Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation
Abstract
1. Introduction
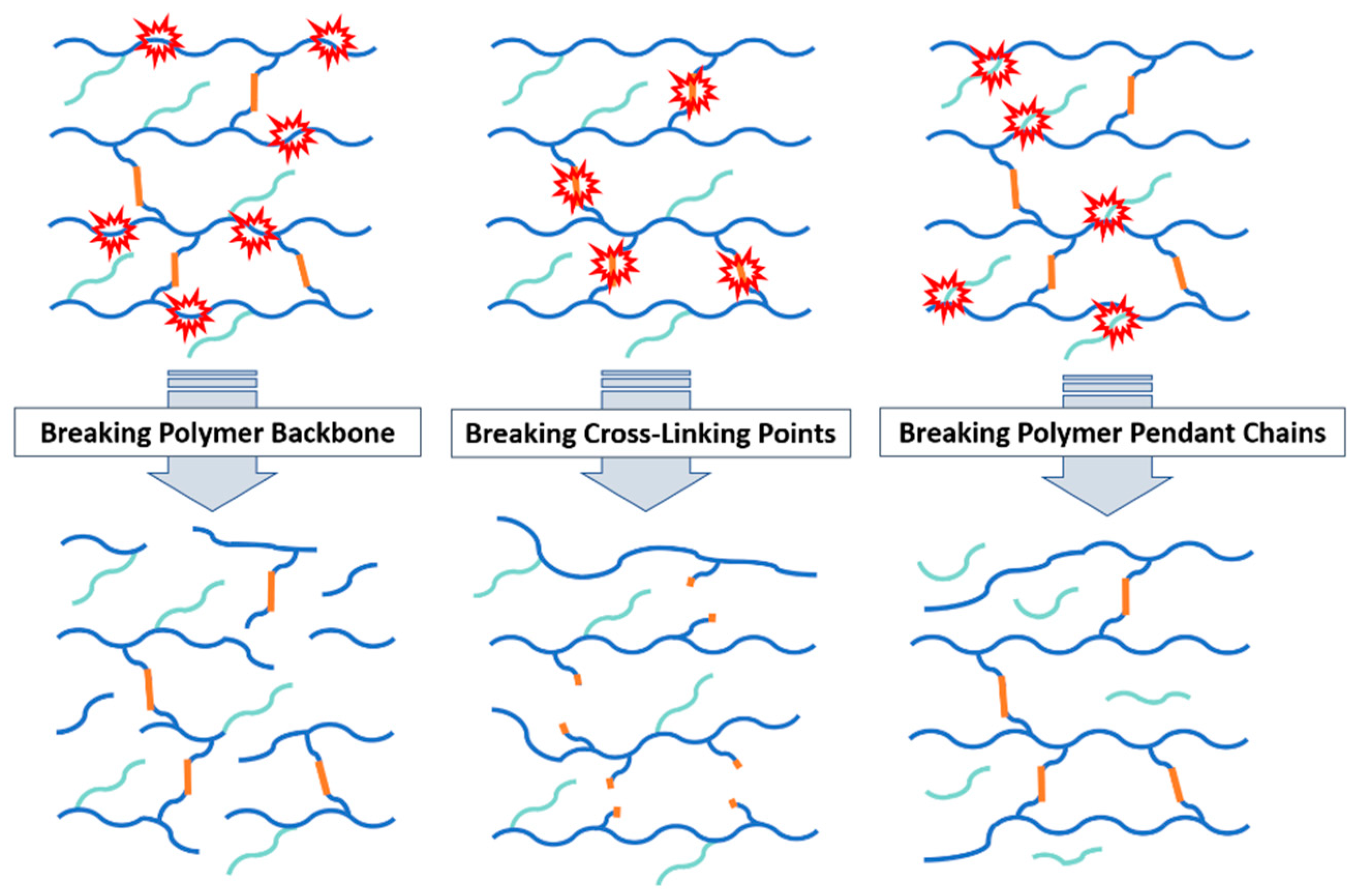
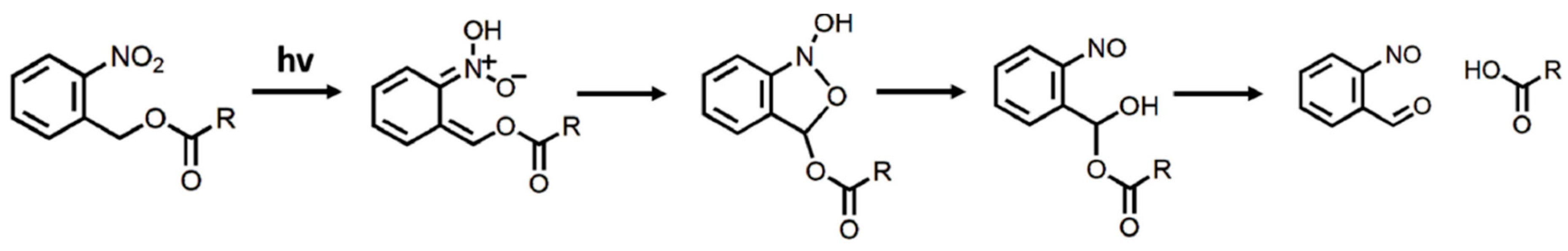
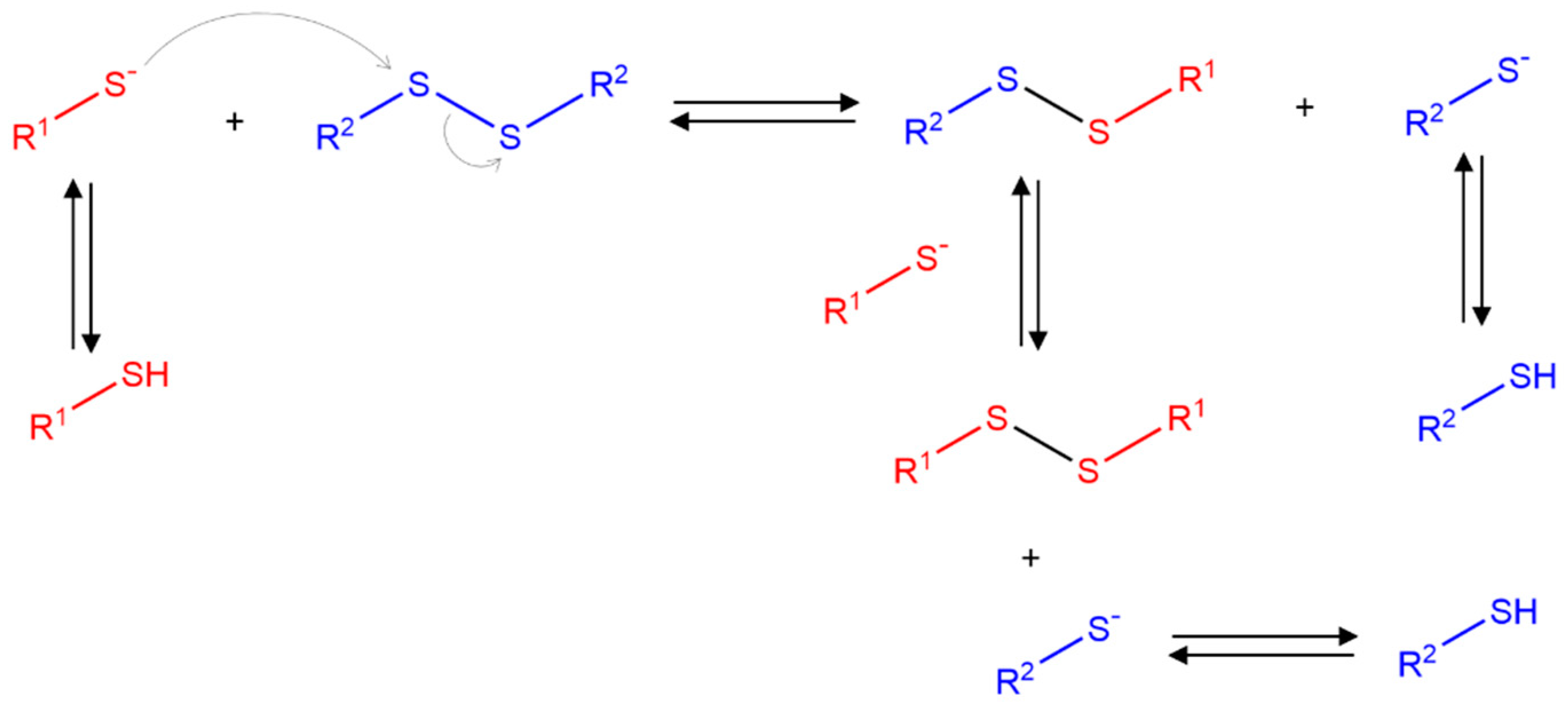
2. Degradation Mechanisms
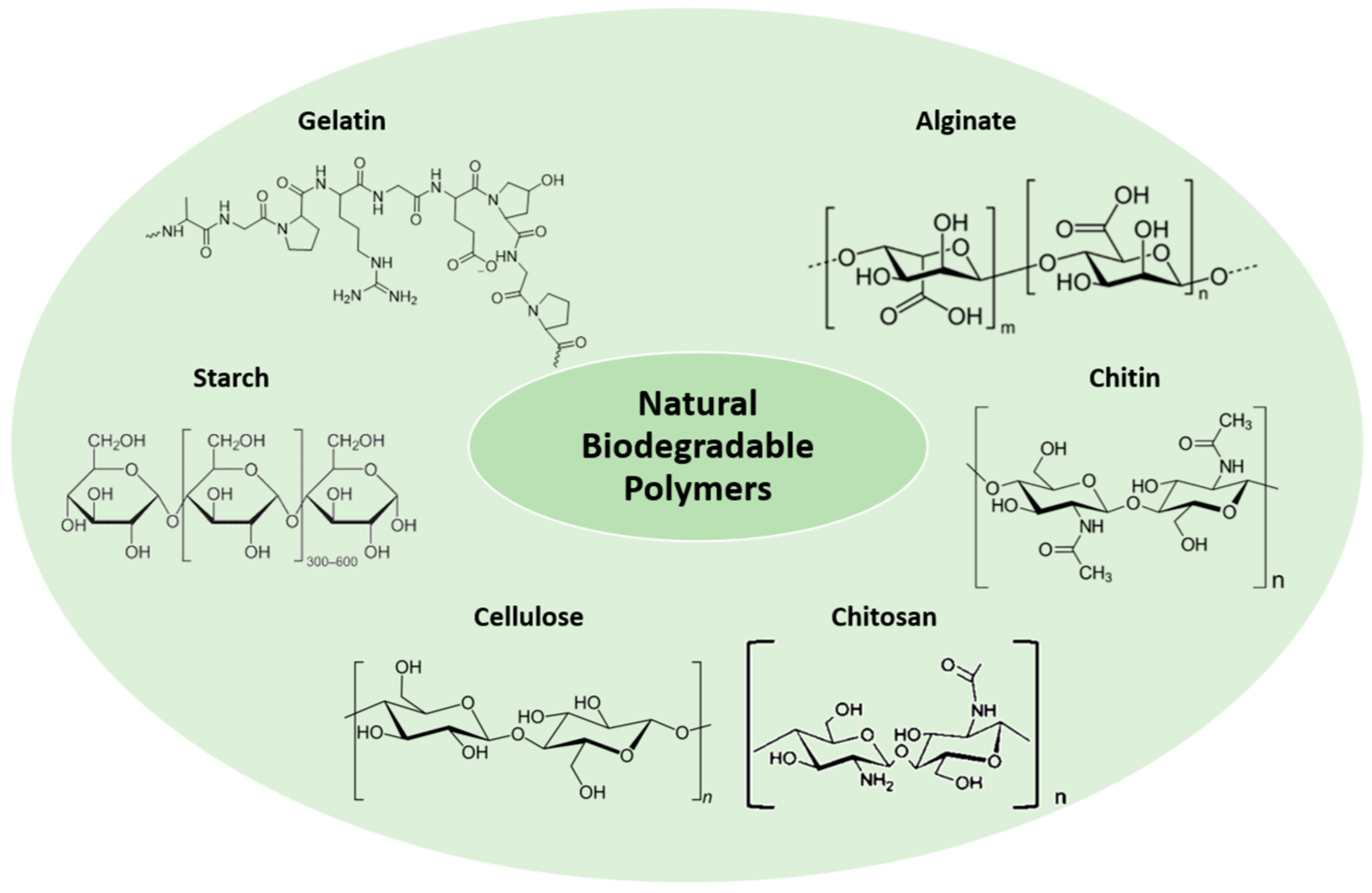
3. Degradable Bio-Based Polymers
3.1. Starch
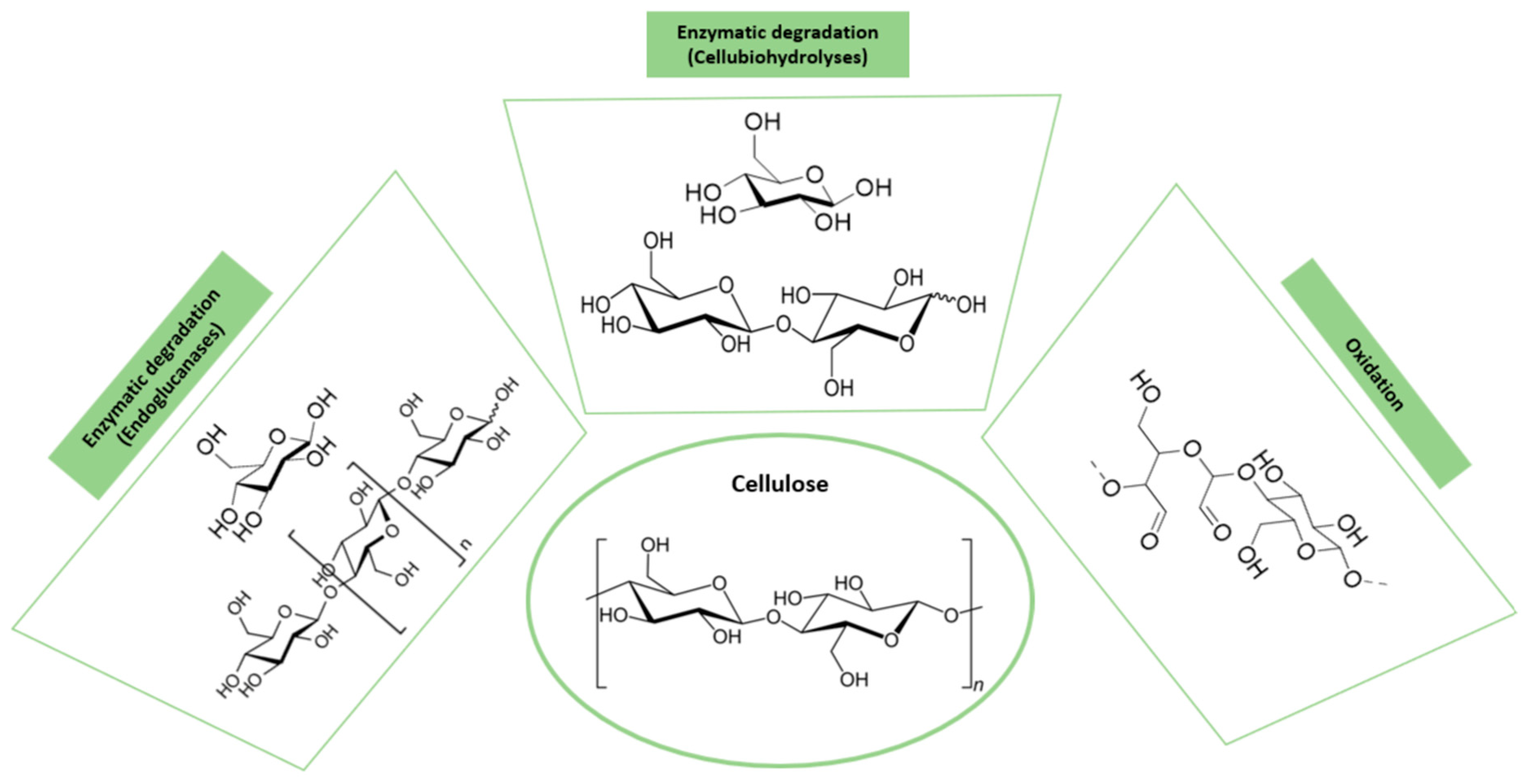
3.2. Cellulose
3.3. Chitosan and Chitin
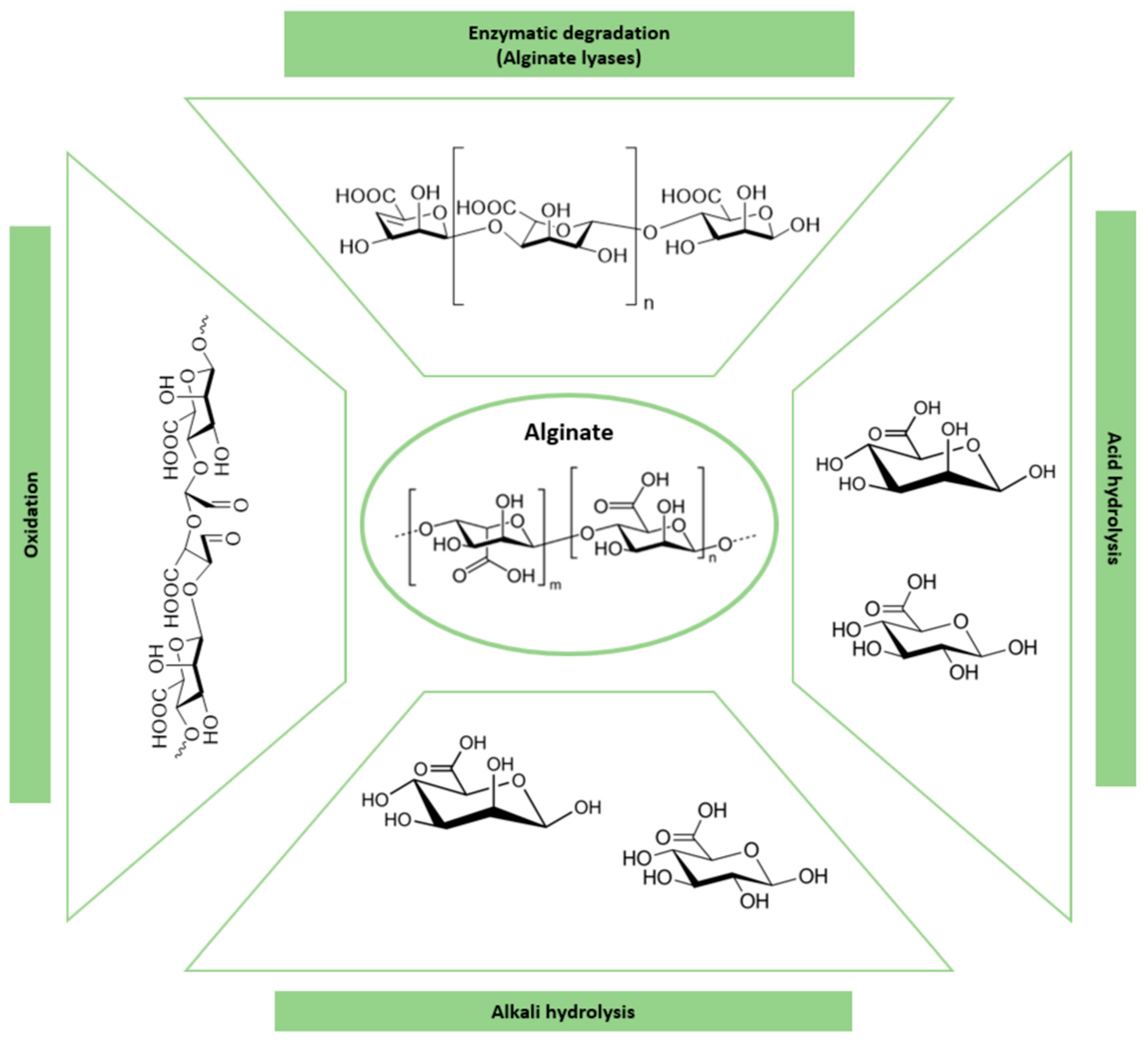
3.4. Alginate
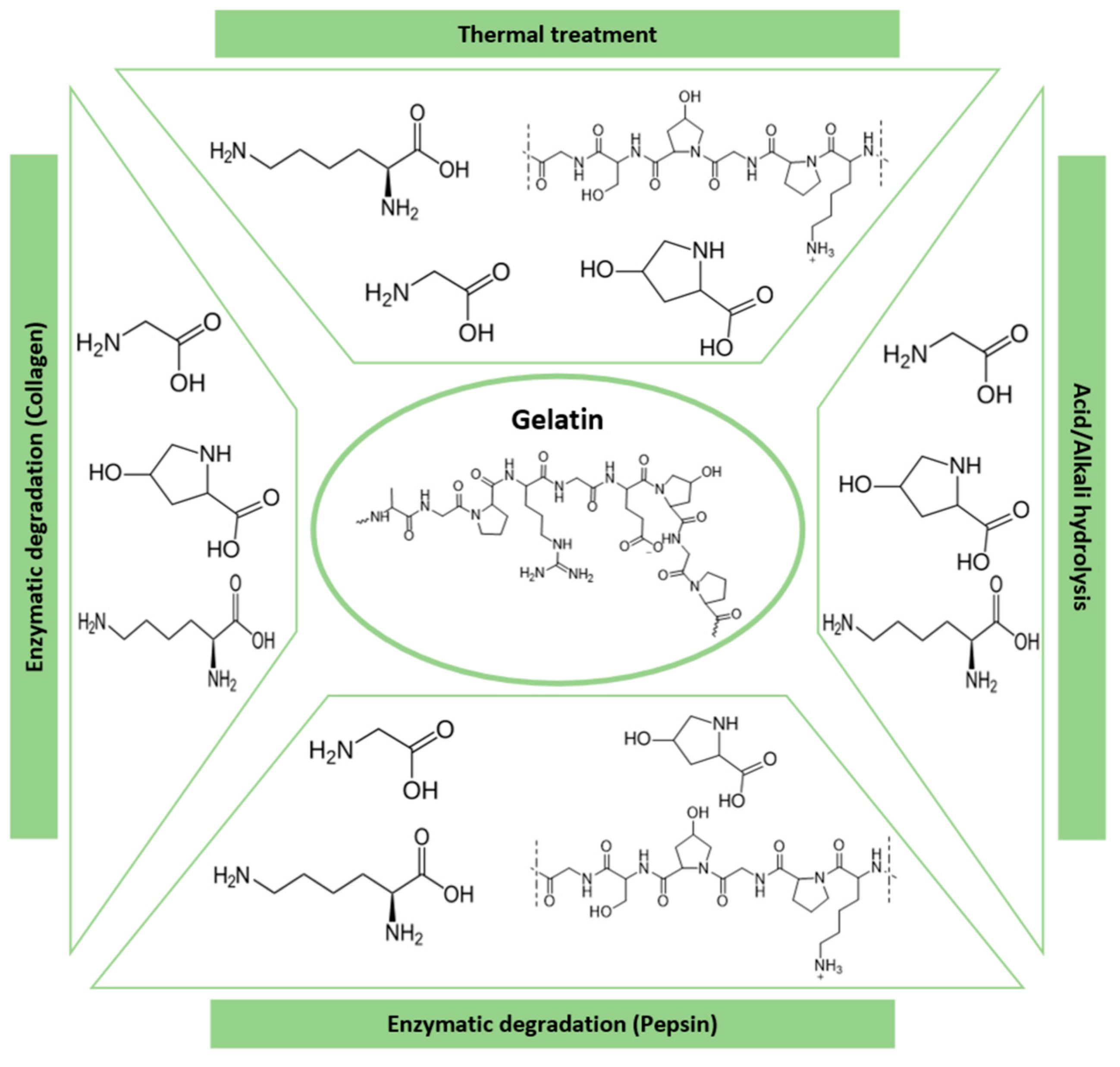
3.5. Gelatin
4. Applications
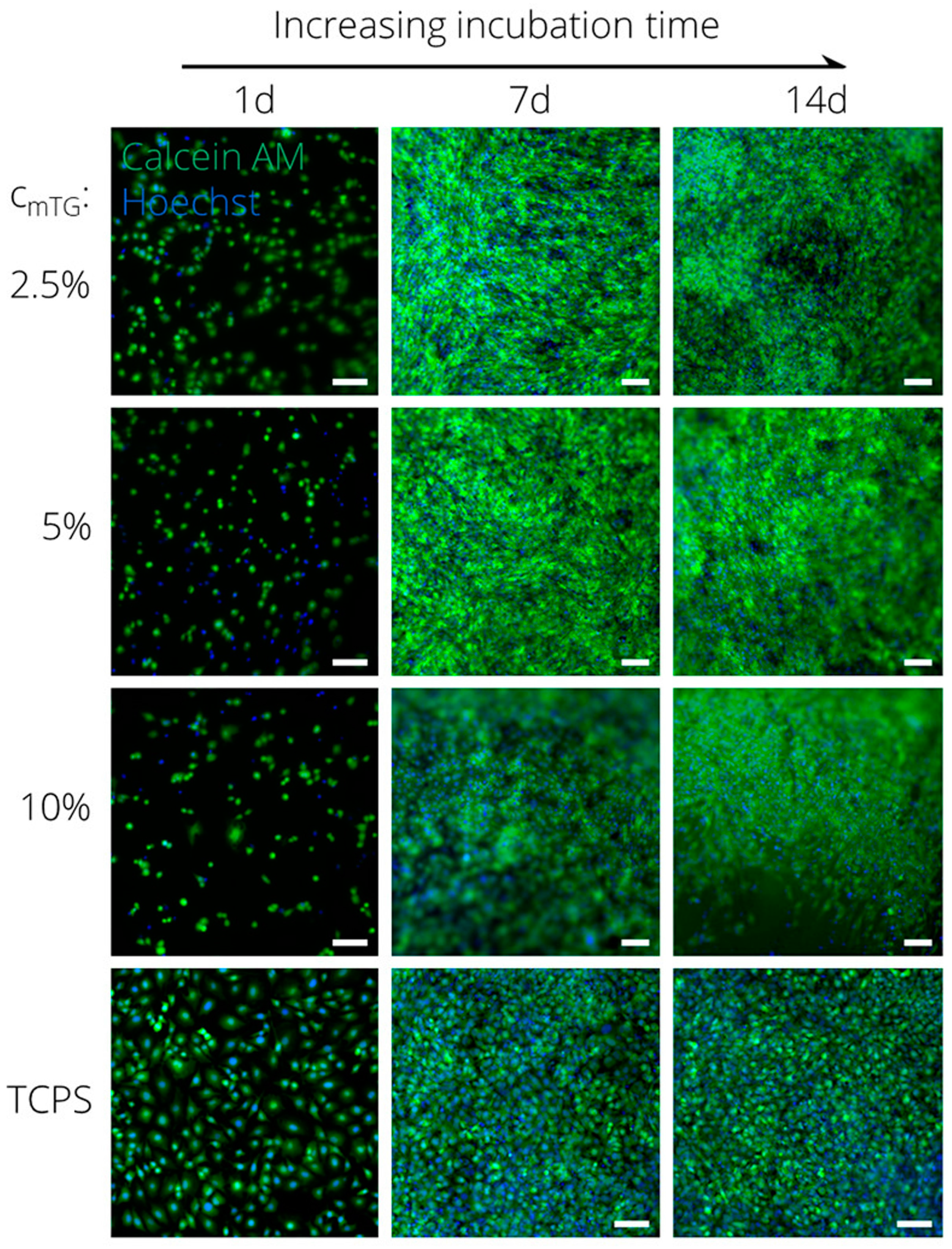
4.1. Tissue Engineering Applications
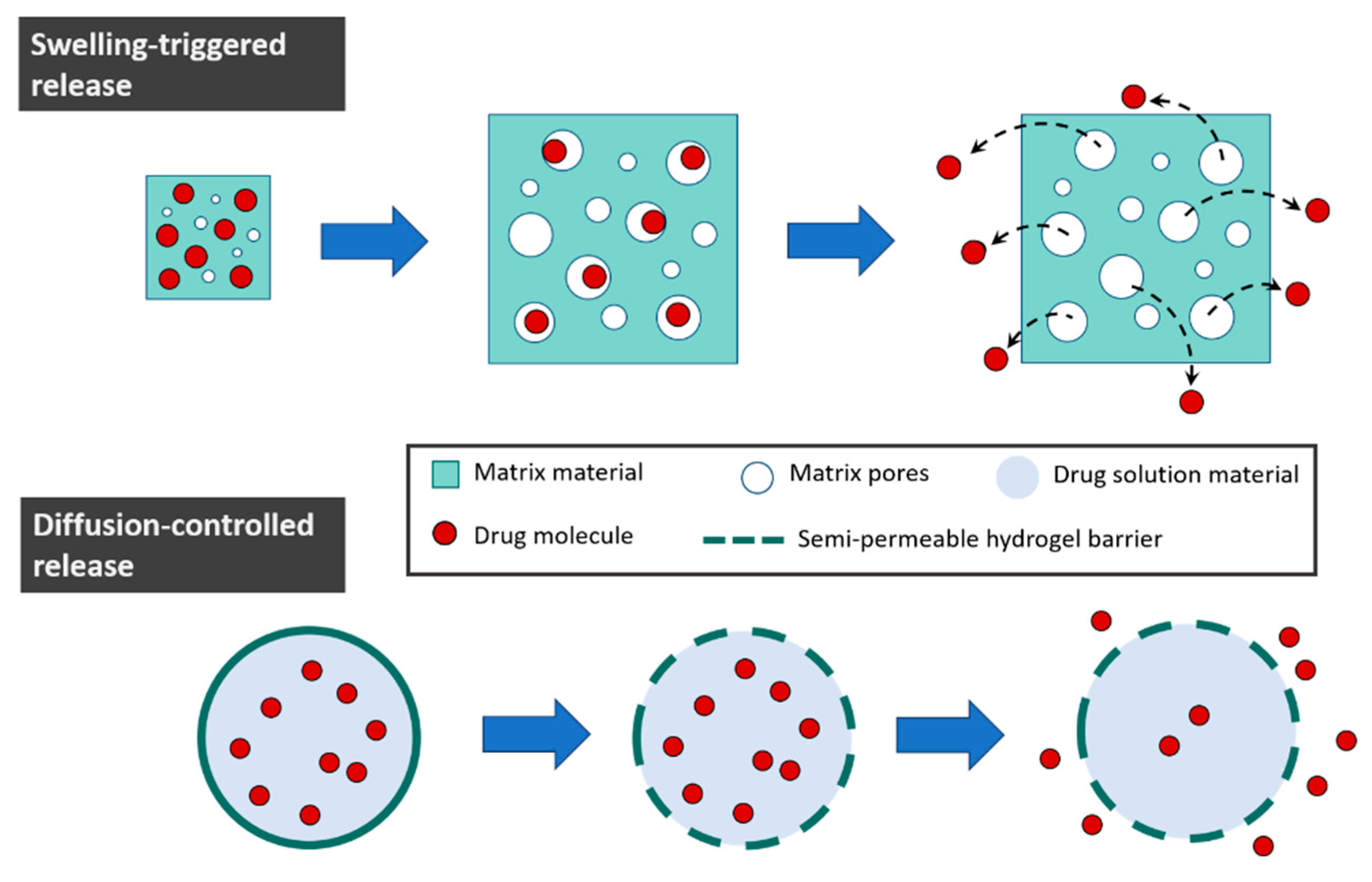
4.2. Controlled Release Applications
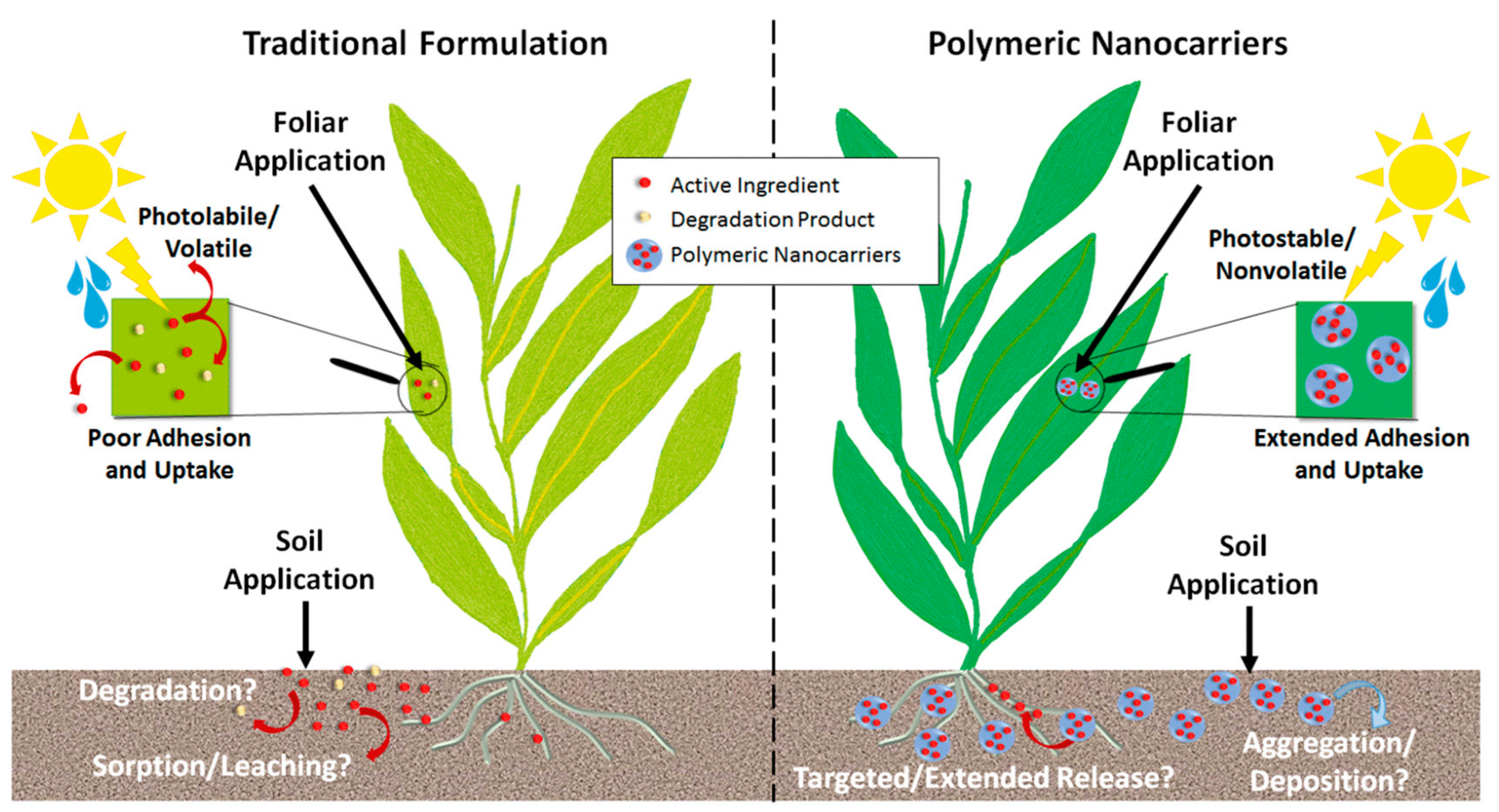
4.3. Soil Remediation and Sustainable Agriculture
| Natural Polymer | Type of Hydrogel | Crosslinking Method | Application | Reference |
|---|---|---|---|---|
| Chitosan | Chitosan-based nanogels | Ionic crosslinking | Controlled release of nanofertilicers | [169] |
| Alginate | Alginate-based microgels | Covalent crosslinking | Controlled release of diuron herbicide | [164] |
| Alginate, lignosulfonate, and konjaku flour-based hydrogels | Ionic crosslinking | Water and nutrient retention | [170] | |
| Cellulose | Carboxymethyl cellulose-based nanogels | Reversible disulfide bonds | Controlled release of agrochemicals | [163] |
| Carboxymethyl cellulose–alginate–hydroxypropyl cellulose-based hydrogels | Ionic crosslinking | Water storage and controlled nutrient release | [167] |
5. Summary and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- MohanKumar, B.S.; Priyanka, G.; Rajalakshmi, S.; Sankar, R.; Sabreen, T.; Ravindran, J. Hydrogels: Potential Aid in Tissue Engineering—A Review. Polym. Bull. 2022, 79, 7009–7039. [Google Scholar] [CrossRef]
- Chamkouri, H. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for Hydrogels in Cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Chen, G. Hydrogels as Water and Nutrient Reservoirs in Agricultural Soil: A Comprehensive Review of Classification, Performance, and Economic Advantages. Environ. Dev. Sustain. 2023. [Google Scholar] [CrossRef]
- Kong, H.J.; Alsberg, E.; Kaigler, D.; Lee, K.Y.; Mooney, D.J. Controlling Degradation of Hydrogels via the Size of Cross-Linked Junctions. Adv. Mater. 2004, 16, 1917–1921. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable Hydrogels: Design Mechanisms and Versatile Applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Bauer, F.; Nielsen, T.D.; Nilsson, L.J.; Palm, E.; Ericsson, K.; Fråne, A.; Cullen, J. Plastics and Climate Change Breaking Carbon Lock-Ins through Three Mitigation Pathways. One Earth 2022, 5, 361–376. [Google Scholar] [CrossRef]
- Rajeswari, S. Natural Polymers: A Recent Review. World J. Pharm. Pharm. Sci. 2017, 6, 472–494. [Google Scholar] [CrossRef][Green Version]
- Jain, S.; Jain, A.; Jain, R.; Chauhan, N.S. Potential of Natural Polymeric Materials in Pharmaceutics. Pharmacol. Res.-Nat. Prod. 2024, 2, 100014. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, H.; Wang, L.; Liu, J.; Liu, X.; Hong, Y.; Huang, Y.; Ren, S. Advances in Adhesive Hydrogels for Tissue Engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Jia, L.; Li, Y.; Ren, A.; Xiang, T.; Zhou, S. Degradable and Recyclable Hydrogels for Sustainable Bioelectronics. ACS Appl. Mater. Interfaces 2024, 16, 32887–32905. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2021, 10, e2001341. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A Review of the Properties and Applications of Bioadhesive Hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Grosjean, M.; Gangolphe, L.; Nottelet, B. Degradable Self-Healable Networks for Use in Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2205315. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in Biodegradable and Injectable Hydrogels for Biomedical Applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Duan, J.; Liu, J.; Yi, H.; Zhang, Z.; Song, H.; Li, Y.; Zhang, K. Stimuli-Responsive Self-Degradable DNA Hydrogels: Design, Synthesis, and Applications. Adv. Healthc. Mater. 2023, 12, e2203031. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of Bio-Based and Synthetic Hydrogels as Sustainable Soil Amendments: A Review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime Prediction of Biodegradable Polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef]
