Alpha-Tocopherol-Infused Flexible Liposomal Nanocomposite Pressure-Sensitive Adhesive: Enhancing Skin Permeation of Retinaldehyde
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of Retinal-Loaded Flexible Liposomes
2.2.1. Preparation of Liposomes
2.2.2. Characterization of Retinal-Loaded Flexible Liposomes
2.2.3. Drug Loading
2.2.4. Stability Study of Retinal-Loaded Flexible Liposomes
2.2.5. HPLC Analysis
2.2.6. In Vitro Skin Permeation Study of Retinal-Loaded Liposome
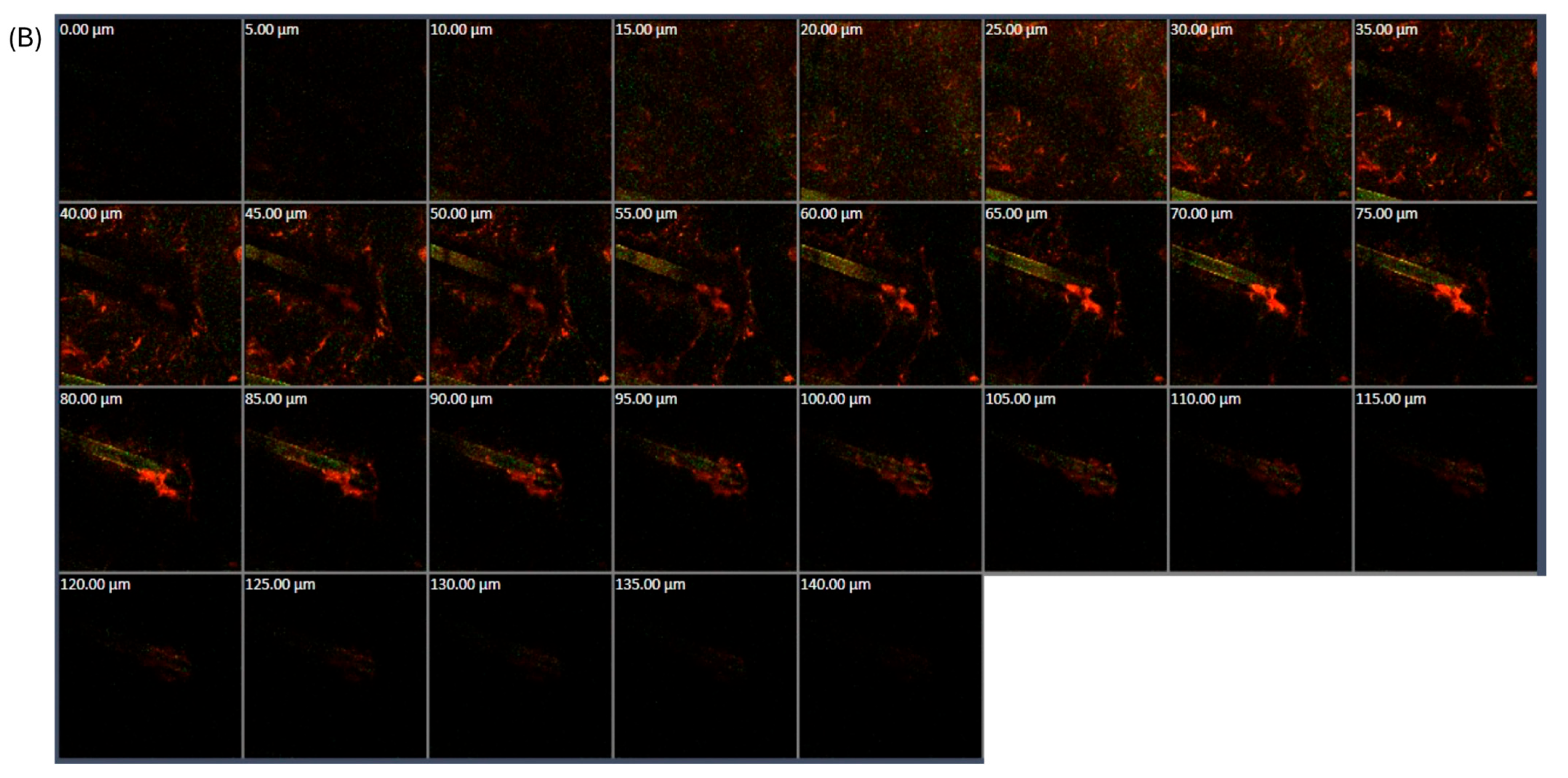
2.2.7. Confocal Laser Scanning Microscope Study
2.3. Development of Retinal-Loaded Flexible Liposome-Embedded Pressure-Sensitive Adhesives
2.3.1. Preparation of Pressure-Sensitive Adhesive Patch
2.3.2. The Evaluation of PSA Patches
Probe Tack Test
Peel Adhesion Measurement
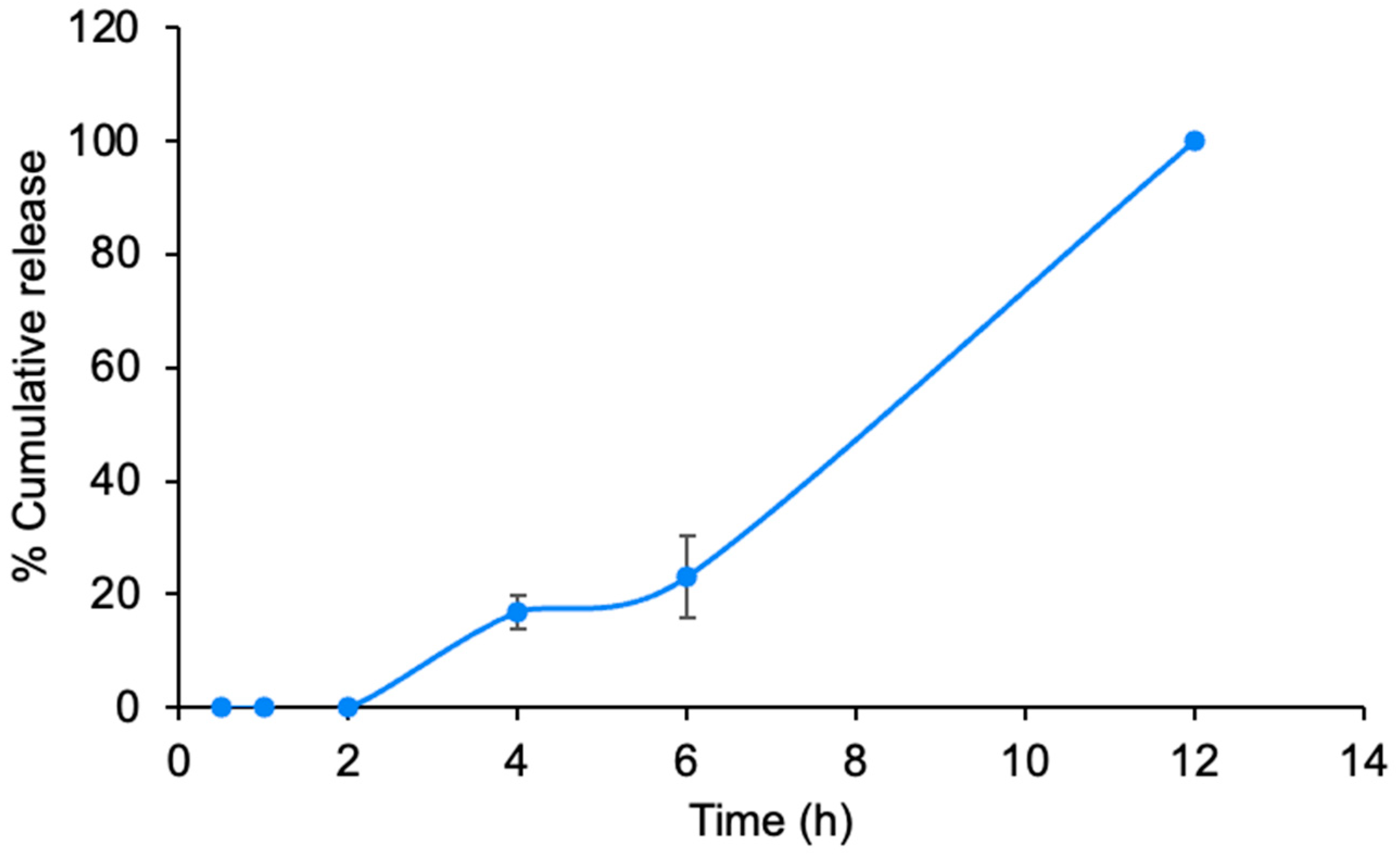
Drug Release Study
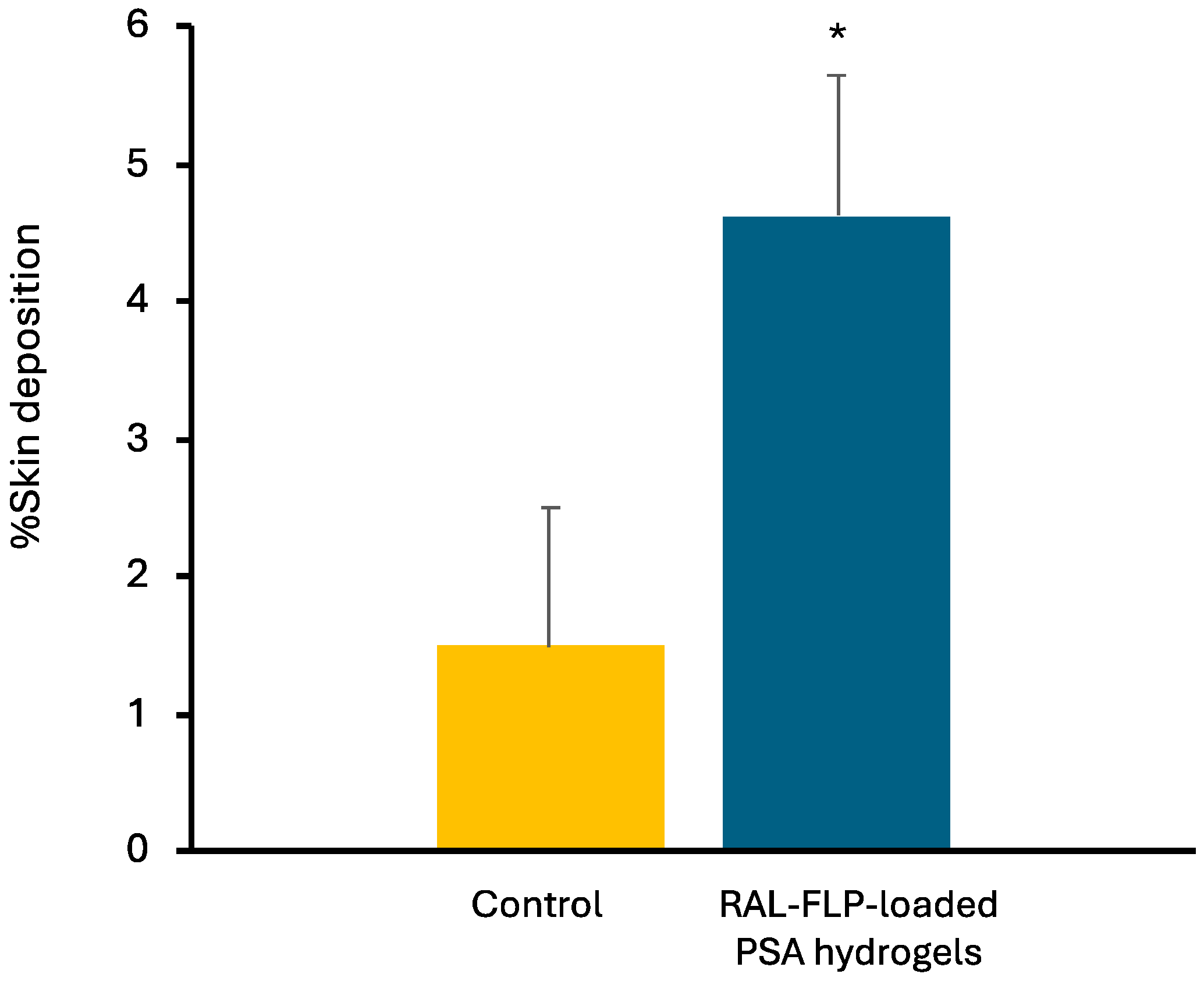
2.3.3. Skin Deposition Study
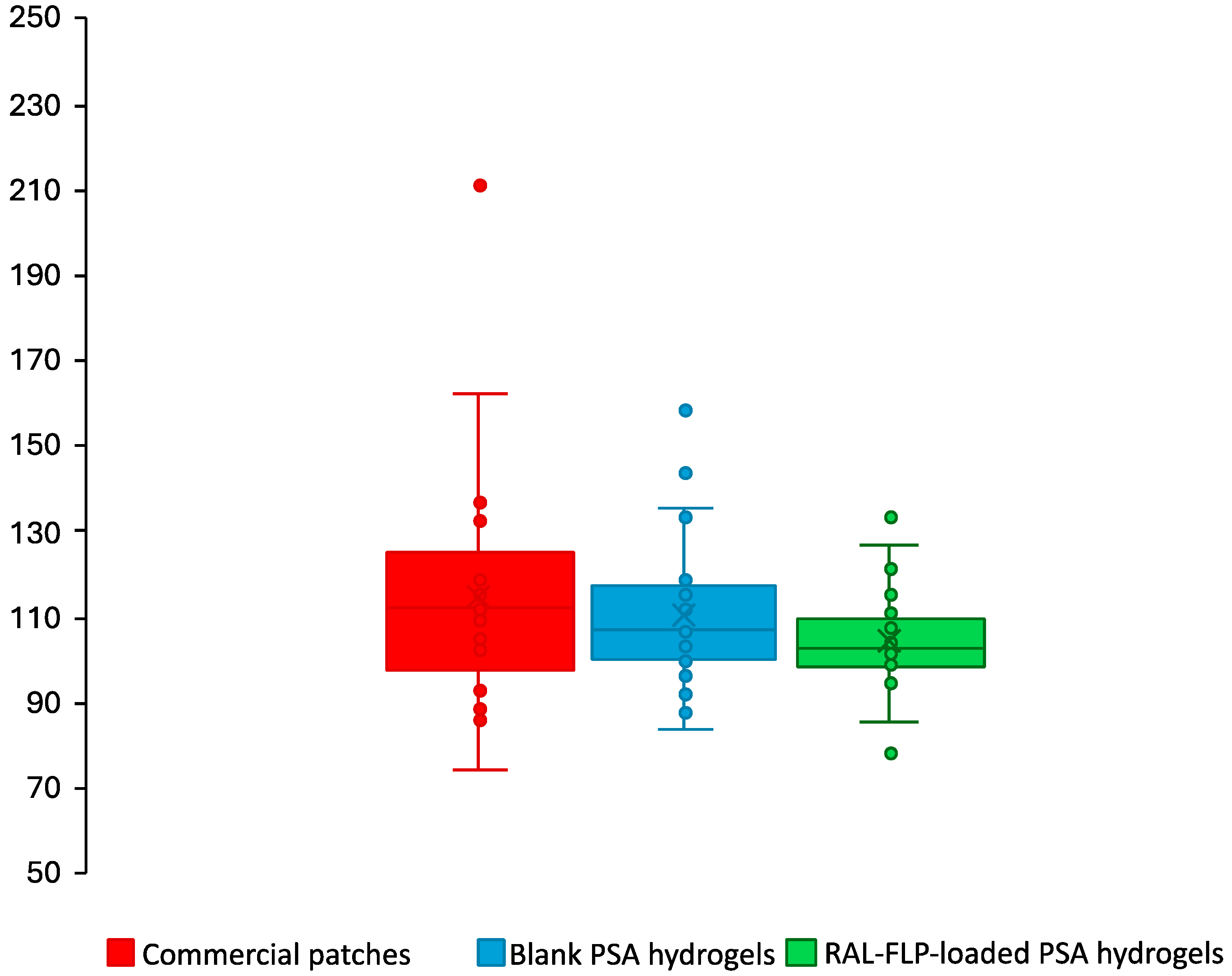
2.3.4. In Vivo Skin Irritation Study and Adhesion Capability in Human Subjects
2.4. Statistical Analysis
3. Results and Discussion
3.1. Development of Retinal-Loaded Flexible Liposomes
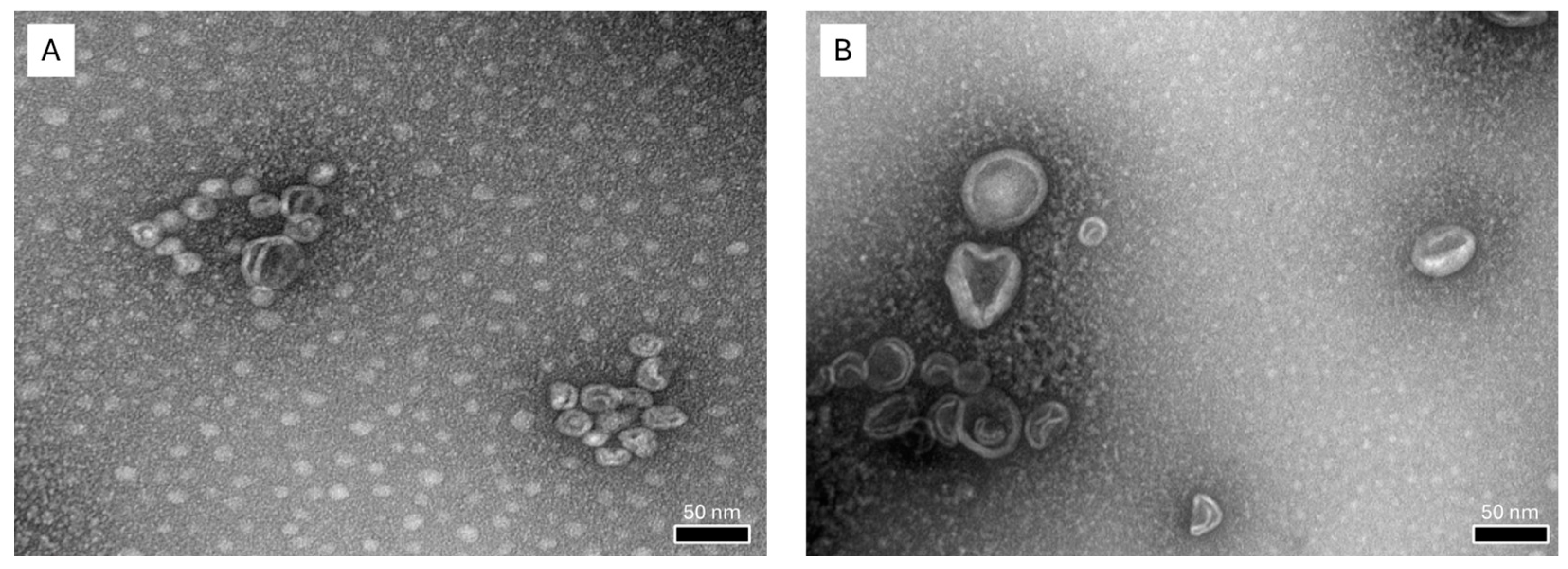
3.1.1. Characterization of Liposomal Formulations
3.1.2. RAL Loading
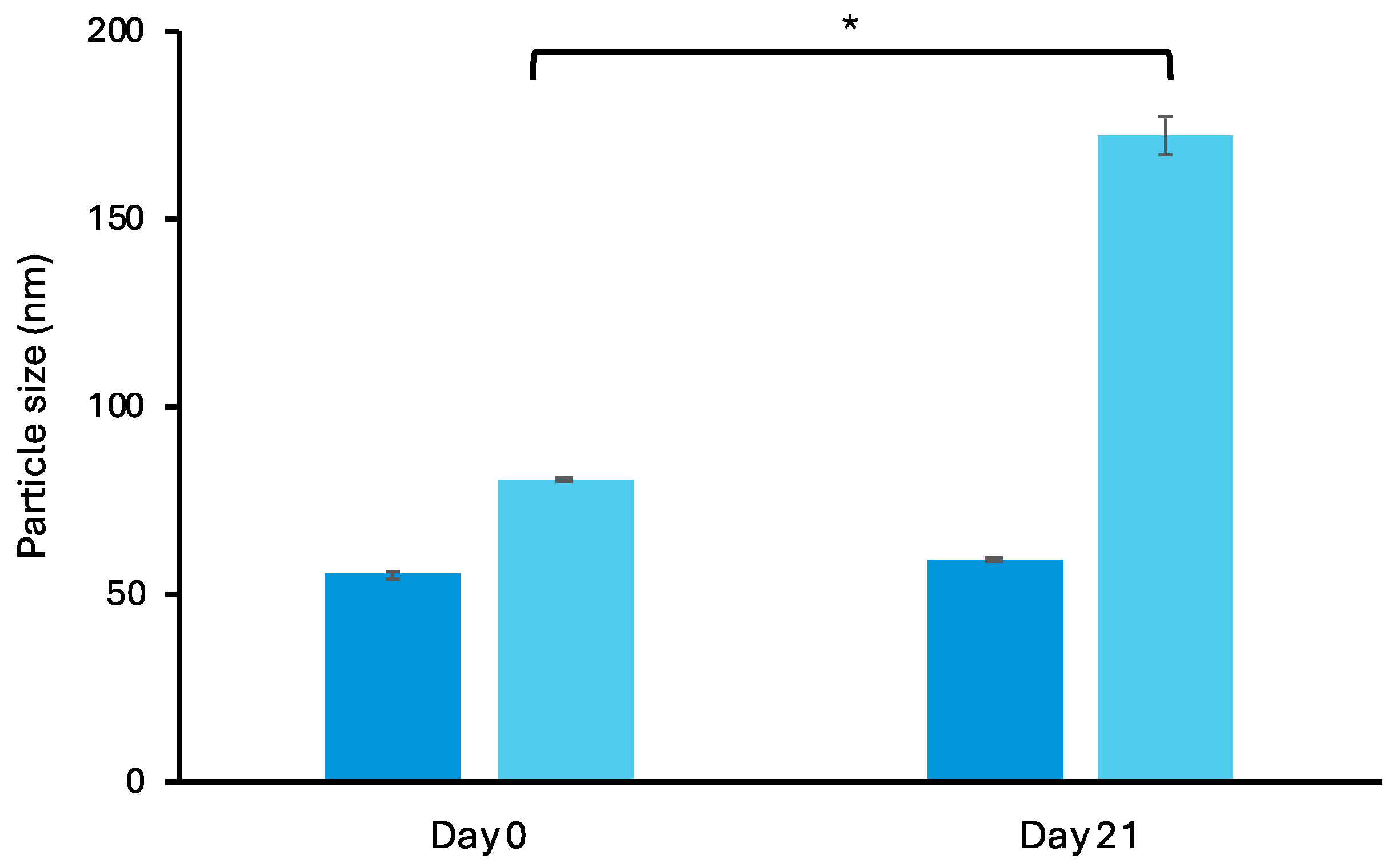
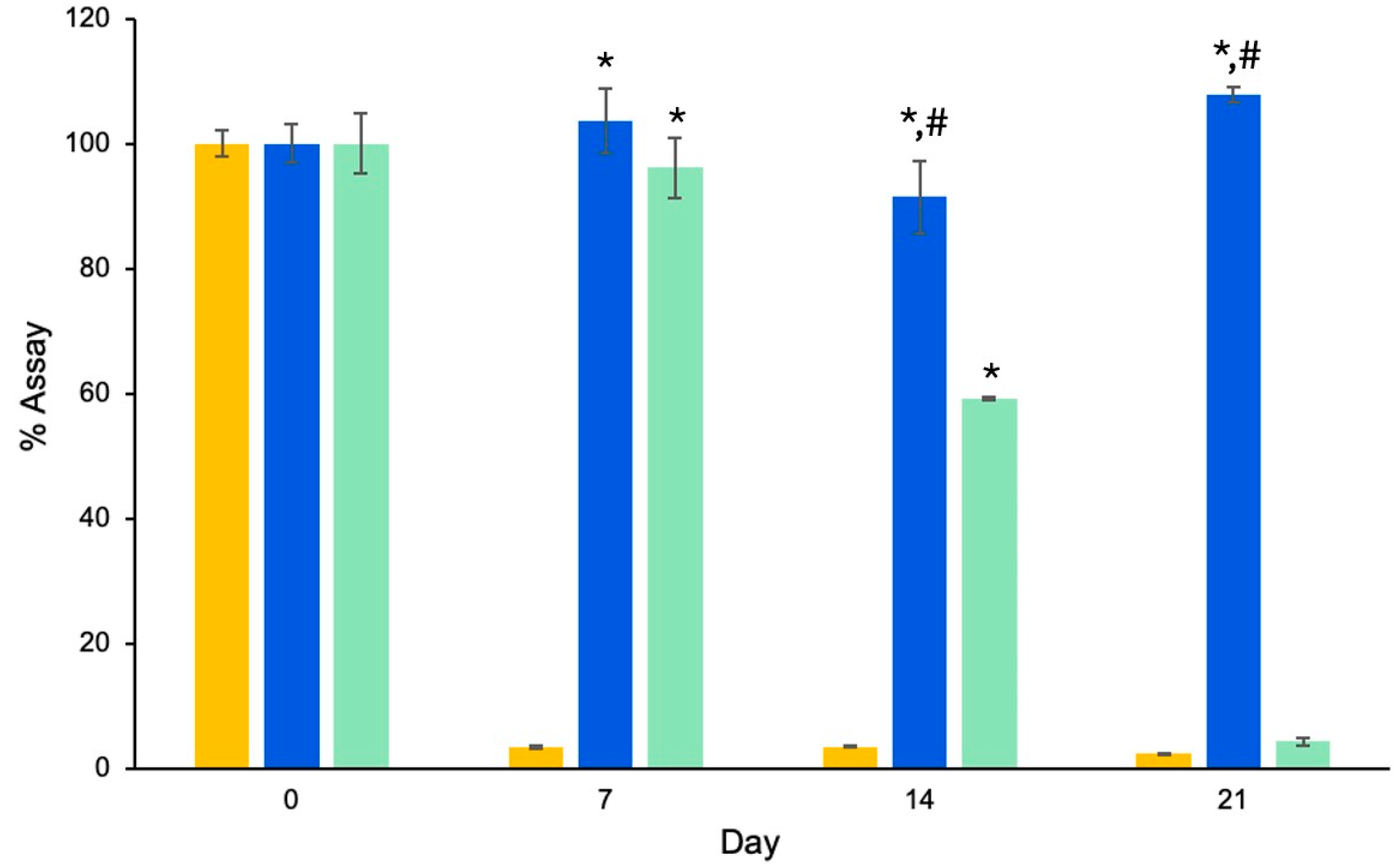
3.1.3. Stability Study of Retinal-Loaded Liposomes
3.1.4. In Vitro Skin Permeation of Retinal-Loaded Liposomes
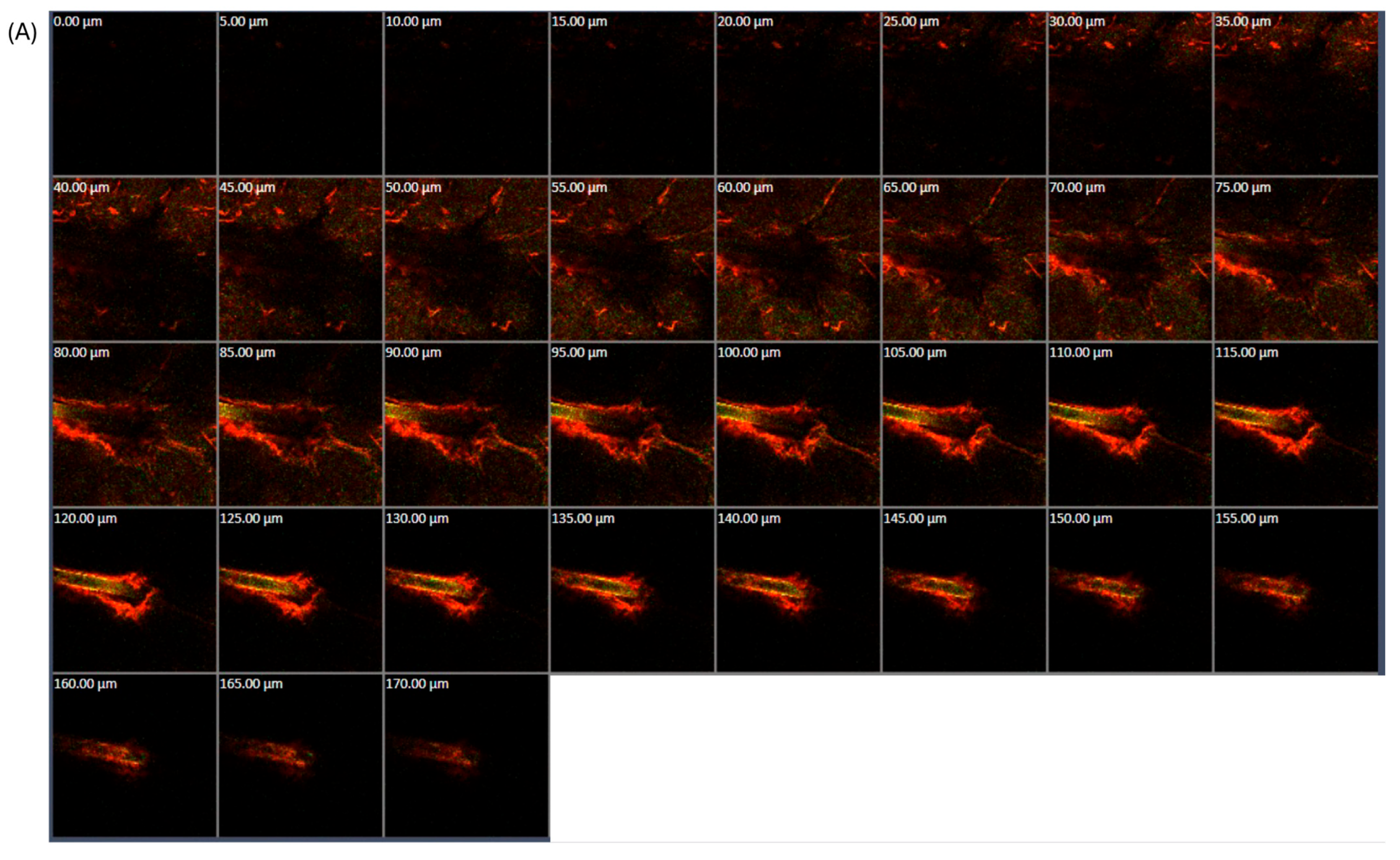
3.1.5. CLSM Study
3.2. Development of Retinal-Loaded Flexible Liposome-Embedded Pressure-Sensitive Adhesive Hydrogels
3.2.1. Characterizations and Release Study of Pressure-Sensitive Adhesive Hydrogels
3.2.2. Skin Deposition Study
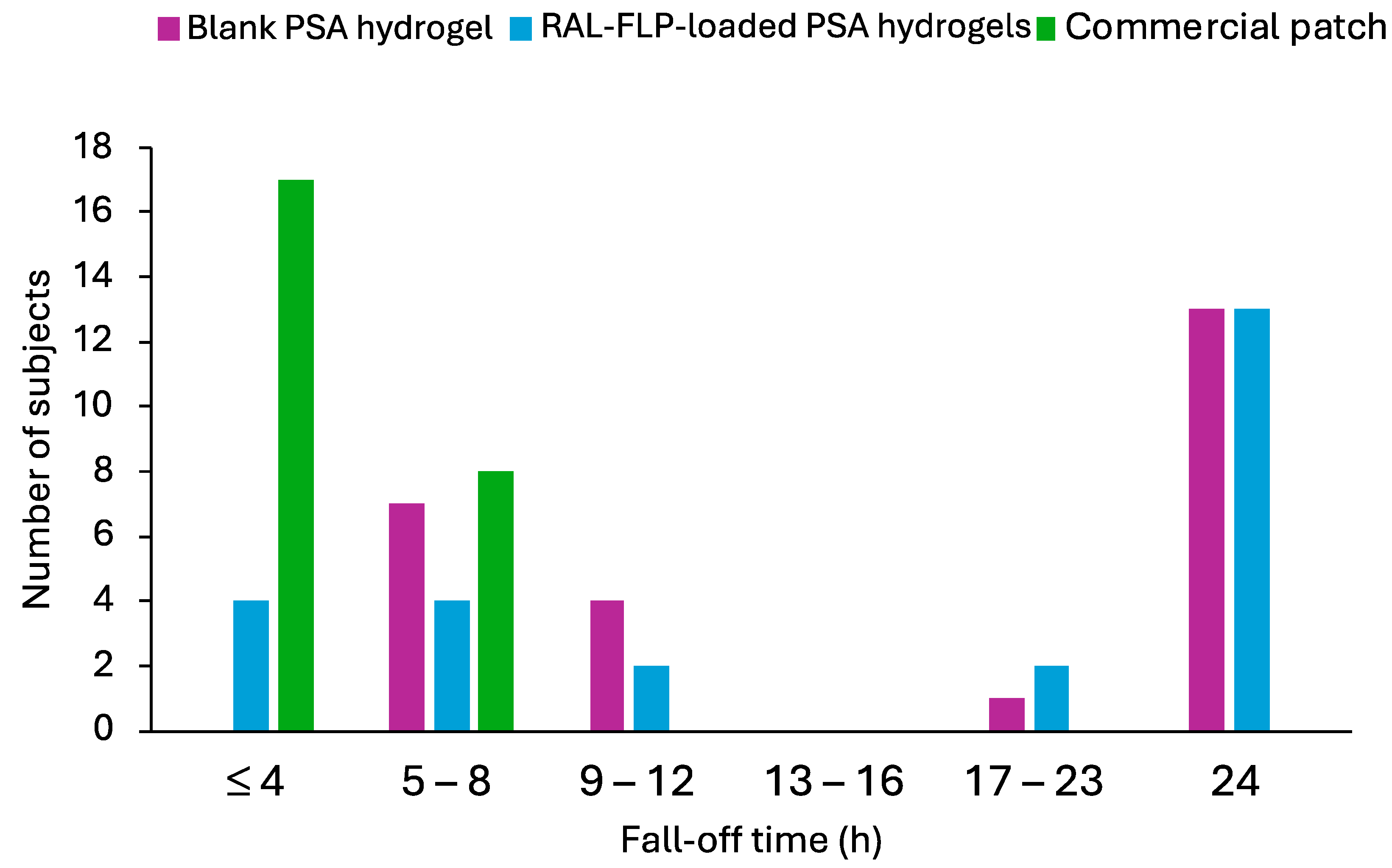
