Chemical Activation Boosted Interface Interaction between Poly(tetrafluoroethylene-co-hexafluoropropylene) Film and Silver Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Activation of FEP
2.3. Ag Coated onto FEP Film
2.4. Characterization
2.5. DFT Calculation
3. Results and Discussion
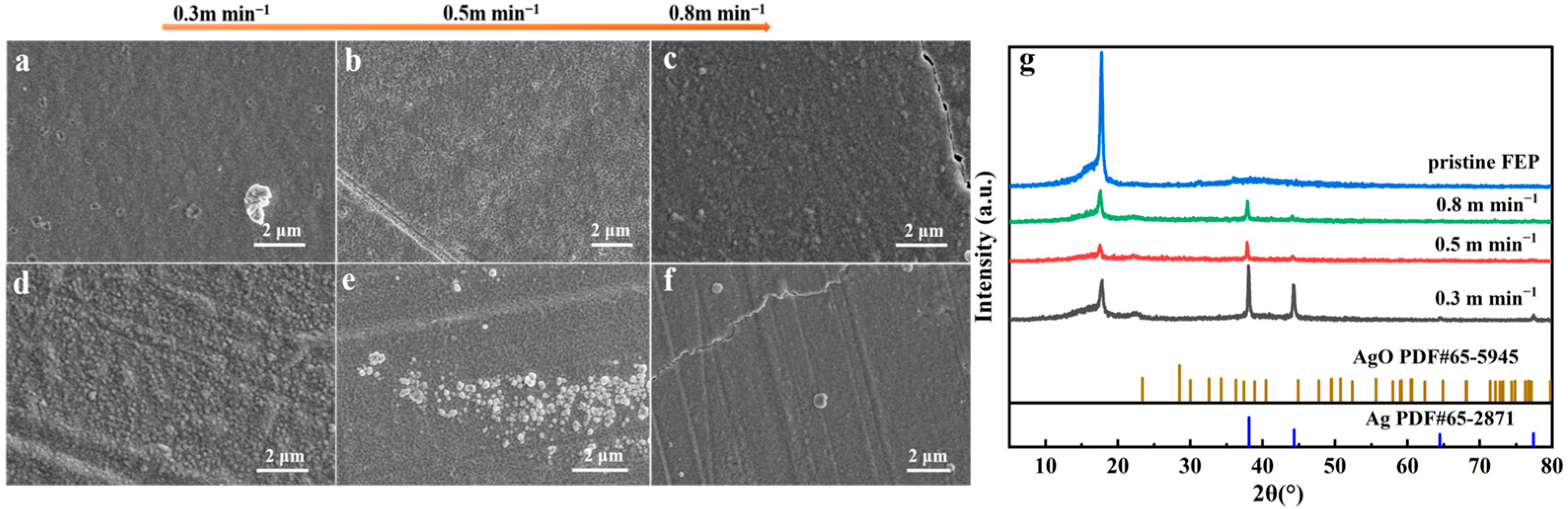
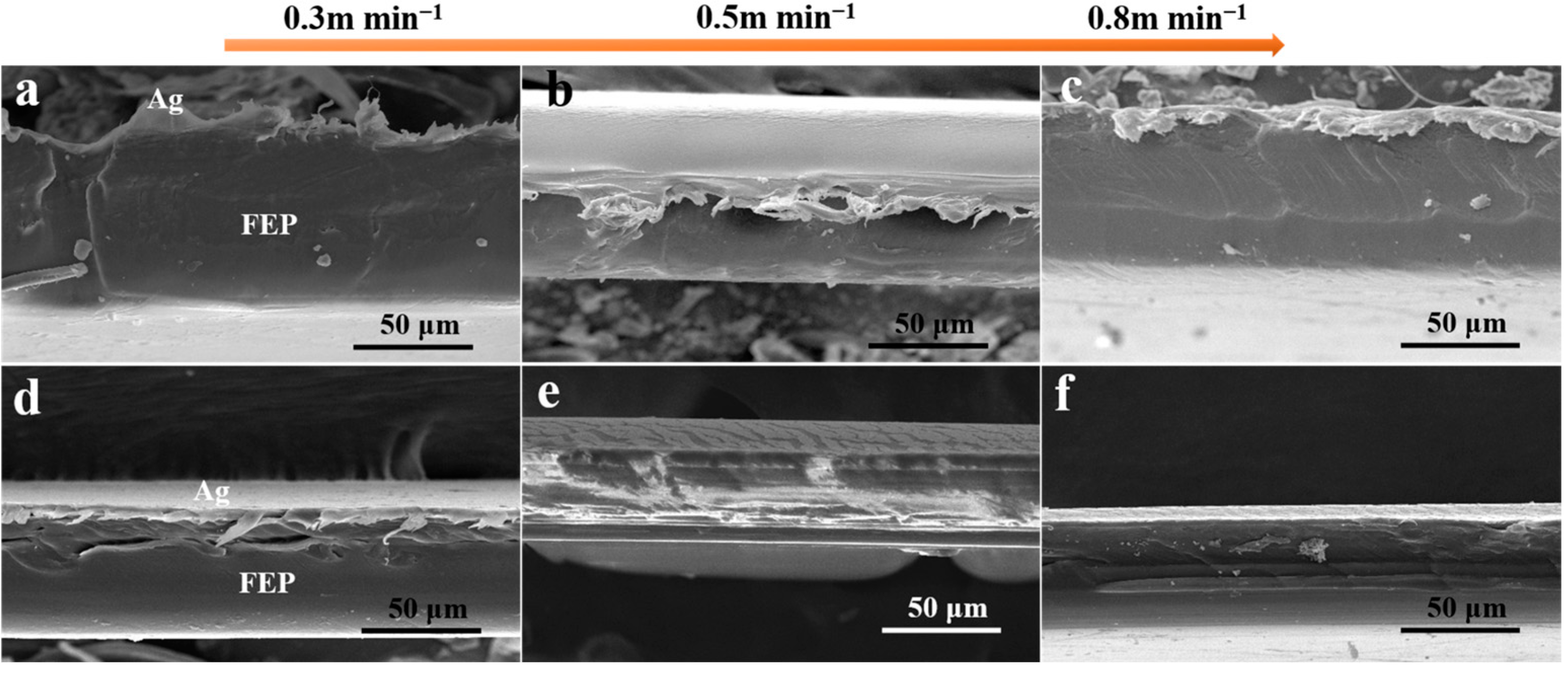
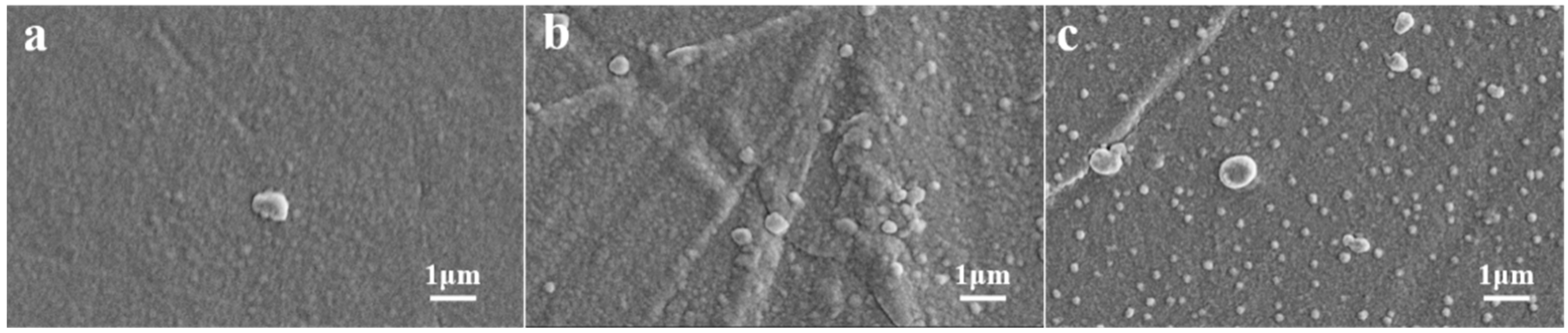
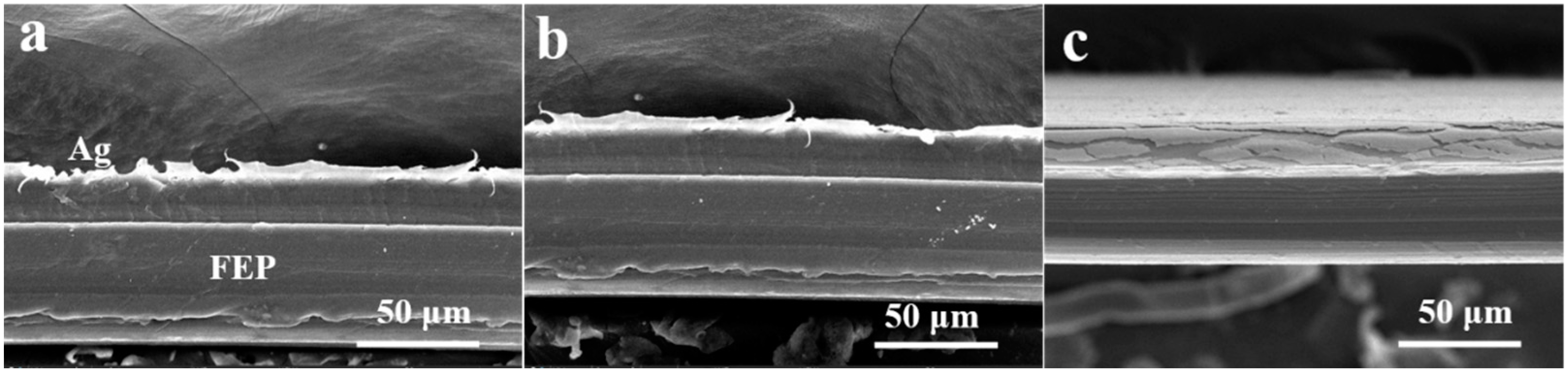
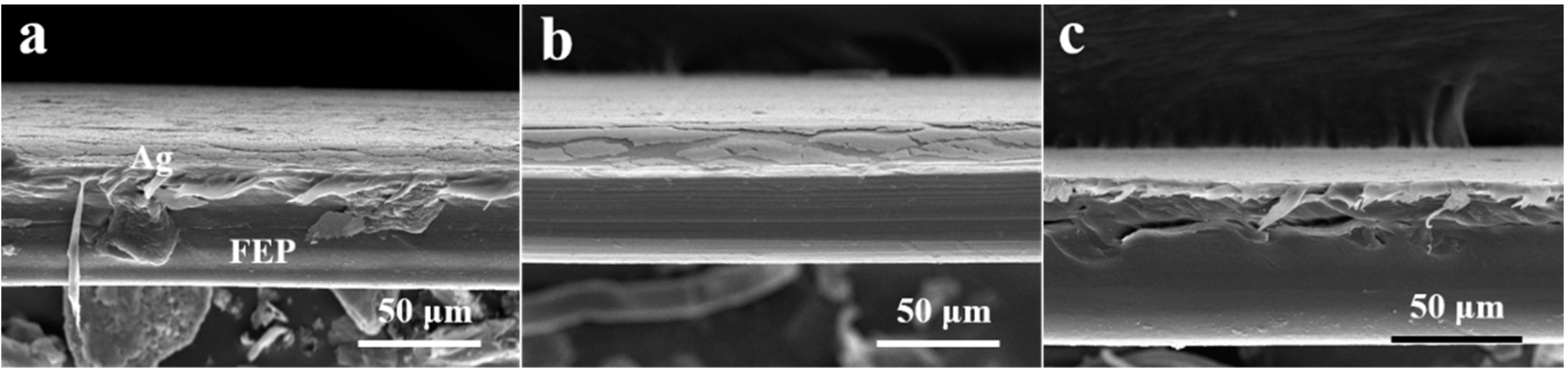
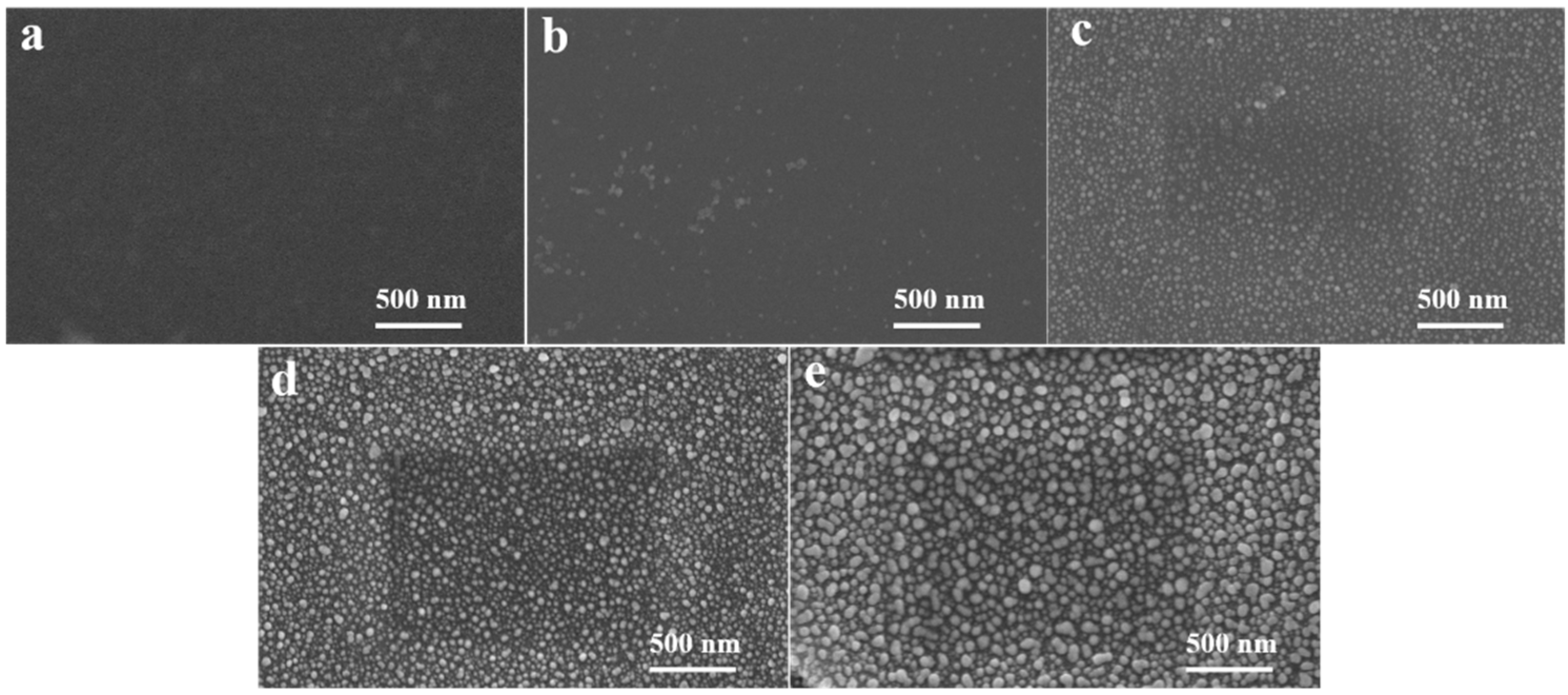
3.1. The Effect of Winding Speed of Vacuum Ag Deposition
3.2. The Effect of Sodium Naphthalene Solution Concentration
3.3. The Effect of Chemical Activation Time
3.4. Mechanism of Enhanced Interface Interaction by Chemical Activation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Angirasa, D.; Ayyaswamy, P.S. Review of evaluation methodologies for satellite exterior materials in low earth orbit. J. Spacecr. Rocket. 2014, 51, 750–761. [Google Scholar] [CrossRef]
- Miyazaki, E.; Yamagata, I. Results of space-environment exposure of the flexible optical solar reflector. J. Spacecr. Rocket. 2009, 46, 28–32. [Google Scholar] [CrossRef]
- Ichino, T.; Sasaki, S.; Hasuda, Y. Development of high-performance flexible optical solar reflectors. Electron. Commun. Jpn. 1986, 69, 199–205. [Google Scholar] [CrossRef]