- Varde, N.K.; Pack, D.W. Microspheres for Controlled Release Drug Delivery. Expert. Opin. Biol. Ther. 2004, 4, 35–51. [Google Scholar] [CrossRef]
- Hahn, S.; Hennecke, D. What Can We Learn from Biodegradation of Natural Polymers for Regulation? Environ. Sci. Eur. 2023, 35, 50. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part. B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Hu, B.; Gao, J.; Lu, Y.; Wang, Y. Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery. Pharmaceutics 2023, 15, 2370. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers. In Biodegradation—Life of Science; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Levalley, P.J.; Neelarapu, R.; Sutherland, B.P.; Dasgupta, S.; Kloxin, C.J.; Kloxin, A.M. Photolabile Linkers: Exploiting Labile Bond Chemistry to Control Mode and Rate of Hydrogel Degradation and Protein Release. J. Am. Chem. Soc. 2020, 142, 4671–4679. [Google Scholar] [CrossRef]
- Liu, T.; Bao, B.; Li, Y.; Lin, Q.; Zhu, L. Photo-Responsive Polymers Based on ο-Nitrobenzyl Derivatives: From Structural Design to Applications. Prog. Polym. Sci. 2023, 146, 101741. [Google Scholar] [CrossRef]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-Sensitive Polymers and Bioconjugates for Biomedical Applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, J.; Wan, J.; Li, Z. Disulfide Based Prodrugs for Cancer Therapy. RSC Adv. 2020, 10, 24397–24409. [Google Scholar] [CrossRef] [PubMed]
- Brülisauer, L.; Gauthier, M.A.; Leroux, J.C. Disulfide-Containing Parenteral Delivery Systems and Their Redox-Biological Fate. J. Control. Release 2014, 195, 147–154. [Google Scholar] [CrossRef]
- Mthembu, S.N.; Sharma, A.; Albericio, F.; de la Torre, B.G. Breaking a Couple: Disulfide Reducing Agents. ChemBioChem 2020, 21, 1947–1954. [Google Scholar] [CrossRef]
- Condò, I.; Giannitelli, S.M.; Lo Presti, D.; Cortese, B.; Ursini, O. Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels 2024, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; An, H.; Li, X.; Zhou, R.; Qin, J.; Tian, Y.; Deng, K. Self-Healable Polymer Gels with Multi-Responsiveness of Gel-Sol-Gel Transition and Degradability. Polym. Chem. 2017, 8, 1263–1271. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric Sustainable Materials and Their Emerging Applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Vinchhi, P.; Rawal, S.U.; Patel, M.M. Biodegradable Hydrogels. In Drug Delivery Devices and Therapeutic Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 395–419. ISBN 9780128198384. [Google Scholar]
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-Based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Li, M.; Witt, T.; Xie, F.; Warren, F.J.; Halley, P.J.; Gilbert, R.G. Biodegradation of Starch Films: The Roles of Molecular and Crystalline Structure. Carbohydr. Polym. 2015, 122, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of Direct Chemical and Biochemical Transformations of Starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef]
- Van Der Maarel, M.J.E.C.; Van Der Veen, B.; Uitdehaag, J.C.M.; Leemhuis, H.; Dijkhuizen, L. Properties and Applications of Starch-Converting Enzymes of the a-Amylase Family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Effect of Acid Hydrolysis on Starch Structure and Functionality: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.; Emeje, M.O. Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-115-1. [Google Scholar]
- Rutenberg, M.W.; Solarek, D. Starch Derivatives: Production and Uses. In Starch: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 311–388. [Google Scholar]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, Y.; Han, L.; Lu, X. Biodegradable Polymer Hydrogel-Based Tissue Adhesives: A Review. Biosurface Biotribol. 2021, 7, 163–179. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from Enzymatic Degradation of Cellulose and Hemicellulose to Fermentable Sugars—A Review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Mondal, M.I.H. Polymers and Polymeric Composites: A Reference Series Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-319-77829-7. [Google Scholar]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.Y.; Farrell, M.; Kostov, Y. Functional Cardiac Cell Constructs on Cellulose-Based Scaffolding. Biomaterials 2004, 25, 5753–5762. [Google Scholar] [CrossRef]
