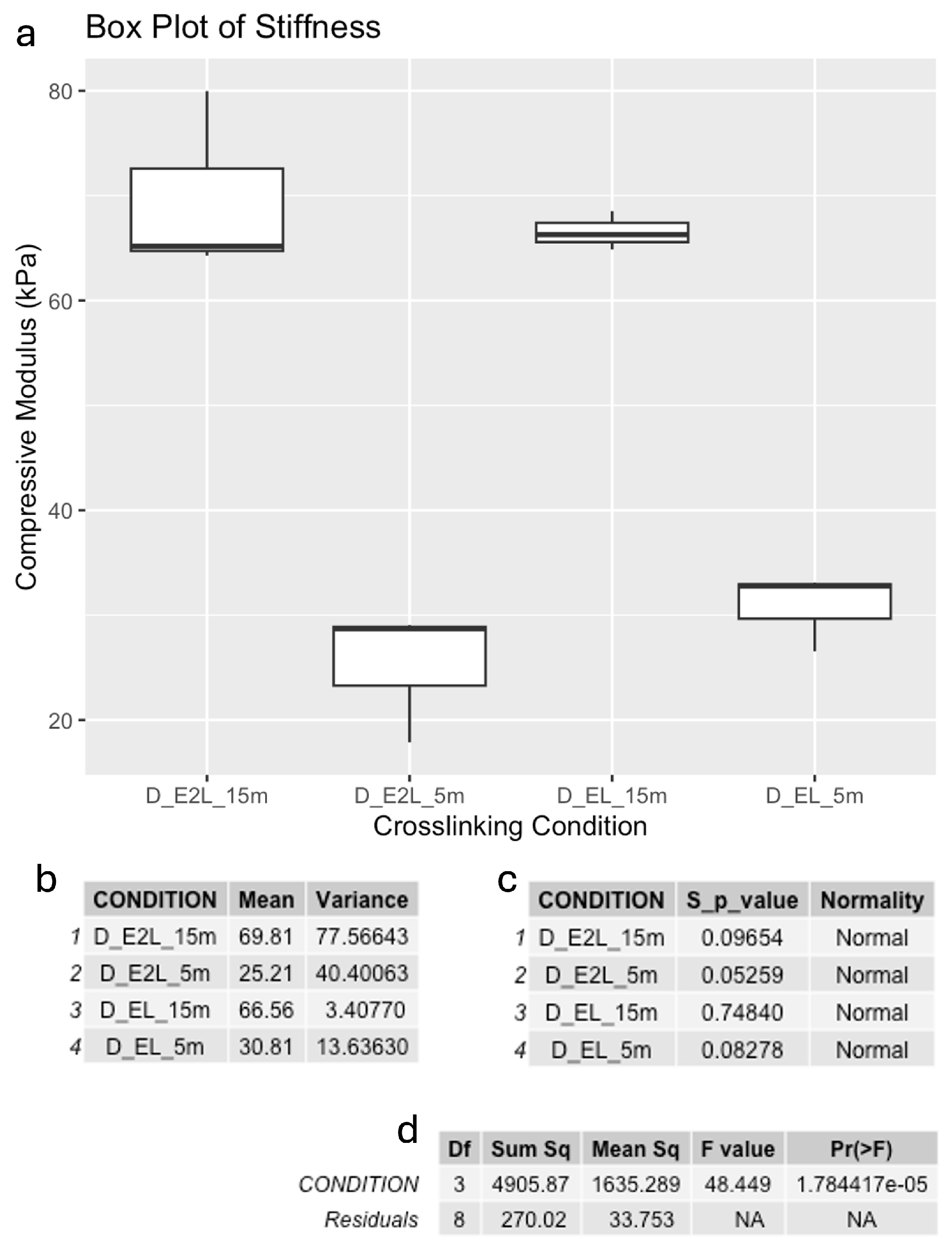
3.1. Mechanical Properties and Construct Stability
Compressive Modulus after Printing
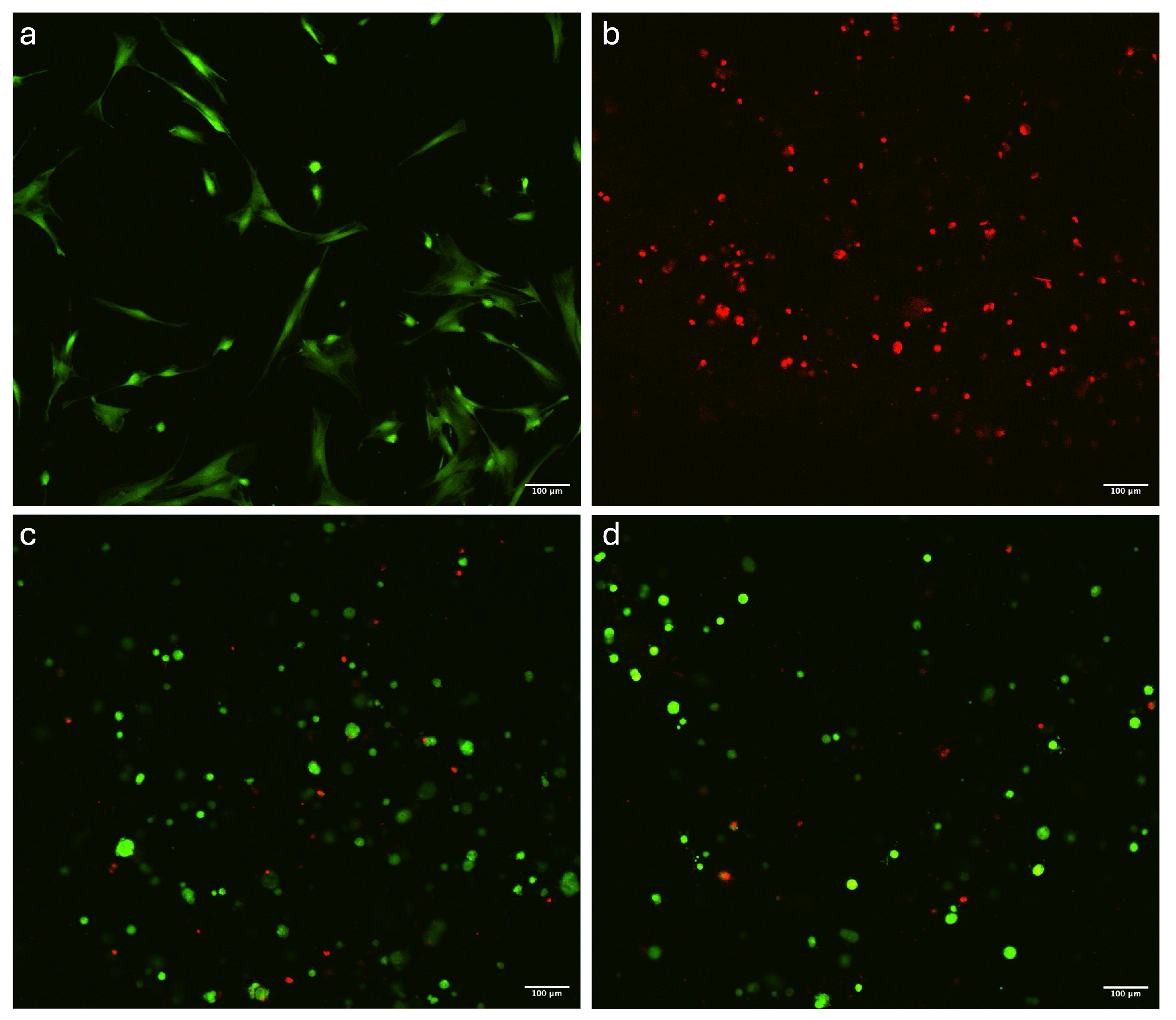
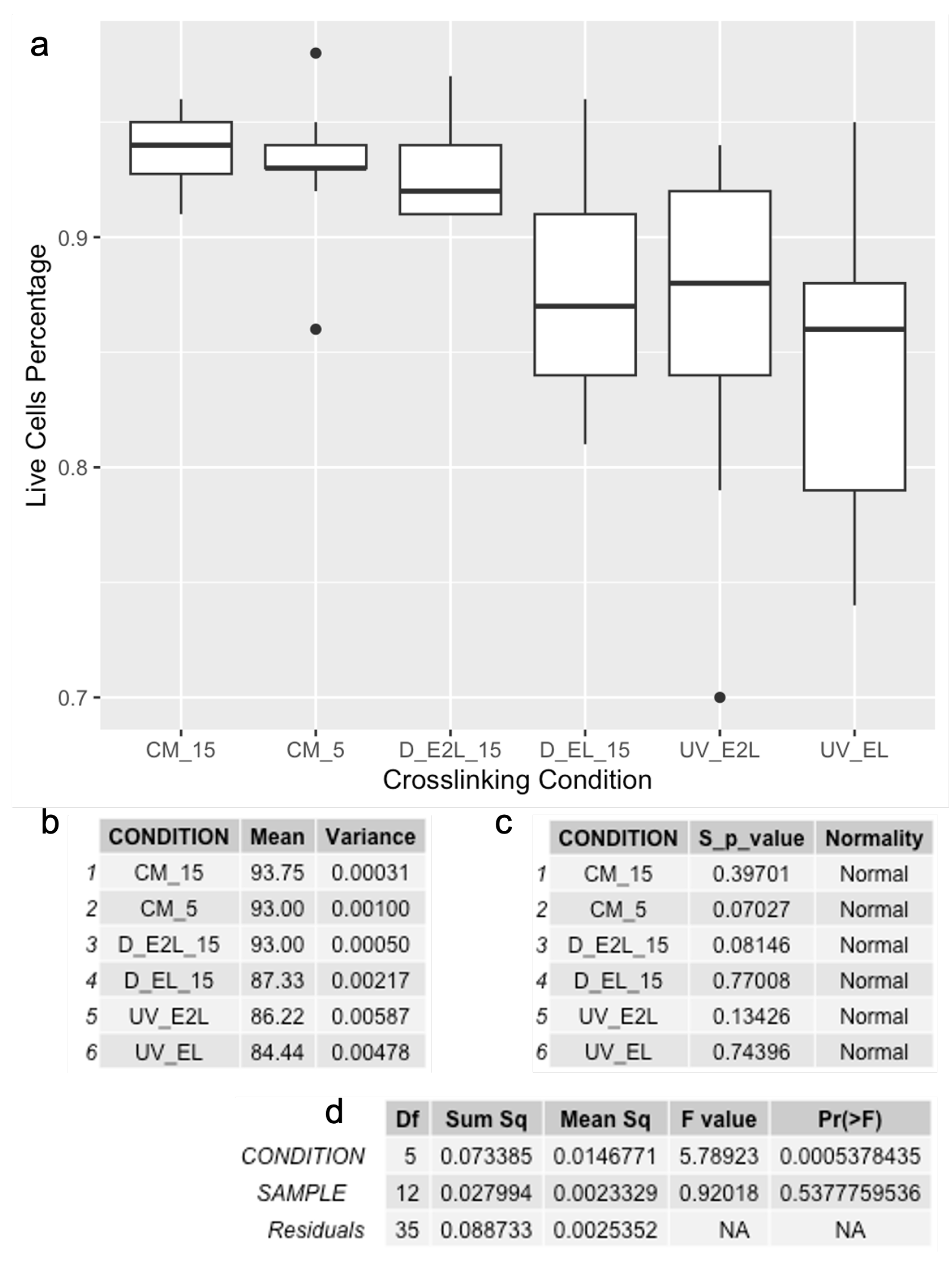
3.2. Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int. J. Mol. Sci. 2023, 24, 9841. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths & misconceptions—Progress & prospects. Osteoarthr. Cartil. 2015, 23, 334–350, ISSN 1063-4584. [Google Scholar] [CrossRef]
- Shen, J.; Song, W.; Liu, J.; Peng, X.; Tan, Z.; Xu, Y.; Liu, S.; Ren, L. 3D bioprinting by reinforced bioink based on photocurable interpenetrating networks for cartilage tissue engineering. Int. J. Biol. Macromol. 2024, 254, 127671. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioact. Mater. 2022, 9, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. Articular cartilage repair biomaterials: Strategies and applications. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, K.; Lokshina, A.; Cruz, M.A.; Karimzadeh, M.; Kemp, R.; Kean, T.J. Biomaterial composition and stiffness as decisive properties of 3D bioprinted constructs for type II collagen stimulation. Acta Biomater. 2022, 152, 221–234. [Google Scholar] [CrossRef]
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering bioinks for 3D bioprinting. Biofabrication 2021, 13, 032001. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Basu, B. An Overview of Hydrogel-Based Bioinks for 3D Bioprinting of Soft Tissues. J. Indian Inst. Sci. 2019, 99, 405–428. [Google Scholar] [CrossRef]
- Das, S.; Jegadeesan, J.T.; Basu, B. Gelatin Methacryloyl (GelMA)-Based Biomaterial Inks: Process Science for 3D/4D Printing and Current Status. Biomacromolecules 2024, 25, 2156–2221. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Nouri Khorasani, S.; Zare, M.; Esmaeely Neisiany, R.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, N.; Canadas, P.; Royer, P.; Noël, D.; Le Floc’h, S. Cartilage biomechanics: From the basic facts to the challenges of tissue engineering. J. Biomed. Mater. Res. 2023, 111, 1067–1089. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite design of injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, P.; D’Este, M.; Tong, W.; Xu, J.; Yao, H.; Stoddart, M.J.; van Osch, G.J.V.M.; Ho, K.K.-W.; Li, Z.; et al. Functionalized Hydrogels for Articular Cartilage Tissue Engineering. Engineering 2022, 13, 71–90. [Google Scholar] [CrossRef]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2022, 4, e194. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37, ISSN 2058-8437. [Google Scholar] [CrossRef]
- Jia, W.; Yang, X.; Liu, Z.; Sun, L.; Shen, Z.; Li, M.; Zhang, H.; An, Y.; Sang, S. Nasal cartilage tissue engineering materials based on 3D bioprinting: Seed cells and dECM. Appl. Mater. Today 2024, 40, 102364, ISSN 2352-9407. [Google Scholar] [CrossRef]
- Abbadessa, A.; Ronca, A.; Salerno, A. Integrating bioprinting, cell therapies and drug delivery towards in vivo regeneration of cartilage, bone and osteochondral tissue. Drug Deliv. Transl. Res. 2024, 14, 858–894. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part B 2019, 107, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, W.; Zhao, F.; Wang, H.; Cheng, J.; Duan, X.; Fu, X.; Zhang, J.; Hu, X.; Ao, Y. Regional specific tunable meniscus decellularized extracellular matrix (MdECM) reinforced bioink promotes anisotropic meniscus regeneration. Ion. Eng. J. 2023, 473, 145209. [Google Scholar] [CrossRef]
- Hafezi, M.; Khorasani, S.N.; Khalili, S.; Neisiany, R.E. Self-healing interpenetrating network hydrogel based on GelMA/alginate/ nano-clay. Int. J. Biol. Macromol. 2023, 242, 124962. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331, ISSN 1742-7061. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976, ISSN 1422-0067. [Google Scholar] [CrossRef] [PubMed]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin-Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moeinzadeh, S.; Kim, C.; Pan, C.-C.; Weale, G.; Kim, S.; Abrams, G.; James, A.W.; Choo, H.; Chan, C.; et al. Development and Systematic Characterization of GelMA/Alginate/PEGDMA/Xanthan Gum Hydrogel Bioink System for Extrusion Bioprinting. Biomaterials 2023, 293, 121969. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The formation mechanism of hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Rashid, A.B.; Showva, N.N.; Hoque, M.E. Gelatin-based scaffolds: An intuitive support structure for regenerative therapy. Curr. Opin. Biomed. Eng. 2023, 26, 100452. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shav, I.A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Higashi, S.; Nakamura, S.; Daigaku, Y.; Furusawa, Y.; Kamei, Y.; Watanabe, M.; Yamamoto, K.; Hieda, K.; Munakata, N.; et al. Action Spectrum Analysis of UVR Genotoxicity for Skin: The Border Wavelengths between UVA and UVB Can Bring Serious Mutation Loads to Skin. J. Investig. Dermatol. 2013, 133, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Nimi, N.; Sivadas, V.P.; Lal, L.M.R.; Nair, P.D. Dual crosslinked pullulan–gelatin cryogel scaffold for chondrocyte-mediated cartilage repair: Synthesis, characterization and in vitro evaluation. Biomed. Mater. 2022, 17, 015001. [Google Scholar] [CrossRef]
- Shehzad, A.; Mukasheva, F.; Moazzam, M.; Sultanova, D.; Abdikhan, B.; Trifonov, A.; Akilbekova, D. Dual-Crosslinking of Gelatin-Based Hydrogels: Promising Compositions for a 3D Printed Organotypic Bone Model. Bioengineering 2023, 10, 704. [Google Scholar] [CrossRef]
- Kong, Y.; Xu, J.; Guan, W.; Sun, S.; Yang, Y.; Li, G. Tailoring the elasticity of nerve implants for regulating peripheral nerve regeneration. Smart Mater. Med. 2023, 4, 266–285, ISSN 2590-1834. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514, ISSN 0144-8617. [Google Scholar] [CrossRef]
- Yan, X.; Huang, H.; Bakry, A.M.; Wu, W.; Liu, X.; Liu, F. Advances in enhancing the mechanical properties of biopolymer hydrogels via multi-strategic approaches. Int. J. Biol. Macromol. 2024, 272, 132583. [Google Scholar] [CrossRef]
- Qin, Z.; Yu, X.; Wu, H.; Yang, L.; Lv, H.; Yang, X. Injectable and Cytocompatible Dual Cross-Linking Hydrogels with Enhanced Mechanical Strength and Stability. ACS Biomater. Sci. Eng. 2020, 6, 3529–3538. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Schmid, R.; Arkudas, A.; Kengelbach-Weig, A.; Bosserhoff, A.K. Tumor Cells Develop Defined Cellular Phenotypes After 3D-Bioprinting in Different Bioinks. Cells 2019, 8, 1295. [Google Scholar] [CrossRef]