3.2.3. In Vivo Skin Irritation Study and Adhesion Capability in Human Subjects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milosheska, D.; Roškar, R. Use of Retinoids in Topical Antiaging Treatments: A Focused Review of Clinical Evidence for Conventional and Nanoformulations. Adv. Ther. 2022, 39, 5351–5375. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Hodak, E.; Lowe, N.J. Adverse effects of retinoids. Med. Toxicol. Advers. Drug Exp. 1988, 3, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.R.; Bush, A.E.; Cohen, P.R. Topical Retinoids: Therapeutic Mechanisms in the Treatment of Photodamaged Skin. Am. J. Clin. Dermatol. 2016, 17, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, O.V.; Adams, M.K.; Popov, K.M.; Kedishvili, N.Y. Generation of Retinaldehyde for Retinoic Acid Biosynthesis. Biomolecules 2019, 10, 5. [Google Scholar] [CrossRef]
- Kwon, H.S.; Lee, J.H.; Kim, G.M.; Bae, J.M. Efficacy and safety of retinaldehyde 0.1% and 0.05% creams used to treat photoaged skin: A randomized double-blind controlled trial. J. Cosmet. Dermatol. 2018, 17, 471–476. [Google Scholar] [CrossRef]
- Katsambas, A.D. RALGA (Diacneal), a retinaldehyde and glycolic acid association and postinflammatory hyperpigmentation in acne--a review. Dermatology 2005, 210 (Suppl. S1), 39–45. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, N.; Song, Q.; Du, Z.; Shu, P. Topical retinoids: Novel derivatives, nano lipid-based carriers, and combinations to improve chemical instability and skin irritation. J. Cosmet. Dermatol. 2024, 23, 3102–3115. [Google Scholar] [CrossRef]
- Pisetpackdeekul, P.; Supmuang, P.; Pan-In, P.; Banlunara, W.; Limcharoen, B.; Kokpol, C.; Wanichwecharungruang, S. Proretinal nanoparticles: Stability, release, efficacy, and irritation. Int. J. Nanomed. 2016, 11, 3277–3286. [Google Scholar] [CrossRef]