- Pipptin, G.; Normand, E.; Woll, S. Analysis of Ag/FEP thermal control blanket performance from multiple satellites. NASA Tech. Rep. 2001, 20010020230, 1–15. [Google Scholar]
- Gilmore, D.G. Spacecraft Thermal Control Handbook, Fundamental Technologies I; The Aerospace Press: Arlington, VA, USA; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2002. [Google Scholar]
- Drobny, J.G. Applications of Fluoropolymer Films; William Andrew: Cambridge, MA, USA, 2020; pp. 151–152. [Google Scholar]
- Sibin, K.P.; Esther, A.C.M.; Shashikala, H.D.; Dey, A.; Sridhara, N.; Sharma, A.K.; Barshilia, H.C. Environmental stability of transparent and conducting ITO thin films coated on flexible FEP and Kapton® substrates for spacecraft applications. Sol. Energy Mater. Sol. Cells 2018, 176, 134–141. [Google Scholar] [CrossRef]
- Sibin, K.P.; Srinivas, G.; Shashikala, H.D.; Dey, A.; Sridhara, N.; Sharma, A.K.; Barshilia, H.C. Highly transparent and conducting ITO/Ag/ITO multilayer thin films on FEP substrates for flexible electronics applications. Sol. Energy Mater. Sol. Cells 2017, 172, 277–284. [Google Scholar] [CrossRef]
- Sibin, K.P.; Swain, N.; Chowdhury, P.; Dey, A.; Sridhara, N.; Shashikala, H.D.; Sharma, A.K.; Barshilia, H.C. Optical and electrical properties of ITO thin films sputtered on flexible FEP substrate as passive thermal control system for space applications. Sol. Energy Mater. Sol. Cells 2016, 145, 314–322. [Google Scholar] [CrossRef]
