Urchin-like WO3 Particles Form Honeycomb-like Structured PLA/WO3 Nanocomposites with Enhanced Crystallinity, Thermal Stability, Rheological, and UV-Blocking and Antifungal Activity
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Preparation of PLA/WO3 Nanocomposite
2.3. Physicochemical Characterization
2.4. Rheological Measurements
2.5. Thermal Analysis
2.6. Antimicrobial Assays
3. Results and Discussions
3.1. Analysis of WO3 Nanoparticles
3.2. Crystallinity and Polymorph of PLA/WO3 Nanocomposites
3.3. Morphological Structure of PLA/WO3 Nanocomposites
3.4. Chemical Bonding in PLA/WO3 Nanocomposites
3.5. UV-Absorbance and Blocking of PLA/WO3 Nanocomposites
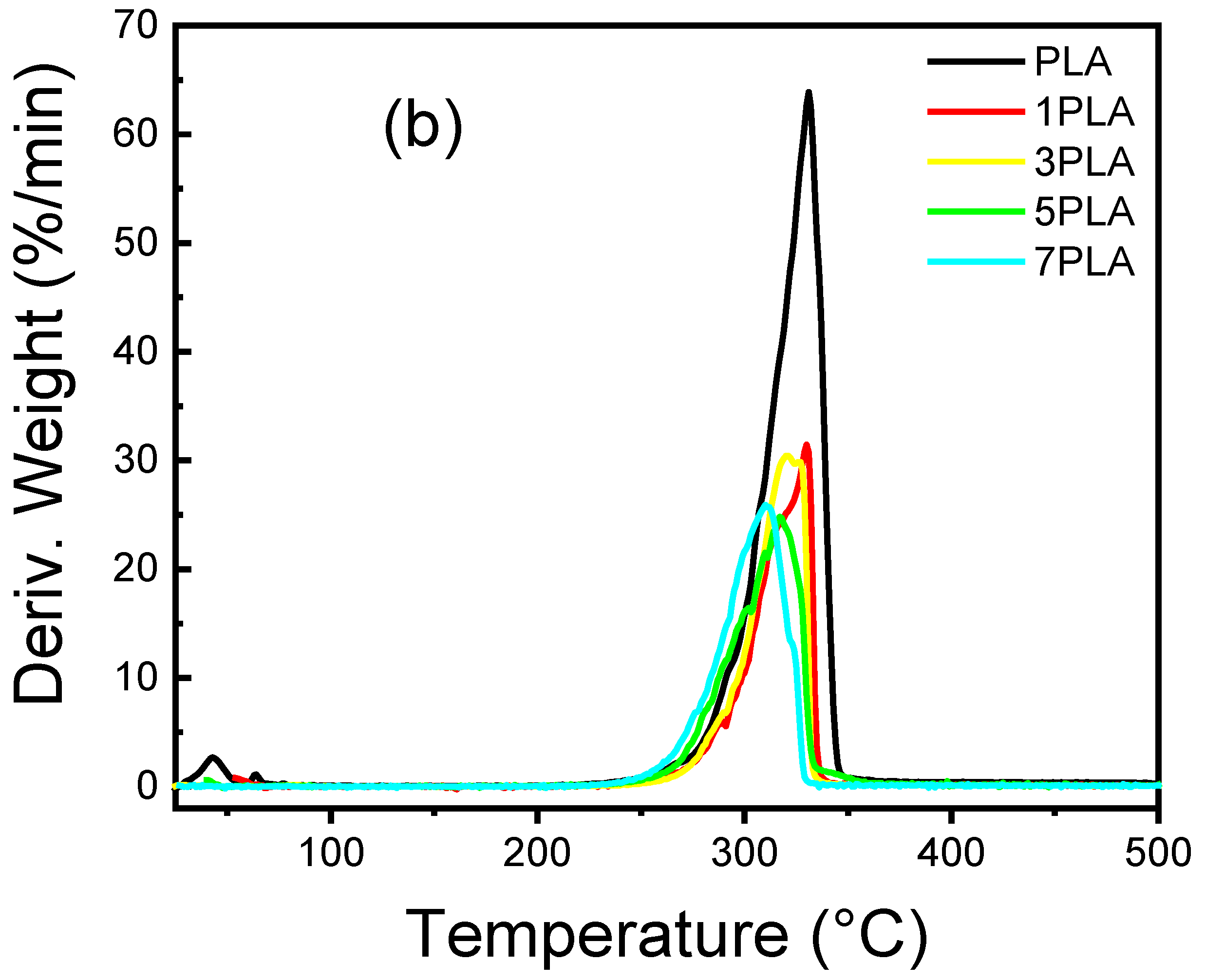
3.6. Thermal Stability of PLA/WO3 Nanocomposites
3.7. DSC Analysis of PLA/WO3 Nanocomposites
3.8. Rheological Properties of PLA/WO3 Nanocomposites
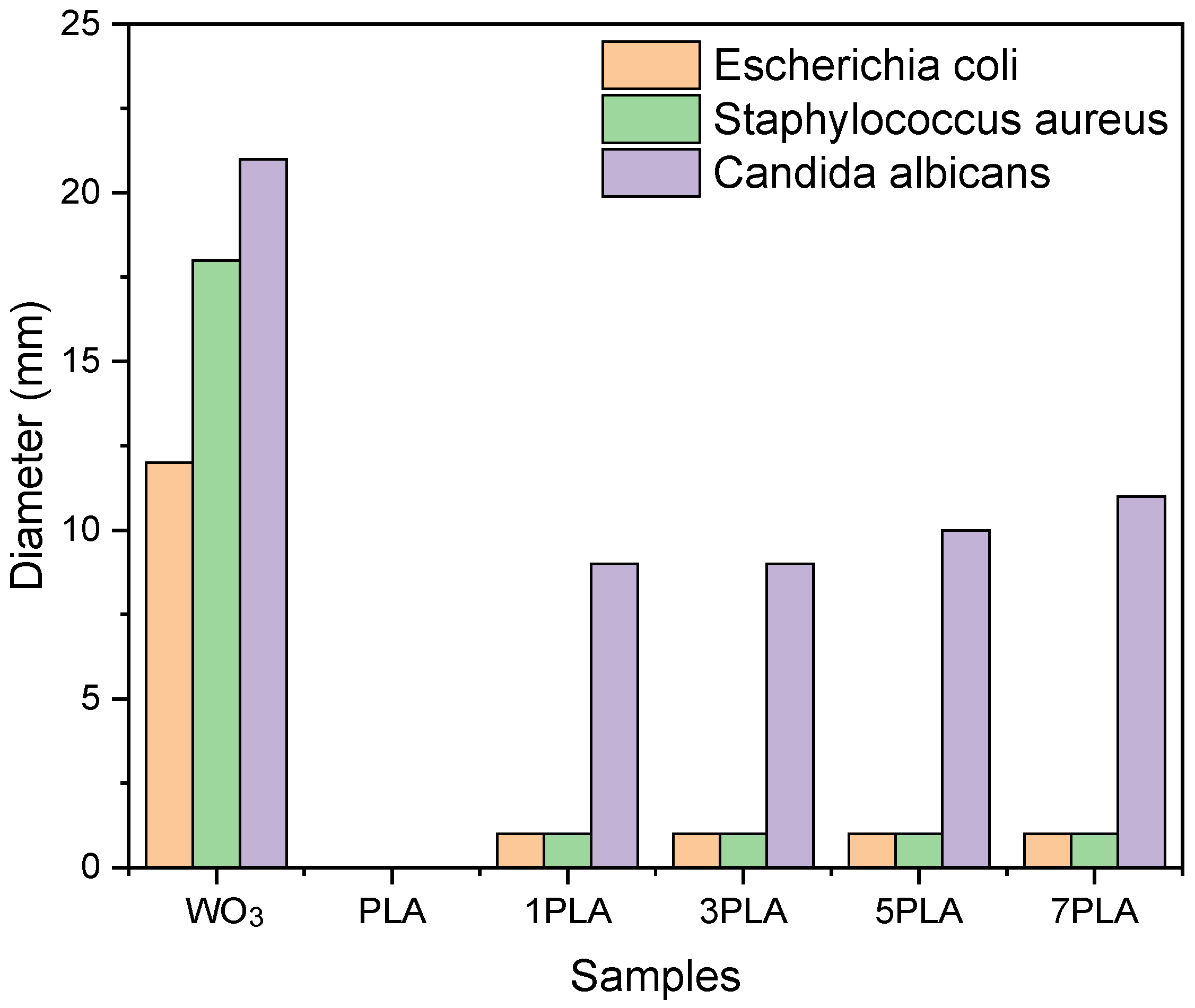
3.9. Antimicrobial Activity of PLA/WO3 Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, I.; Saeed, K.; Ali, N.; Zada, N.; Khan, A.; Ali, F.; Bilal, M.; Akhter, M.S. Polymer nanocomposites: An overview. Smart Polym. Nanocompos. 2023, 167–184. [Google Scholar]
- Barhoum, A.; Van Lokeren, L.; Rahier, H.; Dufresne, A.; Van Assche, G. Roles of in situ surface modification in controlling the growth and crystallization of CaCO3 nanoparticles, and their dispersion in polymeric materials. J. Mater. Sci. 2015, 50, 7908–7918. [Google Scholar] [CrossRef]
- Mishra, R.; Militky, J. Nanotechnology in Textiles: Theory and Application; Woodhead Publishing: Sawston, UK, 2018; p. 387. [Google Scholar]
- Hammani, S.; Barhoum, A.; Nagarajan, S.; Bechelany, M. Toner Waste Powder (TWP) as a Filler for Polymer Blends (LDPE/HIPS) for Enhanced Electrical Conductivity. Materials 2019, 12, 3062. [Google Scholar] [CrossRef]
- Obeid, A.; Balaa, H.E.; Samad, O.E.; Awad, R.; Badawi, M.S. Attenuation parameters of HDPE filled with different nano-size and bulk WO3 for X-ray shielding applications. Eur. Phys. J. Plus 2022, 137, 1229. [Google Scholar] [CrossRef]
- Yang, B.; Tran, T.-T.; Milam-Guerrero, J.; To, D.T.; Stahovich, T.; Myung, N.V. Enhancing gas sensing performance of tungsten trioxide (WO3) nanofibers through diameter and crystallinity control. Sens. Actuators Rep. 2024, 7, 100182. [Google Scholar] [CrossRef]