- Limcharoen, B.; Toprangkobsin, P.; Banlunara, W.; Wanichwecharungruang, S.; Richter, H.; Lademann, J.; Patzelt, A. Increasing the percutaneous absorption and follicular penetration of retinal by topical application of proretinal nanoparticles. Eur. J. Pharm. Biopharm. 2019, 139, 93–100. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; Al-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J. Cosmet. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef] [PubMed]
- Alsabeelah, N.; Arshad, M.F.; Hashmi, S.; Khan, R.A.; Khan, S. Nanocosmeceuticals for the management of ageing: Rigors and Vigors. J. Drug Deliv. Sci. Technol. 2021, 63, 102448. [Google Scholar] [CrossRef]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: A Review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Nayak, K.; Katiyar, S.S.; Kushwah, V.; Jain, S. Coenzyme Q10 and retinaldehyde co-loaded nanostructured lipid carriers for efficacy evaluation in wrinkles. J. Drug Target. 2018, 26, 333–344. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, Y.I.; Suk, J.; Lee, D.; Lee, J.H. A pilot study evaluating the efficacy and safety of retinaldehyde-loaded niosomes against mild-to-moderate acne. J. Cosmet. Dermatol. 2021, 20, 3586–3592. [Google Scholar] [CrossRef]
- Di Ventra, M.; Evoy, S.; Heflin, J. Introduction to Nanoscale Science and Technology; Springer Nature: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Rhim, J.-W. Effect of clay contents on mechanical and water vapor barrier properties of agar-based nanocomposite films. Carbohydr. Polym. 2011, 86, 691–699. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Mendoza-Muñoz, N.; Leyva-Gómez, G.; Piñón-Segundo, E.; Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Prado Audelo, M.L.; Urbán-Morlán, Z. Trends in biopolymer science applied to cosmetics. Int. J. Cosmet. Sci. 2023, 45, 699–724. [Google Scholar] [CrossRef]
