Monolithic Polyepoxide Membranes for Nanofiltration Applications and Sustainable Membrane Manufacture
Abstract
1. Introduction
2. Materials and Methods
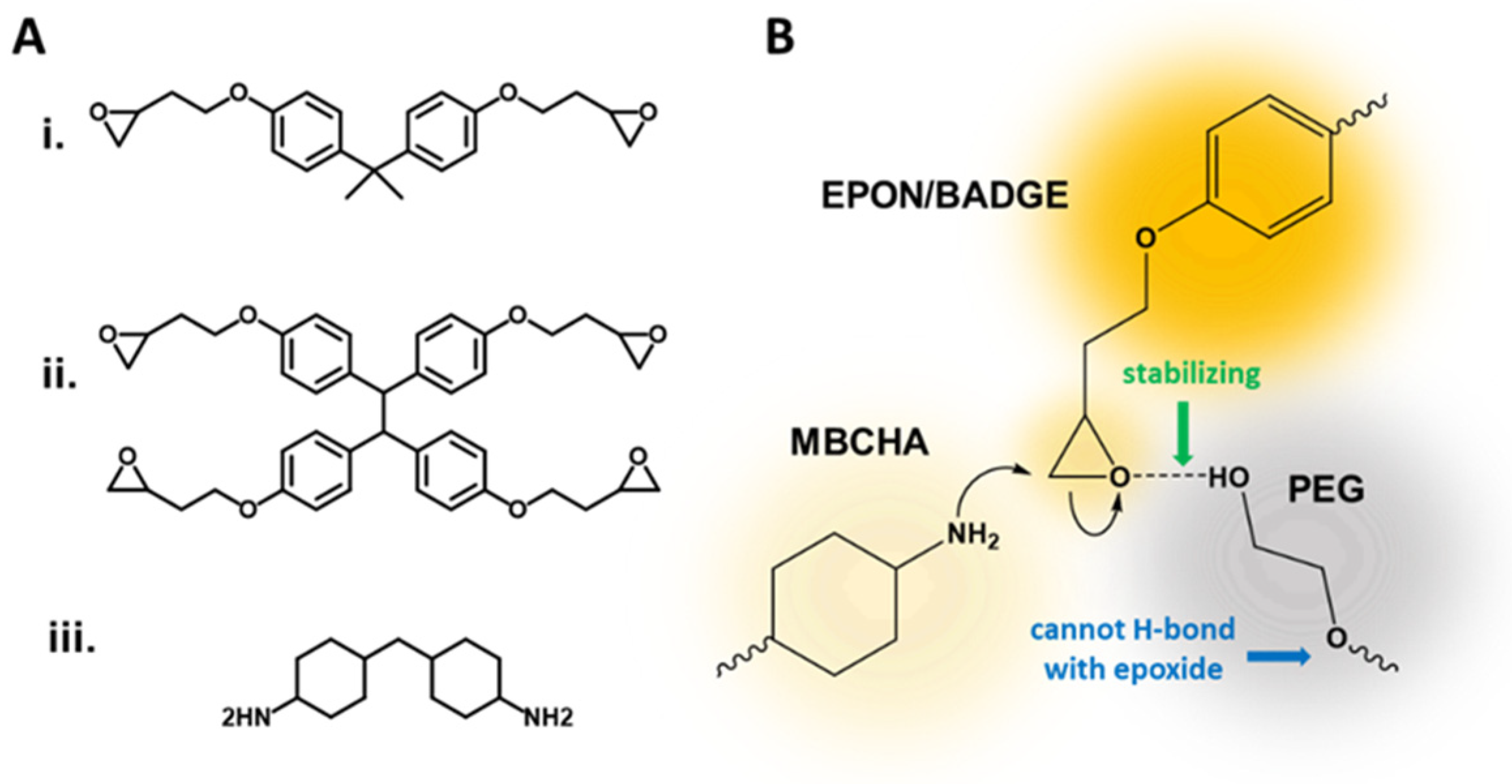
2.1. Materials
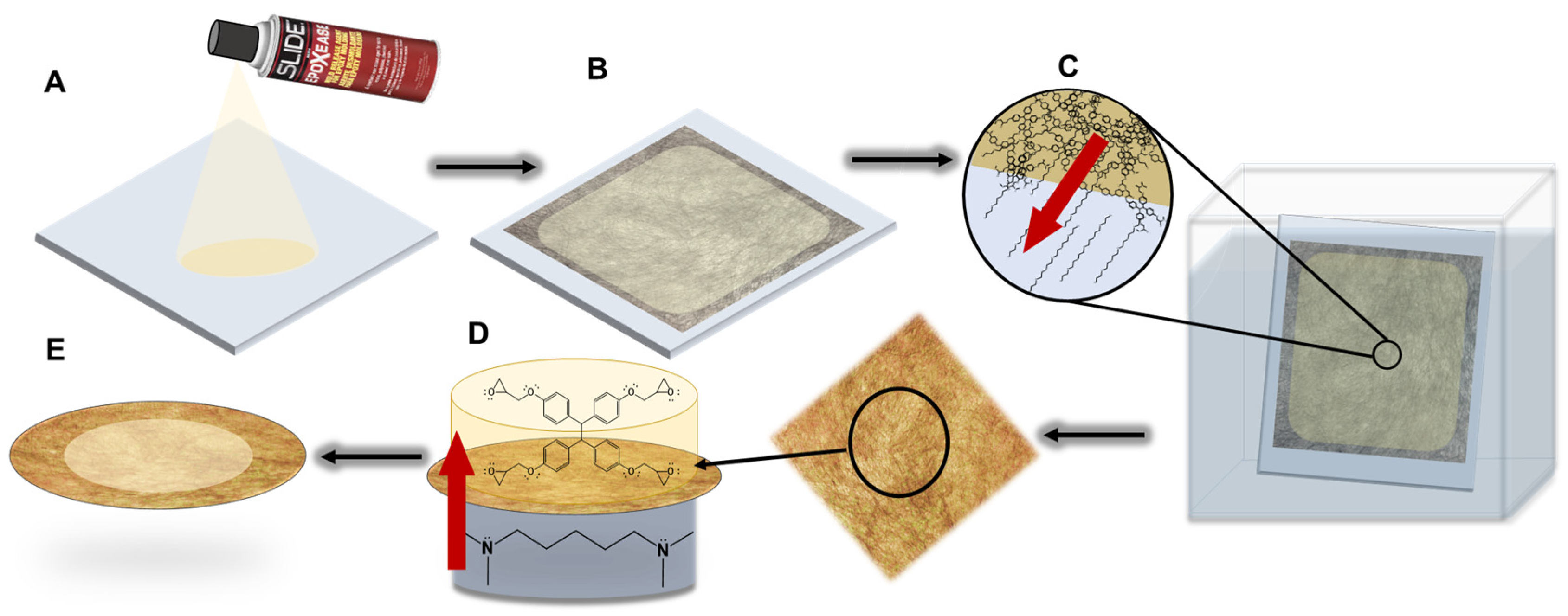
2.2. Membrane Synthesis
2.3. Epoxy Formation
2.4. Membrane Fabrication
2.5. Interfacial Polymerization
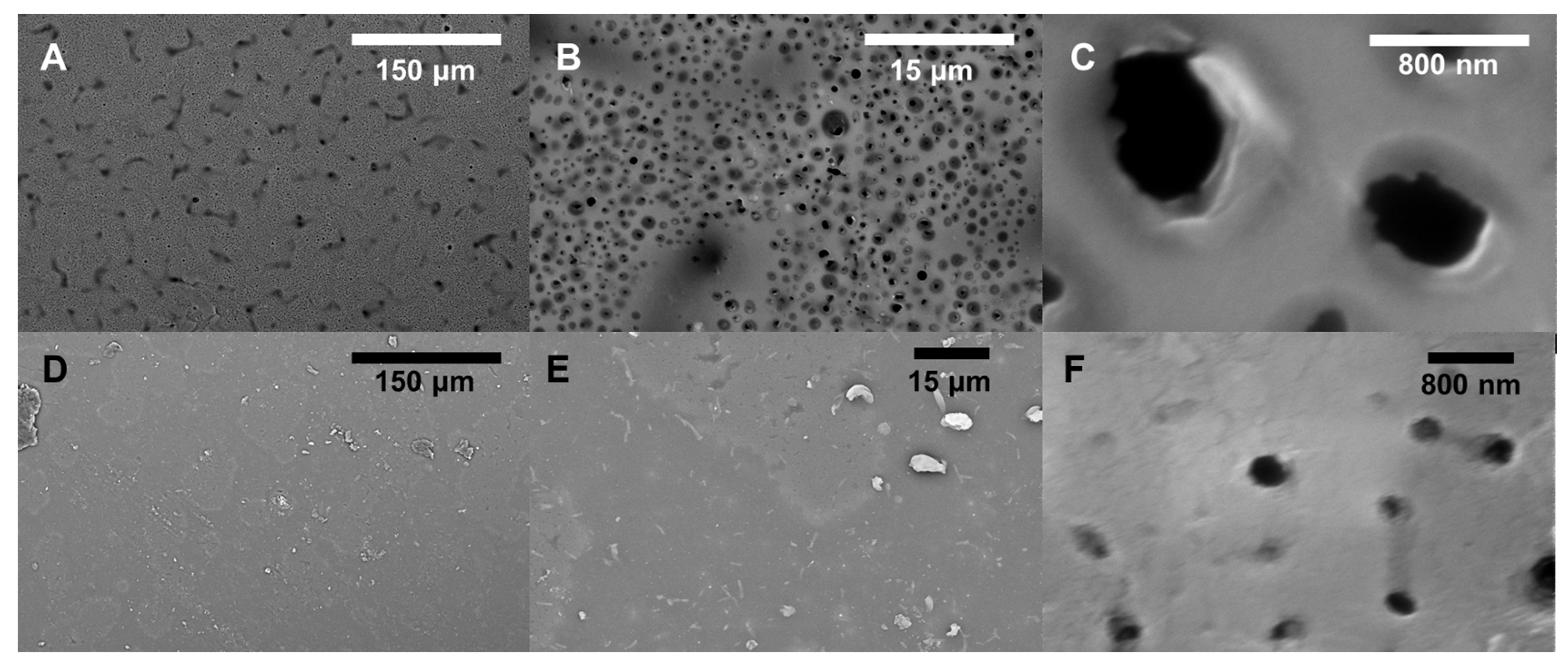
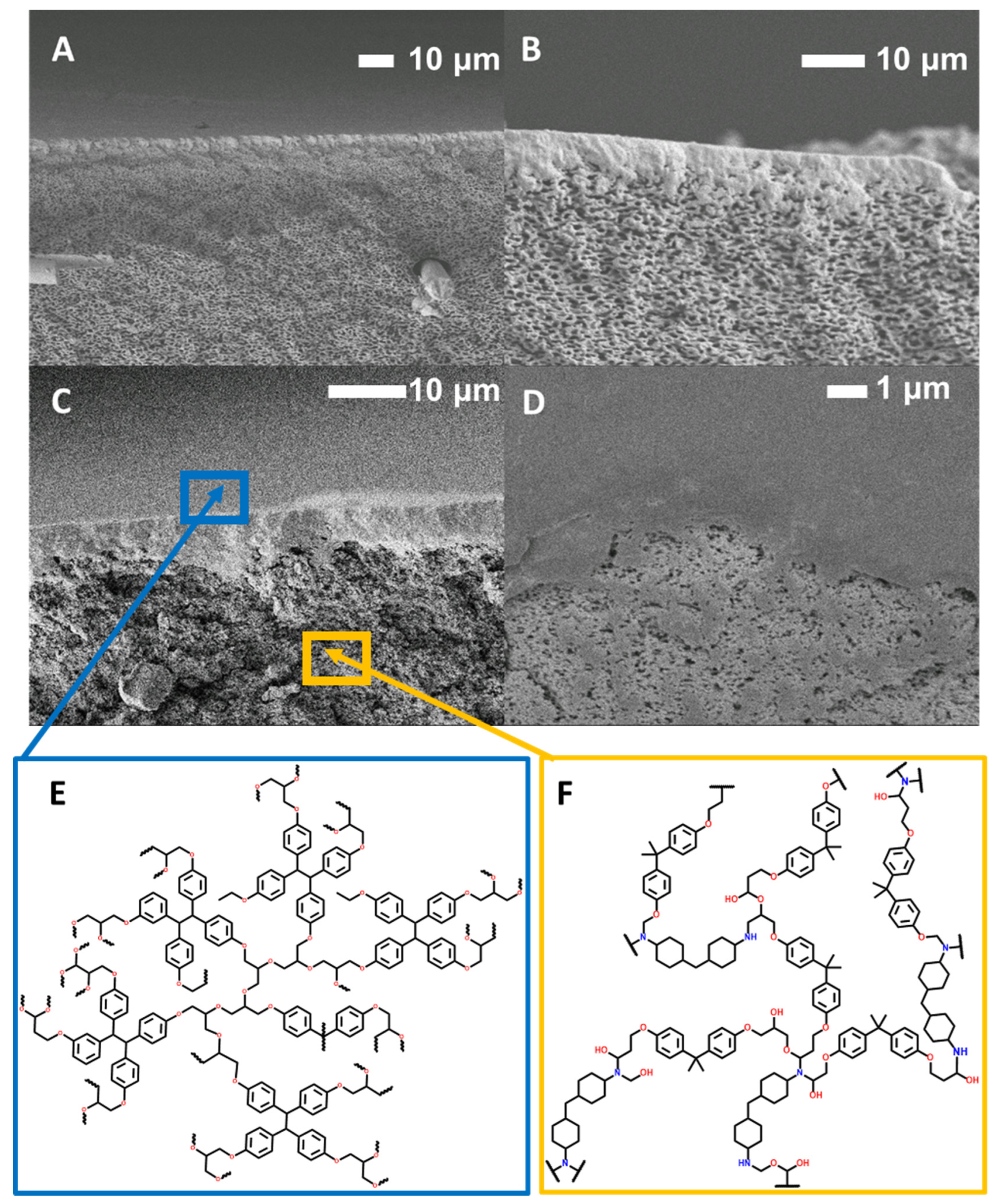
2.6. SEM
2.7. Membrane Testing
2.8. Dye Rejection
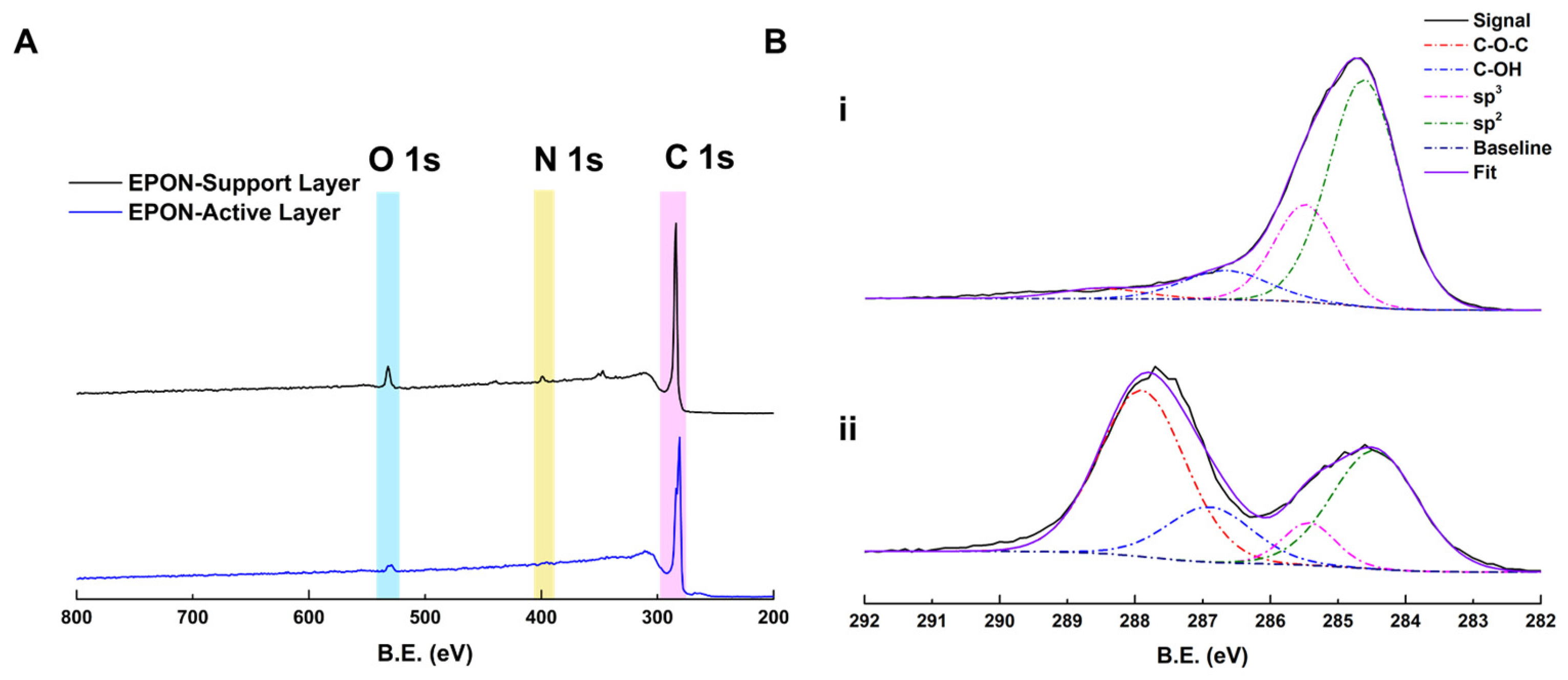
2.9. XPS
2.10. XRD
2.11. Mechanical Testing
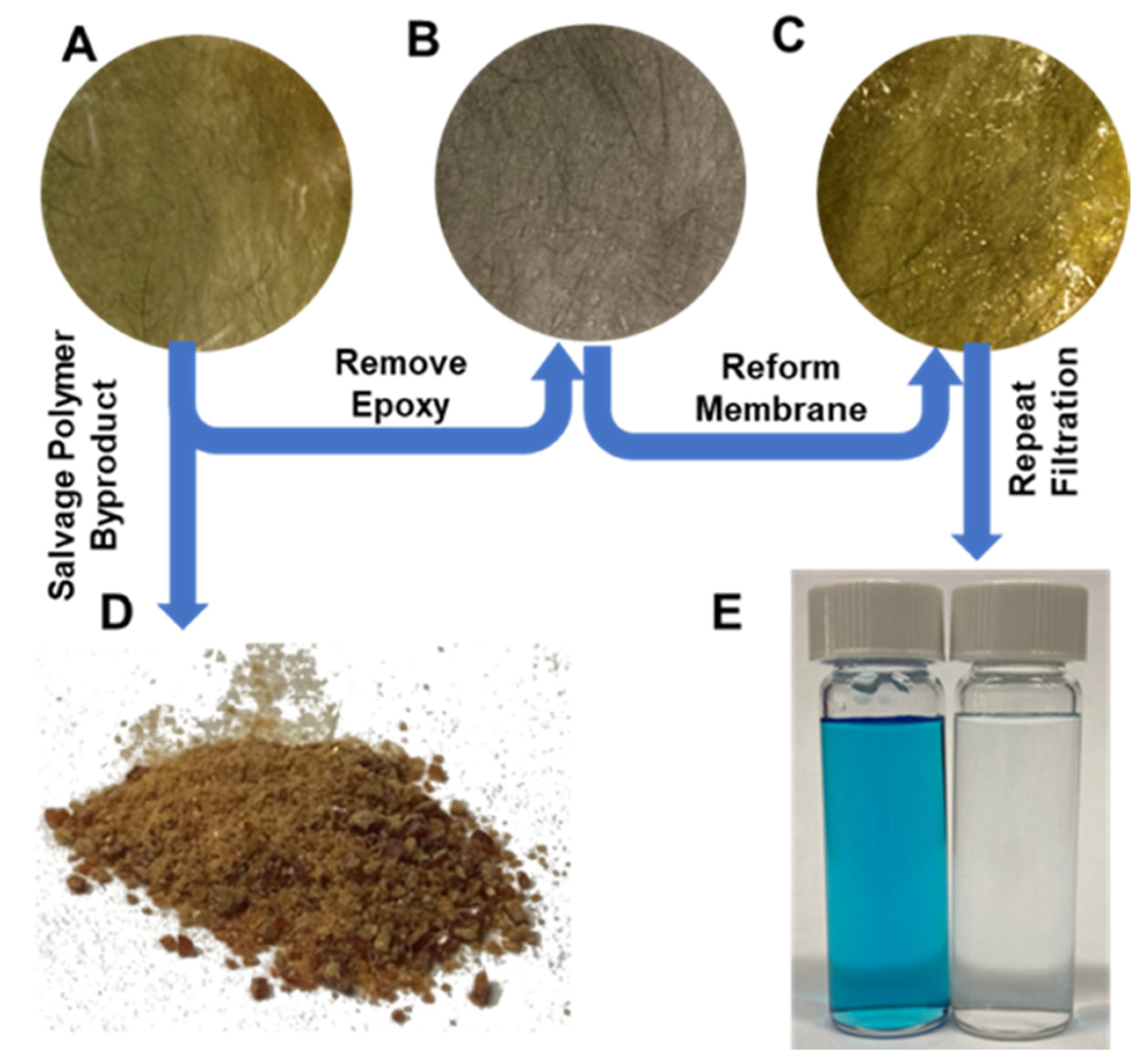
2.12. Membrane Degradation and Fiber Recycling