- Sharmila, B.; Dwivedi, P. Optical sensing and computing memory devices using nanostructured WO3. Mater. Sci. Semicond. Process. 2024, 173, 108106. [Google Scholar]
- Zhuzhelskii, D.; Tolstopyatova, E.; Volkov, A.; Eliseeva, S.; Kondratiev, V. Spectroelectrochemistry of Poly (3,4-Ethylenedioxythiophene)–Tungsten Oxide Composite Films in Dilute Sulfuric Acid Solution. Russ. J. Gen. Chem. 2020, 90, 1949–1954. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, J.; Wang, L.; Yang, X.; Li, X.; Jiang, Y. Facile WO3@ PANI composite film for applications in double-layer photoelectrochromic supercapacitors. Mater. Lett. 2023, 335, 133809. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Enhanced room temperature sulfur dioxide sensing behaviour of in situ polymerized polyaniline–tungsten oxide nanocomposite possessing honeycomb morphology. RSC Adv. 2015, 5, 73535–73544. [Google Scholar] [CrossRef]
- Issa, S.A.; Alrowaily, A.W.; Abulyazied, D.; Ali, E.; Zakaly, H.M. Effects of WO3 reinforcement on the properties of poly (lactic acid) composites for radiation shielding. Radiat. Phys. Chem. 2023, 212, 111121. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Wirzal, M.D.H.; Ali, F.; Roza, L.; Sambudi, N.S. Electrospun polylactic acid/tungsten oxide/amino-functionalized carbon quantum dots (PLA/WO3/N-CQDs) fibers for oil/water separation and photocatalytic decolorization. J. Environ. Chem. Eng. 2021, 9, 106033. [Google Scholar] [CrossRef]
- Liew, W.C.; Muhamad, I.I.; Chew, J.W.; Abd Karim, K.J. Synergistic effect of graphene oxide/zinc oxide nanocomposites on polylactic acid-based active packaging film: Properties, release kinetics and antimicrobial efficiency. Int. J. Biol. Macromol. 2023, 253, 127288. [Google Scholar] [CrossRef]
- Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Sinha Ray, S. Rheology of poly (lactic acid)-based systems. Polym. Rev. 2019, 59, 465–509. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.-B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly (lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly (lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.L.