- Alex, M.; Alsawaftah, N.M.; Husseini, G.A. State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Appl. Sci. 2024, 14, 2926. [Google Scholar] [CrossRef]
- Kang, H.; Zuo, Z.; Lin, R.; Yao, M.; Han, Y.; Han, J. The most promising microneedle device: Present and future of hyaluronic acid microneedle patch. Drug Deliv. 2022, 29, 3087–3110. [Google Scholar] [CrossRef] [PubMed]
- Arbós, P.; Wirth, M.; Arangoa, M.A.; Gabor, F.; Irache, J.M. Gantrez® AN as a new polymer for the preparation of ligand–nanoparticle conjugates. J. Control. Release 2002, 83, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Ogunsola, O.A.; Kraeling, M.E.; Zhong, S.; Pochan, D.J.; Bronaugh, R.L.; Raghavan, S.R. Structural analysis of “flexible” liposome formulations: New insights into the skin-penetrating ability of soft nanostructures. Soft Matter 2012, 8, 10226–10232. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.K. Development of skin-permeable flexible liposome using ergosterol esters containing unsaturated fatty acids. Chem. Phys. Lipids 2023, 250, 105270. [Google Scholar] [CrossRef]
- Kritsanaporn, T.; Koranat, D.; Praneet, O.; Nopparat, N.; Monrudee, S.; Worranan, R. Investigation of lipid nanocarriers and microspicule gel for dermal delivery of porcine placenta extract. J. Curr. Sci. Technol. 2022, 12, 505–516. [Google Scholar]
- Mateos, H.; Palazzo, G. Chapter 3—Colloidal stability. In Colloidal Foundations of Nanoscience, 2nd ed.; Berti, D., Palazzo, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 57–83. [Google Scholar]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Kedishvili, N.Y. Retinoic Acid Synthesis and Degradation. Subcell. Biochem. 2016, 81, 127–161. [Google Scholar] [CrossRef]