- Erdal, N.B.; Hakkarainen, M. Degradation of Cellulose Derivatives in Laboratory, Man-Made, and Natural Environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef]
- Toshikj, E.; Tarbuk, A.; Grgić, K.; Mangovska, B.; Jordanov, I. Influence of Different Oxidizing Systems on Cellulose Oxidation Level: Introduced Groups versus Degradation Model. Cellulose 2019, 26, 777–794. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Determining the Deacetylation Degree of Chitosan: Opportunities to Learn Instrumental Techniques. J. Chem. Educ. 2018, 95, 1022–1028. [Google Scholar] [CrossRef]
- Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Odeniyi, O.A.; Majengbasan, O.S.; Igwaran, A.; Moloantoa, K.M.M.; Khetsha, Z.P.; Iwarere, S.A.; Daramola, M.O. Chitinases: Expanding the Boundaries of Knowledge beyond Routinized Chitin Degradation. Environ. Sci. Pollut. Res. 2024, 31, 38045–38060. [Google Scholar] [CrossRef]
- Churklam, W.; Aunpad, R. Enzymatic Characterization and Structure-Function Relationship of Two Chitinases, LmChiA and LmChiB, from Listeria Monocytogenes. Heliyon 2020, 6, e04252. [Google Scholar] [CrossRef]
- Jiménez-Ortega, E.; Kidibule, P.E.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural Inspection and Protein Motions Modelling of a Fungal Glycoside Hydrolase Family 18 Chitinase by Crystallography Depicts a Dynamic Enzymatic Mechanism. Comput. Struct. Biotechnol. J. 2021, 19, 5466–5478. [Google Scholar] [CrossRef]
- Mushtaq, A.; Li, L.; Anitha, A.; Grøndahl, L. Chitosan Nanomedicine in Cancer Therapy: Targeted Delivery and Cellular Uptake. Macromol. Biosci. 2021, 21, 2100005. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2006, 25, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and Chitosan-Based Derivatives in Plant Protection against Biotic and Abiotic Stresses and in Recovery of Contaminated Soil and Water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.A. Controlling Chitosan Degradation Properties In Vitro and In Vivo. In Chitosan Based Biomaterials: Fundamentals: Volume 1; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 159–182. ISBN 9780081002308. [Google Scholar]
- Boot, R.G.; Blommaart, E.F.C.; Swart, E.; Ghauharali-van der Vlugt, K.; Bijl, N.; Moe, C.; Place, A.; Aerts, J.M.F.G. Identification of a Novel Acidic Mammalian Chitinase Distinct from Chitotriosidase. J. Biol. Chem. 2001, 276, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Eide, K.B.; Norberg, A.L.; Heggset, E.B.; Lindbom, A.R.; Varum, K.M.; Eijsink, V.G.H.; Sørlie, M. Human Chitotriosidase-Catalyzed Hydrolysis of Chitosan. Biochemistry 2012, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Oliveira, J.M.; Sá-Lima, H.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Polymers of Biological Origin. In Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 187–205. [Google Scholar]
- Wardhani, D.H.; Ulya, H.N.; Hafizha, M.; Razani, M.O.A. Effect of Temperatures on Modification of Alginate Hydrophobicity Using Dodecenyl Succinic Anhydride. E3S Web Conf. 2024, 503, 06005. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Lu, C.; Zhou, X.; Chen, L.; Qiu, C.; Jin, Z.; Long, J. Advances in Alginate Lyases and the Potential Application of Enzymatic Prepared Alginate Oligosaccharides: A Mini Review. Int. J. Biol. Macromol. 2024, 260, 129506. [Google Scholar] [CrossRef]
- Chesterman, J.; Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable Polymers. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–342. ISBN 9780128184226. [Google Scholar]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, Properties and Application of Alginic Acid: A Review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Wen, Y.; Li, D.; Sun, X.; Liu, Z.; Yan, H.; Lin, Q. A Study on the Correlation between the Oxidation Degree of Oxidized Sodium Alginate on Its Degradability and Gelation. Polymers 2022, 14, 1679. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H. Alginate Lyase: Review of Major Sources and Classification, Properties, Structure-Function Analysis and Applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical Review of EPS Production, Synthesis and Composition for Sludge Flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Boran, G.; Mulvaney, S.J.; Regenstein, J.M. Rheological Properties of Gelatin from Silver Carp Skin Compared to Commercially Available Gelatins from Different Sources. J. Food Sci. 2010, 75, E565–E571. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. In Proceedings of the Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 42, pp. 240–250. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Van Den Bosch, E.; Gielens, C. Gelatin Degradation at Elevated Temperature. Int. J. Biol. Macromol. 2003, 32, 129–138. [Google Scholar] [CrossRef]