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. Role of Rheology in Morphology Development and Advanced Processing of Thermoplastic Polymer Materials: A Review. ACS Omega 2023, 8, 27969–28001. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Wang, X.; Wang, Z.; Yu, D.; Ji, D. High-hydrophobic ZIF-67@ PLA honeycomb aerogel for efficient oil–water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130768. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Li, P.; Huang, G.; Feng, S.; Shen, C.; Han, B.; Zhang, X.; Jin, F.; Xu, F. Bioinspired engineering of honeycomb structure–Using nature to inspire human innovation. Prog. Mater. Sci. 2015, 74, 332–400. [Google Scholar] [CrossRef]
- Slepička, P.; Siegel, J.; Šlouf, M.; Fajstavr, D.; Fajstavrová, K.; Kolská, Z.; Švorčík, V. The Functionalization of a Honeycomb Polystyrene Pattern by Excimer Treatment in Liquid. Polymers 2022, 14, 4944. [Google Scholar] [CrossRef] [PubMed]
- Korde, J.M.; Shaikh, M.; Kandasubramanian, B. Bionic prototyping of honeycomb patterned polymer composite and its engineering application. Polym.-Plast. Technol. Eng. 2018, 57, 1828–1844. [Google Scholar] [CrossRef]
- Loued, W.; Wéry, J.; Dorlando, A.; Alimi, K. A combined study based on experimental analyses and theoretical calculations on properties of poly (lactic acid) under annealing treatment. J. Mol. Struct. 2015, 1081, 486–493. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag–Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S.; Rahoui, N.; Rahman, M.M.; Abetz, V. Polylactic acid nanocomposites containing functionalized multiwalled carbon nanotubes as antimicrobial packaging materials. Int. J. Biol. Macromol. 2022, 213, 55–69. [Google Scholar] [CrossRef]
- Shafiee Nasab, M.; Tabari, M.; Bidarigh, S. Antifungal activity of nano-composite films based on Poly Lactic Acid. Nanomed. Res. J. 2019, 4, 186–192. [Google Scholar]
- Arshad, M.; Ehtisham-ul-Haque, S.; Bilal, M.; Ahmad, N.; Ahmad, A.; Abbas, M.; Nisar, J.; Khan, M.; Nazir, A.; Ghaffar, A. Synthesis and characterization of Zn doped WO3 nanoparticles: Photocatalytic, antifungal and antibacterial activities evaluation. Mater. Res. Express 2020, 7, 015407. [Google Scholar] [CrossRef]