- Pasarin, D.; Ghizdareanu, A.I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.A. Coating Materials to Increase the Stability of Liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef]
- Musakhanian, J.; Rodier, J.-D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.; Gianeti, M.D.; Camargo, F.B., Jr.; Gaspar, L.R. Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. Eur. J. Pharm. Biopharm. 2012, 82, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Sheraz, M.; Khan, M.; Ahmed, S.; Kazi, S.; Ahmad, I. Stability and Stabilization of Ascorbic Acid. Househ. Pers. Care Today 2015, 10, 20–25. [Google Scholar]
- Baptista, S.; Baptista, F.; Freitas, F. Development of Emulsions Containing L-Ascorbic Acid and α-Tocopherol Based on the Polysaccharide FucoPol: Stability Evaluation and Rheological and Texture Assessment. Cosmetics 2023, 10, 56. [Google Scholar] [CrossRef]
- Guaratini, T.; Gianeti, M.D.; Campos, P.M. Stability of cosmetic formulations containing esters of vitamins E and A: Chemical and physical aspects. Int. J. Pharm. 2006, 327, 12–16. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Gaspar, L.R.; Camargo, F.B., Jr.; Campos, P.M. Benefits of combinations of vitamin A, C and E derivatives in the stability of cosmetic formulations. Molecules 2012, 17, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Pena-Rodríguez, E.; Moreno, M.C.; Blanco-Fernandez, B.; González, J.; Fernández-Campos, F. Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies. Pharmaceutics 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, C. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Final version of the Opinion on Vitamin A (retinol, retinyl acetate and retinyl palmitate) in cosmetic products. Regul. Toxicol. Pharmacol. 2017, 84, 102–104. [Google Scholar] [CrossRef]