- Sessler, G.M.; West, J.E.; Ryan, F.W.; Schonhorn, H. Increase of gold-Teflon FEP joint strength by electron bombardment. J. Appl. Polym. Sci. 1973, 17, 3199–3209. [Google Scholar] [CrossRef]
- Schonhorn, H.; Ryan, F.W. Effect of polymer surface morphology on adhesion and adhesive joint strength. II. FEP Teflon and Nylon 6. J. Polym. Sci. Part A-2 1969, 7, 105–111. [Google Scholar] [CrossRef]
- Brewis, D.M.; Mathieson, I.; Sutherland, I.; Cayless, R.A. Adhesion studies of fluoropolymers. J. Adhes. 1993, 41, 113–128. [Google Scholar] [CrossRef]
- Wang, L.; Duan, R. A study of the joint between fluoroethylenepropylene(FEP) and metal. Aerosp. Mater. Technol. 2003, 33, 49–52. [Google Scholar]
- Kaczinski, M.B.; Dwight, D.W. Enhancement of polymer film adhesion using acid-base interactions determined by contact angle measurements. J. Adhesion Sci. Technol. 1993, 7, 165–177. [Google Scholar] [CrossRef]
- Johansson, K.S. Applied Plastics Engineering Handbook, 2nd ed.; William Andrew: Cambridge, MA, USA, 2017; pp. 443–487. [Google Scholar]
- Allmer, K.; Feiring, A.E. Photochemical modification of a fluoropolymer surface. Macromolecules 1991, 24, 5487–5488. [Google Scholar] [CrossRef]
- Bening, R.C.; McCarthy, T.J. Surface modification of poly(tetrafluoroethylene-co-hexafluoropropylene). Introduction of alcohol functionality. Macromolecules 1990, 23, 2648–2655. [Google Scholar] [CrossRef]