- Ying, Y.; Pung, S.; Sreekantan, S.; Yee, Y.; Ong, M.; Pung, Y. Structural and antibacterial properties of WO3/ZnO hybrid particles against pathogenic bacteria. Mater. Today Proc. 2019, 17, 1008–1017. [Google Scholar] [CrossRef]
- Vijaykumar, B.; Bharati, B.; Priyanka, K.; Manjunatha, B.; Sannakki, B. Investigations on dislocation density and strain of polyaniline with WO3 nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1221, 012017. [Google Scholar] [CrossRef]
- Mane, A.; Navale, S.T.; Pawar, R.C.; Lee, C.; Patil, V.B. Microstructural, optical and electrical transport properties of WO3 nanoparticles coated polypyrrole hybrid nanocomposites. Synth. Met. 2015, 199, 187–195. [Google Scholar] [CrossRef]
- Pang, H.F.; Xiang, X.; Li, Z.J.; Fu, Y.Q.; Zu, X.T. Hydrothermal synthesis and optical properties of hexagonal tungsten oxide nanocrystals assisted by ammonium tartrate. Phys. Status Solidi A 2012, 209, 537–544. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Bahari, A.; Gholipour, S.; Hoseinzadeh, S. Structural, optical and electrical properties of WO3–Ag nanocomposites for the electro-optical devices. Appl. Phys. A 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Cai, G.; Tu, J.; Zhou, D.; Zhang, J.; Wang, X.; Gu, C. Dual electrochromic film based on WO3/polyaniline core/shell nanowire array. Sol. Energy Mater. Sol. Cells 2014, 122, 51–58. [Google Scholar] [CrossRef]
- Cao, S.; Chen, H. Nanorods assembled hierarchical urchin-like WO3 nanostructures: Hydrothermal synthesis, characterization, and their gas sensing properties. J. Alloys Compd. 2017, 702, 644–648. [Google Scholar] [CrossRef]
- Ahmad, N.D.; Wildan, M.W. Preparation and properties of cellulose nanocrystals-reinforced Poly (lactic acid) composite filaments for 3D printing applications. Results Eng. 2023, 17, 100842. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Crasta, V.; Praveen, B. Enhancement of optical, mechanical and micro structural properties in nanocomposite films of PVA doped with WO3 nanoparticles. Int. J. Struct. Integr. 2015, 6, 338–354. [Google Scholar]