- Ronzoni, F.L.; Aliberti, F.; Scocozza, F.; Benedetti, L.; Auricchio, F.; Sampaolesi, M.; Cusella, G.; Redwan, I.N.; Ceccarelli, G.; Conti, M. Myoblast 3D bioprinting to burst in vitro skeletal muscle differentiation. J. Tissue Eng. Regen. Med. 2022, 16, 484–495. [Google Scholar] [CrossRef]
- Calderon, G.A.; Jack, K.; Venkatraman, S.; Wong, J.Y.; Burdick, J.A. Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate gels. Biomater. Sci. 2017, 5, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Cha, H.J. Recent advances in the development of nature-derived photocrosslinkable biomaterials for 3D printing in tissue engineering. Biomater. Res. 2019, 23, 18. [Google Scholar] [CrossRef]
- Olvera, D.; Daly, A.; Kelly, D.J. Mechanical Testing of Cartilage Constructs. In Cartilage Tissue Engineering, Methods in Molecular Biology; Doran, P., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1340, pp. 263–275. [Google Scholar] [CrossRef]
- Juráš, V.; Matejka, J.; Zbyn, S.; Masek, M. The Relationship between MR Parameters and Biomechanical Quantities of Loaded Human Articular Cartilage in Osteoarthritis: An In-Vitro Study. Meas. Sci. Rev. 2009, 9, 127–130. [Google Scholar] [CrossRef][Green Version]
- Juras, V.; Zbyn, S.; Mlynarik, V.; Szomolanyi, P.; Sulzbacher, I.; Trattnig, S. In vitro determination of biomechanical properties of human articular cartilage in osteoarthritis using multi-parametric MRI. J. Magn. Reson. 2009, 197, 40–47. [Google Scholar] [CrossRef]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication 2021, 14, 014107. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Busto, F.; Scalia, A.C.; Castellaneta, A.; Gentile, P.; Cochis, A.; Manfredi, M.; Borrini, V.; Rimondini, L.; De Giglio, E. Effectiveness of gellan gum scaffolds loaded with Boswellia serrata extract for in-situ modulation of pro-inflammatory pathways affecting cartilage healing. Int. J. Biol. Macromol. 2024, 277 Pt 1, 134079. [Google Scholar] [CrossRef]