- Chen, J.X.; Tracy, D.; Zheng, S.; Xiaolu, L.; Brown, S.; VanDerveer, W.; Entenberg, A.; Vukanovic, V.; Takacs, G.A.; Egitto, F.D.; et al. Photoetching and modification of poly(tetrafluoroethylene-co-hexafluoropropylene) polymer surfaces with vacuum UV radiation. Polym. Degrad. Stabil. 2003, 79, 399–404. [Google Scholar] [CrossRef]
- Siperko, L.M.; Thomas, R.R. Chemical and physical modification of fluoropolymer surfaces for adhesion enhancement: A review. J. Adhesion Sci. Technol. 1989, 3, 157–173. [Google Scholar] [CrossRef]
- Kim, S.R. Surface modification of poly(tetrafluoroethylene) film by chemical etching, plasma, and ion beam treatments. J. Appl. Polym. Sci. 2000, 77, 1913–1920. [Google Scholar] [CrossRef]
- Park, Y.W.; Tasaka, S.; Inagaki, N. Surface modification of tetrafluoroethylene–hexafluoropropylene (FEP) copolymer by remote H2, N2, O2, and Ar plasmas. J. Appl. Polym. Sci. 2002, 83, 1258–1267. [Google Scholar] [CrossRef]
- Benderly, A.A. Treatment of Teflon to promote bondability. J. Appl. Polym. Sci. 1962, 6, 221–225. [Google Scholar] [CrossRef]
- Wu, J.K. Surface modification of fluoroplastics. Plastics 1996, 25, 7–12. [Google Scholar]
- Qian, J.; Xu, Z.L. Radiation induced grafting of electron beam pre-irradiated F46 film with 4-vinylpyridine in its saturated vapor phase for preparing anion exchange membranes. J. Radiat. Res. Radiat. Process. 1987, 5, 55–58. [Google Scholar]