- Akshaykranth, A.; Jayarambabu, N.; Venkatappa Rao, T.; Rakesh Kumar, R.; Srinivasa Rao, L. Antibacterial activity study of ZnO incorporated biodegradable poly (lactic acid) films for food packaging applications. Polym. Bull. 2023, 80, 1369–1384. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, F.; Ahmed, S.; Liu, Y. Physico-mechanical and antibacterial properties of PLA/TiO2 composite materials synthesized via electrospinning and solution casting processes. Coatings 2019, 9, 525. [Google Scholar] [CrossRef]
- Ghozali, M.; Fahmiati, S.; Triwulandari, E.; Restu, W.K.; Farhan, D.; Wulansari, M.; Fatriasari, W. PLA/metal oxide bio-composites for antimicrobial packaging application. Polym.-Plast. Technol. Mater. 2020, 59, 1332–1342. [Google Scholar]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly (lactic acid)/ZnO bionanocomposite films with positively charged ZnO as potential antimicrobial food packaging materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef]
- Bocchini, S.; Fukushima, K.; Blasio, A.D.; Fina, A.; Frache, A.; Geobaldo, F. Polylactic acid and polylactic acid-based nanocomposite photooxidation. Biomacromolecules 2010, 11, 2919–2926. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.-T.; Selke, S.E.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, Applications, and End of Life; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Shanshool, H.M.; Yahaya, M.; Yunus, W.M.M.; Abdullah, I.Y. Investigation of energy band gap in polymer/ZnO nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 9804–9811. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of processing conditions and lignin content on thermal, mechanical and degradative behavior of lignin nanoparticles/polylactic (acid) bionanocomposites prepared by melt extrusion and solvent casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Murariu, M.; Benali, S.; Paint, Y.; Dechief, A.-L.; Murariu, O.; Raquez, J.-M.; Dubois, P. Adding value in production of multifunctional polylactide (PLA)–ZnO nanocomposite films through alternative manufacturing methods. Molecules 2021, 26, 2043. [Google Scholar] [CrossRef]
- Clarkson, C.M.; Azrak, S.M.E.A.; Schueneman, G.T.; Snyder, J.F.; Youngblood, J.P. Crystallization kinetics and morphology of small concentrations of cellulose nanofibrils (CNFs) and cellulose nanocrystals (CNCs) melt-compounded into poly (lactic acid) (PLA) with plasticizer. Polymers 2020, 187, 122101. [Google Scholar] [CrossRef]