- Oh, Y.K.; Kim, M.Y.; Shin, J.Y.; Kim, T.W.; Yun, M.O.; Yang, S.J.; Choi, S.S.; Jung, W.W.; Kim, J.A.; Choi, H.G. Skin permeation of retinol in Tween 20-based deformable liposomes: In-vitro evaluation in human skin and keratinocyte models. J. Pharm. Pharmacol. 2006, 58, 161–166. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S. Strategies for vitamin E transdermal delivery. In Handbook of Diet, Nutrition and the Skin; Preedy, V.R., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 128–143. [Google Scholar]
- Trivedi, J.S.; Krill, S.L.; Fort, J.J. Vitamin E as a human skin penetration enhancer. Eur. J. Pharm. Sci. 1995, 3, 241–243. [Google Scholar] [CrossRef]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J. Drug Deliv. 2011, 2011, 418316. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Akhtar, N. Development of stable tocopherol succinate-loaded ethosomes to enhance transdermal permeation: In vitro and in vivo characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, M.; Liu, A. A review on mechanical properties of pressure sensitive adhesives. Int. J. Adhes. Adhes. 2013, 41, 98–106. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Z.; Qi, X. High skin permeation, deposition and whitening activity achieved by xanthan gum string vitamin c flexible liposomes for external application. Int. J. Pharm. 2022, 628, 122290. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Tan, G.; Xu, P.; Lawson, L.B.; He, J.; Freytag, L.C.; Clements, J.D.; John, V.T. Hydration effects on skin microstructure as probed by high-resolution cryo-scanning electron microscopy and mechanistic implications to enhanced transcutaneous delivery of biomacromolecules. J. Pharm. Sci. 2010, 99, 730–740. [Google Scholar] [CrossRef]