- Gray, V.A.; Cole, E.; Riva Toma, J.M.D.; Ghidorsi, L.; Guo, J.H.; Han, J.H.; Han, F.; Hosty, C.T.; Kochling, J.D.; Kraemer, J.; et al. Use of Enzymes in the Dissolution Testing of Gelatin Capsules and Gelatin-Coated Tablets—Revisions to Dissolution <711> and Disintegration and Dissolution of Dietary Supplements <2040>. Dissolut Technol. 2014, 21, 6–19. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, X.; Feng, Y.; Zheng, L.; Zhao, M.; Huang, M. Secretion of Collagenases by Saccharomyces Cerevisiae for Collagen Degradation. Biotechnol. Biofuels Bioprod. 2022, 15, 89. [Google Scholar] [CrossRef]
- Guo, B.; Zhong, Y.; Chen, X.; Yu, S.; Bai, J. 3D Printing of Electrically Conductive and Degradable Hydrogel for Epidermal Strain Sensor. Compos. Commun. 2023, 37, 101454. [Google Scholar] [CrossRef]
- Sagdic, K.; Fernández-Lavado, E.; Mariello, M.; Akouissi, O.; Lacour, S.P. Hydrogels and Conductive Hydrogels for Implantable Bioelectronics. MRS Bull. 2023, 48, 495–505. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Dong, L.; Wang, M.; Wu, J.; Zhang, C.; Shi, J.; Oh, K.; Yao, L.; Zhu, C.; Morikawa, H. Fully Biofriendly, Biodegradable and Recyclable Hydrogels Based on Covalent-like Hydrogen Bond Engineering towards Multimodal Transient Electronics. Chem. Eng. J. 2023, 457, 141276. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically Cross-Linked Chitosan-Based Hydrogels for Tissue Engineering Applications: A State-of-the-Art Review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Rami, L.; Malaise, S.; Delmond, S.; Fricain, J.C.; Siadous, R.; Schlaubitz, S.; Laurichesse, E.; Amédée, J.; Montembault, A.; David, L.; et al. Physicochemical Modulation of Chitosan-Based Hydrogels Induces Different Biological Responses: Interest for Tissue Engineering. J. Biomed. Mater. Res. A 2014, 102, 3666–3676. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A Critical Review on Three Dimensional-Printed Chitosan Hydrogels for Development of Tissue Engineering. Bioprinting 2019, 17, e00063. [Google Scholar] [CrossRef]
- Cao, L.; Werkmeister, J.A.; Wang, J.; Glattauer, V.; McLean, K.M.; Liu, C. Bone Regeneration Using Photocrosslinked Hydrogel Incorporating RhBMP-2 Loaded 2-N, 6-O-Sulfated Chitosan Nanoparticles. Biomaterials 2014, 35, 2730–2742. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive Chitosan-Agarose Hydrogel for Skin Regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef]
- Gnavi, S.; Barwig, C.; Freier, T.; Haastert-Talini, K.; Grothe, C.; Geuna, S. The Use of Chitosan-Based Scaffolds to Enhance Regeneration in the Nervous System. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 109, pp. 1–62. [Google Scholar]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/Chitosan/Alginate Ternary Scaffolds for Cardiac Tissue Engineering Application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Moreira, M.S.; Sarra, G.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.V.; Pedroni, A.C.F.; Lascala, C.A.; Catalani, L.H.; Marques, M.M. Physical and Biological Properties of a Chitosan Hydrogel Scaffold Associated to Photobiomodulation Therapy for Dental Pulp Regeneration: An in Vitro and in Vivo Study. Biomed. Res. Int. 2021, 2021, 6684667. [Google Scholar] [CrossRef] [PubMed]
- Seda Tığlı, R.; Karakeçili, A.; Gümüşderelioğlu, M. In Vitro Characterization of Chitosan Scaffolds: Influence of Composition and Deacetylation Degree. J. Mater. Sci. Mater. Med. 2007, 18, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wei, Y.; Liu, Q.; Yang, B.; Ma, N.; Li, Z.; Zhao, L.; Chen, W.; Huang, D. Silver-Loaded Microspheres Reinforced Chitosan Scaffolds for Skin Tissue Engineering. Eur. Polym. J. 2020, 134, 109861. [Google Scholar] [CrossRef]
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic Fibroblast Growth Factor Promotes Human Dental Pulp Stem Cells Cultured in 3D Porous Chitosan Scaffolds to Neural Differentiation. Int. J. Neurosci. 2021, 131, 625–633. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Alizadeh Sardroud, H.; Yazdanpanah, Z.; Afzal Soltani, Y.; Sernaglia, J.; Chen, X. Extrusion-Based Printing of Chitosan Scaffolds and Their in Vitro Characterization for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef]