- Kang, H.; Kim, D.S. A study on the crystallization and melting of PLA nanocomposites with cellulose nanocrystals by DSC. Polym. Compos. 2023, 44, 7727–7736. [Google Scholar] [CrossRef]
- Vasile, C.; Râpă, M.; Ştefan, M.; Stan, M.; Macavei, S.; Darie-Niţă, R.; Barbu-Tudoran, L.; Vodnar, D.; Popa, E.; Ştefan, R. New PLA/ZnO: Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 1555–1569. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly (L-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Supaphol, P. Crystallization and melting behavior in syndiotactic polypropylene: Origin of multiple melting phenomenon. J. Appl. Polym. Sci. 2001, 82, 1083–1097. [Google Scholar] [CrossRef]
- Benyaya, M.; Bolzinger, M.-A.; Chevalier, Y.; Bordes, C. Rheological properties and stability of Pickering emulsions stabilized with differently charged particles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133514. [Google Scholar] [CrossRef]
- Bek, M.; Gonzalez-Gutierrez, J.; Kukla, C.; Pušnik Črešnar, K.; Maroh, B.; Slemenik Perše, L. Rheological behaviour of highly filled materials for injection moulding and additive manufacturing: Effect of particle material and loading. Appl. Sci. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Liu, L.; Ciftci, O.N. Effects of high oil compositions and printing parameters on food paste properties and printability in a 3D printing food processing model. J. Food Eng. 2021, 288, 110135. [Google Scholar] [CrossRef]
- Yiu, C.; Liang, S.; Mukhtar, K.; Kim, W.; Wang, Y.; Selomulya, C. Food emulsion gels from plant-based ingredients: Formulation, processing, and potential applications. Gels 2023, 9, 366. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ying, R.; Huang, M.; Hayat, K. Protein-stabilized Pickering emulsion interacting with inulin, xanthan gum, and chitosan: Rheological behavior and 3D printing. Carbohydr. Polym. 2024, 326, 121658. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K.; Auras, R. Rheological and thermal properties of polylactide/silicate nanocomposites films. J. Food Sci. 2010, 75, N17–N24. [Google Scholar] [CrossRef]