3. Results and Discussion
3.1. Effects of Different PEG Mw during Synthesis
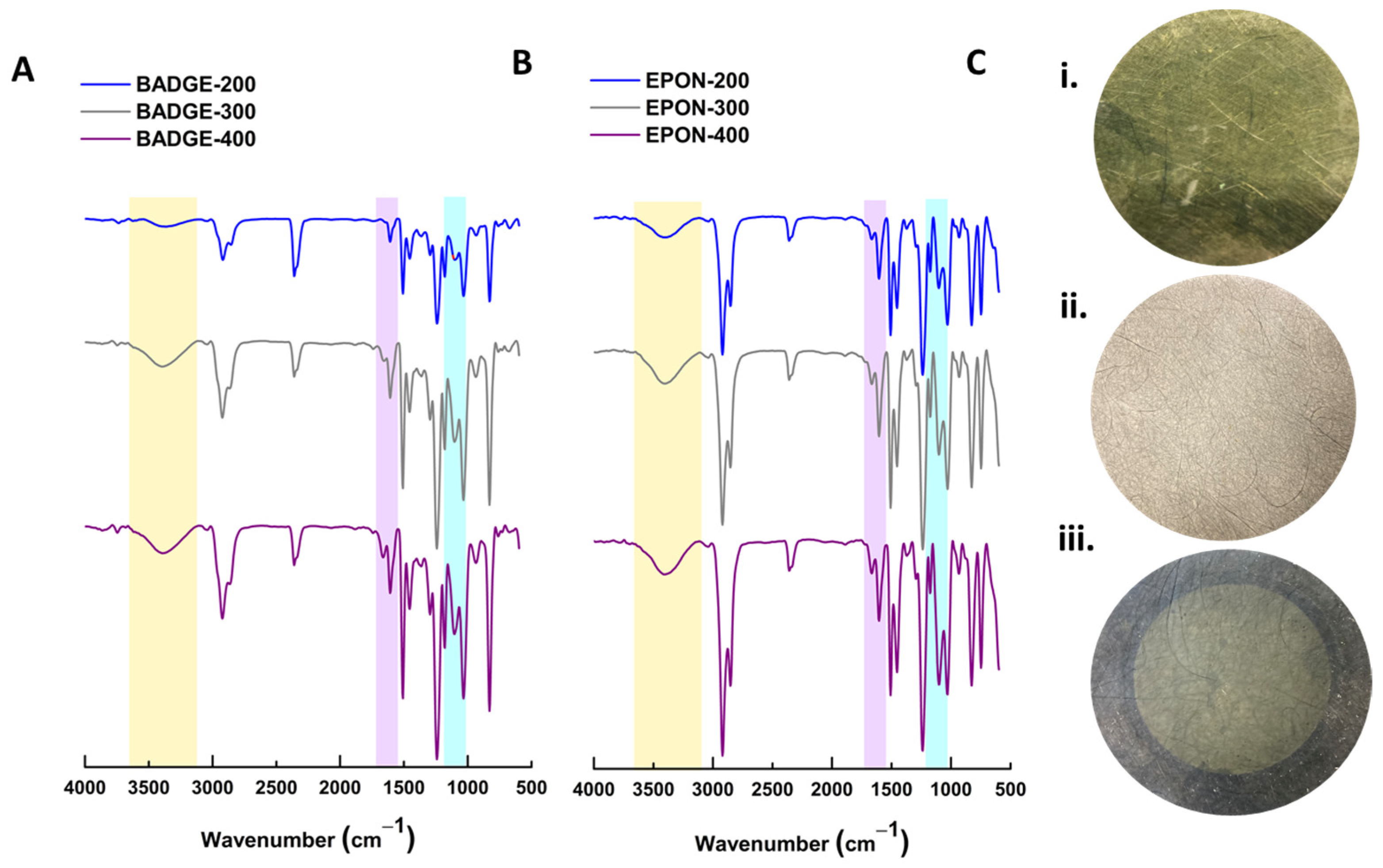
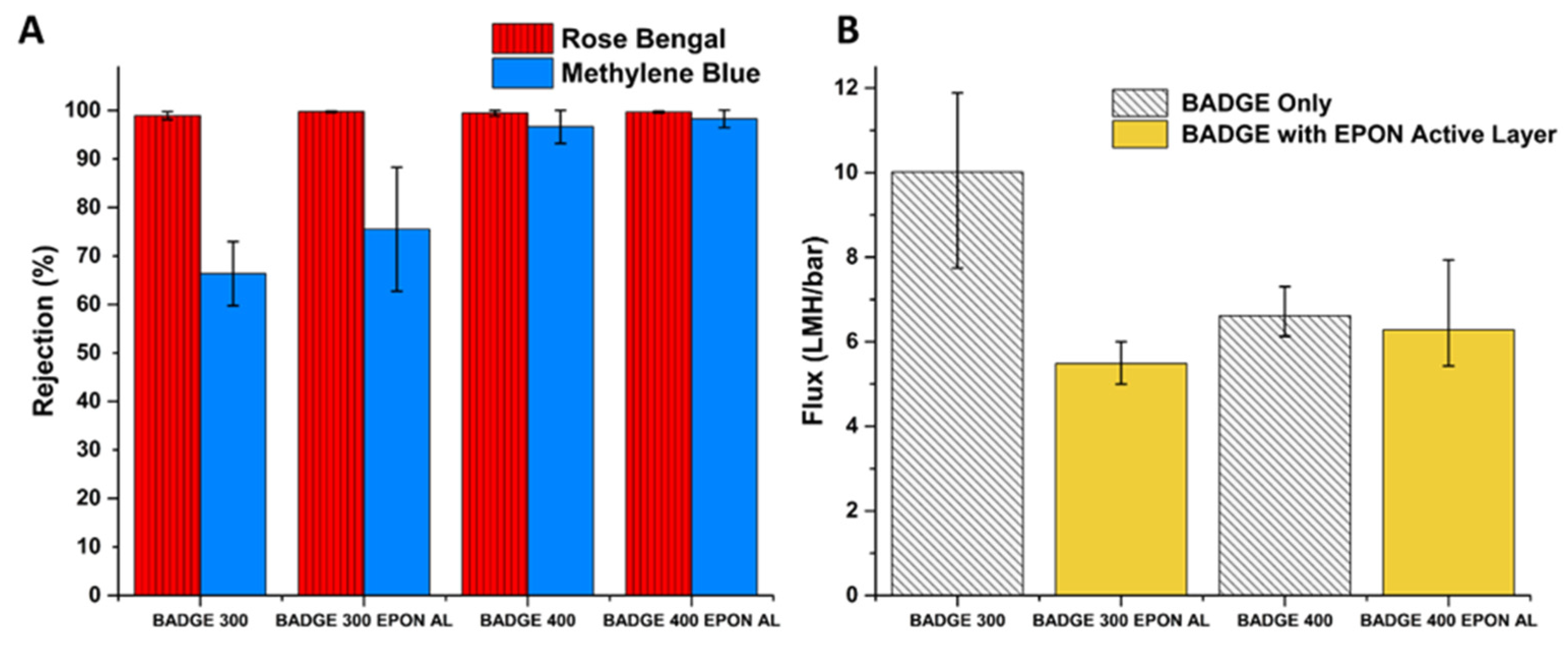
3.2. Performance and Characterization of BADGE-MBCHA Membranes
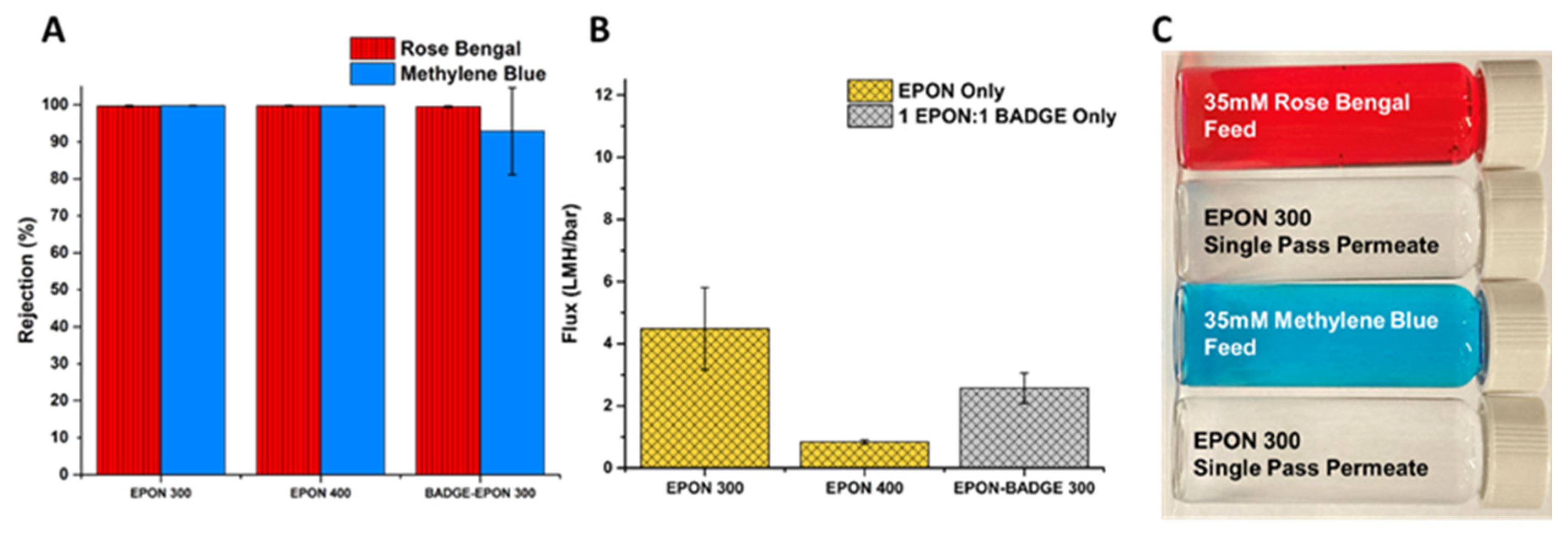
3.3. Performance and Characterization of EPON-MBCHA Membranes
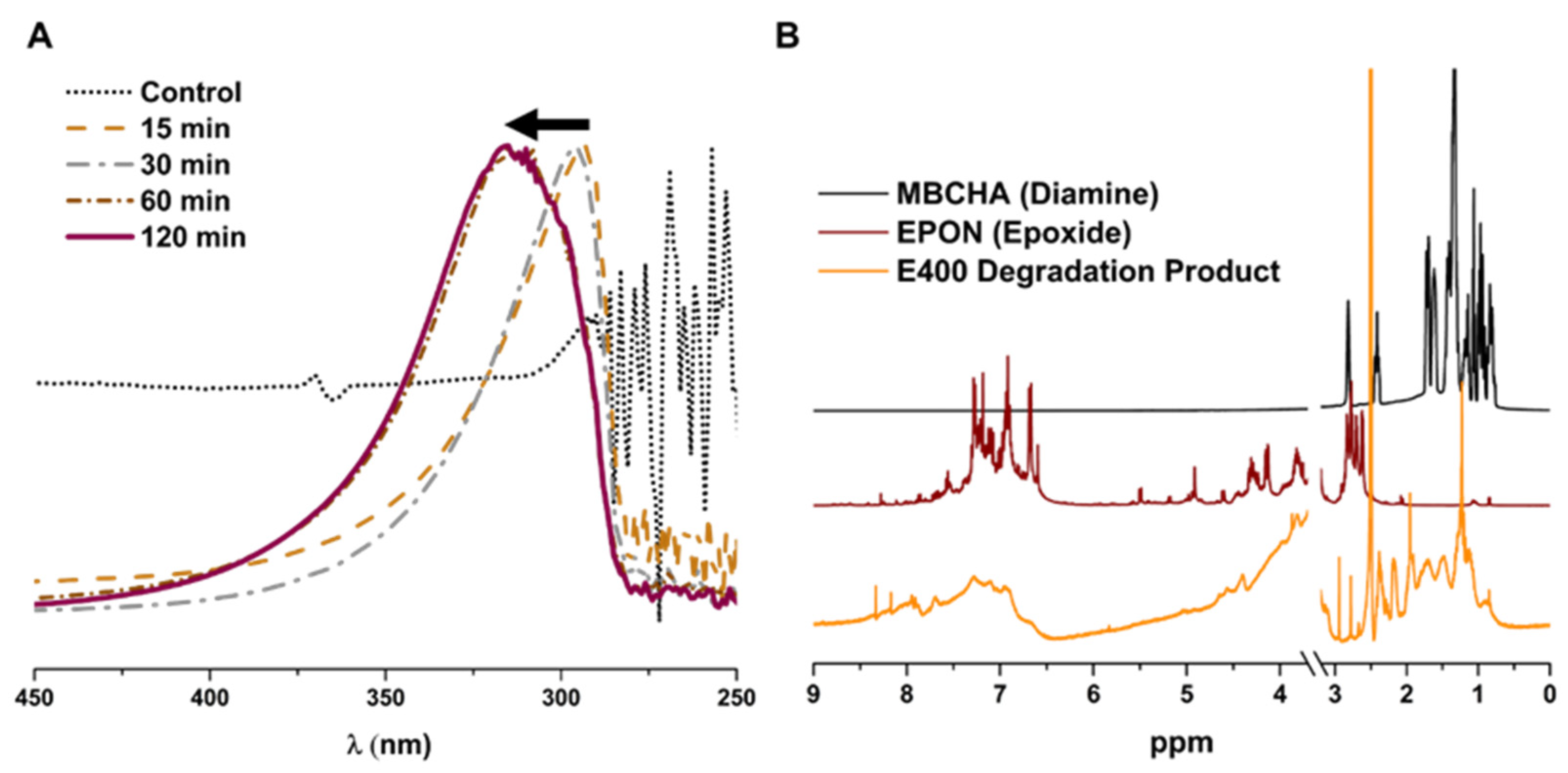
3.4. Chemical and Physical Degradation of Epoxy Membranes for Recycling
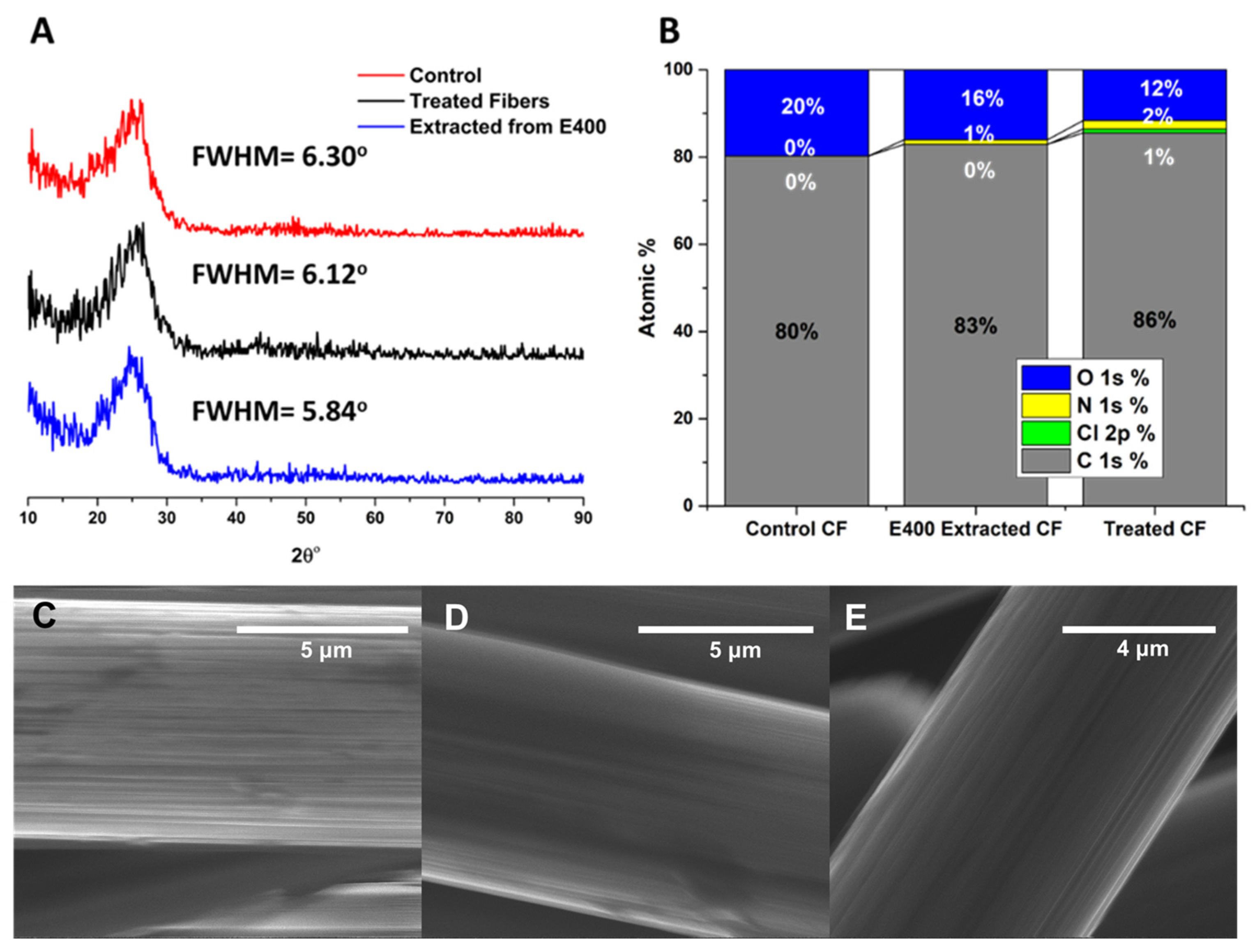
3.5. Evaluation of Recovered Carbon Fibers
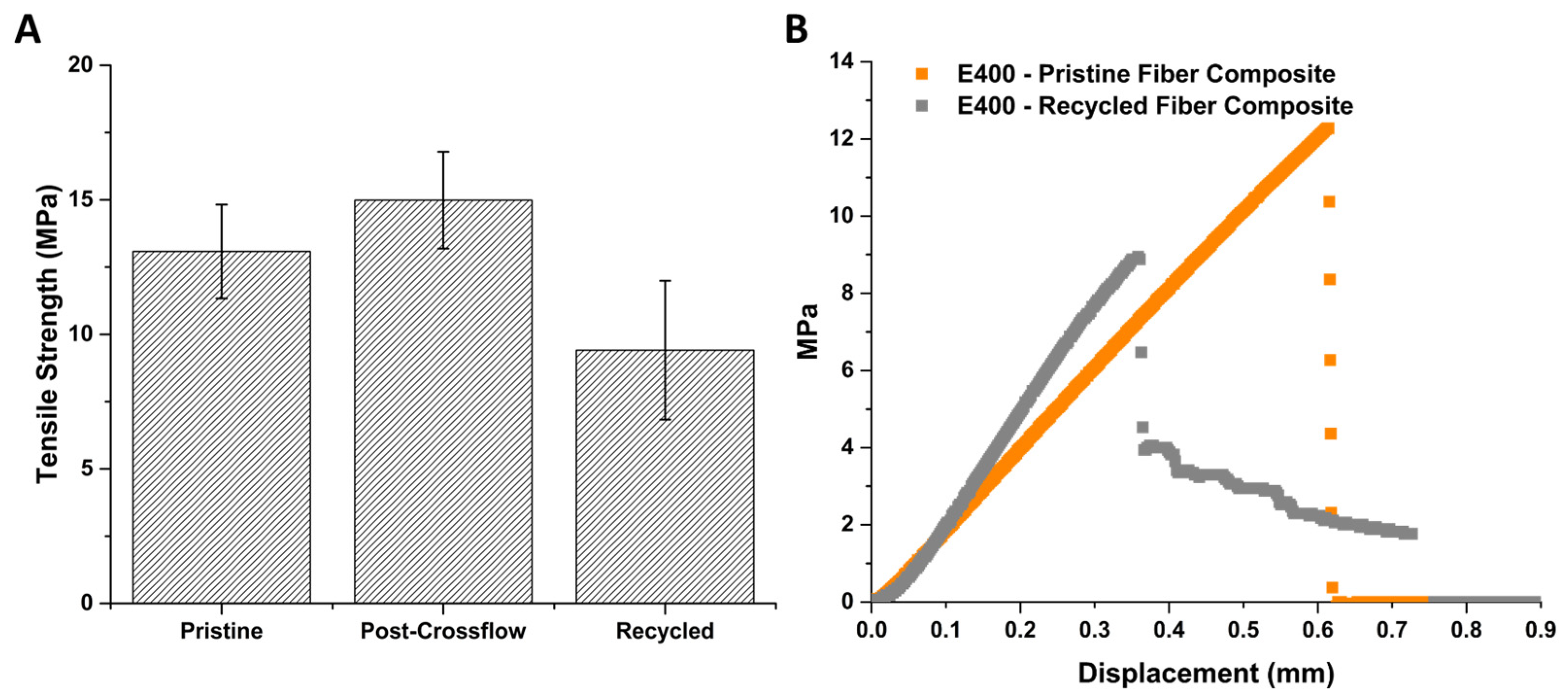