- Ravnihar, K.; Marš, T.; Pirkmajer, S.; Alibegović, A.; Koželj, G.; Stožer, A.; Drobnič, M. The Influence of a Single Intra-Articular Lidocaine Injection on the Viability of Articular Cartilage in the Knee. Cartilage 2021, 13 (Suppl. S1), 456S–463S. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rajabi, N.; Rezaei, A.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Luo, H.; RamaKrishna, S.; Berto, F. Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair. Tissue Eng. Part A 2021, 27, 679–702. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Yu, C.; Zhang, Y. Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Bio-Des. Manuf. 2018, 1, 215–224. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; Francis, S.; O’Connell, C.D.; Aguilar, L.M.C.; Doyle, S.; Yue, Z.; Wallace, G.C.; Choong, P.F.; Di Bella, C. FLASH: Fluorescently LAbelled Sensitive Hydrogel to monitor bioscaffolds degradation during neocartilage generation. Biomaterials 2021, 264, 120383. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhu, M.; Wei, K.; Bian, L. Cell-mediated degradation regulates human mesenchymal stem cell chondrogenesis and hypertrophy in MMP-sensitive hyaluronic acid hydrogels. PLoS ONE 2014, 9, e99587. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.G.; Hodgkinson, T.; Curtin, C.M.; O’Brien, F.J. An injectable and 3D printable pro-chondrogenic hyaluronic acid and collagen type II composite hydrogel for the repair of articular cartilage defects. Biofabrication 2024, 16, 015007. [Google Scholar] [CrossRef]
- Thanh, T.N.; Laowattanatham, N.; Ratanavaraporn, J.; Sereemaspun, A.; Yodmuang, S. Hyaluronic acid crosslinked with alginate hydrogel: A versatile and biocompatible bioink platform for tissue engineering. Eur. Polym. J. 2022, 166, 111027. [Google Scholar] [CrossRef]
- Sang, S.; Mao, X.; Cao, Y.; Liu, Z.; Shen, Z.; Li, M.; Jia, W.; Guo, Z.; Wang, Z.; Xiang, C.; et al. Advances in 3D bioprinting and tissue engineering: A review. ACS Appl. Mater. Interfaces 2023, 15, 8895–8913. [Google Scholar] [CrossRef]