- Ahi, Z.B.; Renkler, N.Z.; Gul Seker, M.; Tuzlakoglu, K. Biodegradable polymer films with a natural antibacterial extract as novel periodontal barrier membranes. Int. J. Biomater. 2019, 2019, 7932470. [Google Scholar] [CrossRef] [PubMed]
- Tajdari, A.; Babaei, A.; Goudarzi, A.; Partovi, R. Preparation and study on the optical, mechanical, and antibacterial properties of polylactic acid/ZnO/TiO2 shared nanocomposites. J. Plast. Film Sheeting 2020, 36, 285–311. [Google Scholar] [CrossRef]
- Restrepo, I.; Flores, P.; Rodríguez-Llamazares, S. Antibacterial nanocomposite of poly (lactic acid) and ZnO nanoparticles stabilized with poly (vinyl alcohol): Thermal and morphological characterization. Polym.-Plast. Technol. Mater. 2019, 58, 105–112. [Google Scholar] [CrossRef]
- Muzaffar, T.; Khosa, R.Y.; Iftikhar, U.; Obodo, R.M.; Sajjad, S.; Usman, M. Synthesis and characterization of WO3/GO nanocomposites for antimicrobial properties. J. Clust. Sci. 2022, 33, 1987–1996. [Google Scholar] [CrossRef]

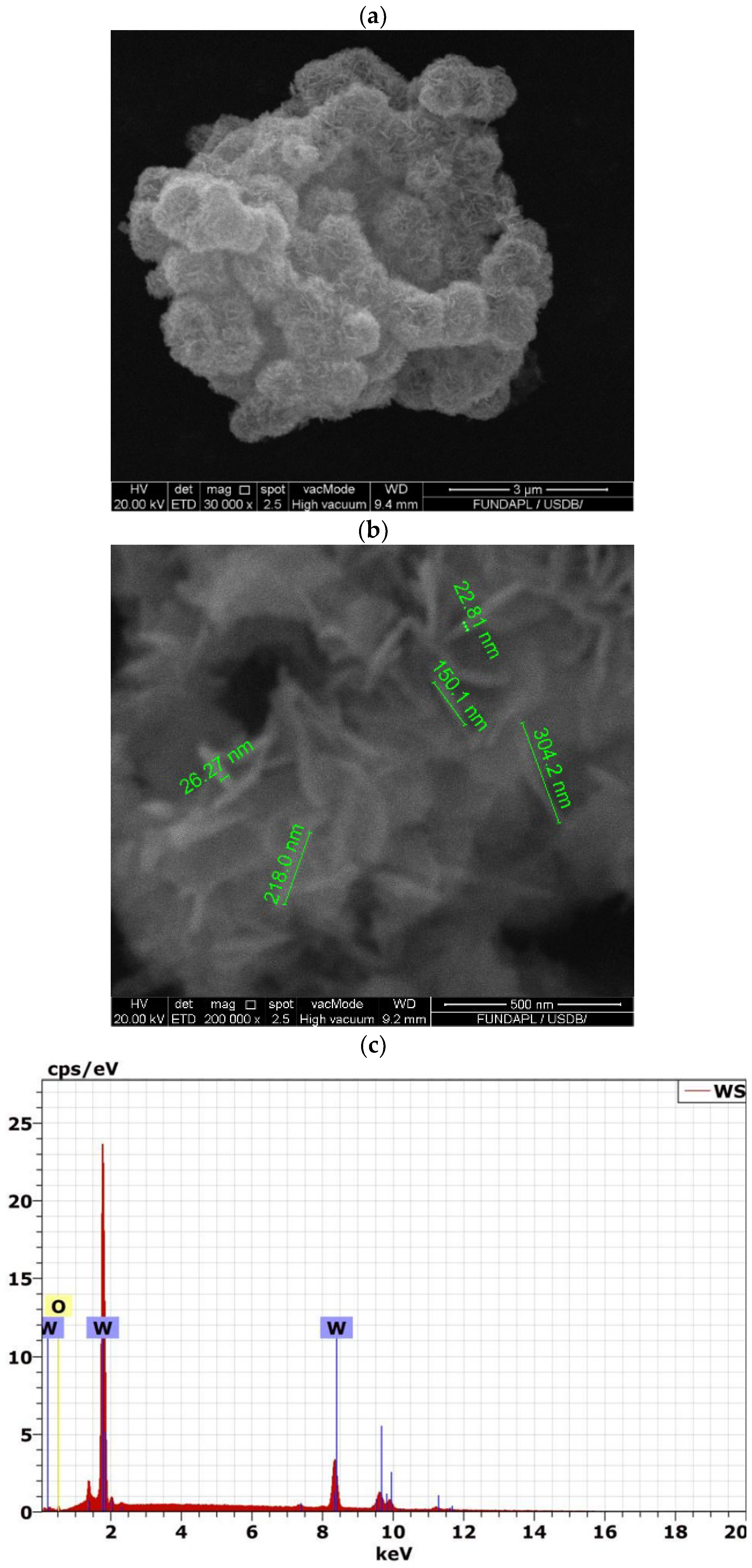
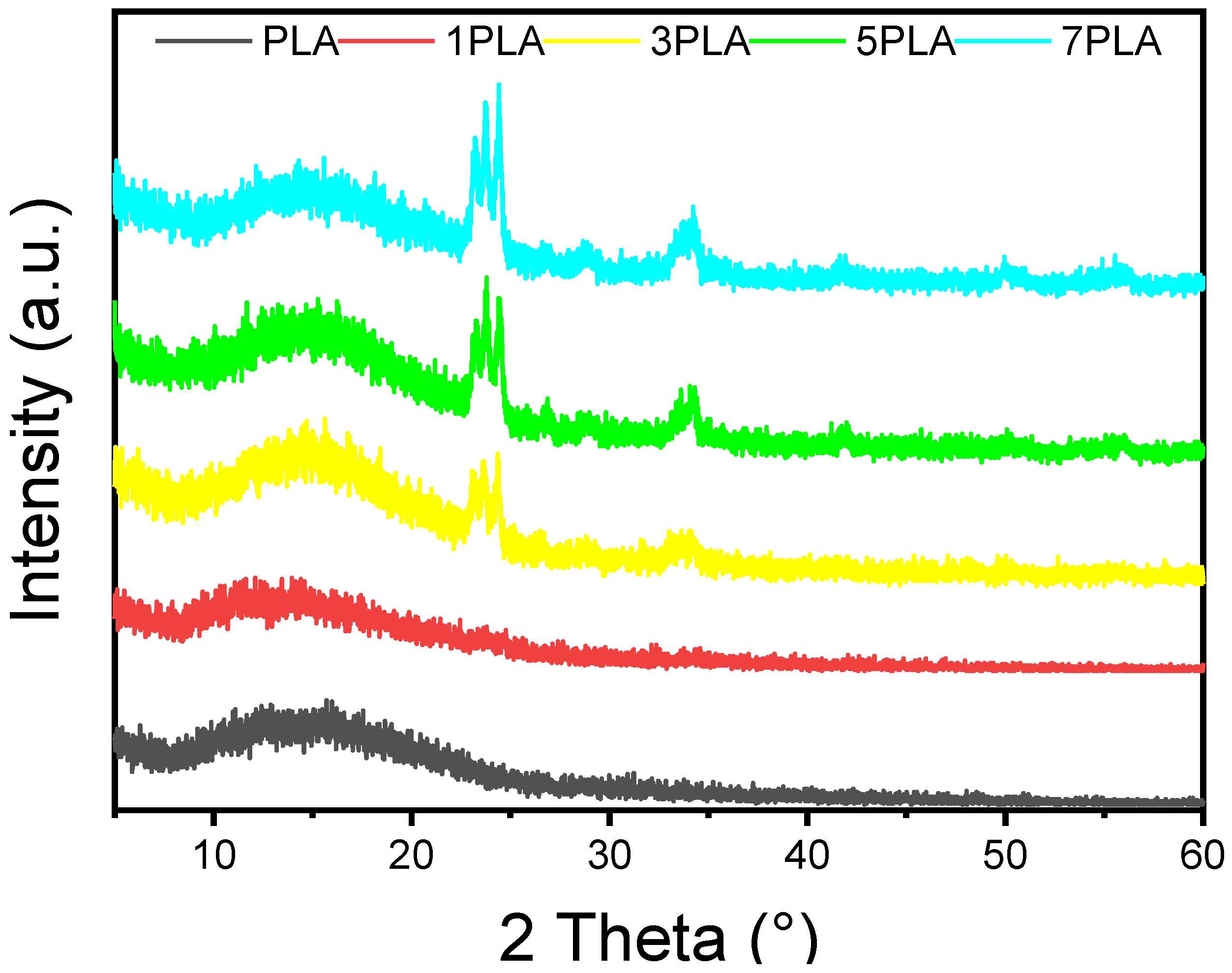



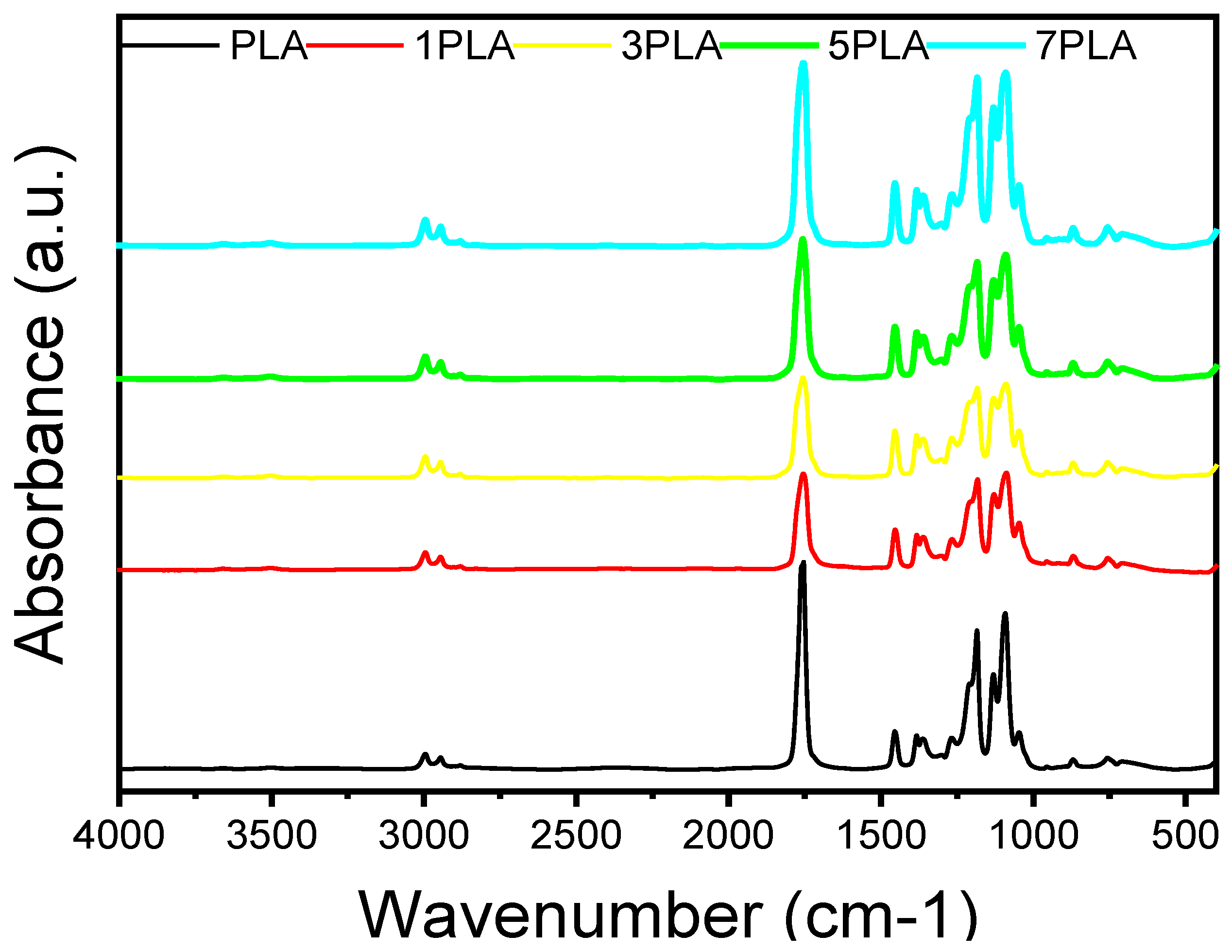

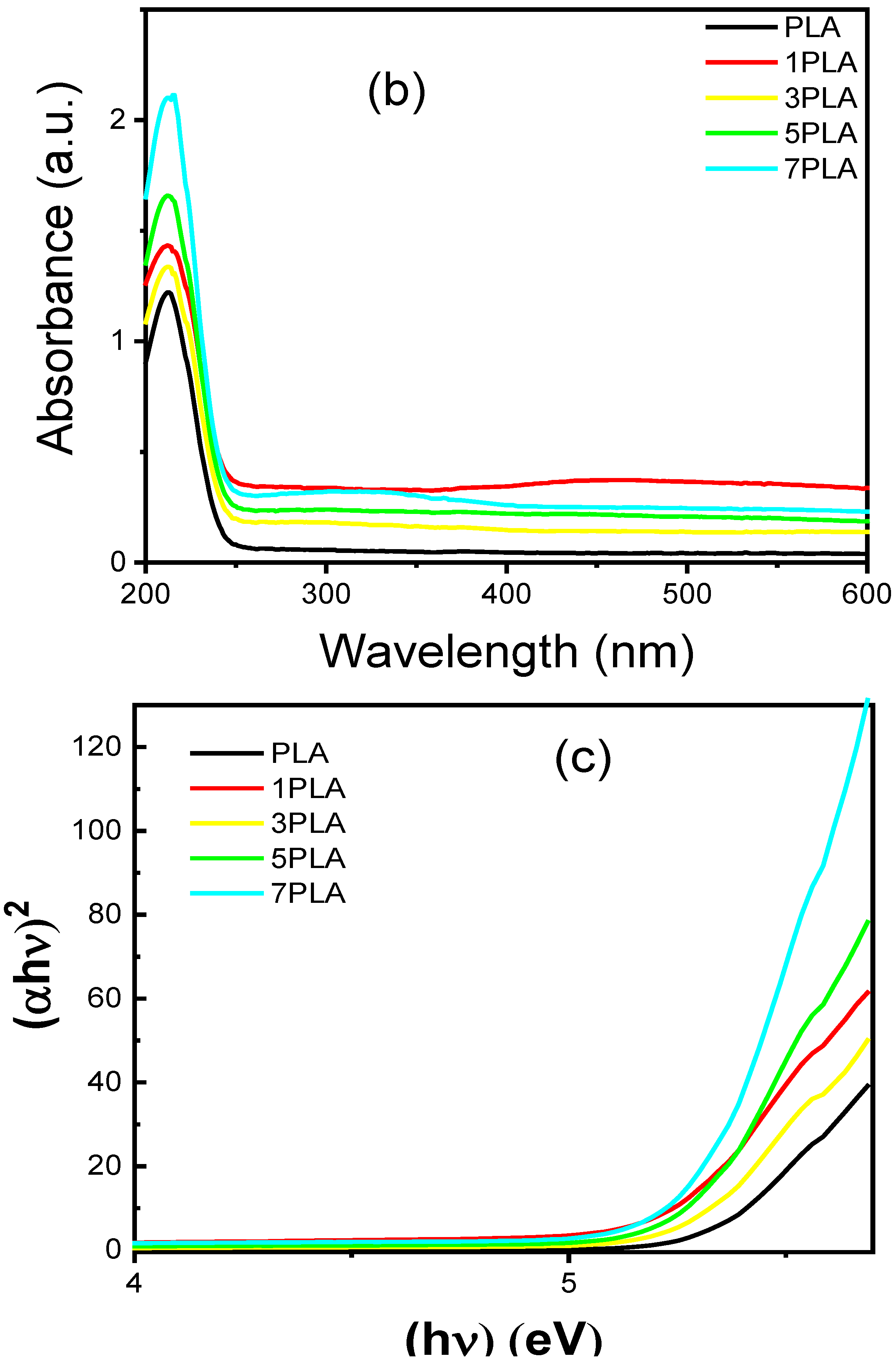




| Samples | WO3 Conc. | Mean Pore Length (µm) | Mean Pore Area (µm) | UV-Blocking (%) |
|---|---|---|---|---|
| PLA | 0 wt% | No pore | No pore | 9 ± 1 |
| 1PLA | 1 wt% | 14.1 ± 1.0 | 4.17 ± 0.5 | 55 ± 2 |
| 3PLA | 3 wt% | 14.8 ± 1.2 | 5.59 ± 0.6 | 28 ± 3 |
| 5PLA | 5 wt% | 19.9 ± 1.5 | 7.40 ± 0.7 | 38 ± 2 |
| 7PLA | 7 wt% | 24.9 ± 1.8 | 9.22 ± 0.8 | 42 ± 2 |
| Samples | WO3 Conc. | T5% (°C) | T50% (°C) | T95% (°C) | Tmax (°C) |
|---|---|---|---|---|---|
| PLA | 0 | 269 ± 1.5 | 322 ± 1.2 | 339 ± 1.8 | 331.0 ± 1.0 |
| 1PLA | 1 | 281 ± 1.3 | 318 ± 1.4 | 389 ± 1.7 | 330.2 ± 1.1 |
| 3PLA | 3 | 281 ± 1.4 | 315 ± 1.3 | 331 ± 1.6 | 320.9 ± 1.2 |
| 5PLA | 5 | 282 ± 1.2 | 310 ± 1.5 | 347 ± 1.8 | 317.4 ± 1.3 |
| 7PLA | 7 | 268 ± 1.6 | 304 ± 1.7 | 326 ± 1.5 | 310.4 ± 1.2 |
| Samples | Tg (°C) | Tc (°C) | Tm1 (°C) | Tm2 (°C) | ΔHc (J/g) | ΔHm (J/g) | Crystallization Rate (%) |
|---|---|---|---|---|---|---|---|
| PLA | 58.0 ± 1.2 | 115.0 ± 1.5 | 164.0 ± 1.3 | 169.0 ± 1.4 | 33.9 ± 1.3 | 37.1 ± 1.2 | 39.9 ± 1.5 |