3.6. “Recycled” CF Composite Membrane Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, G.; Wang, J.; Li, C. Strategies for improving the performance of the polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes: Surface modifications and nanoparticles incorporations. Desalination 2013, 328, 83–100. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater and the Environment: Energy, Technology, and the Environment. Science 2011, 333, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of polyamide nanofiltration and reverse osmosis membranes by hypochlorite. Environ. Sci. Technol. 2012, 46, 852–859. [Google Scholar] [CrossRef]
- Verbeke, R.; Arts, W.; Dom, E.; Dickmann, M.; Egger, W.; Koeckelberghs, G.; Szymczyk, A.; Vankelecom, I.F.J. Transferring bulk chemistry to interfacial synthesis of TFC-membranes to create chemically robust poly(epoxyether)films. J. Membr. Sci. 2019, 582, 442–453. [Google Scholar] [CrossRef]
- Verbeke, R.; Seynaeve, M.; Bastin, M.; Davenport, D.M.; Eyley, S.; Thielemans, W.; Koeckelberghs, G.; Elimelech, M.; Vankelecom, I.F.J. The significant role of support layer solvent annealing in interfacial polymerization: The case of epoxide-based membranes. J. Membr. Sci. 2020, 612, 118438. [Google Scholar] [CrossRef]
- Verbeke, R.; Davenport, D.M.; Stassin, T.; Eyley, S.; Dickmann, M.; Cruz, A.J.; Dara, P.; Ritt, C.L.; Bogaerts, C.; Egger, W.; et al. Chlorine-Resistant