- Boretti, G.; Giordano, E.; Ionita, M.; Vlasceanu, G.M.; Sigurjónsson, Ó.E.; Gargiulo, P.; Lovecchio, J. Human Bone-Marrow-Derived Stem-Cell-Seeded 3D Chitosan–Gelatin–Genipin Scaffolds Show Enhanced Extracellular Matrix Mineralization When Cultured under a Perfusion Flow in Osteogenic Medium. Materials 2023, 16, 5898. [Google Scholar] [CrossRef]
- Bordbar, S.; Li, Z.; Lotfibakhshaiesh, N.; Rahmanian, M. Cartilage tissue engineering using decellularized biomatrix hydrogel containing TGF-β-loaded alginate microspheres in mechanically loaded bioreactor. Sci. Rep. 2024, 14, 11991. [Google Scholar] [CrossRef]
- Strauß, S.; Diemer, M.; Bucan, V.; Kall, H. Spider silk enhanced tissue engineering of cartilage tissue: Approach of a novel bioreactor model using adipose derived stromal cells. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241226656. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xu, Z.; Di, M.; Huang, D.; Li, X. Bioreactor strategies for tissue-engineered osteochondral constructs: Advantages, present situations and future trends. Compos. Part B Eng. 2023, 259, 110736. [Google Scholar] [CrossRef]

| Ionic | Photo | Dual | |||||
|---|---|---|---|---|---|---|---|
| Exposure | N. of Samples | Exposure | N. of Samples | Exposure | N. of Samples | Exposure | N. of Samples |
| 5 min | 6 for mechanical 3 for biological | 15 s every layer | 6 for mechanical 3 for biological | 15 s every layer + 5 min ionic | 6 for mechanical 3 for biological | 15 s every 2 layers + 5 min ionic | 6 for mechanical 3 for biological |
| 15 min | 6 for mechanical 3 for biological | 15 s every 2 layer | 6 for mechanical 3 for biological | 15 s every layer + 15 min ionic | 6 for mechanical 3 for biological | 15 s every 2 layers + 15 min ionic | 6 for mechanical 3 for biological |
| Crosslinking | Compressive Modulus | Cellular Viability | Overall |
|---|---|---|---|
| Ionic 5 m | Not measurable | ~93% | Enhanced cellular compatibility, with reduced structural stability during the printing process |
| Ionic 15 m | Not measurable | ~94% | Enhanced cellular compatibility, with reduced structural stability during the printing process |
| Photo every layer | Not measurable | ~84% | Structural stability during printing, with complete dissolution observed after 4 days and lower viability |