- Romita, P.; Foti, C.; Calogiuri, G.; Cantore, S.; Ballini, A.; Dipalma, G.; Inchingolo, F. Contact dermatitis due to transdermal therapeutic systems: A clinical update. Acta Biomed. 2018, 90, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Rouvrais, C.; Bacqueville, D.; Bogdanowicz, P.; Haure, M.J.; Duprat, L.; Coutanceau, C.; Castex-Rizzi, N.; Duplan, H.; Mengeaud, V.; Bessou-Touya, S. A new dermocosmetic containing retinaldehyde, delta-tocopherol glucoside and glycylglycine oleamide for managing naturally aged skin: Results from in vitro to clinical studies. Clin. Cosmet. Investig. Dermatol. 2017, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Bioinspired ketoprofen-incorporated polyvinylpyrrolidone/polyallylamine/polydopamine hydrophilic pressure-sensitive adhesives patches with improved adhesive performance for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2022, 181, 207–217. [Google Scholar] [CrossRef] [PubMed]

| Formulations | Amount (%w/w) | |||||
|---|---|---|---|---|---|---|
| GantrezTM S-97 | Hya | Al Glycinate | Malic Acid | Glycerin | Water q.s. to | |
| F1 | 20 | 1 | 0.1 | 0.02 | 20 | 100 |
| F2 | 15 | 1 | 0.1 | 0.02 | 20 | 100 |
| F3 | 25 | 1 | 0.1 | 0.02 | 20 | 100 |
| F4 | 20 | 0.5 | 0.1 | 0.02 | 20 | 100 |
| F5 | 20 | 2 | 0.1 | 0.02 | 20 | 100 |
| Formulations | Particle Size (nm) † | PDI | ZP (mV) |
|---|---|---|---|
| α-tocopherol LPs | 55.11 ± 0.51 | 0.29 ± 0.01 | –27.70 ± 1.44 |
| Conventional LPs | 80.71 ± 0.76 | 0.21 ± 0.01 | –4.78 ± 0.44 |
| RAL-FLP Formulation | Initial RAL Concentration (mg/mL) | Particle Size (nm) | PDI | ZP (mV) | %LC | %EE |
|---|---|---|---|---|---|---|
| R1 | 1 | 46.14 ± 1.97 | 0.26 ± 0.01 | –36.32 ± 1.78 | 55.42 ± 3.61 *# | 52.68 ± 3.43 *# |
| R2 | 3 | 59.46 ± 0.54 | 0.33 ± 0.01 | –37.64 ± 6.87 | 42.71 ± 1.80 # | 16.38 ± 0.69 # |
| R3 | 5 | 71.48 ± 0.47 | 0.26 ± 0.02 | –39.50 ± 4.91 | 38.26 ± 2.07 * | 10.33 ± 0.56 * |