| 1PLA | 59.0 ± 0.8 | 112.0 ± 1.5 | 163.0 ± 1.0 | 168.0 ± 1.0 | 35.6 ± 1.7 | 36.2 ± 1.1 | 42.3 ± 1.0 |
| 3PLA | 58.0 ± 1.1 | 110.0 ± 1.8 | 162.0 ± 1.2 | 168.0 ± 1.2 | 35.9 ± 1.5 | 38.7 ± 1.5 | 42.9 ± 1.8 |
| 5PLA | 55.0 ± 1.4 | 104.0 ± 1.8 | -- | 166.0 ± 1.5 | 28.5 ± 1.8 | 44.5 ± 1.7 | 50.5 ± 1.8 |
| Sample | G′ (Pa) | G″ (Pa) | tan(δ) | Gel Temperature (°C) | |
|---|---|---|---|---|---|
| PLA | 3000 ± 150 | 956 ± 50 | 0.318 ± 0.015 | 4730 ± 220 | 74.3 ± 1.0 |
| 1PLA | 8420 ± 250 | 2070 ± 75 | 0.246 ± 0.020 | 13,000 ± 460 | 68.9 ± 1.2 |
| 3PLA | 14,500 ± 300 | 4730 ± 100 | 0.326 ± 0.025 | 21,800 ± 680 | 72.3 ± 1.3 |
| 5PLA | 18,600 ± 400 | 3740 ± 125 | 0.201 ± 0.030 | 28,400 ± 740 | 76.0 ± 1.0 |
| 7PLA | 101,000 ± 500 | 29,900 ± 150 | 0.296 ± 0.035 | 158,000 ± 790 | 78.5 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daikhi, S.; Hammani, S.; Guerziz, S.; Alsaeedi, H.; Sayegh, S.; Bechlany, M.; Barhoum, A. Urchin-like WO3 Particles Form Honeycomb-like Structured PLA/WO3 Nanocomposites with Enhanced Crystallinity, Thermal Stability, Rheological, and UV-Blocking and Antifungal Activity. Polymers 2024, 16, 2702. https://doi.org/10.3390/polym16192702
Daikhi S, Hammani S, Guerziz S, Alsaeedi H, Sayegh S, Bechlany M, Barhoum A. Urchin-like WO3 Particles Form Honeycomb-like Structured PLA/WO3 Nanocomposites with Enhanced Crystallinity, Thermal Stability, Rheological, and UV-Blocking and Antifungal Activity. Polymers. 2024; 16(19):2702. https://doi.org/10.3390/polym16192702
Chicago/Turabian StyleDaikhi, Sihem, Salim Hammani, Soumia Guerziz, Huda Alsaeedi, Syreina Sayegh, Mikhael Bechlany, and Ahmed Barhoum. 2024. "Urchin-like WO3 Particles Form Honeycomb-like Structured PLA/WO3 Nanocomposites with Enhanced Crystallinity, Thermal Stability, Rheological, and UV-Blocking and Antifungal Activity" Polymers 16, no. 19: 2702. https://doi.org/10.3390/polym16192702
APA StyleDaikhi, S., Hammani, S., Guerziz, S., Alsaeedi, H., Sayegh, S., Bechlany, M., & Barhoum, A. (2024). Urchin-like WO3 Particles Form Honeycomb-like Structured PLA/WO3 Nanocomposites with Enhanced Crystallinity, Thermal Stability, Rheological, and UV-Blocking and Antifungal Activity. Polymers, 16(19), 2702. https://doi.org/10.3390/polym16192702