| Photo every 2 layer | Not measurable | ~86% | Structural stability during printing, with complete dissolution observed after 4 days and lower viability |
| Photo every layer + ionic 5 m | ~20 kPa | Not measured | Extended preparation time, with favorable mechanical properties |
| Photo every layer + ionic 15 m | ~43 kPa | ~87% | Extended preparation time, with good mechanical properties |
| Photo every 2 layer + ionic 5 m | ~16 kPa | Not measured | Extended preparation time, with favorable mechanical properties |
| Photo every 2 layer + ionic 15 m | ~45 kPa | ~93% | Extended preparation time, with enhanced mechanical properties and higher cell viability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boretti, G.; Baldursson, H.E.; Buonarrivo, L.; Simonsson, S.; Brynjólfsson, S.; Gargiulo, P.; Sigurjónsson, Ó.E. Mechanical and Biological Characterization of Ionic and Photo-Crosslinking Effects on Gelatin-Based Hydrogel for Cartilage Tissue Engineering Applications. Polymers 2024, 16, 2741. https://doi.org/10.3390/polym16192741
Boretti G, Baldursson HE, Buonarrivo L, Simonsson S, Brynjólfsson S, Gargiulo P, Sigurjónsson ÓE. Mechanical and Biological Characterization of Ionic and Photo-Crosslinking Effects on Gelatin-Based Hydrogel for Cartilage Tissue Engineering Applications. Polymers. 2024; 16(19):2741. https://doi.org/10.3390/polym16192741
Chicago/Turabian StyleBoretti, Gabriele, Hafsteinn Esjar Baldursson, Luca Buonarrivo, Stina Simonsson, Sigurður Brynjólfsson, Paolo Gargiulo, and Ólafur Eysteinn Sigurjónsson. 2024. "Mechanical and Biological Characterization of Ionic and Photo-Crosslinking Effects on Gelatin-Based Hydrogel for Cartilage Tissue Engineering Applications" Polymers 16, no. 19: 2741. https://doi.org/10.3390/polym16192741
APA StyleBoretti, G., Baldursson, H. E., Buonarrivo, L., Simonsson, S., Brynjólfsson, S., Gargiulo, P., & Sigurjónsson, Ó. E. (2024). Mechanical and Biological Characterization of Ionic and Photo-Crosslinking Effects on Gelatin-Based Hydrogel for Cartilage Tissue Engineering Applications. Polymers, 16(19), 2741. https://doi.org/10.3390/polym16192741