| Formulations | Appearance | Tacking Strength (N) | Peeling Force (N) |
|---|---|---|---|
| F1 | Soft and adhesive | 2.44 ± 0.21 * | 3.44 ± 0.50 |
| F2 | Leave residue | 3.05 ± 0.01 * | 0.92 ± 0.02 * |
| F3 | Soft and adhesive | 5.73 ± 0.30 | 3.13 ± 0.90 |
| F4 | Leave residue | 4.01 ± 0.67 * | 2.22 ± 0.36 |
| F5 | Leave residue | 6.37 ± 0.63 | 3.80 ± 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singpanna, K.; Jiratananan, P.; Paiboonwasin, S.; Petcharawuttikrai, N.; Chaksmithanont, P.; Pornpitchanarong, C.; Patrojanasophon, P. Alpha-Tocopherol-Infused Flexible Liposomal Nanocomposite Pressure-Sensitive Adhesive: Enhancing Skin Permeation of Retinaldehyde. Polymers 2024, 16, 2930. https://doi.org/10.3390/polym16202930
Singpanna K, Jiratananan P, Paiboonwasin S, Petcharawuttikrai N, Chaksmithanont P, Pornpitchanarong C, Patrojanasophon P. Alpha-Tocopherol-Infused Flexible Liposomal Nanocomposite Pressure-Sensitive Adhesive: Enhancing Skin Permeation of Retinaldehyde. Polymers. 2024; 16(20):2930. https://doi.org/10.3390/polym16202930
Chicago/Turabian StyleSingpanna, Kanokwan, Puchapong Jiratananan, Santipharp Paiboonwasin, Nawinda Petcharawuttikrai, Prin Chaksmithanont, Chaiyakarn Pornpitchanarong, and Prasopchai Patrojanasophon. 2024. "Alpha-Tocopherol-Infused Flexible Liposomal Nanocomposite Pressure-Sensitive Adhesive: Enhancing Skin Permeation of Retinaldehyde" Polymers 16, no. 20: 2930. https://doi.org/10.3390/polym16202930
APA StyleSingpanna, K., Jiratananan, P., Paiboonwasin, S., Petcharawuttikrai, N., Chaksmithanont, P., Pornpitchanarong, C., & Patrojanasophon, P. (2024). Alpha-Tocopherol-Infused Flexible Liposomal Nanocomposite Pressure-Sensitive Adhesive: Enhancing Skin Permeation of Retinaldehyde. Polymers, 16(20), 2930. https://doi.org/10.3390/polym16202930