- Chu, G.H.; Li, H.; He, Y.Y.; Zhang, S.X. Plasma surface modification of FEP fiber. J. Funct. Mater. 2011, 42, 1332–1334. [Google Scholar]
- Li, X.; Zhang, L.; Wang, H.; Zhao, Y. Effect of chemical activation on surface properties of poly(tetrafluoroethylene-co-hexafluoropropylene) film. Polymers 2022, 14, 4606. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, C.; Xu, X.; Ye, W.; Qiu, G.; Wang, D. Silver mirror films deposited on well plates for SERS detection of multi-analytes: Aiming at 96-well technology. Talanta 2021, 222, 121544. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the Dmol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdrew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long- range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Elstner, M.; Hobza, P.; Frauenheim, T.; Suhai, S.; Kaxiras, E. Hydrogen bonding and stacking interactions of nucleic acid base pairs: A density-functional-theory based treatment. J. Chem. Phys. 2001, 114, 5149–5155. [Google Scholar] [CrossRef]
- Liu, L.; Tong, Z.; Xu, J.; Fan, Z.; Yu, K. Composition heterogeneity of tetrafluoroethylene-hexafluoropropylene random copolymers characterized by successive self-nucleation and annealing. J. Therm. Anal. Calorim. 2013, 114, 573–579. [Google Scholar] [CrossRef]