Epoxide-Based Membranes for Sustainable Water Desalination. Environ. Sci. Technol. Lett. 2021, 8, 818–824. [Google Scholar] [CrossRef]
- McVerry, B.; Anderson, M.; He, N.; Kweon, H.; Ji, C.; Xue, S.; Rao, E.; Lee, C.; Lin, C.-W.; Chen, D.; et al. Next-Generation Asymmetric Membranes Using Thin-Film Liftoff. Nano Lett. 2019, 19, 5036–5043. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Xue, S.; Lin, C.-W.; Mak, W.H.; Mcverry, B.T.; Turner, C.L.; Anderson, M.; Molas, J.C.; Xu, Z.; Kaner, R.B. Ultrapermeable Organic Solvent Nanofiltration Membranes with Precisely Tailored Support Layers Fabricated Using Thin-Film Liftoff. ACS Appl. Mater. Interfaces 2020, 12, 30796–30804. [Google Scholar] [CrossRef]
- Xue, S.; Ji, C.; Kowal, M.D.; Molas, J.C.; Lin, C.-W.; Mcverry, B.T.; Turner, C.L.; Mak, W.H.; Anderson, M.; Muni, M.; et al. Nanostructured Graphene Oxide Composite Membranes with Ultra-permeability and Mechanical Robustness. Nano Lett. 2020, 20, 2209–2218. [Google Scholar] [CrossRef]
- Lawler, W.; Alvarez-Gaitan, J.; Leslie, G.; Le-Clech, P. Comparative life cycle assessment of end-of-life options for reverse osmosis membranes. Desalination 2015, 357, 45–54. [Google Scholar] [CrossRef]
- Ashkelon Seawater Reverse Osmosis (SWRO) Plant, Israel-Water Technology. Available online: https://www.water-technology.net/projects/israel/ (accessed on 3 May 2022).
- Hydranautics to Supply RO Membrane to World’s Biggest Seawater Desalination Plant in Israel. Water World. 2010. Available online: https://www.waterworld.com/print/content/16220856 (accessed on 3 May 2022).
- Synder Filtration. NFG (TFC 600-800Da). Available online: https://synderfiltration.com/2014/wp-content/uploads/2018/11/NFG-TFC-600-800Da-Sanitary-Specsheet.pdf (accessed on 19 August 2024).
- Synder Filtration. NFW (TFC 300-500Da). Available online: https://synderfiltration.com/2014/wp-content/uploads/2018/07/NFW-TFC-300-500Da-Industrial-Specsheet.pdf (accessed on 19 August 2024).