- Goh, C.Y.; Lim, S.S.; Tshai, K.Y.; El Azab, A.W.Z.Z.; Loh, H.S. Fabrication and in Vitro Biocompatibility of Sodium Tripolyphosphate-Crosslinked Chitosan–Hydroxyapatite Scaffolds for Bone Regeneration. J. Mater. Sci. 2019, 54, 3403–3420. [Google Scholar] [CrossRef]
- Hu, J.; Hou, Y.; Park, H.; Choi, B.; Hou, S.; Chung, A.; Lee, M. Visible Light Crosslinkable Chitosan Hydrogels for Tissue Engineering. Acta Biomater. 2012, 8, 1730–1738. [Google Scholar] [CrossRef]
- Chang, H.K.; Yang, D.H.; Ha, M.Y.; Kim, H.J.; Kim, C.H.; Kim, S.H.; Choi, J.W.; Chun, H.J. 3D Printing of Cell-Laden Visible Light Curable Glycol Chitosan Bioink for Bone Tissue Engineering. Carbohydr. Polym. 2022, 287, 119328. [Google Scholar] [CrossRef]
- Vo, N.T.H.; Huang, L.; Lemos, H.; Mellor, A.L.; Novakovic, K. Genipin-Crosslinked Chitosan Hydrogels: Preliminary Evaluation of the in Vitro Biocompatibility and Biodegradation. J. Appl. Polym. Sci. 2021, 138, 50848. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez-Martínez, V.; Benito-Cid, S.; Ruiz-Rubio, L.; Pérez-González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Wound Healing and Antibacterial Chitosan-Genipin Hydrogels with Controlled Drug Delivery for Synergistic Anti-Inflammatory Activity. Int. J. Biol. Macromol. 2022, 203, 679–694. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.T.; Stilhano, R.S.; Silva, E.A. Enzymatically Degradable Alginate Hydrogel Systems to Deliver Endothelial Progenitor Cells for Potential Revasculature Applications. Biomaterials 2018, 179, 109–121. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating Degradation of Sodium Alginate/Bioglass Hydrogel for Improving Tissue Infiltration and Promoting Wound Healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef] [PubMed]
- Reakasame, S.; Boccaccini, A.R. Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Jalali Kandeloos, A.; Bastani, S.; Mashayekhan, S. Architecting Oxidized Alginate Methacrylate Hydrogels with Tunable Characteristics by Altering the Sequence of the Cross-Linking Steps, Methacrylation Reaction Time, and Polymer Concentration. J. Biomater. Appl. 2023, 38, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Sonaye, S.Y.; Ertugral, E.G.; Kothapalli, C.R.; Sikder, P. Extrusion 3D (Bio)Printing of Alginate-Gelatin-Based Composite Scaffolds for Skeletal Muscle Tissue Engineering. Materials 2022, 15, 7945. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Chimpibul, W.; Nakaji-Hirabayashi, T.; Yuan, X.; Matsumura, K. Controlling the Degradation of Cellulose Scaffolds with Malaprade Oxidation for Tissue Engineering. J. Mater. Chem. B 2020, 8, 7904–7913. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Baldwin, A.; Tanideh, N.; Zarei, M.; Muthuraj, R.; Cao, Y.; Zhao, G.; Geng, J.; Jin, H.; Luo, J. Crosslinked Porous Three-Dimensional Cellulose Nanofibers-Gelatine Biocomposite Scaffolds for Tissue Regeneration. Int. J. Biol. Macromol. 2020, 164, 1949–1959. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and Enzymatically Dual Cross-Linked Oxidized Alginate Gelatin Hydrogels with Tunable Stiffness and Degradation Behavior for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Shehzad, A.; Mukasheva, F.; Moazzam, M.; Sultanova, D.; Abdikhan, B.; Trifonov, A.; Akilbekova, D. Dual-Crosslinking of Gelatin-Based Hydrogels: Promising Compositions for a 3D Printed Organotypic Bone Model. Bioengineering 2023, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Paravyan, G.; Shrivastava, R.; Benhabbour, S.R. Thermo-/PH-Responsive Chitosan-Cellulose Nanocrystals Based Hydrogel with Tunable Mechanical Properties for Tissue Regeneration Applications. Materialia 2020, 12, 100681. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Z.; Liang, Y.; Huang, Z.; Rong, Z.; Xiong, J.; Wang, D. Pulse Electromagnetic Fields Enhance the Repair of Rabbit Articular Cartilage Defects with Magnetic Nano-Hydrogel. RSC Adv. 2019, 10, 541–550. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.I.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl–Riboflavin (Gelma–Rf) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/Stimuli-Responsive Hydrogels: State-of-the-Art Platforms for Bone Tissue Engineering. Appl. Mater. Today 2022, 29, 101560. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Brandl, F.; Hammer, N.; Blunk, T.; Tessmar, J.; Goepferich, A. Biodegradable Hydrogels for Time-Controlled Release of Tethered Peptides or Proteins. Biomacromolecules 2010, 11, 496–504. [Google Scholar] [CrossRef]