- Flores-Rojas, G.; López-Saucedo, F.; Vera-Graziano, R.; Magaña, H.; Mendizábal, E.; Bucio, E. Silver nanoparticles loaded on polyethylene terephthalate films grafted with chitosan. Polymers 2023, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, R. Physisorption of benzene derivatives on graphene: Critical roles of steric and stereoelectronic effects of the substituent. Phys. Chem. Chem. Phys. 2015, 17, 12185. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Guo, X.; Li, X.; Gong, C.; Zhao, Y. Chemical Activation Boosted Interface Interaction between Poly(tetrafluoroethylene-co-hexafluoropropylene) Film and Silver Coating. Polymers 2024, 16, 2730. https://doi.org/10.3390/polym16192730
Wang H, Guo X, Li X, Gong C, Zhao Y. Chemical Activation Boosted Interface Interaction between Poly(tetrafluoroethylene-co-hexafluoropropylene) Film and Silver Coating. Polymers. 2024; 16(19):2730. https://doi.org/10.3390/polym16192730
Chicago/Turabian StyleWang, Hu, Xiuqi Guo, Xuelei Li, Chenliang Gong, and Yongqing Zhao. 2024. "Chemical Activation Boosted Interface Interaction between Poly(tetrafluoroethylene-co-hexafluoropropylene) Film and Silver Coating" Polymers 16, no. 19: 2730. https://doi.org/10.3390/polym16192730
APA StyleWang, H., Guo, X., Li, X., Gong, C., & Zhao, Y. (2024). Chemical Activation Boosted Interface Interaction between Poly(tetrafluoroethylene-co-hexafluoropropylene) Film and Silver Coating. Polymers, 16(19), 2730. https://doi.org/10.3390/polym16192730