- Bastin, M.; Bogaert, K.; Dom, E.; Verbeke, R.; Vankelecom, I.F.J. Towards fully epoxy-based thin film composite membranes for solvent-resistant and solvent-tolerant nanofiltration. J. Membr. Sci. 2023, 683, 121813. [Google Scholar] [CrossRef]
- Souza-Chaves, B.M.; Alhussaini, M.A.; Felix, V.; Presson, L.K.; Betancourt, W.Q.; Hickenbottom, K.L.; Achilli, A. Extending the life of water reuse reverse osmosis membranes using chlorination. J. Membr. Sci. 2022, 642, 119897. [Google Scholar] [CrossRef]
- McKeen, L.W. Markets and Applications for Films, Containers, and Membranes. Permeability Prop. Plast. Elastomers 2017, 4, 61–82. [Google Scholar]
- Pakdel, E.; Kashi, S.; Varley, R.; Wang, X. Recent progress in recycling carbon fibre reinforced composites and dry carbon fibre wastes. Resour. Conserv. Recycl. 2021, 166, 105340. [Google Scholar] [CrossRef]
- Wölling, J.; Schmieg, M.; Manis, F.; Drechsler, K. Nonwovens from Recycled Carbon Fibres-Comparison of Processing Technologies. Procedia CIRP 2017, 66, 271–276. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Alqaheem, Y.; Alomair, A.A. Minimizing Solvent Toxicity in Preparation of Polymeric Membranes for Gas Separation. ACS Omega 2020, 5, 630–6335. [Google Scholar] [CrossRef]
- Ong, C.; Falca, G.; Huang, T.; Liu, J.; Manchanda, P.; Chisca, S.; Nunes, S.P. Green Synthesis of Thin-Film Composite Membranes for Organic Solvent Nanofiltration. ACS Sustain. Chem. Eng. 2020, 8, 11541–11548. [Google Scholar] [CrossRef]
- Cheng, C.; Gupta, M. Solvent-Free Synthesis of Selectively Wetting Multilayer and Janus Membranes. Adv. Mater. Interfaces 2020, 7, 2001103. [Google Scholar] [CrossRef]
- Jang, H.-J.; Shin, C.Y.; Kim, K.-B. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef]
- Dipalma, J.A.; Deridder, P.H.; Orlando, R.C.; Kolts, B.E.; Cleveland, M.V. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am. J. Gastroenterol. 2000, 95, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, Y.P.; Alele, N.; Galleguillos, H.R.; Ulbricht, M. Nanofiltration Separation of Aqueous Polyethyleneglycol–Salt Mixtures. Sep. Sci. Technol. 2013, 48, 1298–1307. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S. Recycling of carbon fiber with epoxy composites by chemical recycling for future perspective: A review. Chem. Pap. 2020, 74, 3785–3807. [Google Scholar] [CrossRef]
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728. [Google Scholar] [CrossRef]
- Baker, D.A.; Gallego, N.C.; Baker, F.S. On the characterization and spinning of an organic-purified lignin toward the manufacture of low-cost carbon fiber. J. Appl. Polym. Sci. 2012, 124, 227–234. [Google Scholar] [CrossRef]
- Jody, B.J.; Pomykala, J.A.; Daniels, E.J.; Greminger, J.L. A process to recover carbon fibers from polymer-matrix composites in end-of-life vehicles. JOM 2004, 56, 43–47. [Google Scholar] [CrossRef]
- Ma, Y.; Navarro, C.A.; Williams, T.J.; Nutt, S.R. Recovery and reuse of acid digested amine/epoxy-based composite matrices. Polym. Degrad. Stab. 2020, 175, 109125. [Google Scholar] [CrossRef]
- Mohan, T.P.; Kanny, K. Reuse of Cured Epoxy as a Reinforcement in an Epoxy Composite. Polym. Eng. Sci. 2013, 53, 1839–1844. [Google Scholar] [CrossRef]
- Kizaki, T.; Zhang, J.; Yao, Q.; Yanagimoto, J. Continuous manufacturing of CFRP sheets by rolling for rapid fabrication of long CFRP products. Compos. Part B Eng. 2020, 189, 107896. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, M.; Quezada-Renteria, J.A.; Hou, Z.; Hoek, E.M.V. Sample preparation matters: Scanning electron microscopic characterization of polymeric membranes. J. Membr. Sci. Lett. 2024, 4, 100073. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Li, D.; Liu, Q.; Deng, B. A review: The effect of the microporous support during interfacial polymerization on the morphology and performances of a thin film composite membrane for liquid purification. RSC Adv. 2019, 9, 35417–35428. [Google Scholar] [CrossRef]
- Singh, P.S.; Joshi, S.V.; Trivedi, J.J.; Devmurari, C.V.; Rao, A.P.; Ghosh, P.K. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Hermans, S.; Bernstein, R.; Volodin, A.; Vankelecom, I.F.J. Study of synthesis parameters and active layer morphology of interfacially polymerized polyamide-polysulfone membranes. React. Funct. Polym. 2015, 86, 199–208. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M.V. Impacts of support membrane structure and chemistry on polyamide-polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Anderson, M.B. Next Generation Membranes for Selective Water Purification. UCLA. ProQuest ID: Anderson_ucla_0031D_20682. Merritt ID: Ark:/13030/m5s25685. 2022. Available online: https://escholarship.org/uc/item/9b51b580 (accessed on 11 July 2023).
- Sharabati, J.A.D.; Erkoc-Ilter, S.; Guclu, S.; Koseoglu-Imer, D.Y.; Unal, S.; Menceloglu, Y.Z.; Ozturk, I.; Koyuncu, I. Zwitterionic polysiloxane-polyamide hybrid active layer for high performance and chlorine resistant TFC desalination membranes. Sep. Purif. Technol. 2022, 282, 119965. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water. Mater Des 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Lim, J.; Gan, C.; Hu, X. Unraveling the Mechanistic Origins of Epoxy Degradation in Acids. ACS Omega 2019, 4, 10799–10808. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An approach to chemical recycling of epoxy resin cured with amine using nitric acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, M.B.; Danna, R.A.; French, C.; Wu, J.; Thiel, M.N.; Yang, Z.; Hoek, E.M.V.; Kaner, R.B. Monolithic Polyepoxide Membranes for Nanofiltration Applications and Sustainable Membrane Manufacture. Polymers 2024, 16, 2569. https://doi.org/10.3390/polym16182569
Anderson MB, Danna RA, French C, Wu J, Thiel MN, Yang Z, Hoek EMV, Kaner RB. Monolithic Polyepoxide Membranes for Nanofiltration Applications and Sustainable Membrane Manufacture. Polymers. 2024; 16(18):2569. https://doi.org/10.3390/polym16182569
Chicago/Turabian StyleAnderson, Mackenzie Babetta, Riley A. Danna, Clayton French, Jishan Wu, Markus N. Thiel, Zhiyin Yang, Eric M. V. Hoek, and Richard B. Kaner. 2024. "Monolithic Polyepoxide Membranes for Nanofiltration Applications and Sustainable Membrane Manufacture" Polymers 16, no. 18: 2569. https://doi.org/10.3390/polym16182569
APA StyleAnderson, M. B., Danna, R. A., French, C., Wu, J., Thiel, M. N., Yang, Z., Hoek, E. M. V., & Kaner, R. B. (2024). Monolithic Polyepoxide Membranes for Nanofiltration Applications and Sustainable Membrane Manufacture. Polymers, 16(18), 2569. https://doi.org/10.3390/polym16182569