- Nambiar, M.; Schneider, J.P. Cargo: Current and Potential Strategies. J. Peptide Sci. 2023, 28, 1–27. [Google Scholar] [CrossRef]
- Peattie, R.A.; Pike, D.B.; Yu, B.; Cai, S.; Shu, X.Z.; Prestwich, G.D.; Firpo, M.A.; Fisher, R.J. Effect of Gelatin on Heparin Regulation of Cytokine Release from Hyaluronan-Based Hydrogels. Drug Deliv. 2008, 15, 389–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, H.J.; Kim, H.K.; Yoon, J.J.; Park, T.G. Heparin Immobilized Porous PLGA Microspheres for Angiogenic Growth Factor Delivery. Pharm. Res. 2006, 23, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Wang, X.; Chen, B.; Xiao, Z.; Shi, C.; Wei, Z.; Hou, X.; Wang, Q.; Dai, J. The Osteogenic Effect of Bone Morphogenetic Protein-2 on the Collagen Scaffold Conjugated with Antibodies. J. Control. Release 2010, 141, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Enhanced Protein Delivery from Photopolymerized Hydrogels Using a Pseudospecific Metal Chelating Ligand. Pharm. Res. 2006, 23, 614–622. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. PH-Responsive Injectable Hydrogels with Mucosal Adhesiveness Based on Chitosan-Grafted-Dihydrocaffeic Acid and Oxidized Pullulan for Localized Drug Delivery. J. Colloid. Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef] [PubMed]
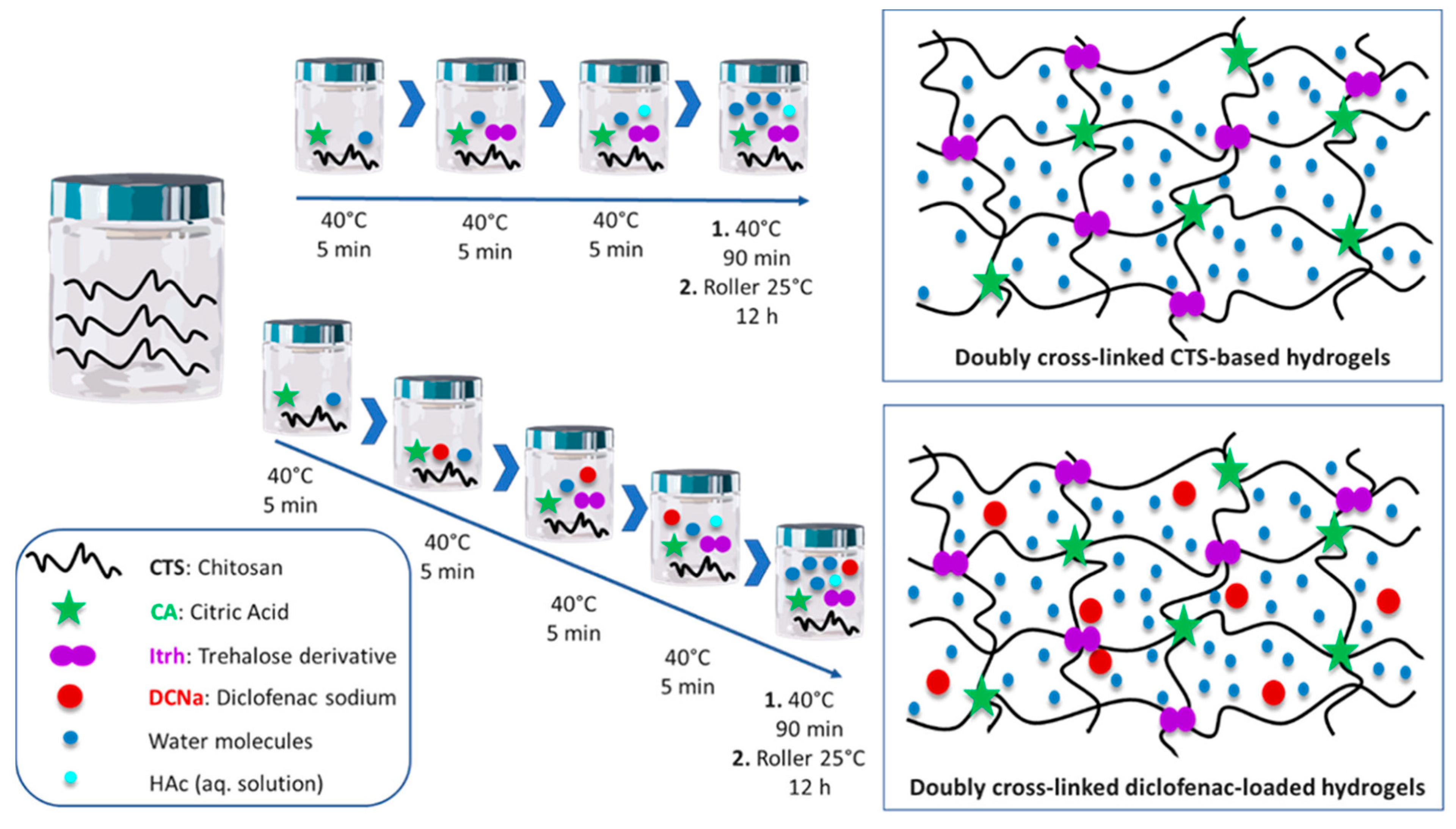
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.V. Biodegradable Double Cross-Linked Chitosan Hydrogels for Drug Delivery: Impact of Chemistry on Rheological and Pharmacological Performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Preman, N.K.; Barki, R.R.; Vijayan, A.; Sanjeeva, S.G.; Johnson, R.P. Recent Developments in Stimuli-Responsive Polymer Nanogels for Drug Delivery and Diagnostics: A Review. Eur. J. Pharm. Biopharm. 2020, 157, 121–153. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S.; Sharma, P.; Kumar, B. Single-, Dual-, and Multi-Stimuli-Responsive Nanogels for Biomedical Applications. Gels 2024, 10, 61. [Google Scholar] [CrossRef]
- Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and Injectable Hydrogel for Drug Delivery in Soft Tissues. Biomacromolecules 2019, 20, 149–163. [Google Scholar] [CrossRef]
- Hussain AL-Mayahy, M.; Imad Hameed, H. Hydrogels and Nanogels as a Promising Carrier for Drug Delivery. In Hydrogels and Nanogels—Applications in Medicine; IntechOpen: London, UK, 2023. [Google Scholar]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New Progress and Prospects: The Application of Nanogel in Drug Delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Myint, S.S.; Laomeephol, C.; Thamnium, S.; Chamni, S.; Luckanagul, J.A. Hyaluronic Acid Nanogels: A Promising Platform for Therapeutic and Theranostic Applications. Pharmaceutics 2023, 15, 2671. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.; Zou, Y.; Zhong, Y.; Zhong, Z.; Meng, F. NIR and UV-Responsive Degradable Hyaluronic Acid Nanogels for CD44-Targeted and Remotely Triggered Intracellular Doxorubicin Delivery. Colloids Surf. B Biointerfaces 2017, 158, 547–555. [Google Scholar] [CrossRef]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; Van Der Sanden, B.; et al. Coumarin-Containing Thermoresponsive Hyaluronic Acid-Based Nanogels as Delivery Systems for Anticancer Chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef]
- Pedrosa, S.S.; Pereira, P.; Correia, A.; Gama, F.M. Targetability of Hyaluronic Acid Nanogel to Cancer Cells: In Vitro and in Vivo Studies. Eur. J. Pharm. Sci. 2017, 104, 102–113. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Chen, J.; Zhang, J.; Meng, F.; Deng, C.; Cheng, R.; Feijen, J.; Zhong, Z. Bioresponsive and Fluorescent Hyaluronic Acid-Iodixanol Nanogels for Targeted X-Ray Computed Tomography Imaging and Chemotherapy of Breast Tumors. J. Control. Release 2016, 244, 229–239. [Google Scholar] [CrossRef]
- Coninx, S.; Kalot, G.; Godard, A.; Bodio, E.; Goze, C.; Sancey, L.; Auzély-Velty, R. Tailored Hyaluronic Acid-Based Nanogels as Theranostic Boron Delivery Systems for Boron Neutron Cancer Therapy. Int. J. Pharm. X 2022, 4, 100134. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Liu, A.; Zhen, X.; Gao, J.; Wu, W.; Cai, W.; Jiang, X. Responsive Hyaluronic Acid-Gold Cluster Hybrid Nanogel Theranostic Systems. Biomater. Sci. 2021, 9, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, S.; Zhang, S.; Dong, S.; Hu, J.; Kang, L.; Yang, X. A Double-Layer Hydrogel Based on Alginate-Carboxymethyl Cellulose and Synthetic Polymer as Sustained Drug Delivery System. Sci. Rep. 2021, 11, 9142. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Fang, C.W.; Chiu, I.H.; Khan, A.; Wu, Y.C.; Lin, I.L.; Tsai, M.J.; Wu, P.C. Synthesis and Evaluation of Alginate-Based Nanogels as Sustained Drug Carriers for Caffeine. ACS Omega 2023, 8, 23991–24002. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Barrett, A.; Pishnamazi, M.; Walker, G.M.; Collins, M.N. Wood-Derived Hydrogels as a Platform for Drug-Release Systems. ACS Sustain. Chem. Eng. 2021, 9, 2515–2522. [Google Scholar] [CrossRef]
- Mehdi-Sefiani, H.; Granados-Carrera, C.M.; Romero, A.; Chicardi, E.; Domínguez-Robles, J.; Perez-Puyana, V.M. Chitosan–Type-A-Gelatin Hydrogels Used as Potential Platforms in Tissue Engineering for Drug Delivery. Gels 2024, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Ogbeh, G.O.; Tsokar, T.O.; Salifu, E. Optimization of Nutrients Requirements for Bioremediation of Spent-Engine Oil Contaminated Soils. Environ. Eng. Res. 2019, 24, 484–494. [Google Scholar] [CrossRef]
- Mujtaba, M.; Sharif, R.; Ali, Q.; Rehman, R.; Khawar, K.M. Biopolymer Based Nanofertilizers Applications in Abiotic Stress (Drought and Salinity) Control. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture: A Smart Delivery System for Crop Improvement; Elsevier: Amsterdam, The Netherlands, 2020; pp. 85–110. ISBN 9780128200926. [Google Scholar]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging Investigator Series: Polymeric Nanocarriers for Agricultural Applications: Synthesis, Characterization, and Environmental and Biological Interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Otey, F.H.; Trimnell, D.; Westhoff, R.P.; Shasha, B.S. Starch Matrix for Controlled Release of Urea Fertilizer. J. Agric. Food Chem. 1984, 32, 1095–1098. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Paterson, W.J.; Dum, E.J.; Li, Q.; Hunter, B.K.; Goosen, M.F.A. Assessment of Chitosan Gels for the Controlled Release of Agrochemicals. Ind. Eng. Chem. Res. 1990, 29, 1205–1209. [Google Scholar] [CrossRef]
- García, M.C.; Díez, J.A.; Vallejo, A.; García, L.; Cartagena, M.C. Use of Kraft Pine Lignin in Controlled-Release Fertilizer Formulations. Ind. Eng. Chem. Res. 1996, 35, 245–249. [Google Scholar] [CrossRef]
- Fertahi, S.; Ilsouk, M.; Zeroual, Y.; Oukarroum, A.; Barakat, A. Recent Trends in Organic Coating Based on Biopolymers and Biomass for Controlled and Slow Release Fertilizers. J. Control. Release 2021, 330, 341–361. [Google Scholar] [CrossRef]
- Hou, X.; Pan, Y.; Xiao, H.; Liu, J. Controlled Release of Agrochemicals Using PH and Redox Dual-Responsive Cellulose Nanogels. J. Agric. Food Chem. 2019, 67, 6700–6707. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Sharma, A.K.; Bhardwaj, D.; Agrawal, G. Biodegradable Dual Stimuli Responsive Alginate Based Microgels for Controlled Agrochemicals Release and Soil Remediation. Int. J. Biol. Macromol. 2023, 228, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and Development of Guar Gum Based Novel, Superabsorbent and Moisture Retaining Hydrogels for Agricultural Applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel Biodegradable Hydrogel Based on Natural Polymers: Synthesis, Characterization, Swelling/Reswelling and Biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Gabriel, G.R.; Joshua, G.B.; Ashley, Y.C.; Jake, N.P.; Terence, T. Synthesis and Characterization of Sodium Carboxymethyl Cellulose/Sodium Alginate/Hydroxypropyl Cellulose Hydrogel for Agricultural Water Storage and Controlled Nutrient Release. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 304 SSP, pp. 51–57. [Google Scholar]
- Passauer, L.; Hallas, T.; Bäucker, E.; Ciesielski, G.; Lebioda, S.; Hamer, U. Biodegradation of Hydrogels from Oxyethylated Lignins in Model Soils. ACS Sustain. Chem. Eng. 2015, 3, 1955–1964. [Google Scholar] [CrossRef]
- Sharma, G.; Saharan, V.; Pal, A.; Sharma, S.S. Chitosan Nanofertilizer to Strengthen Sink Strength and Provide Resistance against PFSR (Post Flowering Stalk Rot) Disease in Maize. Biocatal. Agric. Biotechnol. 2024, 60, 103303. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel Synthesis Based on Lignin/Sodium Alginate and Application in Agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef]


| Characteristic | Natural Biopolymers | Synthetic Polymers |
|---|---|---|
| Biocompatibility | High | Polymer-dependent |
| Inherent biodegradability | High | Limited (polymer-dependent) |
| Degradation products | Non-toxic | Potentially harmful |
| Synthesis and modification flexibility | Medium | High |
| Tunable properties and kinetics | Medium | High |
| Working range (pH, T, and ionic strength, etc.) | Limited | Wide |
| Reproducibility | Likely | Controlled |
| Ecological impact | Low | Medium–high (polymer-dependent) |
| Natural Polymer | Type of Hydrogel | Crosslinking Method | Tissue | Reference |
|---|---|---|---|---|
| Chitosan | Chitosan-based hydrogels | Physical crosslinking | Soft tissues | [102] |
| Chitosan-based hydrogels | Physical crosslinking | Bone | [95] | |
| Chitosan-based hydrogel modified with glycidyl methacrylate | Chemical/Free-radical photo-crosslinking | Cartilage | [107] | |
| Methacrylated glycol chitosan hydrogels | Chemical/Free-radical photo-crosslinking | Bone | [108] | |
| Chitosan–genipin hydrogels | Chemical crosslinking | Skin | [110] | |
| Chitosan–cellulose nanocrystals-based hydrogels | Physical crosslinking | Bone | [124] | |
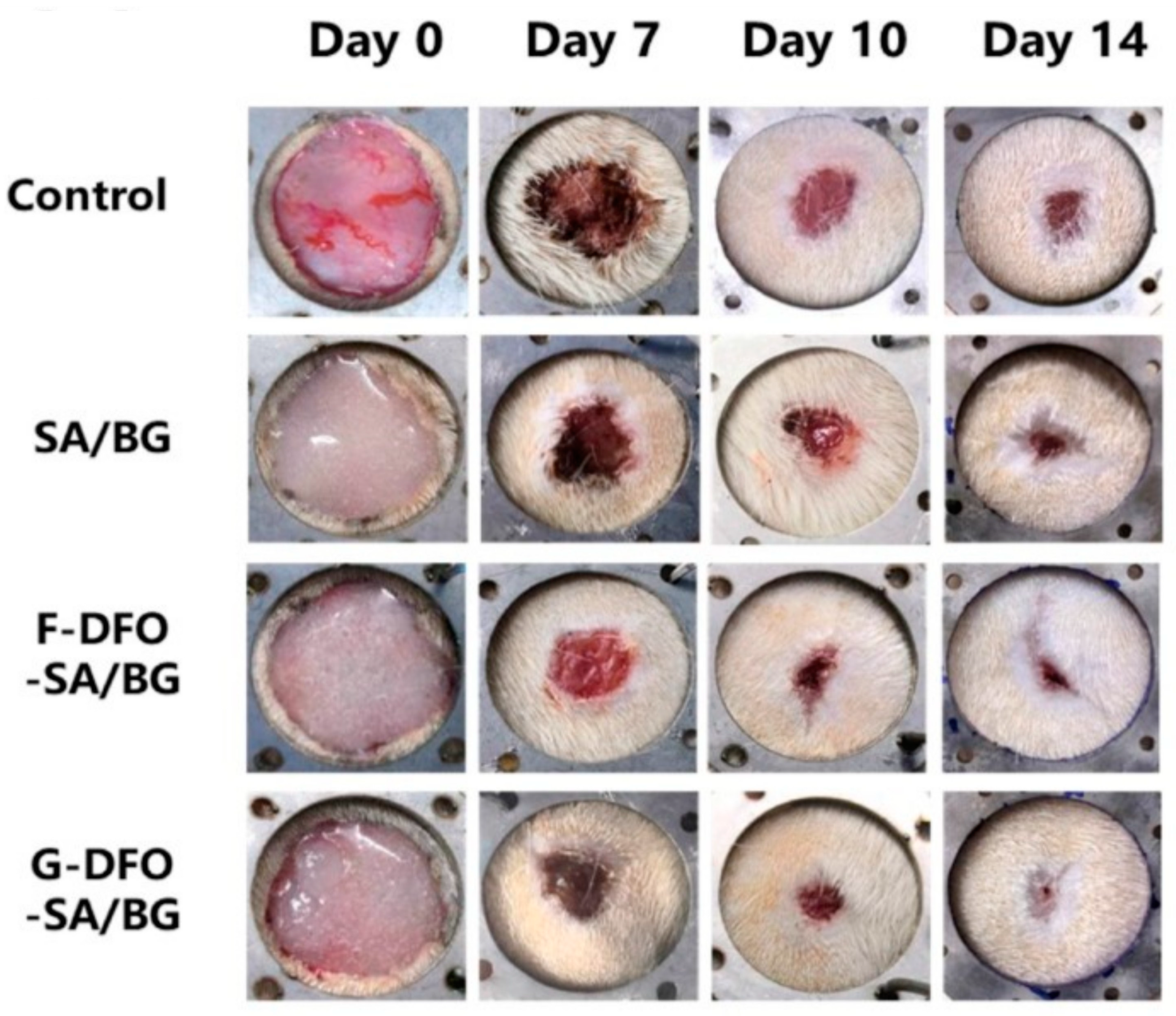
| Alginate | Sodium alginate/bioglass hydrogel (SA/BG) grafted with deferoxamine (DFO) | Chemical crosslinking | Skin | [113] |
| Oxidized alginate-based hydrogels | Physical crosslinking | Cartilage | [115] | |
| Oxidized alginate-based hydrogels | Physical crosslinking | General | [76] | |
| Oxidized methacrylated alginate (OMA) hydrogels | Physical and chemical crosslinking | General | [116] | |
| Alginate–gelatin-based hydrogels | Physical and chemical crosslinking | Skeletal muscle | [117] | |
| Cellulose | Oxidized cellulose-based scaffolds | Chemical crosslinking | General | [119] |
| Cellulose nanofibers-gelatin based scaffolds with epichlorohydrin | Physical and chemical crosslinking | General | [120] | |
| Gelatin | Gelatin and oxidized alginate-based hydrogels | Physical and chemical crosslinking | Cartilage, bone, and blood vessels | [122] |
| Methacrylated gelatin-alginate-based hydrogels | Physical and chemical crosslinking | Bone | [123] | |
| Gelatin with Fe3O4 magnetic nanoparticles | Chemical crosslinking | Cartilage | [125] | |
| Gelatin methacryloyl with riboflavin | Chemical crosslinking | Bone | [126] |
| Natural Polymer | Type of Hydrogel | Crosslinking Method | Delivered Drug | Reference |
|---|---|---|---|---|
| Chitosan | Chitosan-grafted-dihydrocaffeic acid-based hydrogel | Covalent crosslinking | Doxorubicin | [135] |
| Chitosan-based double crosslinked hydrogel | Ionic and covalent crosslinking | Diclofenac sodium | [136] | |
| Alginate | Alginate-carboxymethyl cellulose-based double-layer hydrogel | Ionic and covalent crosslinking | BSA and indomethacin | [151] |
| Alginate-based nanogels | Covalent crosslinking | Caffeine | [152] | |
| Cellulose | Cellulose–lignin-based hydrogels | Physical crosslinking | Paracetamol | [153] |
| Gelatin | Type-A gelatin and chitosan-based hydrogels | Ionic and thermal crosslinking | Tetracycline | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. https://doi.org/10.3390/polym16182599
Garcia-Garcia A, Muñana-González S, Lanceros-Mendez S, Ruiz-Rubio L, Alvarez LP, Vilas-Vilela JL. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers. 2024; 16(18):2599. https://doi.org/10.3390/polym16182599
Chicago/Turabian StyleGarcia-Garcia, Ane, Sara Muñana-González, Senentxu Lanceros-Mendez, Leire Ruiz-Rubio, Leyre Perez Alvarez, and José Luis Vilas-Vilela. 2024. "Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation" Polymers 16, no. 18: 2599. https://doi.org/10.3390/polym16182599
APA StyleGarcia-Garcia, A., Muñana-González, S., Lanceros-Mendez, S., Ruiz-Rubio, L., Alvarez, L. P., & Vilas-Vilela, J. L. (2024). Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers, 16(18), 2599. https://doi.org/10.3390/polym16182599









